Effects of Through-Bond and Through-Space Conjugations on the Photoluminescence of Small Aromatic and Aliphatic Aldimines
Abstract
1. Introduction
2. Results and Discussion
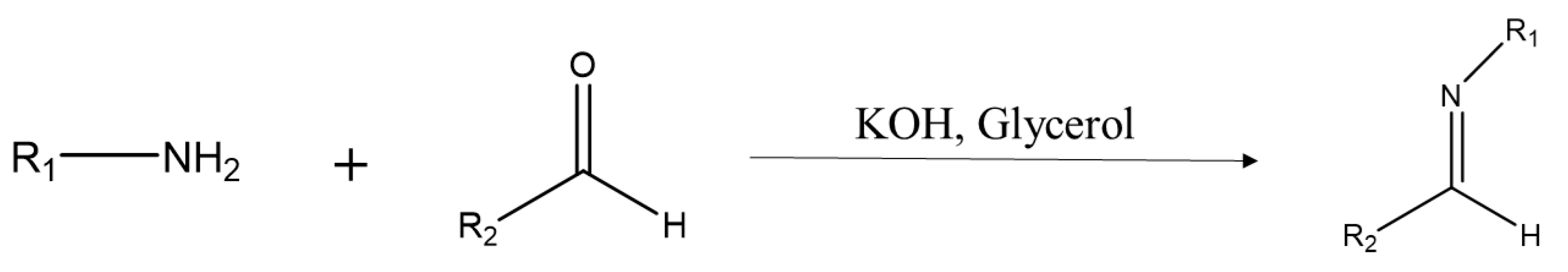
2.1. Synthesis and Characterization of Aldimines
2.2. Photoluminescence Behaviors of Aldimines
2.3. PL Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Aldimines
3.3. Characterization
3.4. Fluorescence Spectroscopy
3.5. Theoretical Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, H.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, S.; Wen, Y.; Zheng, S.; Yang, B.; Yuan, W.Z. Nonconventional luminophores with unprecedented efficiencies and color-tunable afterglows. Mater. Horiz. 2020, 7, 215–2112. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Klajnert-Maculewicz, B.; Johnson, K.A.M.; Brinkman, H.F.; Janaszewska, A.; Hedstrand, D.M. Non-traditional intrinsic luminescence: Inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Prog. Polym. Sci. 2019, 90, 35–117. [Google Scholar] [CrossRef]
- Zhang, H.K.; Zhao, Z.; McGonigal, P.R.; Ye, R.Q.; Liu, S.J.; Lam, J.W.Y.; Kwok, R.T.K.; Yuan, W.Z.; Xie, J.P.; Rogach, A.L.; et al. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 2020, 32, 275–292. [Google Scholar] [CrossRef]
- Tang, S.; Yang, T.; Zhao, Z.; Zhu, T.; Zhang, Q.; Hou, W.; Yuan, W.Z. Nonconventional luminophores: Characteristics, advancements and perspectives. Chem. Soc. Rev. 2021, 50, 12616–12655. [Google Scholar] [CrossRef]
- Lee, W.I.; Bae, Y.J.; Bard, A.J. Strong blue photoluminescence and ECL from OH-terminated PAMAM dendrimers in the absence of gold nanoparticles. J. Am. Chem. Soc. 2004, 126, 8358–8359. [Google Scholar] [CrossRef]
- Xing, C.M.; Lam, J.W.Y.; Qin, A.J.; Dong, Y.Q.; Haussler, M.; Yang, W.T.; Tang, B.Z. Unique photoluminescence from nonconjugated alternating copolymer poly[(maleic anhydride)-alt-(vinyl acetate). Polym. Mater. Sci. Eng. 2007, 96, 418–419. [Google Scholar]
- Pucci, A.; Rausa, R.; Ciardelli, F. Aggregation-induced luminescence of polyisobutene succinic anhydrides and imides. Macromol. Chem. Phys. 2008, 209, 900–906. [Google Scholar] [CrossRef]
- Gong, Y.; Tan, Y.; Mei, J.; Zhang, Y.; Yuan, W.; Zhang, Y.; Sun, J.; Tang, B.Z. Room temperature phosphorescence from natural products: Crystallization matters. Sci. China-Chem. 2013, 56, 1178–1182. [Google Scholar] [CrossRef]
- Lu, H.; Feng, L.; Li, S.; Zhang, J.; Lu, H.; Feng, S. Unexpected strong blue photoluminescence produced from the aggregation of unconventional chromophores in novel siloxane-poly(amidoamine) dendrimers. Macromolecules 2015, 48, 476–482. [Google Scholar] [CrossRef]
- Shang, C.; Wei, N.; Zhuo, H.; Shao, Y.; Zhang, Q.; Zhang, Z.; Wang, H. Highly emissive poly(maleic anhydride-alt-vinyl pyrrolidone) with molecular weight-dependent and excitation-dependent fluorescence. J. Mater. Chem. C 2017, 5, 8082–8090. [Google Scholar] [CrossRef]
- Zhen, S.J.; Mao, J.C.; Chen, L.; Ding, S.Y.; Luo, W.W.; Zhou, X.S.; Qin, A.J.; Zhao, Z.J.; Tang, B.Z. Remarkable multichannel conductance of novel single-molecule wires built on through-space conjugated hexaphenylbenzene. Nano Lett. 2018, 18, 4200–4205. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, G.F.; Zhang, B.H.; Zhu, D.X.; Su, Z.M.; Bryce, M.R. Aggregation-induced long-lived phosphorescence in nonconjugated polyurethane derivatives at 77 K. Macromolecules 2018, 51, 4178–4184. [Google Scholar] [CrossRef]
- Bai, L.; Yan, H.; Bai, T.; Feng, Y.; Zhao, Y.; Ji, Y.; Feng, W.; Lu, T.; Nie, Y. High fluorescent hyperbranched polysiloxane containing beta-cyclodextrin for cell imaging and drug delivery. Biomacromolecules 2019, 20, 4230–4240. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, Y.; Cao, P.; Wu, P. Color-tunable ultralong room temperature phosphorescence from EDTA. Chem. Commun. 2021, 57, 3575–3578. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Zhao, Y.; Long, J.; Ji, Y.; Wang, H. Orange-red and white-emitting nonconventional luminescent polymers containing cyclic acid anhydride and lactam groups. J. Mater. Chem. C 2020, 8, 1017–1024. [Google Scholar] [CrossRef]
- Deng, J.; Wu, H.; Xie, W.; Jia, H.; Xia, Z.; Wang, H. Metal cation-responsive and excitation-dependent nontraditional multicolor fluorescent hydrogels for multidimensional information encryption. Acs Appl. Mater. Interfaces 2021, 13, 39967–39975. [Google Scholar] [CrossRef]
- Deng, J.; Jia, H.; Xie, W.; Wu, H.; Li, J.; Wang, H. Nontraditional organic/polymeric luminogens with red-shifted fluorescence emissions. Macromol. Chem. Phys. 2022, 223, 2100425. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, B.Z. Through-space interactions in clusteroluminescence. JACS Au 2021, 1, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Cram, D.J.; Steinberg, H. Macro rings. I. Preparation and spectra of the paracyclophanes. J. Am. Chem. Soc. 1952, 73, 5691–5704. [Google Scholar] [CrossRef]
- Cram, D.J.; Allinger, N.L.; Steinberg, H. Macro rings. VII. The spectral consequences of bringing two benzene rings face. J. Am. Chem. Soc. 1952, 76, 6132–6141. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Xie, N.; He, Z.; Liu, J.; Leung, N.L.C.; Niu, Y.; Huang, X.; Wong, K.S.; Kwok, R.T.K.; et al. Why do simple molecules with "isolated" phenyl rings emit visible light? J. Am. Chem. Soc. 2017, 139, 16264–16272. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, L.; Zhang, K.; Liu, J.; Li, X.; Wang, H.; Wang, Z.; Sung, H.H.Y.; Williams, I.D.; Zeng, Z.; et al. How to manipulate through-space conjugation and clusteroluminescence of simple aiegens with isolated phenyl rings. J. Am. Chem. Soc. 2021, 143, 9565–9574. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, W.; Nie, H.; Xu, L.; Hu, R.; Zhao, Z.; Qin, A.; Tang, B.Z. Oligo(maleic anhydride)s: A platform for unveiling the mechanism of clusteroluminescence of non-aromatic polymers. J. Mater. Chem. C 2017, 5, 4775–4779. [Google Scholar] [CrossRef]
- Zhou, Q.; Cao, B.; Zhu, C.; Xu, S.; Gong, Y.; Yuan, W.Z.; Zhang, Y. Clustering-triggered emission of nonconjugated polyacrylonitrile. Small 2016, 12, 6586–6592. [Google Scholar] [CrossRef]
- Han, T.; Hong, Y.; Xie, N.; Chen, S.; Zhao, N.; Zhao, E.; Lam, J.W.Y.; Sung, H.H.Y.; Dong, Y.; Tong, B.; et al. Defect-sensitive crystals based on diaminomaleonitrile-functionalized Schiff base with aggregation-enhanced emission. J. Mater. Chem. C 2013, 1, 7314–7320. [Google Scholar] [CrossRef]
- Chai, J.; Wu, Y.; Yang, B.; Liu, B. The photochromism, light harvesting and self-assembly activity of a multi-function Schiff-base compound based on the AIE effect. J. Mater. Chem. C 2018, 6, 4057–4064. [Google Scholar] [CrossRef]
- Liu, H.; Lu, Z.; Tang, B.; Zhang, Z.; Wang, Y.; Zhang, H. AIE-active organic polymorphs displaying molecular conformation-dependent amplified spontaneous emissions (ASE). Dyes Pigments 2018, 149, 284–289. [Google Scholar] [CrossRef]
- Lee, K.; Choi, S.; Yang, C.; Wu, H.C.; Yu, J. Autofluorescence generation and elimination: A lesson from glutaraldehyde. Chem. Commun. 2013, 49, 3028–3030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bai, Y.; Zhuang, P.; Zhao, Y.; Wang, H. Photoluminescence behaviors of small aliphatic ketimines. Sci. Sin. Chim. 2022, 52, 980–988. [Google Scholar] [CrossRef]
- Bürgi, H.B.; Dunitz, J.D. Crystal and Molecular structures of benzylideneaniline, benzylideneaniline-p-carboxylic acid and p-methylbenzylidene-p-nitroaniline. Helv. Chim. Acta 1970, 53, 1747–1764. [Google Scholar] [CrossRef]
- Azua, A.; Finn, M.; Yi, H.; Beatriz Dantas, A.; Voutchkova-Kostal, A. Transfer hydrogenation from glycerol: Activity and recyclability of iridium and ruthenium sulfonate-functionalized n-heterocyclic carbene catalysts. ACS Sustain. Chem. Eng. 2017, 5, 3963–3972. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis set exchange: A community database for computational sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]

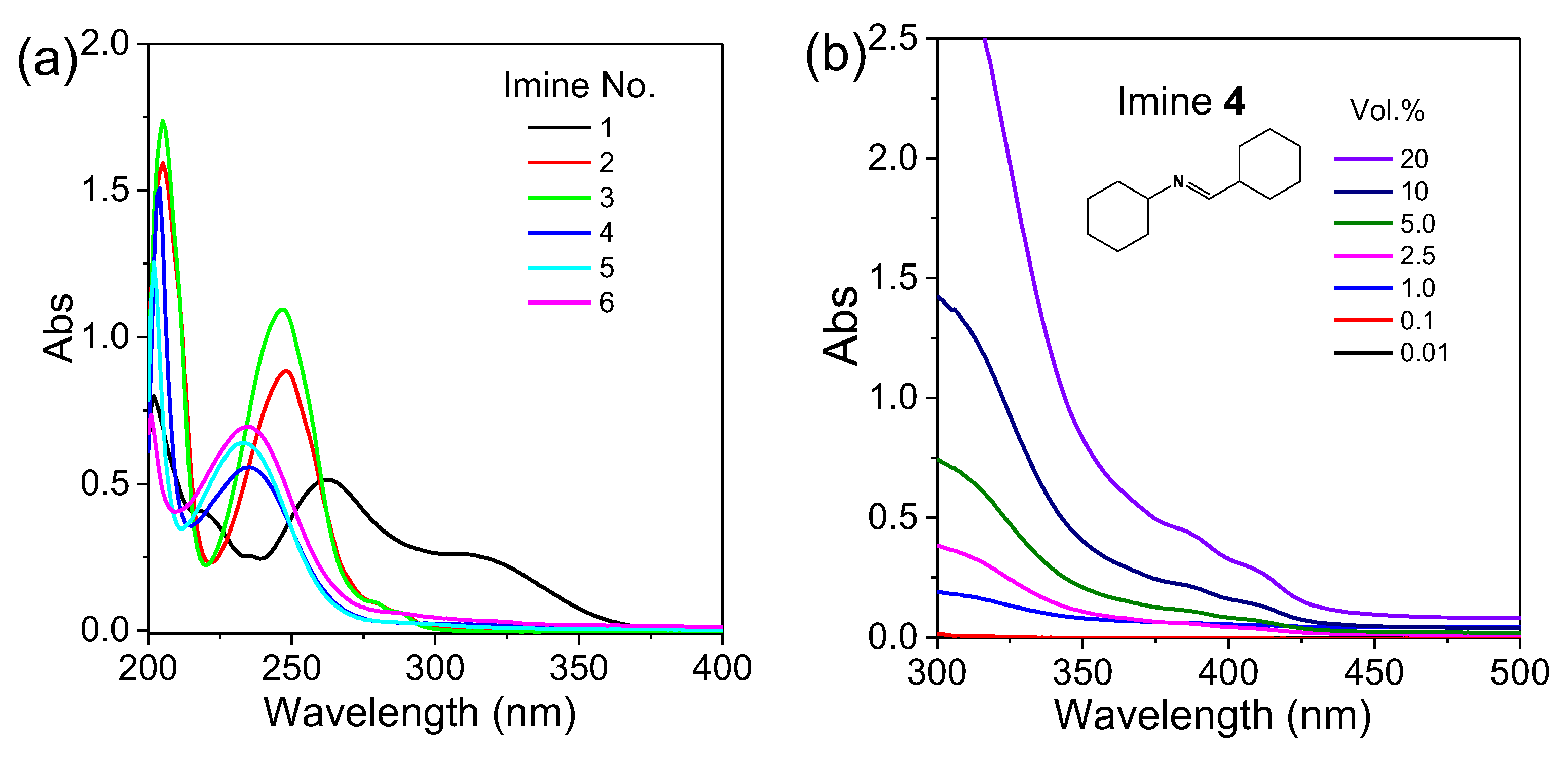
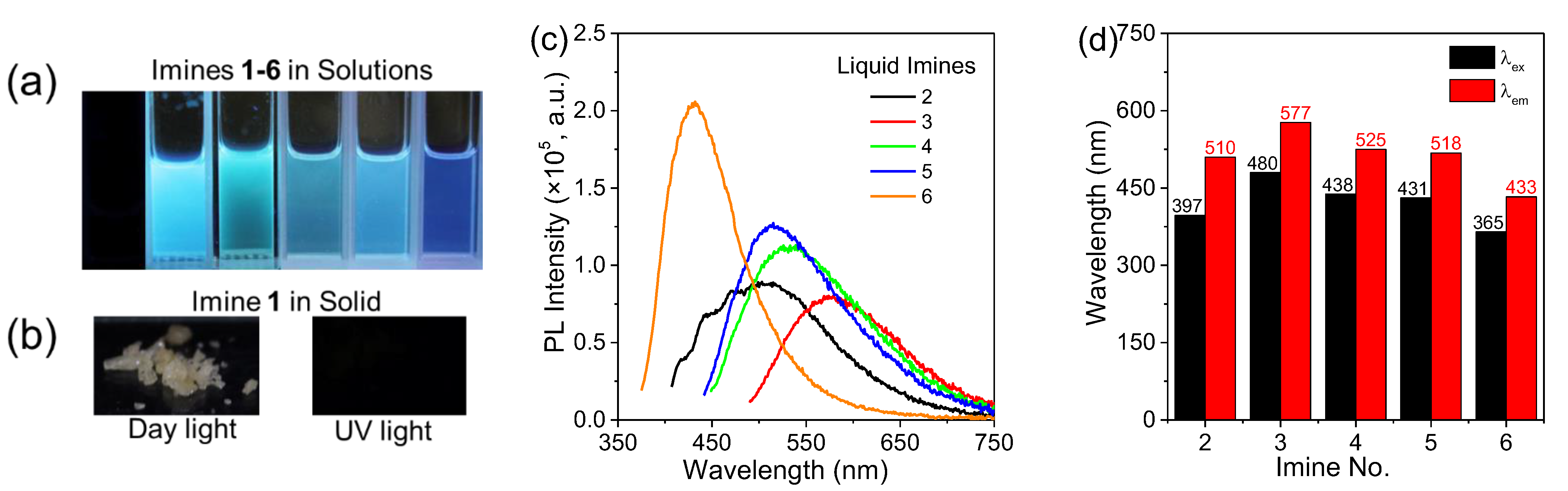
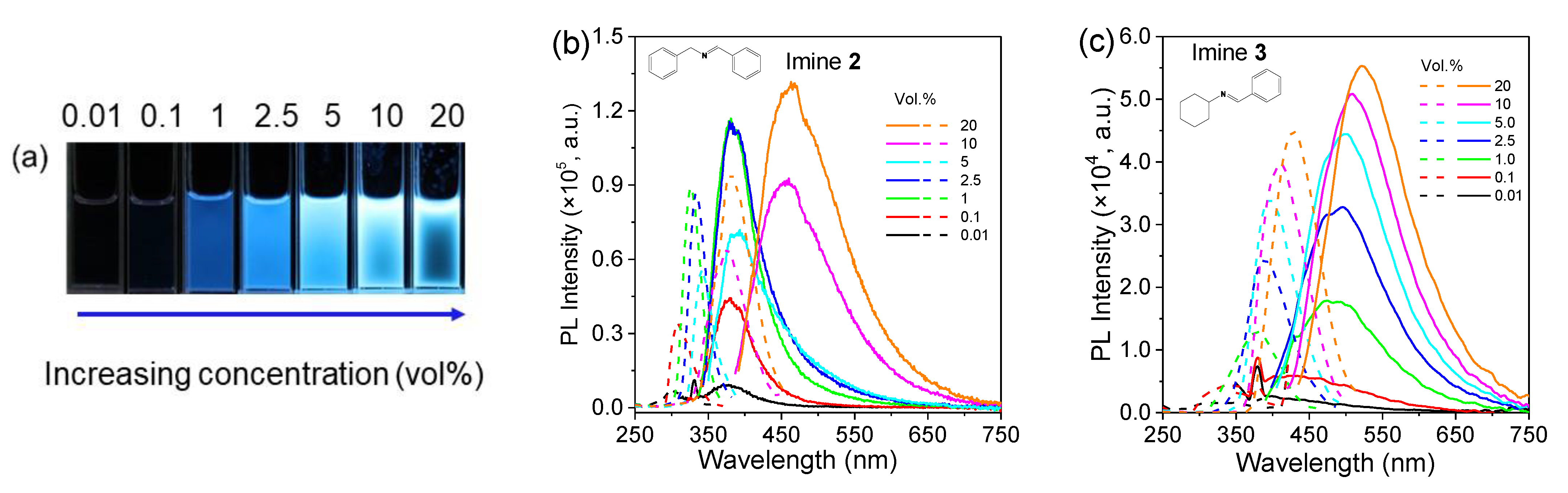
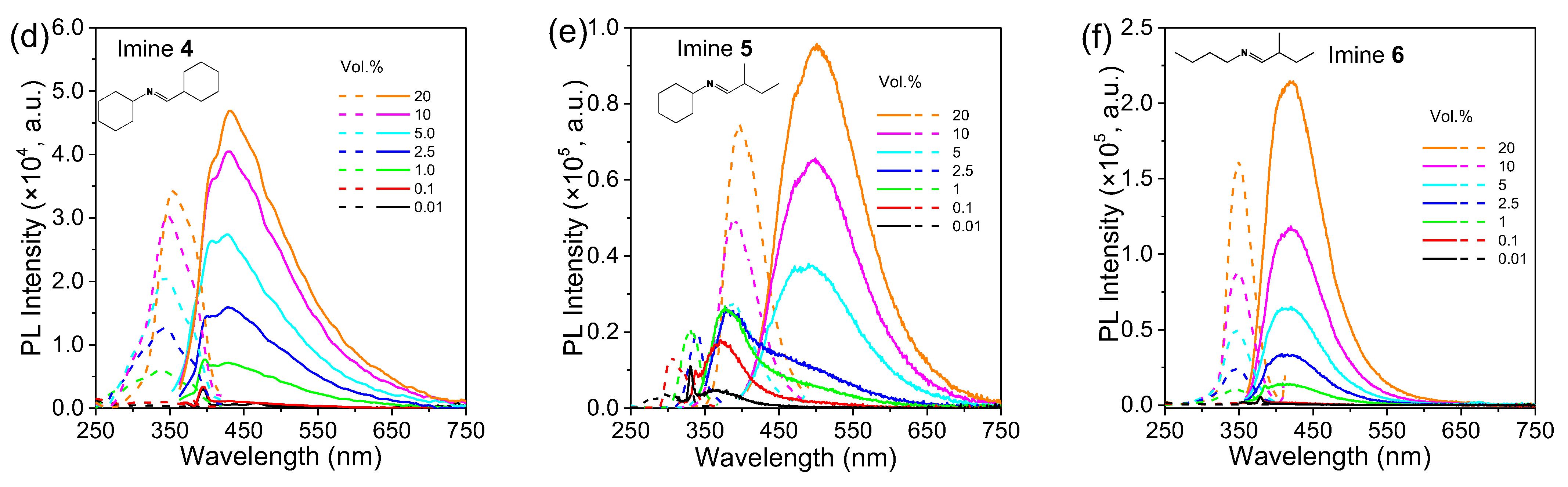
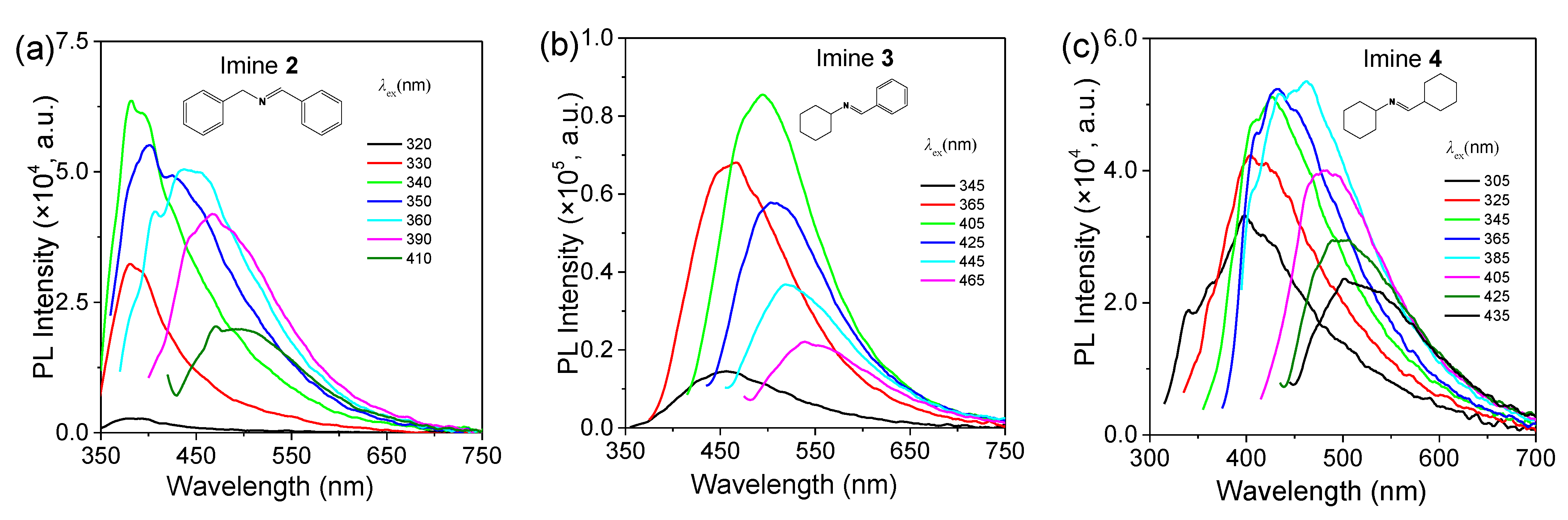


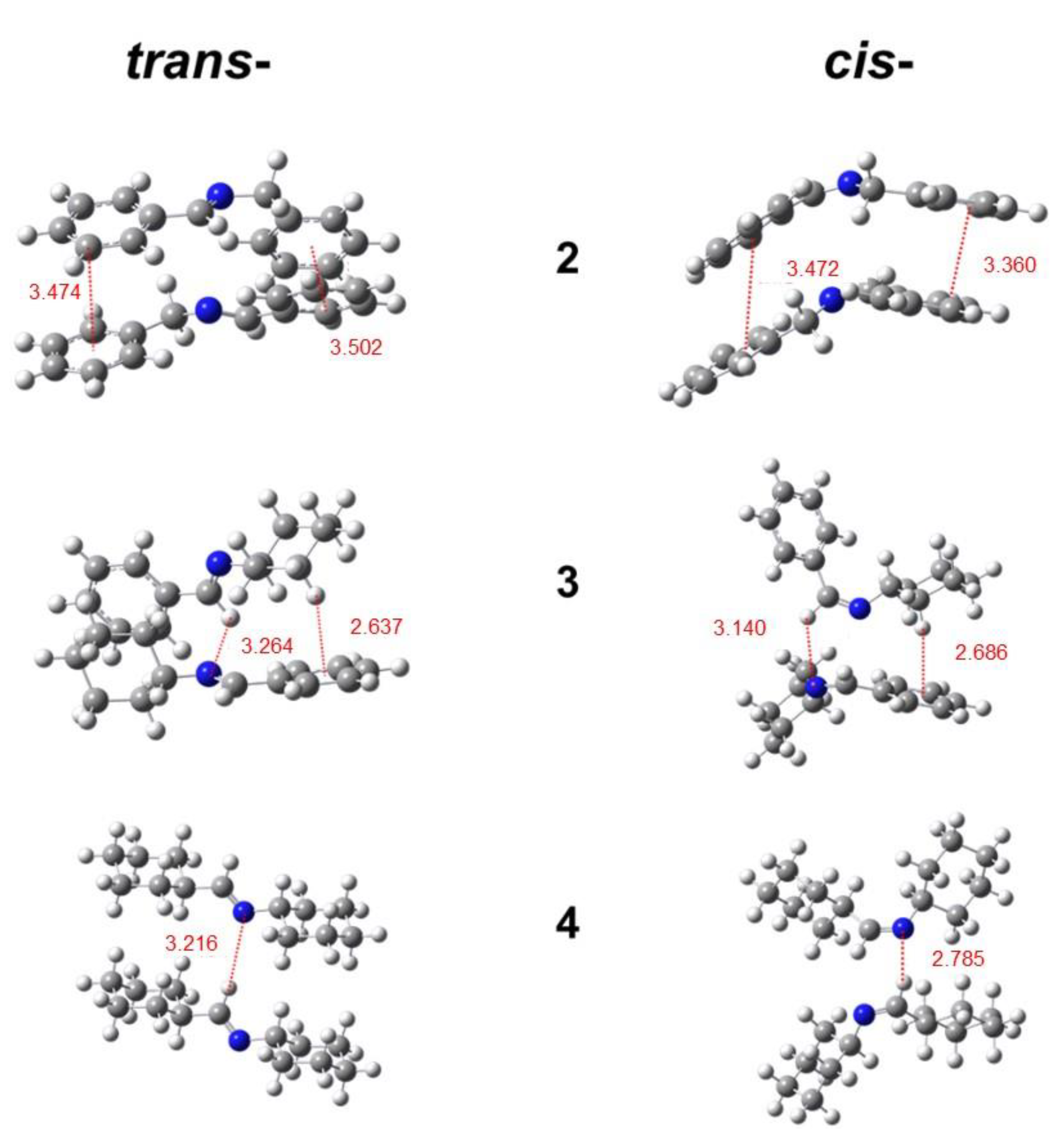
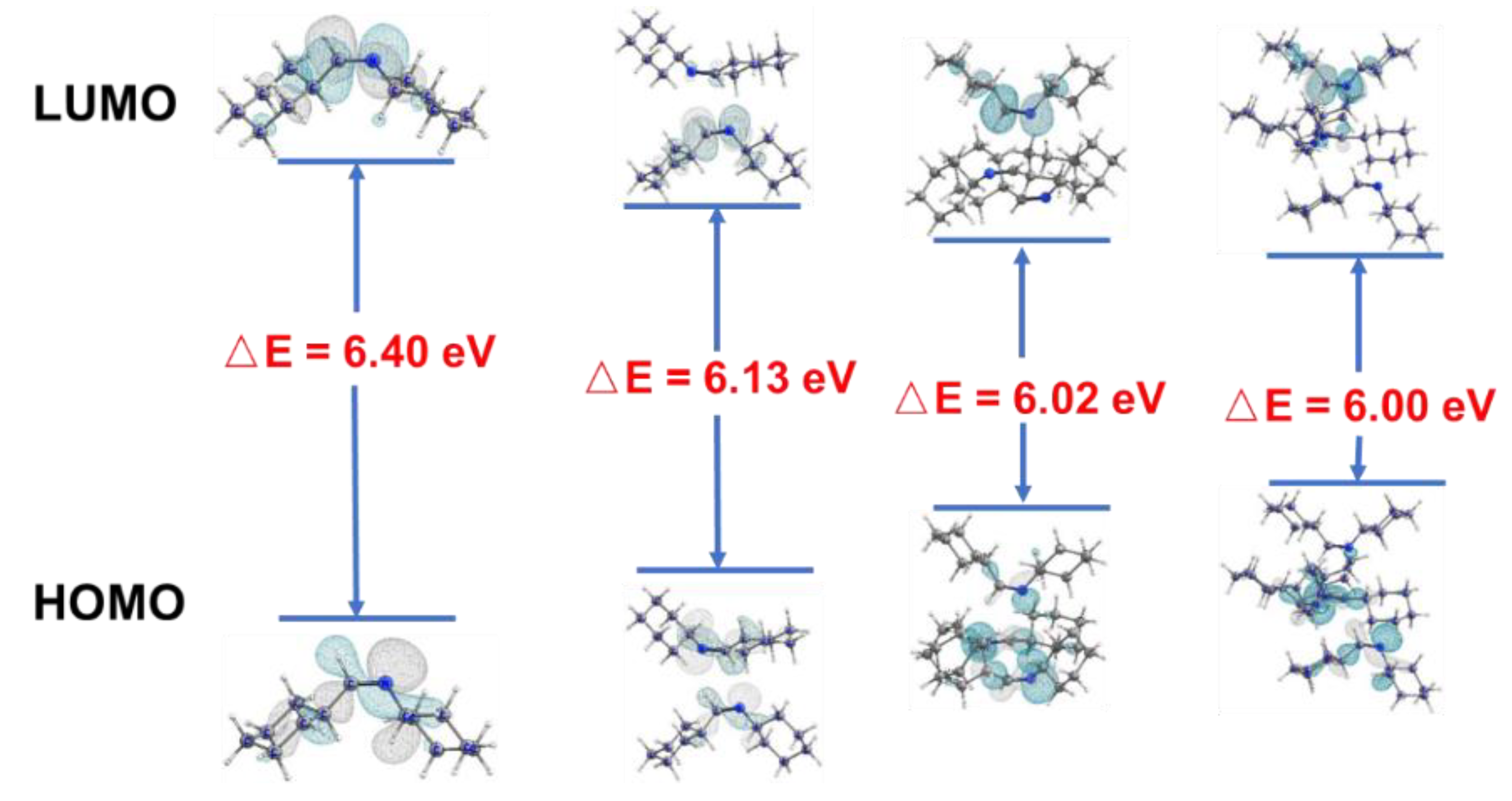
| Imine No. | Solution (0.1 vol.%) λem (nm) | Solution (20 vol.%) λem (nm) | Pure Liquid λem (nm) | Solution Φ (%) |
|---|---|---|---|---|
| 2 | 378 | 467 | 510 | 2.0 |
| 3 | 426 | 523 | 577 | 1.1 |
| 4 | 428 | 434 | 525 | 0.8 |
| 5 | 370 | 501 | 518 | 1.7 |
| 6 | 416 | 421 | 433 | 2.8 |
| Imine No. | Monomer (eV) | Dimer (eV) | Trimer (eV) | Tetramer (eV) |
|---|---|---|---|---|
| 2 | 5.12 | 5.04 | 4.84 | 4.76 |
| 3 | 5.40 | 5.13 | 5.11 | 4.96 |
| 4 | 6.40 | 6.13 | 6.02 | 6.00 |
| 5 | 6.48 | 6.18 | 6.09 | 5.91 |
| 6 | 6.51 | 6.09 | 6.08 | 5.94 |
| Imine No. | R1 | R2 | t (h) | T (°C) | Appearance |
|---|---|---|---|---|---|
| 1 | phenyl | phenyl | 3 | 120 | yellow, s |
| 2 | benzyl | phenyl | 1 | 120 | yellow, l |
| 3 | cyclohexyl | phenyl | 3 | 120 | brown, l |
| 4 | cyclohexyl | cyclohexyl | 4 | r. t. | brown, l |
| 5 | cyclohexyl | isobutyl | 4 | r. t | brown, l |
| 6 | n-butyl | isobutyl | 12 | r. t | colorless, l |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, P.; Yuan, C.; Bai, Y.; He, C.; Long, J.; Tan, H.; Wang, H. Effects of Through-Bond and Through-Space Conjugations on the Photoluminescence of Small Aromatic and Aliphatic Aldimines. Molecules 2022, 27, 8046. https://doi.org/10.3390/molecules27228046
Zhuang P, Yuan C, Bai Y, He C, Long J, Tan H, Wang H. Effects of Through-Bond and Through-Space Conjugations on the Photoluminescence of Small Aromatic and Aliphatic Aldimines. Molecules. 2022; 27(22):8046. https://doi.org/10.3390/molecules27228046
Chicago/Turabian StyleZhuang, Peifeng, Chang Yuan, Yunhao Bai, Changcheng He, Jiayu Long, Hongwei Tan, and Huiliang Wang. 2022. "Effects of Through-Bond and Through-Space Conjugations on the Photoluminescence of Small Aromatic and Aliphatic Aldimines" Molecules 27, no. 22: 8046. https://doi.org/10.3390/molecules27228046
APA StyleZhuang, P., Yuan, C., Bai, Y., He, C., Long, J., Tan, H., & Wang, H. (2022). Effects of Through-Bond and Through-Space Conjugations on the Photoluminescence of Small Aromatic and Aliphatic Aldimines. Molecules, 27(22), 8046. https://doi.org/10.3390/molecules27228046







