A Series of Green Oxovanadium(IV) Precatalysts with O, N and S Donor Ligands in a Sustainable Olefins Oligomerization Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Oxovanadium(IV) Precatalysts
2.2. Analysis of FTIR Spectra of Olefin Oligomers
2.3. MALDI-TOF-MS Spectra Analysis of the Oligomers
2.3.1. Reaction of Oligomerization Catalyzed by [VO(TDA)phen] • 1.5 H2O
2.3.2. Reaction of Oligomerization Catalyzed by [VOO(dipic)(2-phepyH)] • H2O
2.3.3. Reaction of Oligomerization Catalyzed by [VO(dipic)(dmbipy)] • 2 H2O
2.3.4. Reaction of Oligomerization Catalyzed by [VO(ODA)bipy] • 2 H2O
2.4. Thermogravimetric Analysis (TGA) of the Oligomers
2.4.1. Reactions of Oligomerization Catalyzed by [VO(TDA)phen] • 1.5 H2O
2.4.2. Reactions of Oligomerization Catalyzed by [VOO(dipic)(2-phepyH)] • H2O
2.4.3. Reactions of Oligomerization Catalyzed by [VO(dipic)(dmbipy)] • 2 H2O
2.4.4. Reactions of Oligomerization Catalyzed by [VO(ODA)bipy] • 2 H2O
2.5. Differential Scanning Calorimetry (DSC) Analysis of the Oligomers
2.5.1. Reactions of Oligomerization Catalyzed by [VO(TDA)phen] • 1.5 H2O
2.5.2. Reactions of Oligomerization Catalyzed by [VOO(dipic)(2-phepyH)] • H2O
2.5.3. Reactions of Oligomerization Catalyzed by [VO(dipic)(dmbipy)] • 2 H2O
2.5.4. Reactions of Oligomerization Catalyzed by [VO(ODA)bipy] • 2 H2O
2.6. Catalytic Activity of Green Precatalysts Based on Oxovanadium(IV) Coordination Compounds
3. Materials and Methods
3.1. Materials
3.2. Elemental Analysis
3.3. FTIR Spectra
3.4. MALDI-TOF-MS Spectra
3.5. Thermogravimetric Analysis (TGA)
3.6. The Differential Scanning Calorimetry (DSC)
3.7. Synthesis of Oxovanadium(IV) Coordination Complex Compounds
3.7.1. Synthesis of [VO(TDA)phen] • 1.5 H2O
3.7.2. Synthesis of Oxovanadium(IV) Cage (Oxovanadium(IV) Microclusters with 2-phenylpyridine)
3.7.3. Synthesis of [VOO(dipic)(2-phepyH)] • H2O
3.7.4. Synthesis of [VO(dipic)(dmbipy)] • 2 H2O
3.7.5. Synthesis of [VO(ODA)bipy] • 2 H2O
3.8. Oligomerization Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, G.; Zhang, X.; Zhao, M.; Wang, L.; Jing, C.; Wang, P.; Wang, X.; Wang, Q. Influences of Fluorine Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers 2018, 10, 934. [Google Scholar] [CrossRef] [PubMed]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The Influence of Ziegler-Natta and Metallocene Catalysts on Polyolefin Structure, Properties, and Processing Ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Sauter, D.W.; Taoufik, M.; Boisson, C. Polyolefins, a Success Story. Polymers 2017, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef]
- Kurtz, M.S. The UHMWPE Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement, 1st ed.; Elsevier Academic Press: New York, NY, USA, 2004; ISBN 9780080481463. [Google Scholar]
- Serenko, O.A.; Buzin, M.I.; Tuskaev, V.A.; Gagieva, S.C.; Kolosov, N.A.; Kurmaev, D.A.; Savel’eva, T.F.; Golubev, E.K.; Zubkevich, S.V.; Vasil’ev, V.G.; et al. A Novel Ziegler–Natta-Type Catalytic System—TiCl4/2,2′-Dimethoxy-1,1′-Binaphthalene/Et3Al2Cl3/Bu2Mg for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solvent-Free Processing. Polymers 2018, 10, 1281. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Mink, R.I.; Brandolini, A.J.; Nowlin, T.E. AlR2Cl/MgR2 combinations as universal cocatalysts for Ziegler–Natta, metallocene, and post-metallocene catalysts. J. Polym. Sci. A 2009, 47, 3271–3285. [Google Scholar] [CrossRef]
- Seenivasan, K.; Sommazzi, A.; Bonino, F.; Bordiga, S.; Groppo, E. Spectroscopic Investigation of Heterogeneous Ziegler–Natta Catalysts: Ti and Mg Chloride Tetrahydrofuranates, Their Interaction Compound, and the Role of the Activator. Chem. Eur. J. 2011, 17, 8648–8656. [Google Scholar] [CrossRef]
- Murdzek, J.S.; Schrock, R.R. Well-characterized olefin metathesis catalysts that contain molybdenum. Organomet 1987, 6, 1373–1374. [Google Scholar] [CrossRef]
- Scholl, M.; Ding, S.; Lee, C.W.; Grubbs, R.H. Synthesis and Activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands. Org. Lett. Publication Date: 13 August 1999. 1999, 1, 953–956. [Google Scholar] [CrossRef]
- Hanik, N.; Kilbinger, A.F.M. Telechelic Polymers. In Handbook of Metathesis, 2nd ed.; Grubbs, R.H., Wenzel, A.G., O’Leary, D.J., Khosravi, E., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Volume 3. [Google Scholar]
- Guillaume, S.M. Handbook of Telechelic Polyesters, Polycarbonates and Polyethers; Pan Stanford Publishing Pte. Ltd.: Singapore, 2017. [Google Scholar]
- Chung, T.C. Functionalization of Polyolefins; Academic Press: London, UK, 2002. [Google Scholar]
- Antonova, A.S.; Vinokurova, M.A.; Kumandin, P.A.; Merkulova, N.L.; Sinelshchikova, A.A.; Grigoriev, M.S.; Novikov, R.A.; Kouznetsov, V.V.; Polyanskii, K.B.; Zubkov, F.I. Application of New Efficient Hoveyda–Grubbs Catalysts Comprising an N→Ru Coordinate Bond in a Six-Membered Ring for the Synthesis of Natural Product-Like Cyclopenta[b]furo [2,3-c]pyrroles. Molecules 2020, 25, 5379. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Kolosov, N.A.; Mikhaylik, E.S.; Golubev, E.K.; Sizov, A.I.; Zubkevich, S.V.; Vasil’ev, V.G.; Nikiforova, G.G.; et al. Titanium(III, IV)-Containing Catalytic Systems for Production of Ultrahigh Molecular Weight Polyethylene Nascent Reactor Powders, Suitable for Solventless Processing—Impact of Oxidation States of Transition Metal. Polymers 2018, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Ronca, S.; Rastogi, S. A Hemi-metallocene Chromium Catalyst with Trimethylaluminum-Free Methylaluminoxane for the Synthesis of Disentangled Ultra-High Molecular Weight Polyethylene. Macromol. Rapid Commun. 2015, 36, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Tuskaev, V.A.; Gagieva, S.C.; Maleev, V.I.; Borissova, A.O.; Solov’ev, M.V.; Starikova, Z.A.; Bulychev, B.M. Titanium (IV) and zirconium (IV) chloride complexes on the base of chiral tetraaryl-1,3-dioxolane-4,5-dimetanol ligands in the polymerization of ethylene: The promoting role of lithium and magnesium chloride. Polymer 2013, 54, 4455–4462. [Google Scholar] [CrossRef]
- Del Sole, R.; Mele, G.; Bloise, E.; Mergola, L. Green Aspects in Molecularly Imprinted Polymers by Biomass Waste Utilization. Polymers 2021, 13, 2430. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Kim, Y.T.; Lee, J. Recent Advances in Renewable Polymer Production from Lignin-Derived Aldehydes. Polymers 2021, 13, 364. [Google Scholar] [CrossRef]
- Grigora, M.-E.; Terzopoulou, Z.; Tsongas, K.; Klonos, P.; Kalafatakis, N.; Bikiaris, D.N.; Kyritsis, A.; Tzetzis, D. Influence of Reactive Chain Extension on the Properties of 3D Printed Poly(Lactic Acid) Constructs. Polymers 2021, 13, 1381. [Google Scholar] [CrossRef]
- Viveiros, R.; Rebocho, S.; Casimiro, T. Green Strategies for Molecularly Imprinted Polymer Development. Polymers 2018, 10, 306. [Google Scholar] [CrossRef]
- Lima, R.C.; Carvalho, A.P.A.d.; Vieira, C.P.; Moreira, R.V.; Conte-Junior, C.A. Green and Healthier Alternatives to Chemical Additives as Cheese Preservative: Natural Antimicrobials in Active Nanopackaging/Coatings. Polymers 2021, 13, 2675. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, B.; Zhong, W.; Fu, Z.; Fan, Z. Modification of the Acyl Chloride Quench-Labeling Method for Counting Active Sites in Catalytic Olefin Polymerization. Catalysts 2021, 11, 683. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, P.; Zhang, Y.; Zan, X.; Zhu, W.; Jiang, Y.; Zhang, L.; Yasin, A. Improved Ozonation Efficiency for Polymerization Mother Liquid from Polyvinyl Chloride Production Using Tandem Reactors. Molecules 2019, 24, 4436. [Google Scholar] [CrossRef]
- Pobłocki, K.; Drzeżdżon, J.; Kostrzewa, T.; Jacewicz, D. Coordination Complexes as a New Generation Photosensitizer for Photodynamic Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 8052. [Google Scholar] [CrossRef] [PubMed]
- Pranczk, J.; Tesmar, A.; Wyrzykowski, D.; Inkielewicz-Stępniak, I.; Jacewicz, D.; Chmurzyński, L. Influence of primary ligands (ODA, TDA) on physicochemical and biological properties of oxovanadium(IV) complexes with bipy and phen as auxiliary ligands. Biol. Trace Elem. Res. 2016, 174, 251. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, L.; Grirrane, A.; Moyano, R.; Álvarez, E.; Pastor, A.; Galindo, A. Comparison of the coordination capabilities of thiodiacetate and oxydiacetate ligands through the X-ray characterization and DFT studies of [V (O)(tda)(phen)]· 4H2O and [V (O)(oda)(phen)]· 1.5 H2O. Polyhedron 2010, 29, 3028. [Google Scholar] [CrossRef]
- Gawdzik, B.; Drzeżdżon, J.; Siarhei, T.; Sikorski, A.; Malankowska, A.; Kowalczyk, P.; Jacewicz, D. Catalytic Activity of New Oxovanadium(IV) Microclusters with 2-Phenylpyridine in Olefin Oligomerization. Materials 2021, 14, 7670. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Pawlak, M.; Matyka, N.; Sikorski, A.; Gawdzik, B.; Jacewicz, D. Relationship between Antioxidant Activity and Ligand Basicity in the Dipicolinate Series of Oxovanadium(IV) and Dioxovanadium(V) Complexes. Int. J. Mol. Sci. 2021, 22, 9886. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, Z.A.; Sharma, P.K.; Shahid, M.; Khalid, M.; Kumar, S. Synthesis, spectral characterizations and biological studies of transition metal mixed ligand complexes: X-ray crystal structures of [Cu(oda)(Bipy)(H2O)]. J. Mol. Struct. 2011, 994, 295–301. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Inkielewicz-Stępniak, I.; Pranczk, J.; Żamojć, K.; Zięba, P.; Tesmar, A.; Chmurzyński, L. Physicochemical properties of ternary oxovanadium(IV) complexes with oxydiacetate and 1,10-phenanthroline or 2,2-bipyridine. Cytoprotective activity in hippocampal neuronal HT22 cells. BioMetals 2015, 28, 307. [Google Scholar] [CrossRef]
- Rabek, J.F. Współczesna Wiedza o Polimerach Tom 1; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2017. [Google Scholar]
- Coleman, M.M.; Skrovanek, D.J.; Hu, J.; Painter, P.C. Hydrogen bonding in polymer blends. 1. FTIR studies of urethane-ether blends. Macromolecules 1988, 21, 59. [Google Scholar] [CrossRef]
- He, Y.; Zhu, B.; Inoue, Y. Hydrogen bonds in polymer blends. Prog. Polym. Sci. 2004, 29, 1021. [Google Scholar] [CrossRef]
- Pobłocki, K.; Jacewicz, D.; Walczak, J.; Gawdzik, B.; Kramkowski, K.; Drzeżdżon, J.; Kowalczyk, P. Preparation of Allyl Alcohol Oligomers Using Dipicolinate Oxovanadium(IV) Coordination Compound. Materials 2022, 15, 695. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Jacewicz, D.; Sielicka, A.; Chmurzyński, L. MALDI-MS for polymer characterization–recent developments and future prospects. Trends. Analyt. Chem. 2019, 115, 121. [Google Scholar] [CrossRef]
- Rybiński, P.; Janowska, G.; Jóźwiak, M.; Pająk, A. Thermal stability and flammability of butadiene–styrene rubber nanocomposites. J. Therm. Anal. 2012, 109, 561. [Google Scholar] [CrossRef]
- Rybiński, P.; Janowska, G. Thermal properties and flammability of nanocomposites based on nitrile rubbers and activated halloysite nanotubes and carbon nanofibers. Thermochim Acta. 2012, 549, 6. [Google Scholar] [CrossRef]
- Jankowska, G.; Ślusarski, L.; Rybiński, P. Analiza termiczna spęcznionych siarkowych wulkanizatów kauczuku butadienowo-akrylonitrylowego. Polimery 2005, 50, 196. [Google Scholar]
- Pobłocki, K.; Walczak, J.; Drzeżdżon, J.; Jacewicz, D. Katalizatory wykorzystywane w syntezie biodiesla. Wiad. Chem. 2022, 76, 3–4. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. 1999, 38, 428. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Pawlak, M.; Gawdzik, B.; Wypych, A.; Kramkowski, K.; Kowalczyk, P.; Jacewicz, D. Dipicolinate Complexes of Oxovanadium(IV) and Dioxovanadium(V) with 2-Phenylpyridine and 4,4′-Dimethoxy-2,2′-bipyridyl as New Precatalysts for Olefin Oligomerization. Materials 2022, 15, 1379. [Google Scholar] [CrossRef]
- Phillips, A.M.F.; Suo, H.; Maria de Fátima, C.; Pombeiro, A.J.; Sun, W.H. Recent developments in vanadium-catalyzed olefin coordination polymerization. Coord. Chem. Rev. 2020, 416, 213332. [Google Scholar] [CrossRef]
- Białek, M.; Leksza, A.; Piechota, A.; Kurzak, K.; Koprek, K. Oxovanadium(IV) complexes with [ONNO]-chelating ligands as catalysts for ethylene homo-and copolymerization. J. Polym. Res. 2014, 21, 1. [Google Scholar] [CrossRef][Green Version]

| Complex Compound | Wavenumber [cm−1] | Type of Vibration with a Function Group |
|---|---|---|
| [VO(TDA)phen] • 1.5 H2O | 3454 | Strong -OH stretching vibrations |
| 2943 | Weak asymmetric -CH stretching vibrations | |
| 1389 | Very weak -CH2 bending vibrations | |
| 1074 | Strong C-O stretching vibrations | |
| 764 | Very weak -CH2 rocking vibrations | |
| [VOO(dipic)(2-phepyH)] • H2O | 3488 | Strong symmetric -OH stretching vibrations |
| 2988 | Strong asymmetric -CH stretching vibrations | |
| 1458 | Strong -CH2 bending vibrations | |
| 1088 | Strong C-O stretching vibrations | |
| 746 | Weak -CH2 rocking vibrations | |
| [VO(dipic)(dmbipy)] • 2 H2O | 3468 | Strong symmetric -OH stretching vibrations |
| 2966 | Strong asymmetric -CH stretching vibrations | |
| 1492 | Strong -CH2 bending vibrations | |
| 1098 | Strong C-O stretching vibrations | |
| 733 | Weak -CH2 rocking vibrations | |
| [VO(ODA)bipy] • 2 H2O | 3399 | Symmetric -OH stretching vibrations |
| 2998 | Strong asymmetric -CH stretching vibrations | |
| 1488 | Weak -CH2 bending vibrations | |
| 1092 | Strong C-O stretching vibrations | |
| 734 | Very weak -CH2 rocking vibrations |
| Complex Compound | Wavenumber [cm−1] | Type of Vibration with Function Group |
|---|---|---|
| [VO(TDA)phen] • 1.5 H2O | 3488 | Strong symmetric -OH stretching vibrations |
| 2988 | Strong asymmetric -CH stretching vibrations | |
| 1420 | -CH2 bending vibrations | |
| 1101 | Strong C-O stretching vibrations | |
| 724 | Very weak -CH2 rocking vibrations | |
| [VOO(dipic)(2-phepyH)] • H2O | 2998 | Strong asymmetric -CH stretching vibrations |
| 1436 | Strong -CH2 bending vibrations | |
| 1079 | Strong C-O stretching vibrations | |
| 746 | Weak -CH2 rocking vibrations | |
| [VO(dipic)(dmbipy)] • 2 H2O | 3449 | Strong symmetric -OH stretching vibrations |
| 2985 | Strong asymmetric -CH stretching vibrations | |
| 1435 | Strong -CH2 bending vibrations | |
| 1104 | Strong C-O stretching vibrations | |
| 721 | Weak -CH2 rocking vibrations | |
| [VO(ODA)bipy] • 2 H2O | 3217 | Weak -OH stretching vibrations |
| 2998 | Asymmetric -CH stretching vibrations | |
| 1477 | Weak -CH2 bending vibrations | |
| 1091 | Strong C-O stretching vibrations | |
| 718 | Very weak -CH2 rocking vibrations |
| Complex Compound | Wavenumber [cm−1] | Type of Vibration with a Function Group |
|---|---|---|
| [VO(TDA)phen] • 1.5 H2O | 3482 | Symmetric -OH stretching vibrations |
| 2945 | Strong asymmetric -CH stretching vibrations | |
| 1419 | -CH2 bending vibrations | |
| 1093 | Strong C-O stretching vibrations | |
| 731 | Very weak -CH2 rocking vibrations | |
| [VOO(dipic)(2-phepyH)] • H2O | 3432 | Strong symmetric -OH stretching vibrations |
| 2935 | Strong asymmetric -CH stretching vibrations | |
| 1476 | Strong -CH2 bending vibrations | |
| 1096 | Strong C-O stretching vibrations | |
| 755 | Very weak -CH2 rocking vibrations | |
| [VO(dipic)(dmbipy)] • 2 H2O | 3566 | Symmetric -OH stretching vibrations |
| 2921 | Asymmetric -CH stretching vibrations | |
| 1411 | Very weak -CH2 bending vibrations | |
| 1081 | Strong C-O stretching vibrations | |
| 762 | Very weak -CH2 rocking vibrations | |
| [VO(ODA)bipy] • 2 H2O | 3279 | Symmetric -OH stretching vibrations |
| 2963 | Strong asymmetric -CH stretching vibrations | |
| 1421 | -CH2 bending vibrations | |
| 1093 | Strong C-O stretching vibrations | |
| 713 | Very weak -CH2 rocking vibrations |
| Complex Compound | Degree of Oligomerization (n) |
|---|---|
| [VO(TDA)phen] • 1.5 H2O | 5, 9, 11, 14 |
| [VOO(dipic)(2-phepyH)] • H2O | 3, 6 |
| [VO(dipic)(dmbipy)] • 2 H2O | 3, 5, 6 |
| [VO(ODA)bipy] • 2 H2O | 5, 6, 9, 12, 16 |
| Complex Compound | Degree of Oligomerization (n) |
|---|---|
| [VO(TDA)phen] • 1.5 H2O | 7, 11, 14, 17, 20 |
| [VOO(dipic)(2-phepyH)] • H2O | 7, 11, 14, 17 |
| [VO(dipic)(dmbipy)] • 2 H2O | 2, 4, 6, 7, 8, 9, 12 |
| [VO(ODA)bipy] • 2 H2O | 7, 9, 10, 11, 14, 17, 20 |
| Complex Compound | Degree of Oligomerization (n) |
|---|---|
| [VO(TDA)phen] • 1.5 H2O | 2, 3, 4 |
| [VOO(dipic)(2-phepyH)] • H2O | 2, 3, 4 |
| [VO(dipic)(dmbipy)] • 2 H2O | 2, 3, 4 |
| [VO(ODA)bipy] • 2 H2O | 2, 3, 4, 5 |
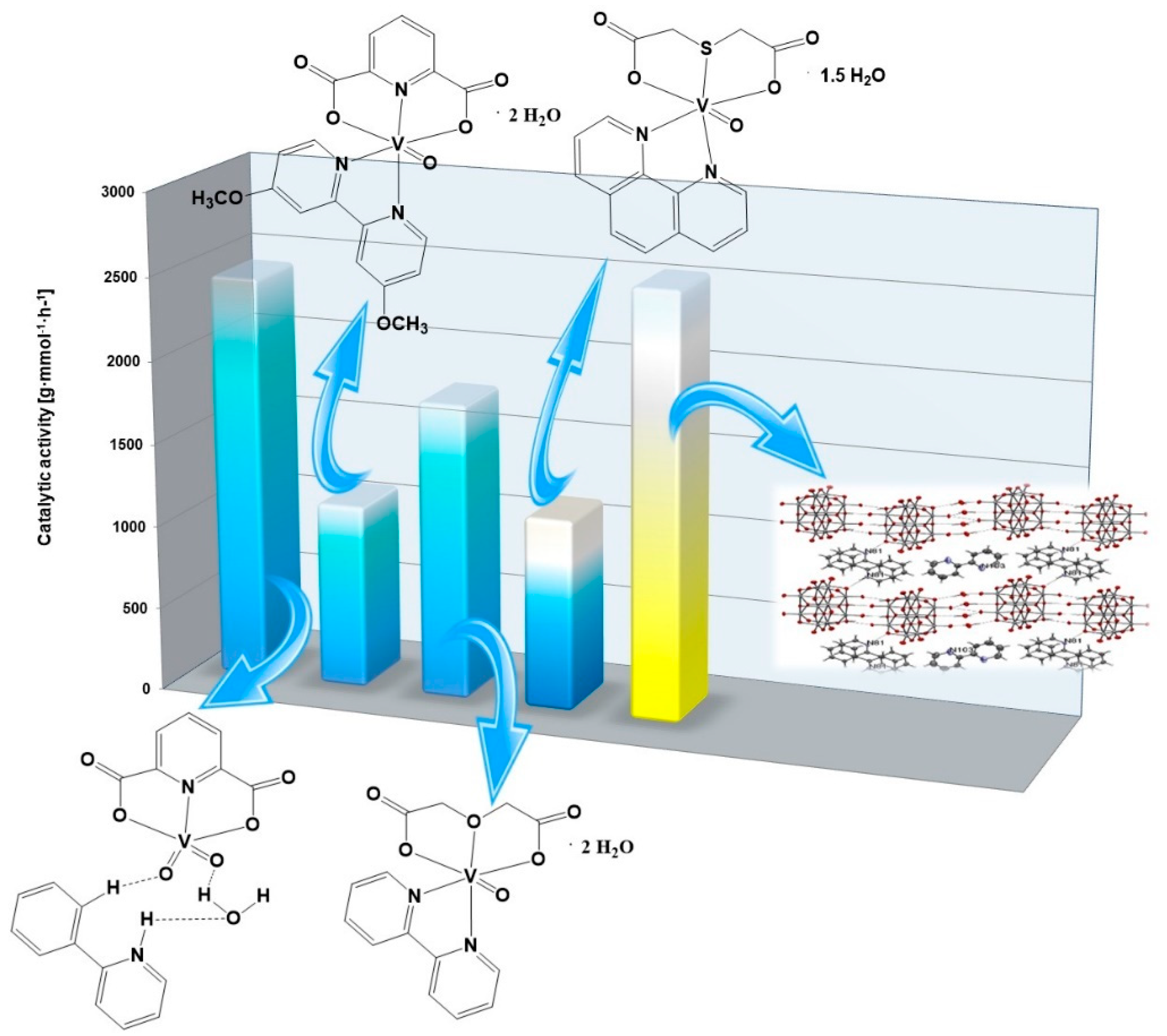
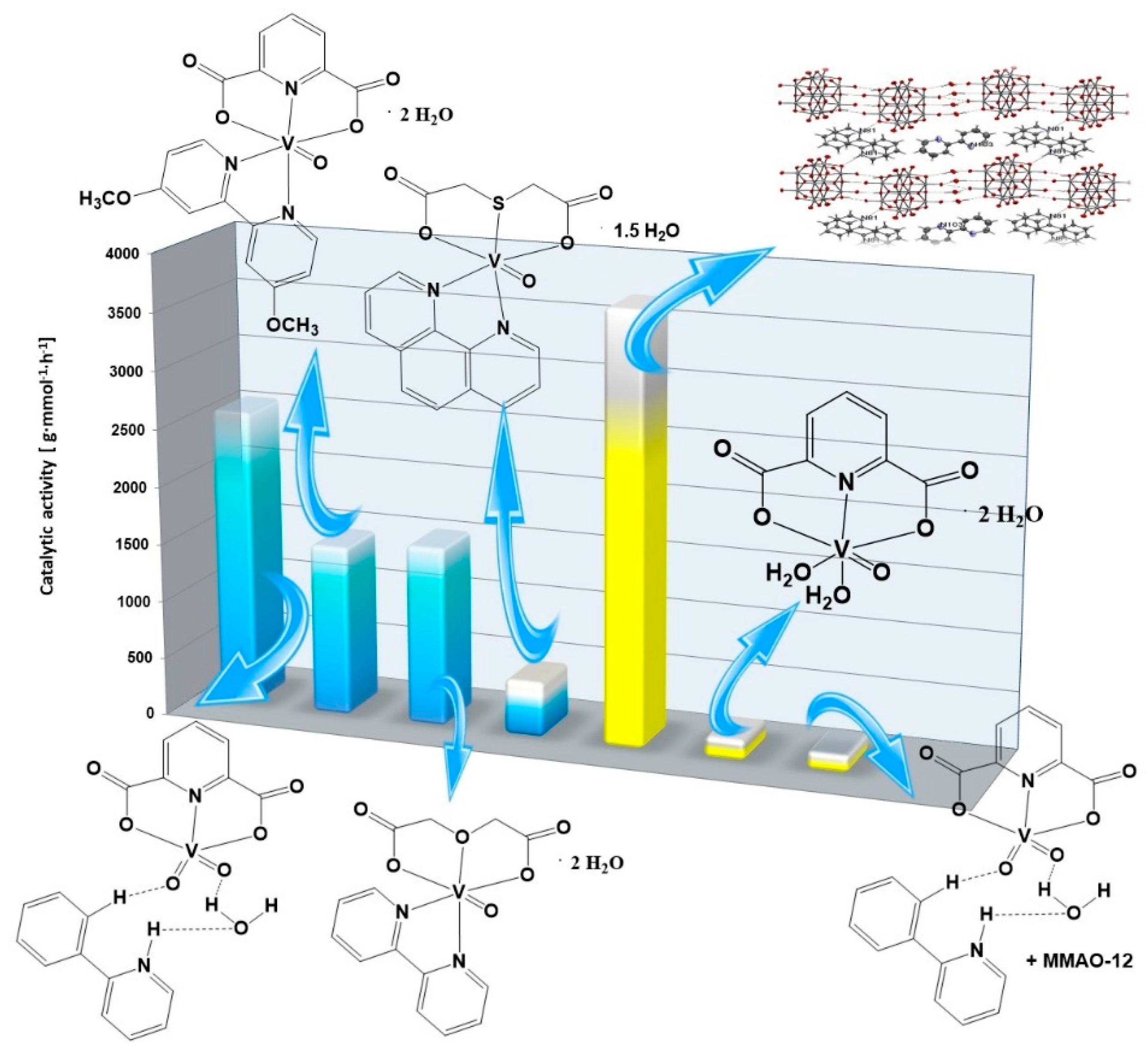
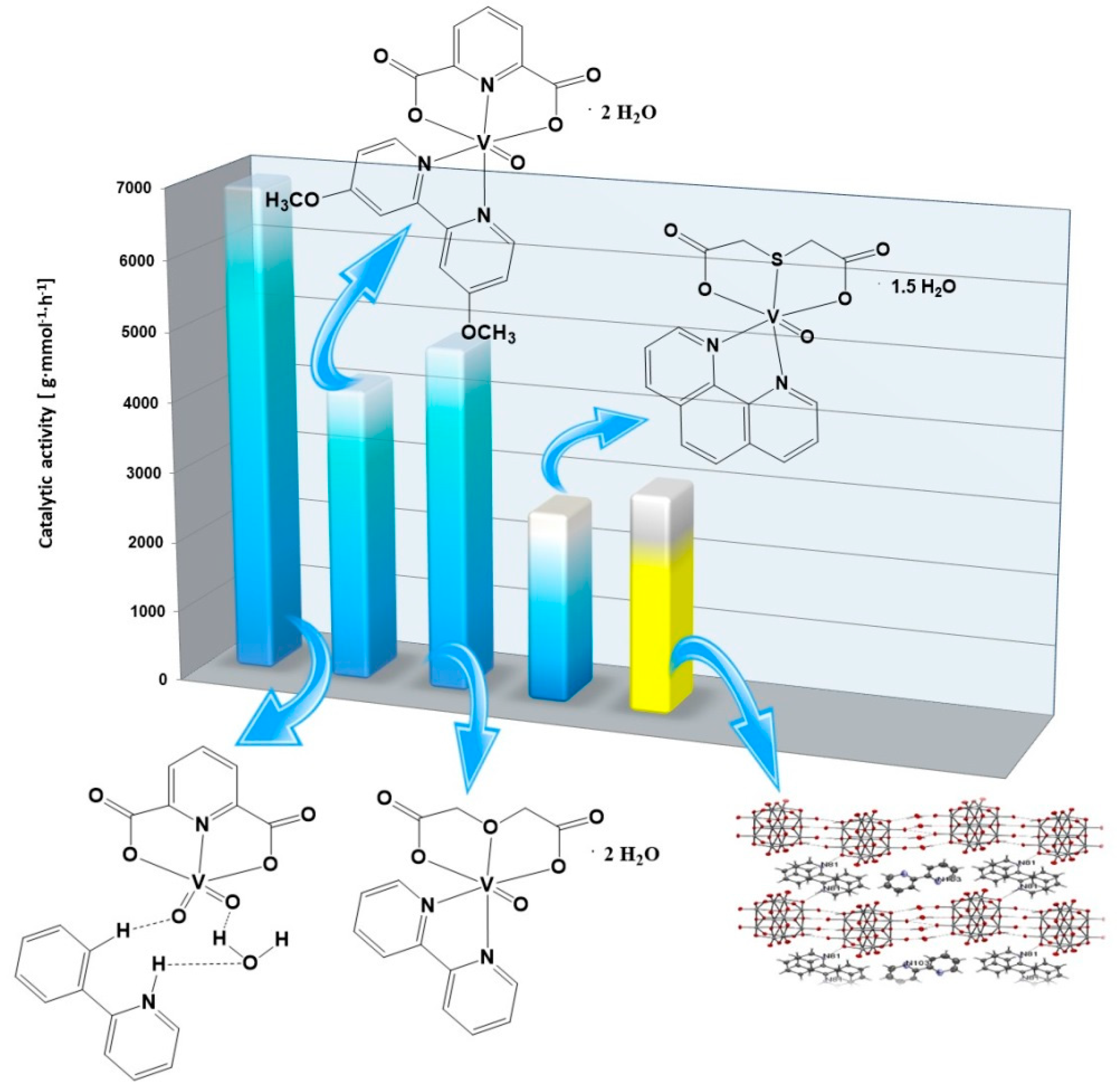
| Complex Compounds | Olefins | Activator | Amount of Oxovanadium(IV) [mmol] | The Molar Ratio of the Complex Compound to the Activator | Temperature [°C] | Catalytic Activity [g mmol−1 h−1] |
|---|---|---|---|---|---|---|
| [VO(TDA)phen] • 1.5 H2O | Allyl alcohol | AlEtCl2 | 0.003 | 1:1000 | 30 | 492 |
| 3-buten-2-ol | 1160 | |||||
| 2,3-dibromo-2-propen-1-ol | 2707 | |||||
| [VOO(dipic)(2-phepyH)] • H2O | Allyl alcohol | 2591 | ||||
| 3-buten-2-ol | 2441 | |||||
| 2,3-dibromo-2-propen-1-ol | 6933 | |||||
| [VO(dipic)(dmbipy)] • 2H2O | Allyl alcohol | 1494 | ||||
| 3-buten-2-ol | 1121 | |||||
| 2,3-dibromo-2-propen-1-ol | 4188 | |||||
| [VO(ODA)(bipy)] • 2 H2O | Allyl alcohol | 1572 | ||||
| 3-buten-2-ol | 1792 | |||||
| 2,3-dibromo-2-propen-1-ol | 4894 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbaniak, M.; Pobłocki, K.; Kowalczyk, P.; Kramkowski, K.; Drzeżdżon, J.; Gawdzik, B.; Świtała, P.; Miler, M.; Heleniak, D.; Rybiński, P.; et al. A Series of Green Oxovanadium(IV) Precatalysts with O, N and S Donor Ligands in a Sustainable Olefins Oligomerization Process. Molecules 2022, 27, 8038. https://doi.org/10.3390/molecules27228038
Urbaniak M, Pobłocki K, Kowalczyk P, Kramkowski K, Drzeżdżon J, Gawdzik B, Świtała P, Miler M, Heleniak D, Rybiński P, et al. A Series of Green Oxovanadium(IV) Precatalysts with O, N and S Donor Ligands in a Sustainable Olefins Oligomerization Process. Molecules. 2022; 27(22):8038. https://doi.org/10.3390/molecules27228038
Chicago/Turabian StyleUrbaniak, Mariusz, Kacper Pobłocki, Paweł Kowalczyk, Karol Kramkowski, Joanna Drzeżdżon, Barbara Gawdzik, Patrycja Świtała, Maja Miler, Daria Heleniak, Przemysław Rybiński, and et al. 2022. "A Series of Green Oxovanadium(IV) Precatalysts with O, N and S Donor Ligands in a Sustainable Olefins Oligomerization Process" Molecules 27, no. 22: 8038. https://doi.org/10.3390/molecules27228038
APA StyleUrbaniak, M., Pobłocki, K., Kowalczyk, P., Kramkowski, K., Drzeżdżon, J., Gawdzik, B., Świtała, P., Miler, M., Heleniak, D., Rybiński, P., & Jacewicz, D. (2022). A Series of Green Oxovanadium(IV) Precatalysts with O, N and S Donor Ligands in a Sustainable Olefins Oligomerization Process. Molecules, 27(22), 8038. https://doi.org/10.3390/molecules27228038








