The Dietary Flavonol Kaempferol Inhibits Epstein–Barr Virus Reactivation in Nasopharyngeal Carcinoma Cells
Abstract
1. Introduction
2. Results
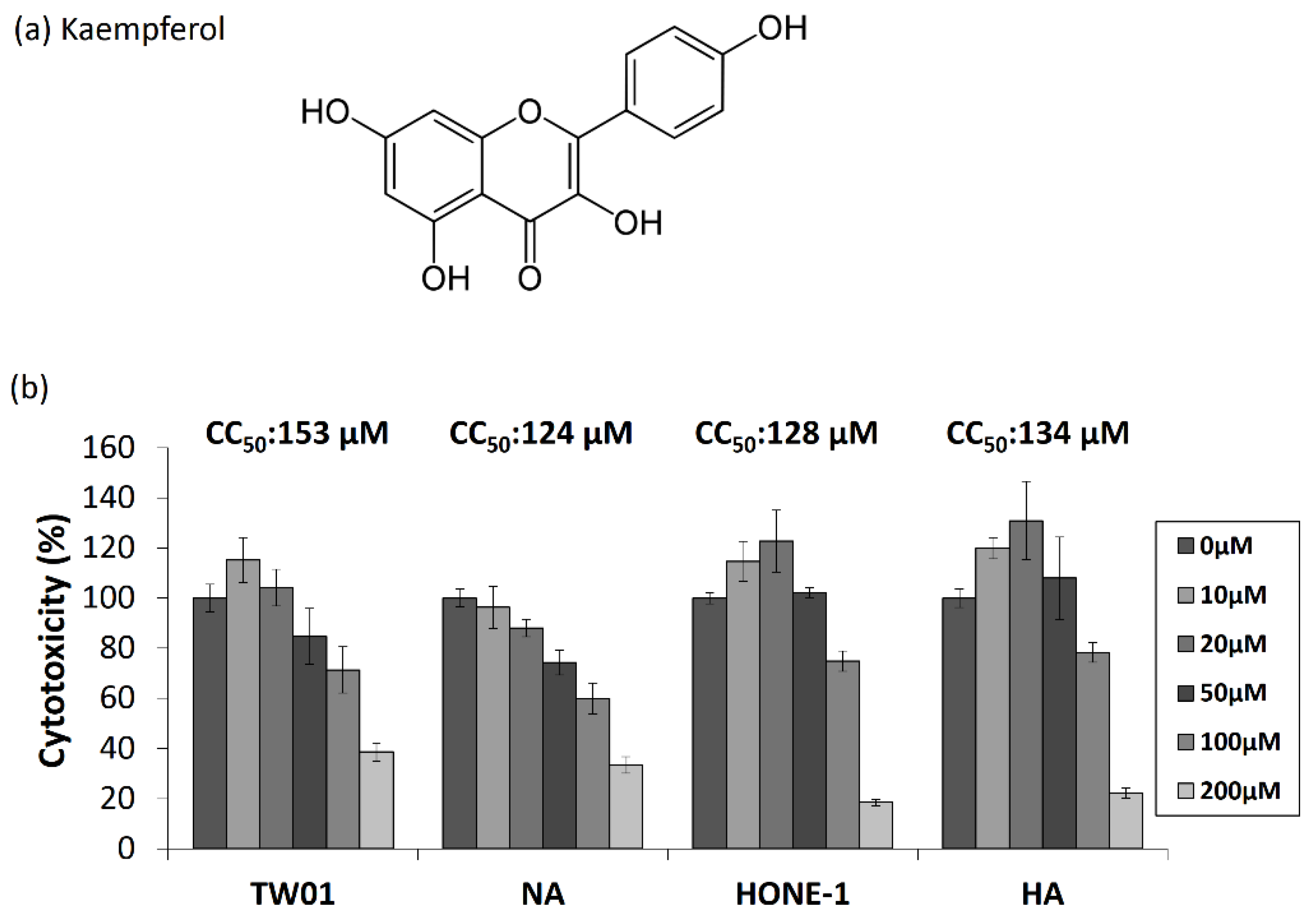
2.1. The Cytotoxic Effects of Kaempferol in EBV-Positive NPC Cell Lines
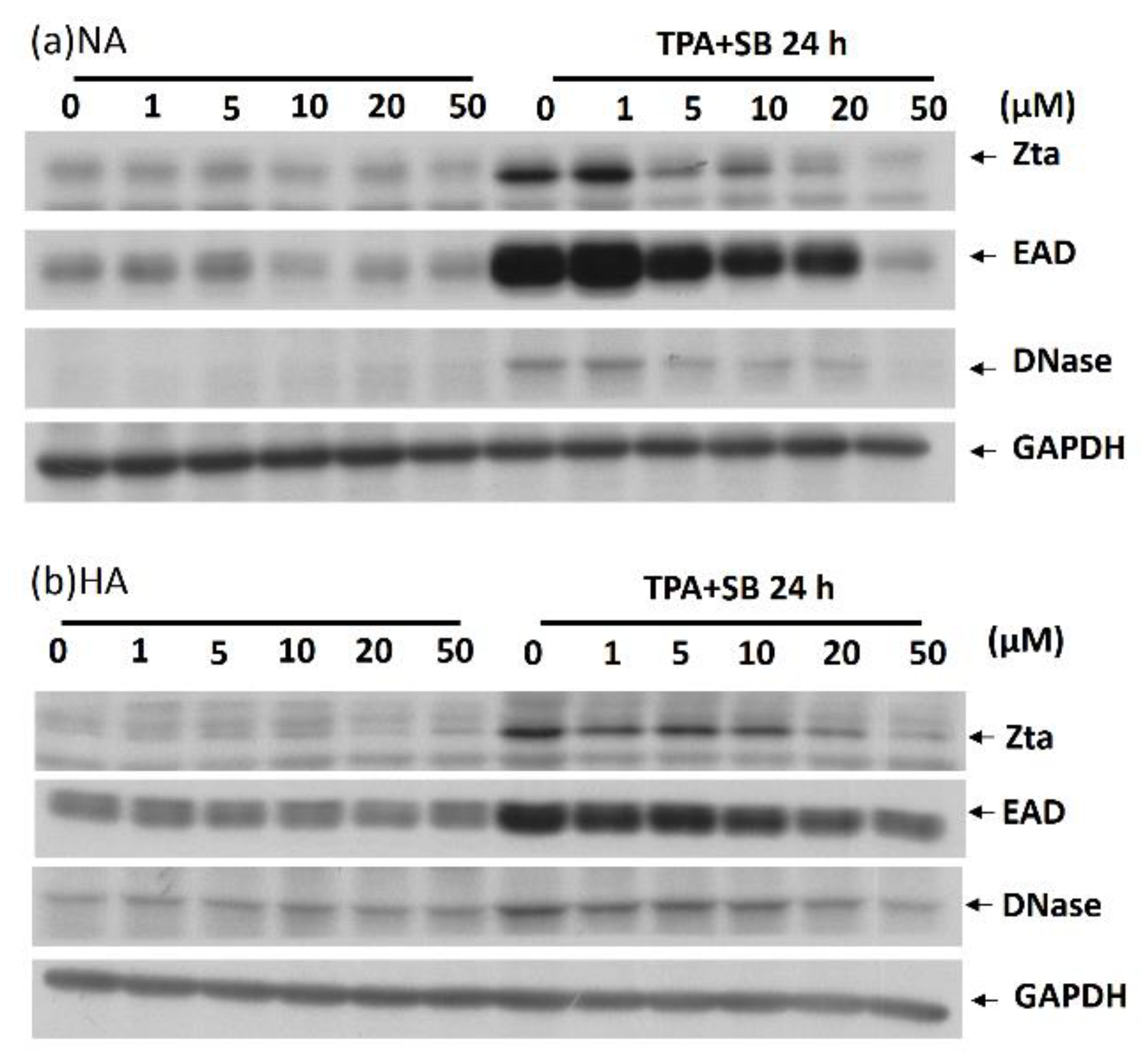
2.2. Kaempferol Treatment Inhibited EBV Lytic Protein Expression in NPC Cell Lines
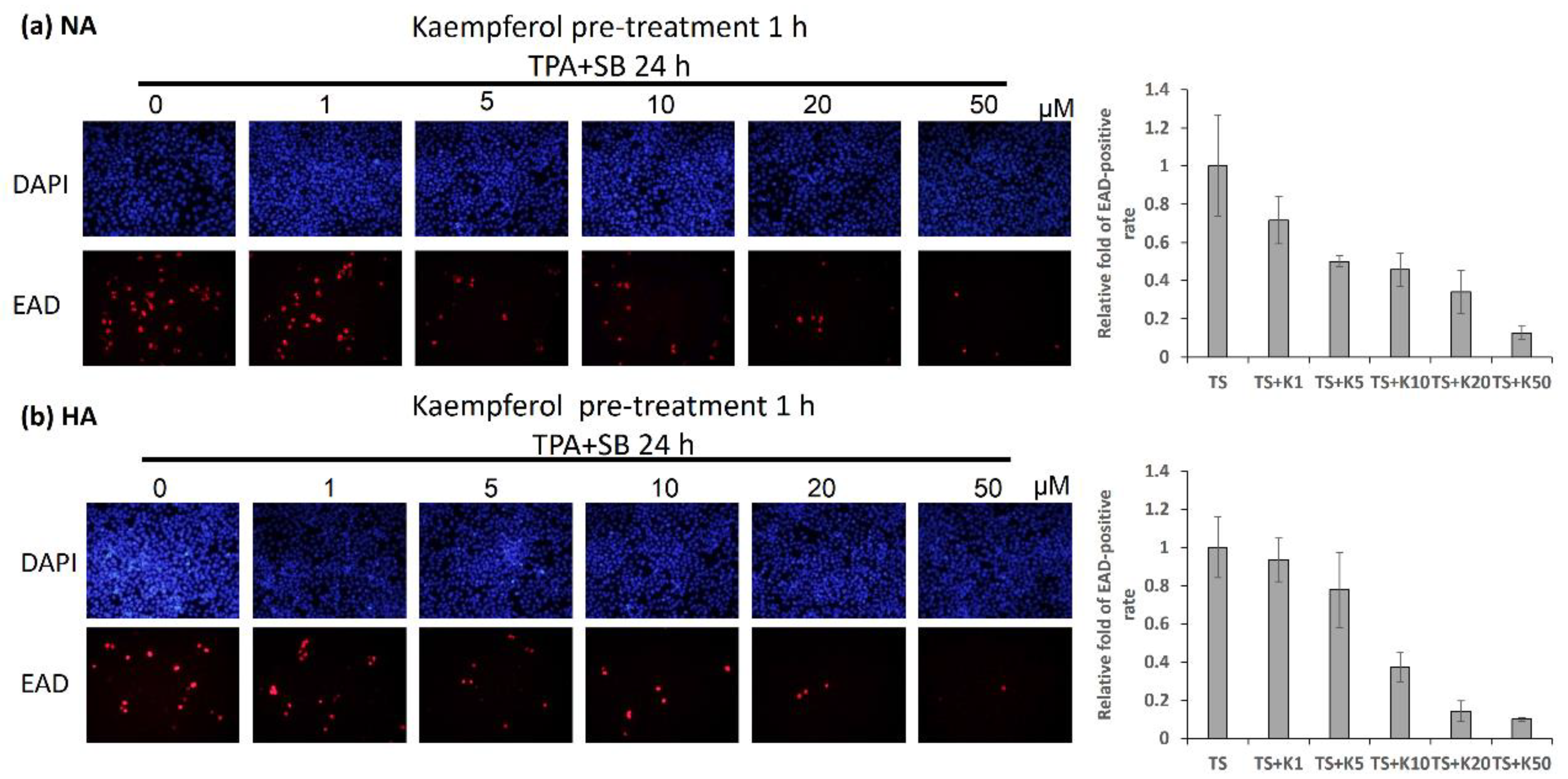
2.3. Kaempferol Treatment Decreased EAD-Expressing NPC Cells in Immunofluorescence (IFA) Analysis
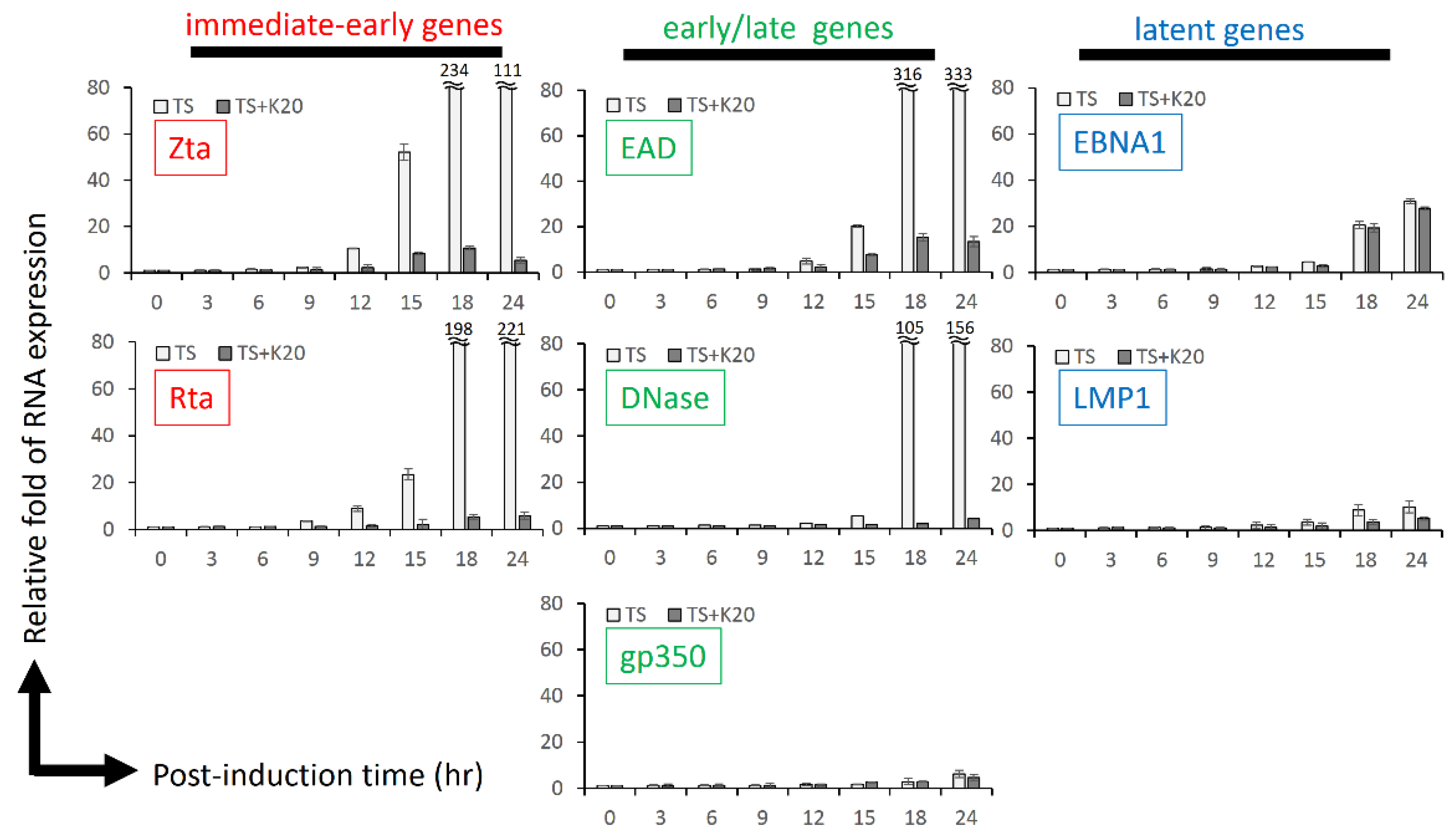
2.4. Kaempferol Treatment Inhibited EBV Reactivation in a Time-Course Analysis
2.5. Kaempferol Treatment Decreased Viral Production of EBV
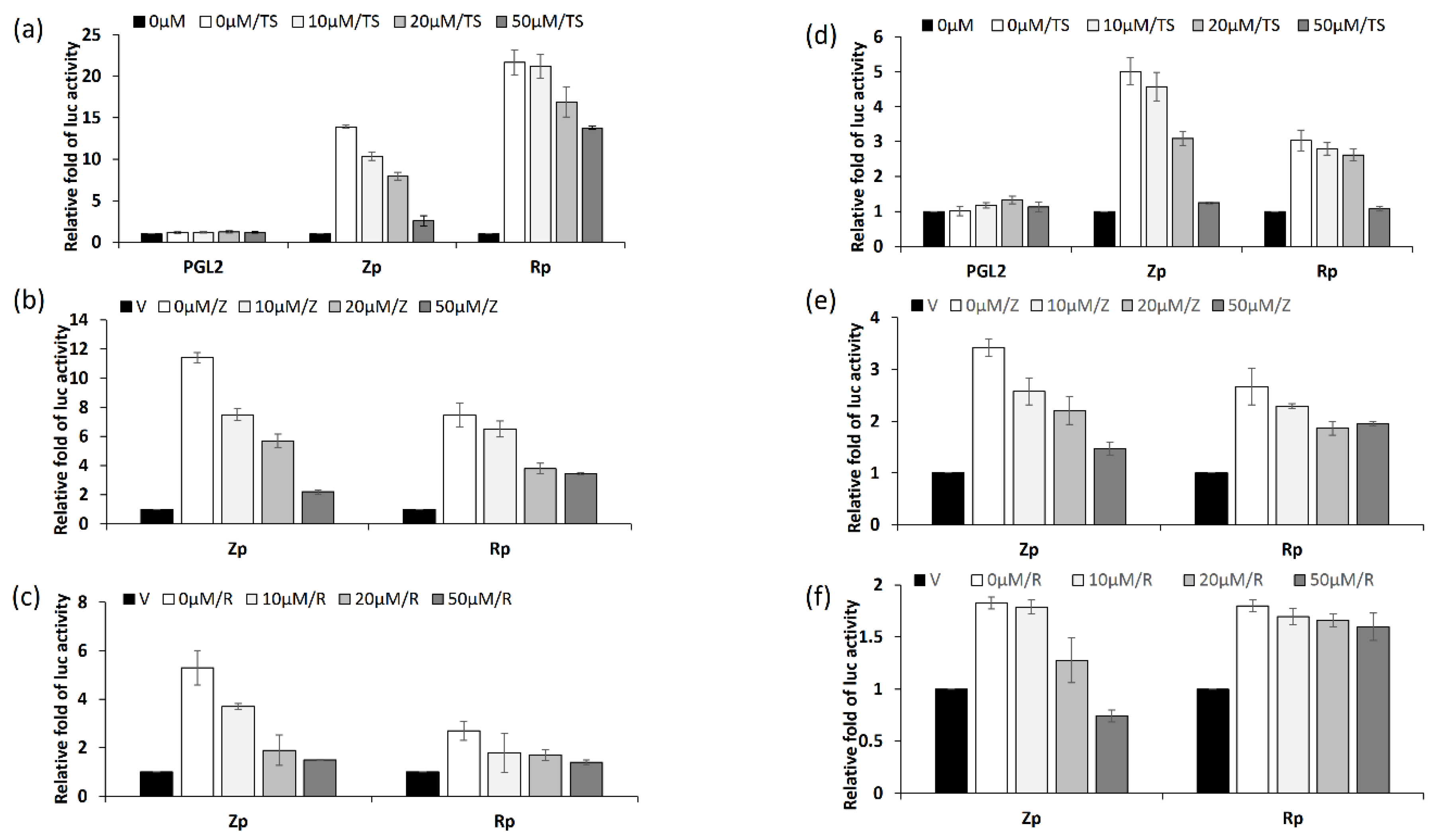
2.6. Kaempferol Repressed Promoter Activities of EBV Immediate-Early Genes
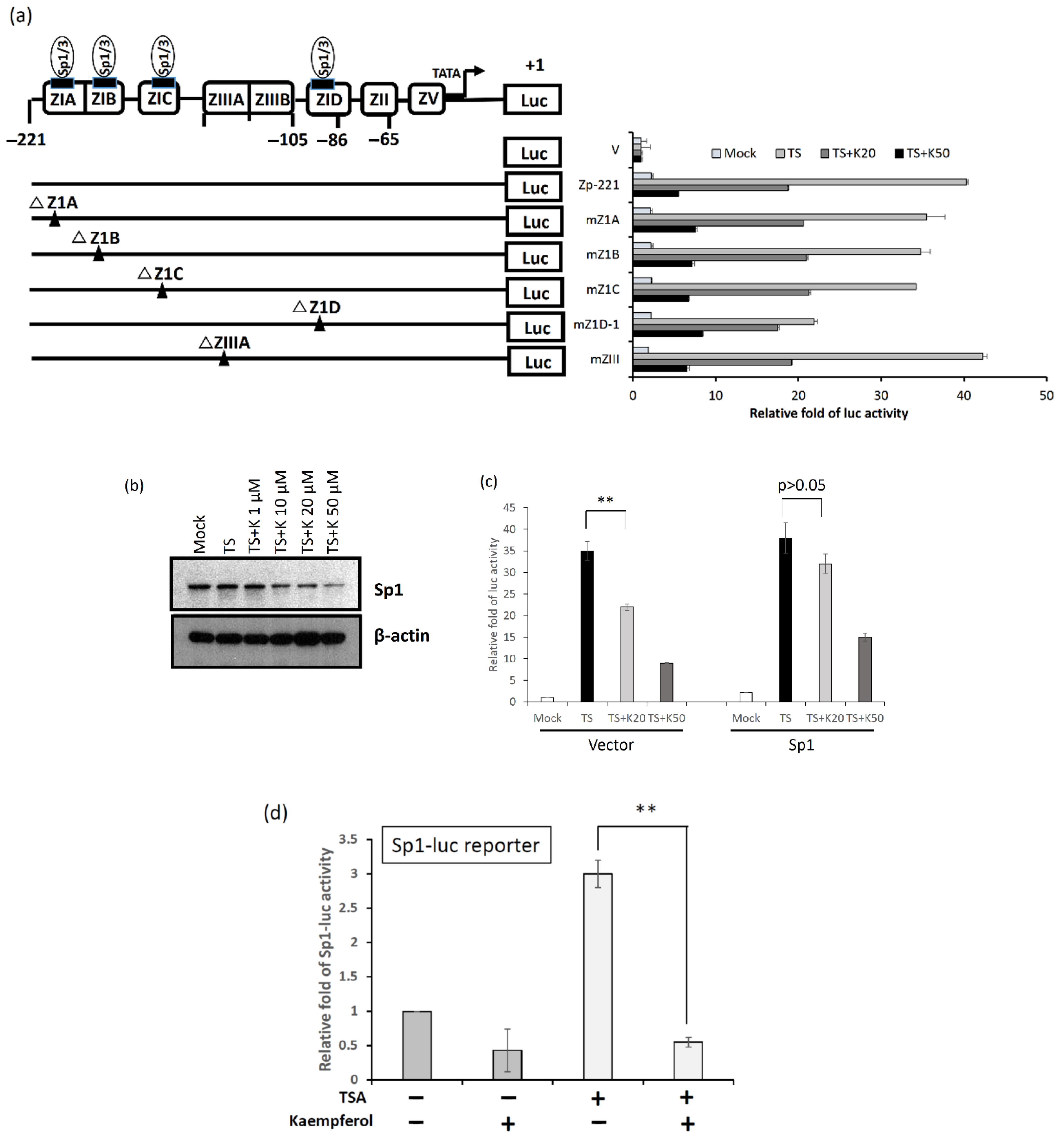
2.7. Kaempferol Inhibited Zta Promoter Activity through Repressing Sp1 Expression
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Chemicals and Antibodies
4.3. WST-1 Assay
4.4. EBV Induction and Kaempferol Treatment
4.5. Western Blotting
4.6. Luciferase Reporter Assay
4.7. The Detection of Copy Number of the EBV Genome
4.8. Quantitative PCR Analysis
4.9. Immunofluorescence Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdal Dayem, A.; Choi, H.Y.; Kim, Y.B.; Cho, S.G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Alzaabi, M.M.; Hamdy, R.; Ashmawy, N.S.; Hamoda, A.M.; Alkhayat, F.; Khademi, N.N.; Al Joud, S.M.A.; El-Keblawy, A.A.; Soliman, S.S.M. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 2022, 21, 291–312. [Google Scholar] [CrossRef]
- Bilger, A.; Plowshay, J.; Ma, S.; Nawandar, D.; Barlow, E.A.; Romero-Masters, J.C.; Bristol, J.A.; Li, Z.; Tsai, M.H.; Delecluse, H.J.; et al. Leflunomide/teriflunomide inhibit Epstein-Barr virus (EBV)-induced lymphoproliferative disease and lytic viral replication. Oncotarget 2017, 8, 44266–44280. [Google Scholar] [CrossRef] [PubMed]
- Care, C.; Sornjai, W.; Jaratsittisin, J.; Hitakarun, A.; Wikan, N.; Triwitayakorn, K.; Smith, D.R. Discordant Activity of Kaempferol towards Dengue Virus and Japanese Encephalitis Virus. Molecules 2020, 25, 1246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Tsai, W.H.; Chen, Y.L.; Ko, Y.C.; Chou, S.P.; Chen, J.Y.; Lin, S.F. Epstein-Barr virus (EBV) Rta-mediated EBV and Kaposi’s sarcoma-associated herpesvirus lytic reactivations in 293 cells. PLoS ONE 2011, 6, e17809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, Y.C. Potential use of antiviral L(-)nucleoside analogues for the prevention or treatment of viral associated cancers. Cancer Lett. 2001, 162 (Suppl. S1), S33–S37. [Google Scholar] [CrossRef]
- Chou, S.C.; Huang, T.J.; Lin, E.H.; Huang, C.H.; Chou, C.H. Antihepatitis B virus constituents of Solanum erianthum. Nat. Prod. Commun. 2012, 7, 153–156. [Google Scholar] [CrossRef]
- da Silva, F.M.A.; da Silva, K.P.A.; de Oliveira, L.P.M.; Costa, E.V.; Koolen, H.H.; Pinheiro, M.L.B.; de Souza, A.Q.L.; de Souza, A.D.L. Flavonoid glycosides and their putative human metabolites as potential inhibitors of the SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp). Mem. Inst. Oswaldo Cruz 2020, 115, e200207. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orru, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Disanto, G.; Meier, U.; Giovannoni, G.; Ramagopalan, S.V. Vitamin D: A link between Epstein-Barr virus and multiple sclerosis development? Expert Rev. Neurother. 2011, 11, 1221–1224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef]
- Evers, D.L.; Chao, C.F.; Wang, X.; Zhang, Z.; Huong, S.M.; Huang, E.S. Human cytomegalovirus-inhibitory flavonoids: Studies on antiviral activity and mechanism of action. Antiviral Res. 2005, 68, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Lee, C.H.; Wu, C.C.; Chang, Y.T.; Yu, S.L.; Chou, S.P.; Huang, P.T.; Chen, C.L.; Hou, J.W.; Chang, Y.; et al. Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int. J. Cancer 2009, 124, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Zhang, H.Y.; Yao, K.T.; Zhu, H.C.; Wang, F.X.; Li, G.Y.; Wen, D.S.; Li, Y.P. Two epithelial tumor cell lines (HNE-1 and HONE-1) latently infected with Epstein-Barr virus that were derived from nasopharyngeal carcinomas. Proc. Natl. Acad. Sci. USA 1989, 86, 9524–9528. [Google Scholar] [CrossRef] [PubMed]
- Gorres, K.L.; Daigle, D.; Mohanram, S.; McInerney, G.E.; Lyons, D.E.; Miller, G. Valpromide Inhibits Lytic Cycle Reactivation of Epstein-Barr Virus. mBio 2016, 7, e00113. [Google Scholar] [CrossRef]
- Greger, I.H.; Demarchi, F.; Giacca, M.; Proudfoot, N.J. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res. 1998, 26, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Hergenhahn, M.; Soto, U.; Weninger, A.; Polack, A.; Hsu, C.H.; Cheng, A.L.; Rosl, F. The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BZLF1 transcription in Raji DR-LUC cells. Mol. Carcinog. 2002, 33, 137–145. [Google Scholar] [CrossRef]
- Hong, M.H.; Chou, Y.C.; Wu, Y.C.; Tsai, K.N.; Hu, C.P.; Jeng, K.S.; Chen, M.L.; Chang, C. Transforming growth factor-beta1 suppresses hepatitis B virus replication by the reduction of hepatocyte nuclear factor-4alpha expression. PLoS ONE 2012, 7, e30360. [Google Scholar] [CrossRef]
- Huang, S.Y.; Fang, C.Y.; Wu, C.C.; Tsai, C.H.; Lin, S.F.; Chen, J.Y. Reactive oxygen species mediate Epstein-Barr virus reactivation by N-methyl-N’-nitro-N-nitrosoguanidine. PLoS ONE 2013, 8, e84919. [Google Scholar] [CrossRef]
- Huang, S.Y.; Wu, C.C.; Cheng, Y.J.; Chou, S.P.; Jiang, Y.J.; Chu, K.C.; Tsai, C.H.; Lin, S.F.; Chen, J.Y. Epstein-Barr virus BRLF1 induces genomic instability and progressive malignancy in nasopharyngeal carcinoma cells. Oncotarget 2017, 8, 78948–78964. [Google Scholar] [CrossRef]
- Javed, T.; Ashfaq, U.A.; Riaz, S.; Rehman, S.; Riazuddin, S. In-vitro antiviral activity of Solanum nigrum against Hepatitis C Virus. Virol. J. 2011, 8, 26. [Google Scholar] [CrossRef]
- Jones, K.A.; Tjian, R. Sp1 binds to promoter sequences and activates herpes simplex virus ‘immediate-early’ gene transcription in vitro. Nature 1985, 317, 179–182. [Google Scholar] [CrossRef]
- Kerr, J.R. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J. Clin. Pathol. 2019, 72, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Heng, W.; Wang, Y.; Qiu, J.; Wei, X.; Peng, S.; Saleem, S.; Khan, M.; Ali, S.S.; Wei, D.Q. In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS-CoV-2 main protease (3CLpro). Phytother. Res. 2021, 35, 2841–2845. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Wu, H.; Chen, S.; Zhu, Q.; Gao, H.; Yu, X.; Wang, Y.; Su, W.; Yao, X.; et al. Anti-herpes simplex virus type 1 activity of Houttuynoid A, a flavonoid from Houttuynia cordata Thunb. Antiviral Res. 2017, 144, 273–280. [Google Scholar] [CrossRef]
- Li, X.; Jin, F.; Lee, H.J.; Lee, C.J. Kaempferol Regulates the Expression of Airway MUC5AC Mucin Gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 Signaling Pathways. Biomol. Ther. 2021, 29, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Wong, C.I.; Chan, W.Y.; Tzung, K.W.; Ho, J.K.; Hsu, M.M.; Chuang, S.M. Establishment and characterization of two nasopharyngeal carcinoma cell lines. Lab. Investig. 1990, 62, 713–724. [Google Scholar] [PubMed]
- Liu, Z.; Zhao, J.; Li, W.; Shen, L.; Huang, S.; Tang, J.; Duan, J.; Fang, F.; Huang, Y.; Chang, H.; et al. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci. Rep. 2016, 6, 19095. [Google Scholar] [CrossRef]
- Longnecker, R.; Kieff, R.; Cohen, J.I. Epstein-Barr Virus, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Lung, J.; Lin, Y.S.; Yang, Y.H.; Chou, Y.L.; Shu, L.H.; Cheng, Y.C.; Liu, H.T.; Wu, C.Y. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J. Med. Virol. 2020, 92, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Flores, O. Binding of SP1 to the immediate-early protein-responsive element of the human cytomegalovirus DNA polymerase promoter. J. Virol. 1997, 71, 6683–6691. [Google Scholar] [CrossRef]
- Meerbach, A.; Holy, A.; Wutzler, P.; De Clercq, E.; Neyts, J. Inhibitory effects of novel nucleoside and nucleotide analogues on Epstein-Barr virus replication. Antivir. Chem. Chemother. 1998, 9, 275–282. [Google Scholar] [CrossRef]
- Mikirova, N.; Hunninghake, R. Effect of high dose vitamin C on Epstein-Barr viral infection. Med. Sci. Monit. 2014, 20, 725–732. [Google Scholar] [PubMed]
- Mitrocotsa, D.; Mitaku, S.; Axarlis, S.; Harvala, C.; Malamas, M. Evaluation of the antiviral activity of kaempferol and its glycosides against human cytomegalovirus. Planta Med. 2000, 66, 377–379. [Google Scholar] [CrossRef]
- Ortega, J.T.; Serrano, M.L.; Suarez, A.I.; Baptista, J.; Pujol, F.H.; Cavallaro, L.V.; Campos, H.R.; Rangel, H.R. Antiviral activity of flavonoids present in aerial parts of Marcetia taxifolia against Hepatitis B virus, Poliovirus, and Herpes Simplex Virus in vitro. EXCLI J. 2019, 18, 1037–1048. [Google Scholar]
- Ozcelik, B.; Orhan, I.; Toker, G. Antiviral and antimicrobial assessment of some selected flavonoids. Z. Naturforsch. C J. Biosci. 2006, 61, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Parvez, M.K.; Ahmed, S.; Al-Dosari, M.S.; Abdelwahid, M.A.S.; Arbab, A.H.; Al-Rehaily, A.J.; Al-Oqail, M.M. Novel Anti-Hepatitis B Virus Activity of Euphorbia schimperi and Its Quercetin and Kaempferol Derivatives. ACS Omega 2021, 6, 29100–29110. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Rickinson, A.B.; Kieff, E. Epstein-Barr Virus, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Rojas, A.; Del Campo, J.A.; Clement, S.; Lemasson, M.; Garcia-Valdecasas, M.; Gil-Gomez, A.; Ranchal, I.; Bartosch, B.; Bautista, J.D.; Rosenberg, A.R.; et al. Effect of Quercetin on Hepatitis C Virus Life Cycle: From Viral to Host Targets. Sci. Rep. 2016, 6, 31777. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef]
- Schwarz, S.; Sauter, D.; Wang, K.; Zhang, R.; Sun, B.; Karioti, A.; Bilia, A.R.; Efferth, T.; Schwarz, W. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014, 80, 177–182. [Google Scholar] [CrossRef]
- Tsai, C.H.; Liu, M.T.; Chen, M.R.; Lu, J.; Yang, H.L.; Chen, J.Y.; Yang, C.S. Characterization of monoclonal antibodies to the Zta and DNase proteins of Epstein-Barr virus. J. Biomed. Sci. 1997, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Williams, M.V.; Glaser, R. Characterization of two monoclonal antibodies to Epstein-Barr virus diffuse early antigen which react to two different epitopes and have different biological function. J. Virol. Methods 1991, 33, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.J.; Lin, C.W.; Lai, C.C.; Lan, Y.C.; Lai, C.H.; Hung, C.H.; Hsueh, K.C.; Lin, T.H.; Chang, H.C.; Wan, L.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef]
- Wang, F.Z.; Roy, D.; Gershburg, E.; Whitehurst, C.B.; Dittmer, D.P.; Pagano, J.S. Maribavir inhibits epstein-barr virus transcription in addition to viral DNA replication. J. Virol. 2009, 83, 12108–12117. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chen, M.C.; Chang, Y.R.; Hsu, T.Y.; Chen, J.Y. Identification and characterization of the conserved nucleoside-binding sites in the Epstein-Barr virus thymidine kinase. Biochem. J. 2004, 379, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chen, M.S.; Cheng, Y.J.; Ko, Y.C.; Lin, S.F.; Chiu, I.M.; Chen, J.Y. Emodin Inhibits EBV Reactivation and Represses NPC Tumorigenesis. Cancers 2019, 11, 1795. [Google Scholar] [CrossRef]
- Wu, C.C.; Chuang, H.Y.; Lin, C.Y.; Chen, Y.J.; Tsai, W.H.; Fang, C.Y.; Huang, S.Y.; Chuang, F.Y.; Lin, S.F.; Chang, Y.; et al. Inhibition of Epstein-Barr virus reactivation in nasopharyngeal carcinoma cells by dietary sulforaphane. Mol. Carcinog. 2013, 52, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Fang, C.Y.; Cheng, Y.J.; Hsu, H.Y.; Chou, S.P.; Huang, S.Y.; Tsai, C.H.; Chen, J.Y. Inhibition of Epstein-Barr virus reactivation by the flavonoid apigenin. J. Biomed. Sci. 2017, 24, 2. [Google Scholar] [CrossRef]
- Wu, C.C.; Fang, C.Y.; Hsu, H.Y.; Chen, Y.J.; Chou, S.P.; Huang, S.Y.; Cheng, Y.J.; Lin, S.F.; Chang, Y.; Tsai, C.H.; et al. Luteolin inhibits Epstein-Barr virus lytic reactivation by repressing the promoter activities of immediate-early genes. Antivir. Res. 2016, 132, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Fang, C.Y.; Hsu, H.Y.; Chuang, H.Y.; Chen, Y.J.; Chou, S.P.; Huang, S.Y.; Cheng, Y.J.; Lin, S.F.; Chang, Y.; et al. EBV reactivation as a target of luteolin to repress NPC tumorigenesis. Oncotarget 2016, 7, 18999–19017. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Liu, M.T.; Chang, Y.T.; Fang, C.Y.; Chou, S.P.; Liao, H.W.; Kuo, K.L.; Hsu, S.L.; Chen, Y.R.; Wang, P.W.; et al. Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res. 2010, 38, 1932–1949. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, S.; Huang, Q.; Ming, Z.; Wang, M.; Li, R.; Zhao, Y. Kaempferol attenuates liver fibrosis by inhibiting activin receptor-like kinase 5. J. Cell. Mol. Med. 2019, 23, 6403–6410. [Google Scholar] [CrossRef]
- Ye, J.; Shedd, D.; Miller, G. An Sp1 response element in the Kaposi’s sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J. Virol. 2005, 79, 1397–1408. [Google Scholar] [CrossRef]
- Yiu, C.Y.; Chen, S.Y.; Chang, L.K.; Chiu, Y.F.; Lin, T.P. Inhibitory effects of resveratrol on the Epstein-Barr virus lytic cycle. Molecules 2010, 15, 7115–7124. [Google Scholar] [CrossRef]
- Yiu, S.P.T.; Dorothea, M.; Hui, K.F.; Chiang, A.K.S. Lytic Induction Therapy against Epstein-Barr Virus-Associated Malignancies: Past, Present, and Future. Cancers 2020, 12, 2142. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, Z.; Li, X.; Zhang, X. Kaempferol’s Protective Effect on Ethanol-Induced Mouse Primary Hepatocytes Injury Involved in the Synchronous Inhibition of SP1, Hsp70 and CYP2E1. Am. J. Chin. Med. 2018, 46, 1093–1110. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.G.; Lee, J.; Yang, W.S.; Park, G.W.; Kim, H.G.; Yi, Y.S.; Baek, K.S.; Sung, N.Y.; Hossen, M.J.; et al. The dietary flavonoid Kaempferol mediates anti-inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediat. Inflamm. 2015, 2015, 904142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Lee, M.; Son, M.; Ryu, E.; Shin, Y.S.; Kim, J.G.; Kang, B.W.; Cho, H.; Kang, H. Quercetin-induced apoptosis prevents EBV infection. Oncotarget 2015, 6, 12603–12624. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.H.; Shin, Y.S.; Kang, H.; Cho, H. The anti-HSV-1 effect of quercetin is dependent on the suppression of TLR-3 in Raw 264.7 cells. Arch. Pharm. Res. 2017, 40, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, R.; Hu, H.; Chen, X.; Yin, Z.; Liang, X.; He, C.; Yin, L.; Ye, G.; Zou, Y.; et al. The antiviral activity of kaempferol against pseudorabies virus in mice. BMC Vet. Res. 2021, 17, 247. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Oligonucleotide Sequence (5′→3′) | |
|---|---|---|
| Zta | Forward | 5′-GAGTCAACATCCAGGCTTGG-3′ |
| Reverse | 5′-GCAGCACTACCGTGAGGTG-3′ | |
| Rta | Forward | 5′AGAGACCAGAGAGCCCAGC-3′ |
| Reverse | 5′-ACACAAACAGACGCAGATGAG-3′ | |
| EAD | Forward | 5′-AGAGACACCCTCGCCTGC-3′ |
| Reverse | 5′-GCTTCTGCTTCTGCCTGG-3′ | |
| DNase | Forward | 5′-AGAAGGCCGTCACAATGTTC-3′ |
| Reverse | 5′-TCCACGGCACAACTACTTCT-3′ | |
| gp350 | Forward | 5′-TTTCTGTGCCGTTGTCCC-3′ |
| Reverse | 5′-CCCCACTGTATCCACCGC-3′ | |
| EBNA1 | Forward | 5′-GACAAAGCCCGCTCCTACCT-3′ |
| Reverse | 5′-AGCCCCTTCCACCATAGGTG-3′ | |
| LMP1 | Forward | 5′-GGGTCGTCATCATCTCCACC-3′ |
| Reverse | 5′-CCACACCTTCCTACGCTGC-3′ | |
| GAPDH | Forward | 5′-CCTGCCAAATATTGATGACATCAAG-3′ |
| Reverse | 5′-ACCCTGTTGCTGTAGCCAAA-3′ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Lee, T.-Y.; Cheng, Y.-J.; Cho, D.-Y.; Chen, J.-Y. The Dietary Flavonol Kaempferol Inhibits Epstein–Barr Virus Reactivation in Nasopharyngeal Carcinoma Cells. Molecules 2022, 27, 8158. https://doi.org/10.3390/molecules27238158
Wu C-C, Lee T-Y, Cheng Y-J, Cho D-Y, Chen J-Y. The Dietary Flavonol Kaempferol Inhibits Epstein–Barr Virus Reactivation in Nasopharyngeal Carcinoma Cells. Molecules. 2022; 27(23):8158. https://doi.org/10.3390/molecules27238158
Chicago/Turabian StyleWu, Chung-Chun, Ting-Ying Lee, Yu-Jhen Cheng, Der-Yang Cho, and Jen-Yang Chen. 2022. "The Dietary Flavonol Kaempferol Inhibits Epstein–Barr Virus Reactivation in Nasopharyngeal Carcinoma Cells" Molecules 27, no. 23: 8158. https://doi.org/10.3390/molecules27238158
APA StyleWu, C.-C., Lee, T.-Y., Cheng, Y.-J., Cho, D.-Y., & Chen, J.-Y. (2022). The Dietary Flavonol Kaempferol Inhibits Epstein–Barr Virus Reactivation in Nasopharyngeal Carcinoma Cells. Molecules, 27(23), 8158. https://doi.org/10.3390/molecules27238158






