4-(Aryl)-Benzo[4,5]imidazo[1,2-a]pyrimidine-3-Carbonitrile-Based Fluorophores: Povarov Reaction-Based Synthesis, Photophysical Studies, and DFT Calculations
Abstract
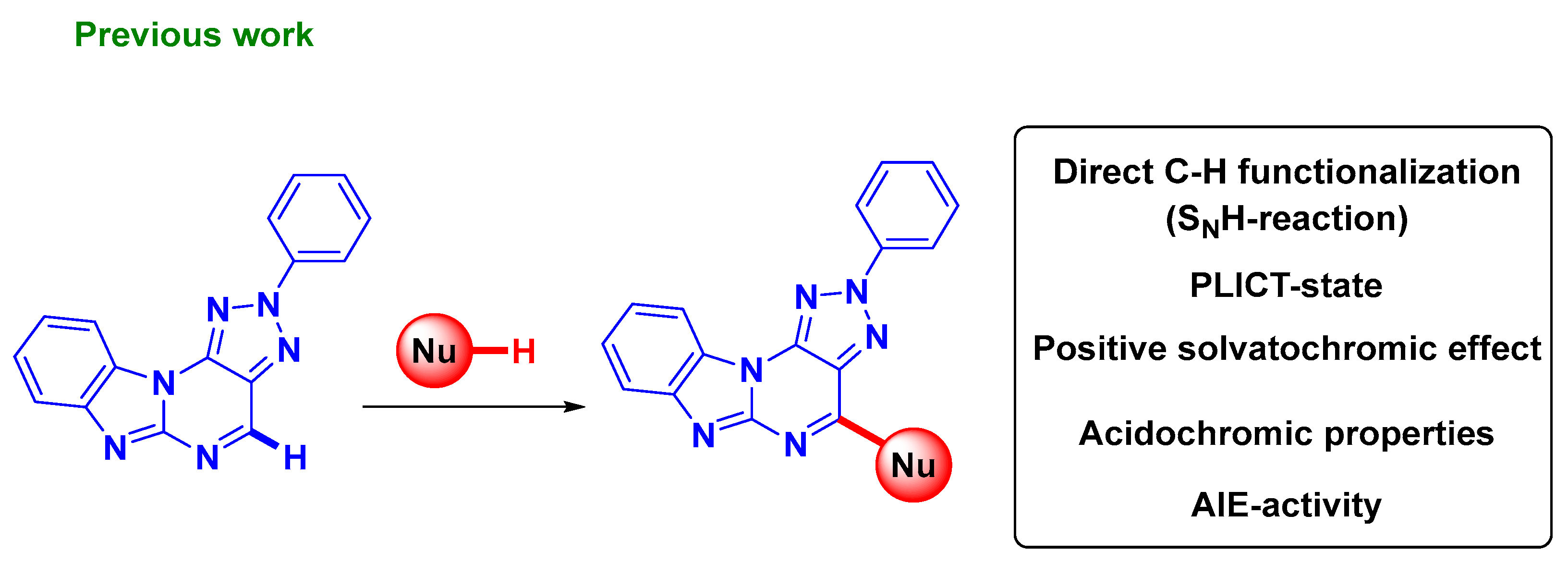
1. Introduction
2. Results
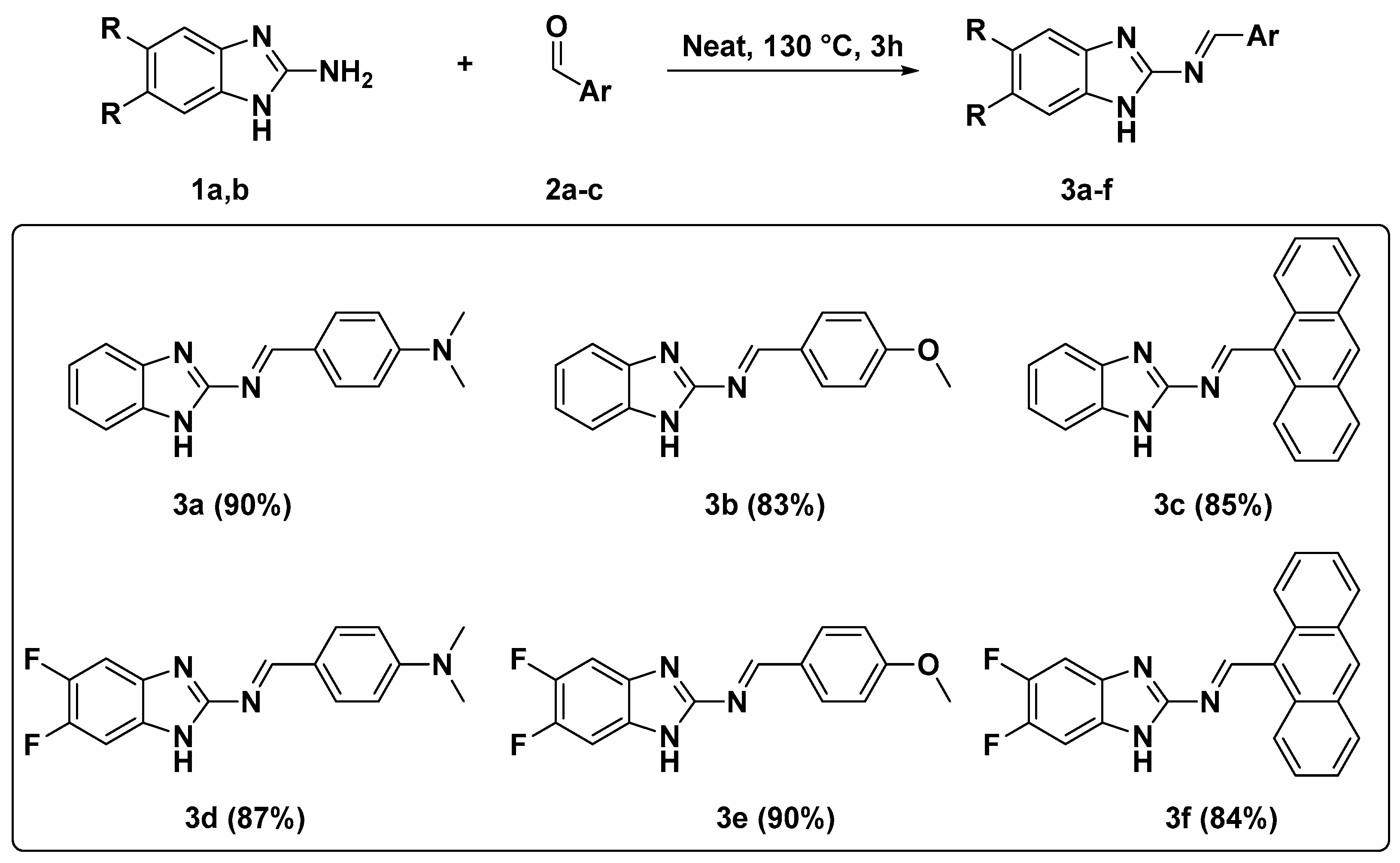
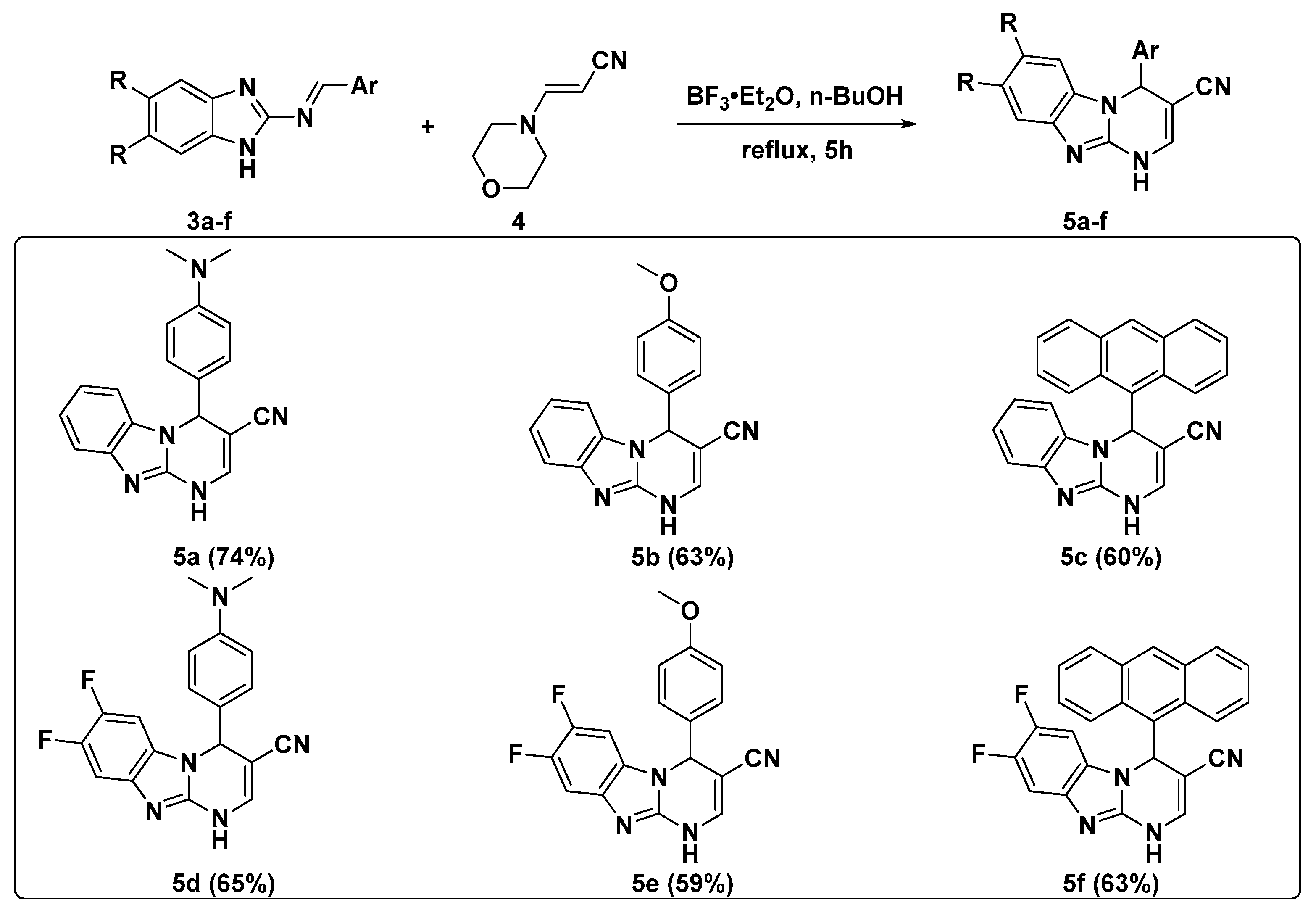
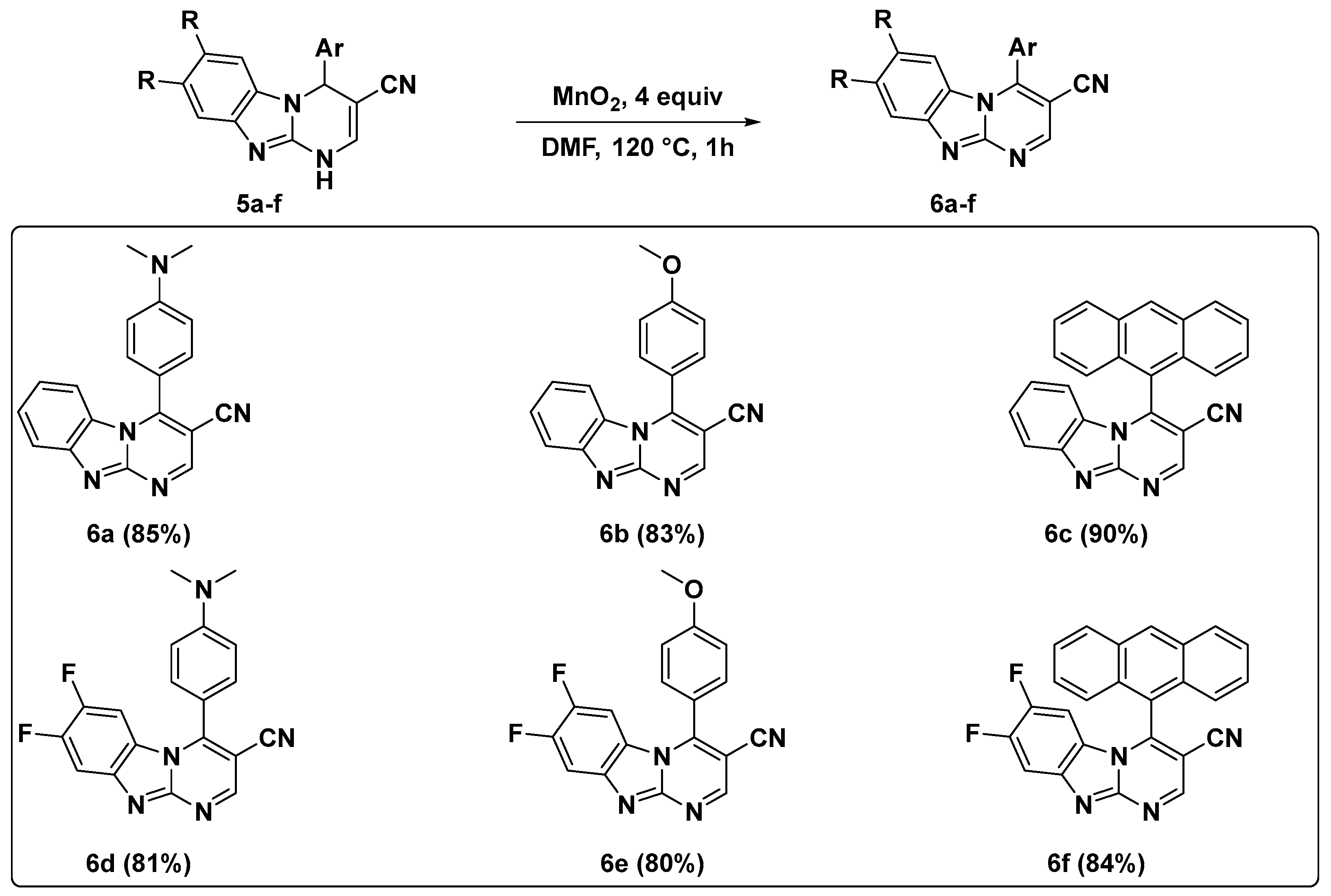
2.1. Synthesis
2.2. Photophysical Studies
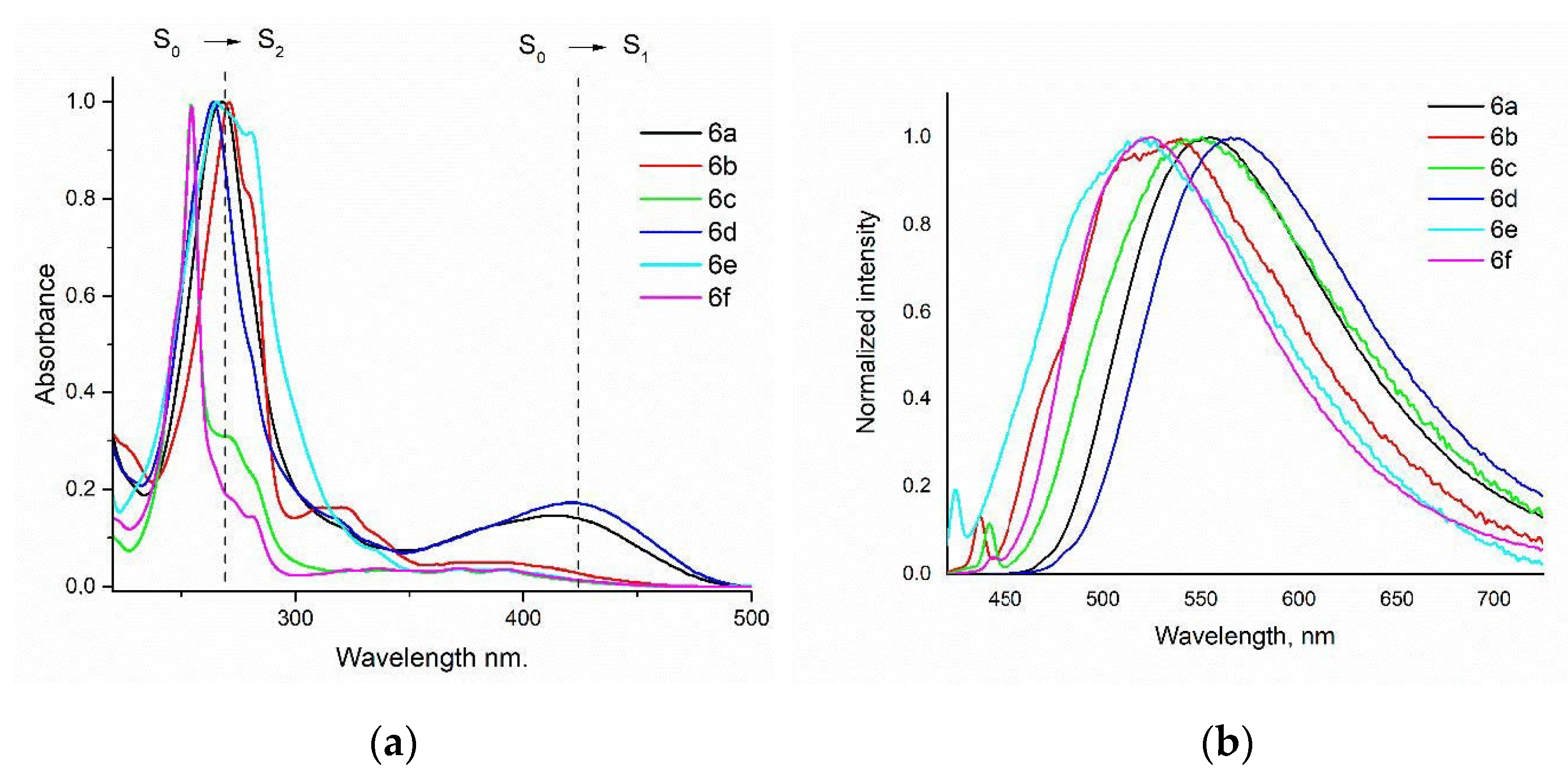
2.2.1. Absorption/Fluorescence Studies in Solution and Solvent Effect
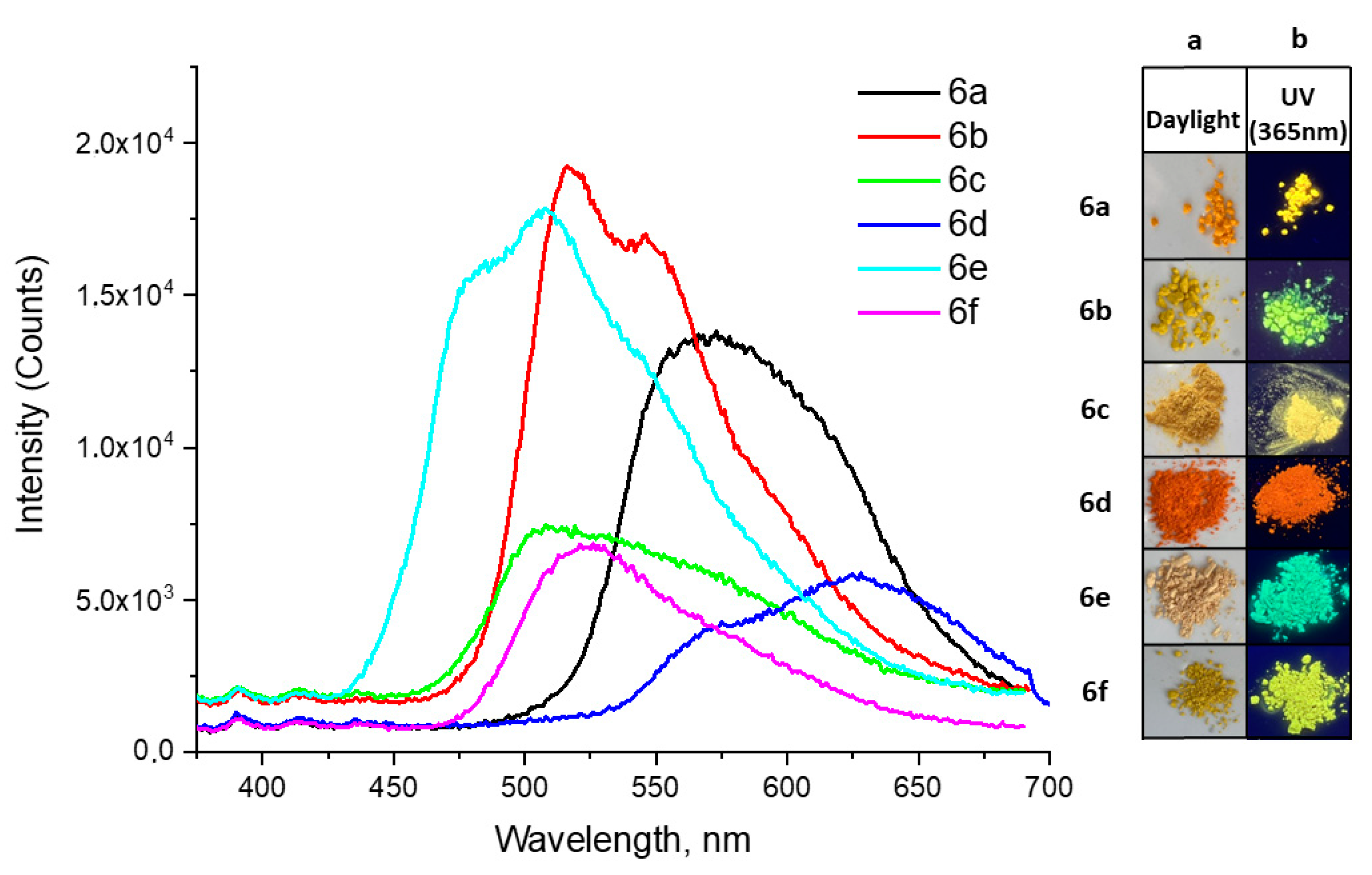
2.2.2. Solid State Fluorescence Studies
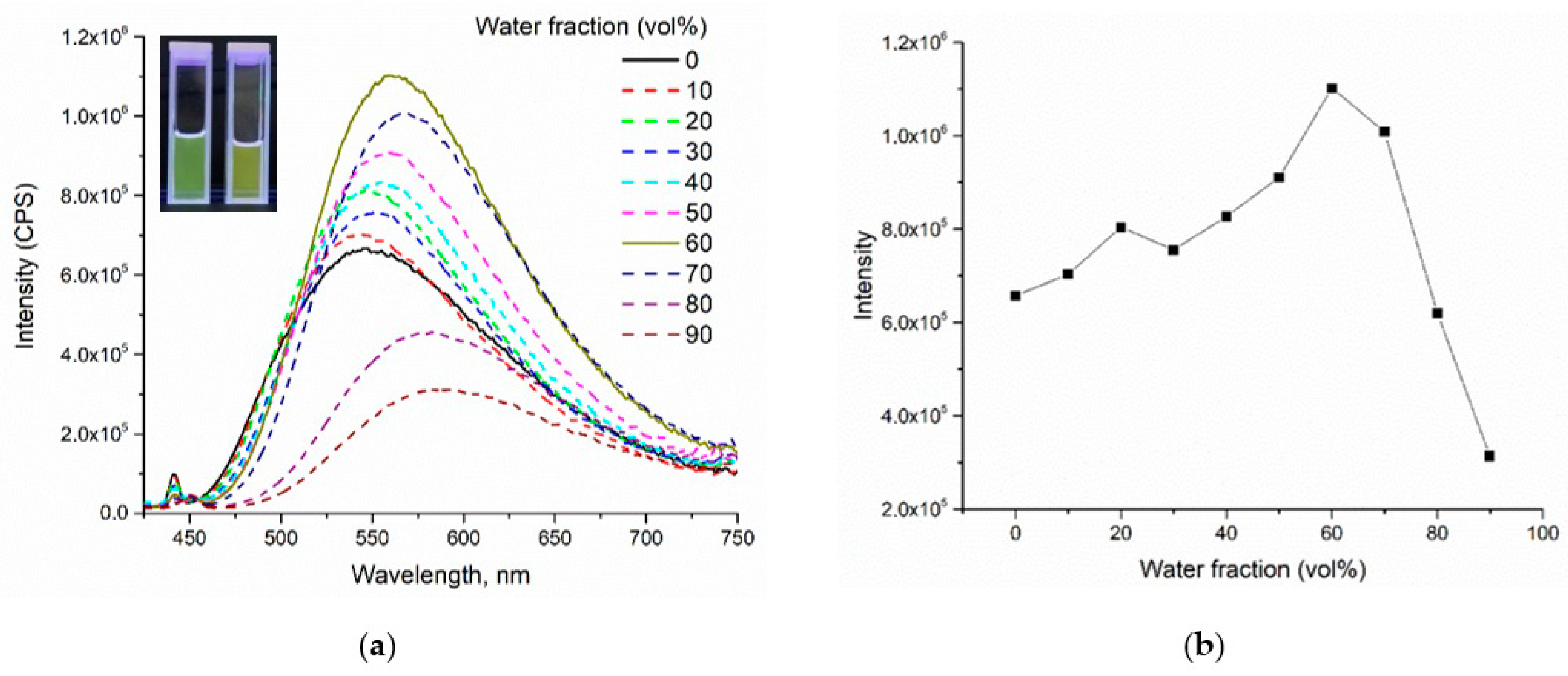
2.2.3. Aggregation Studies
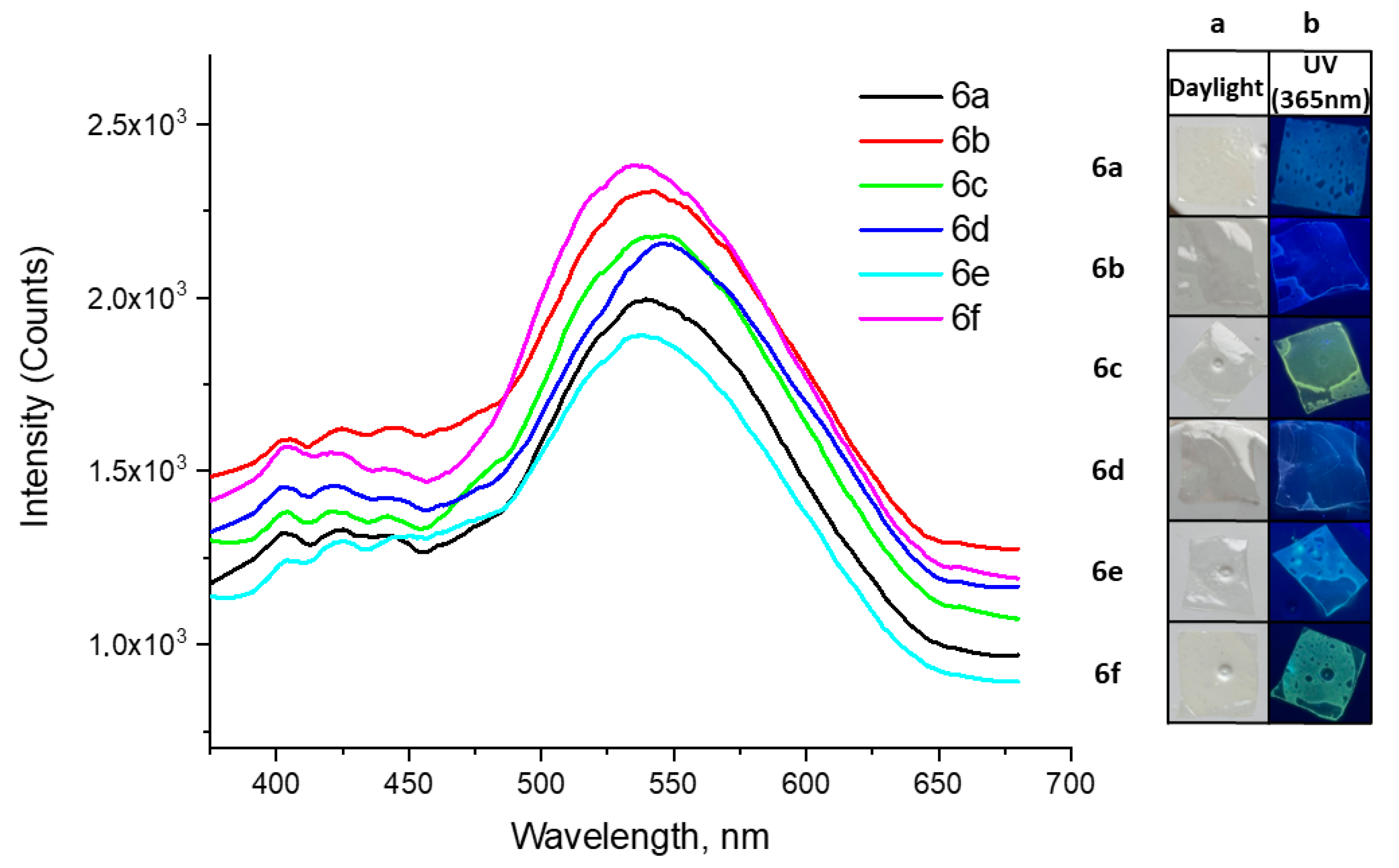
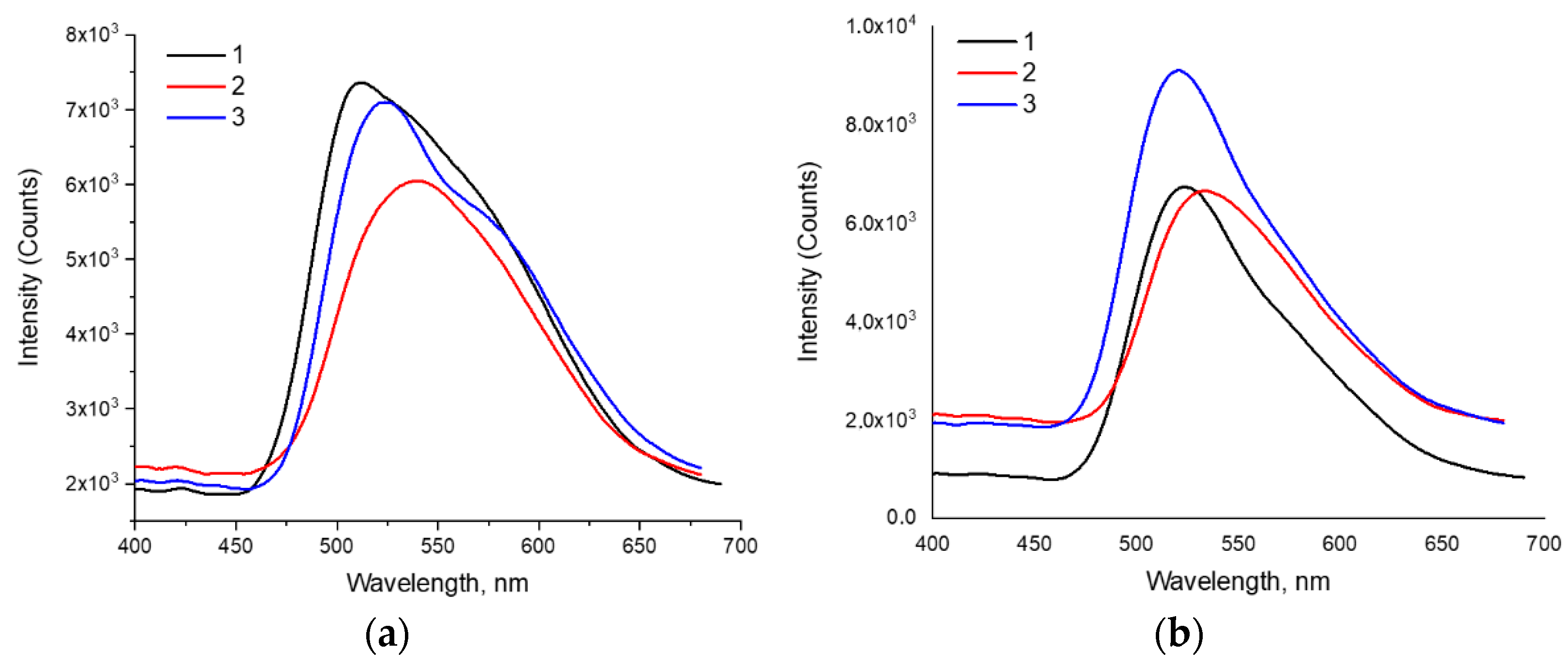
2.2.4. Mechanochromic Properties
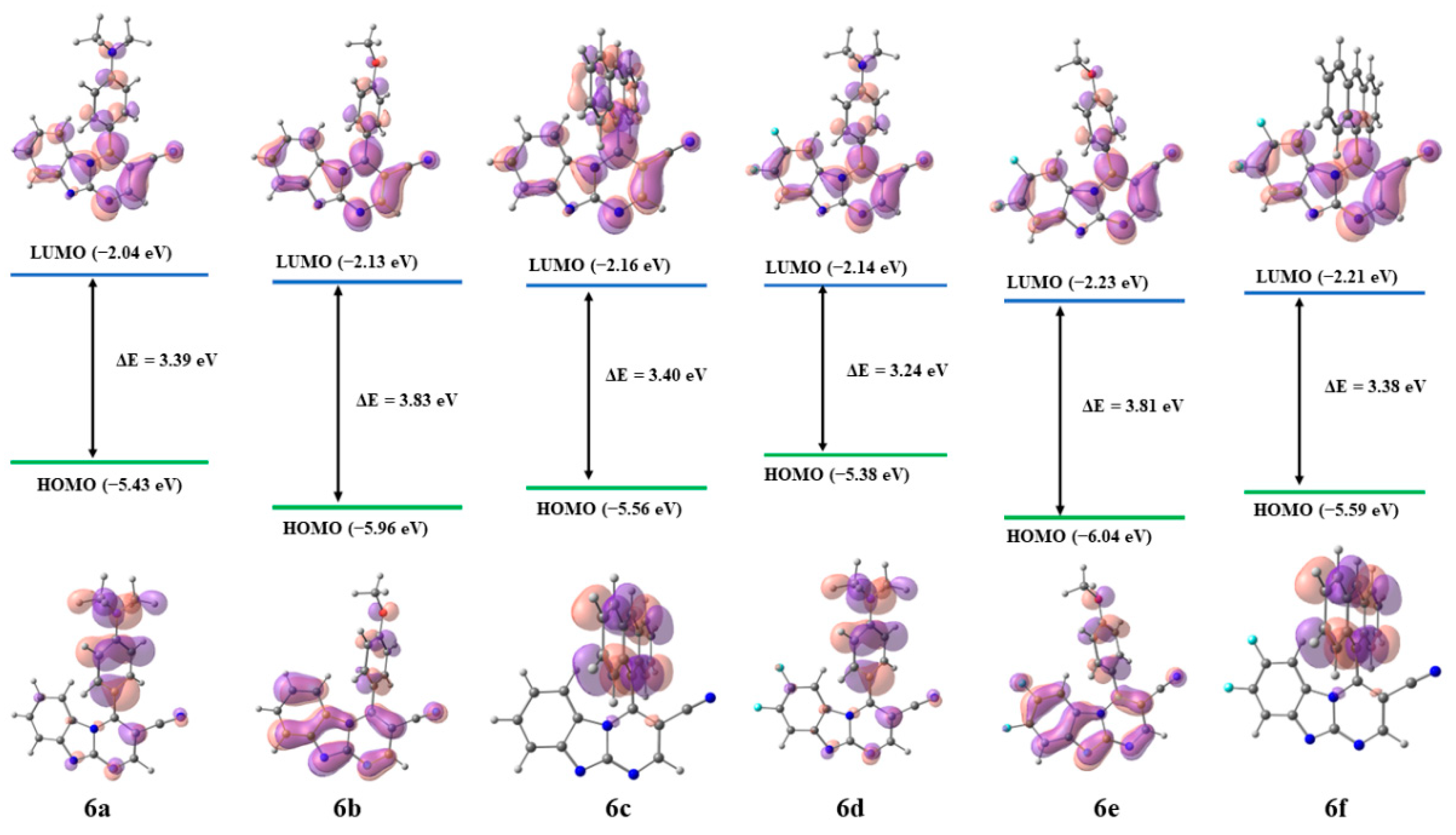
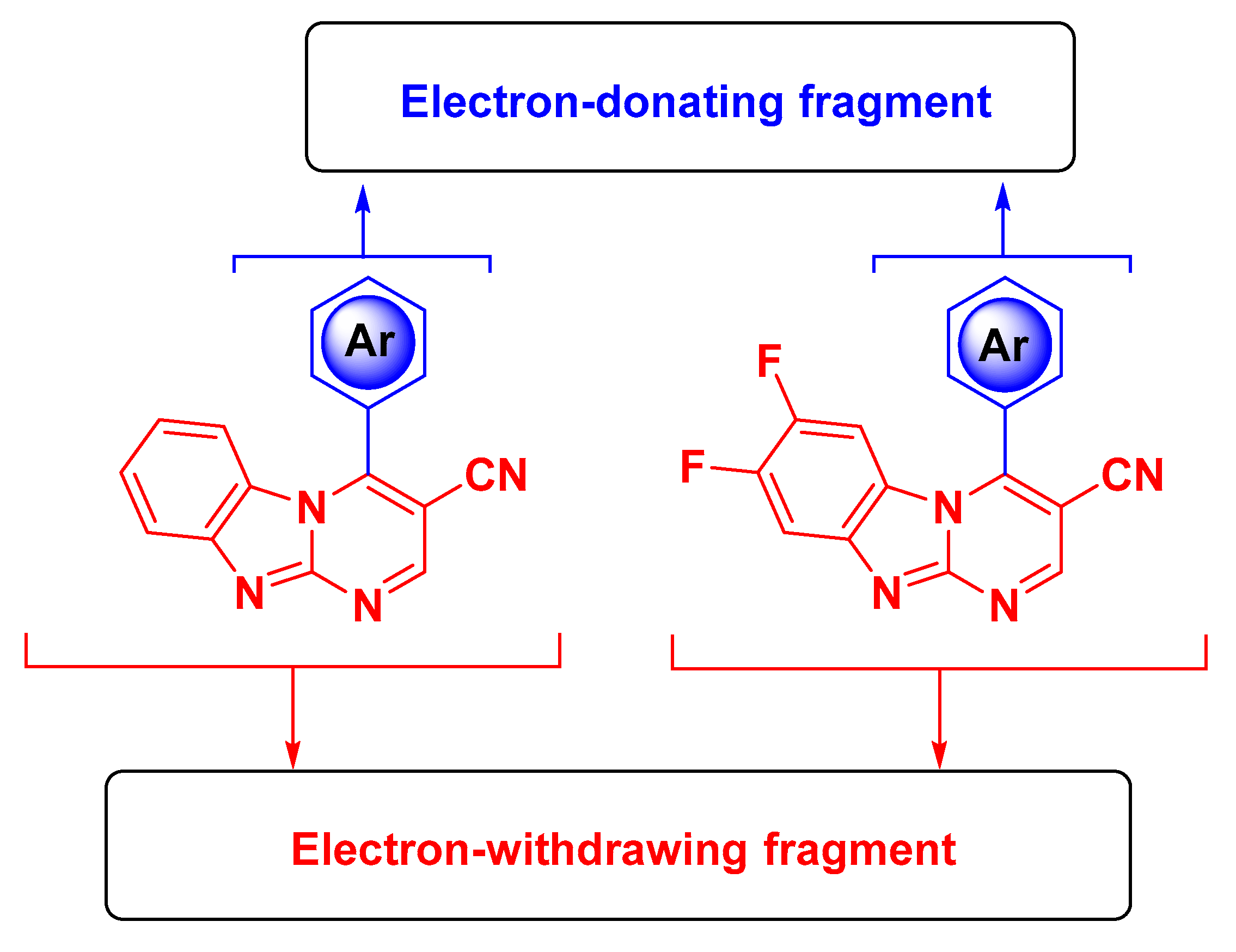
2.2.5. Theoretical Calculations
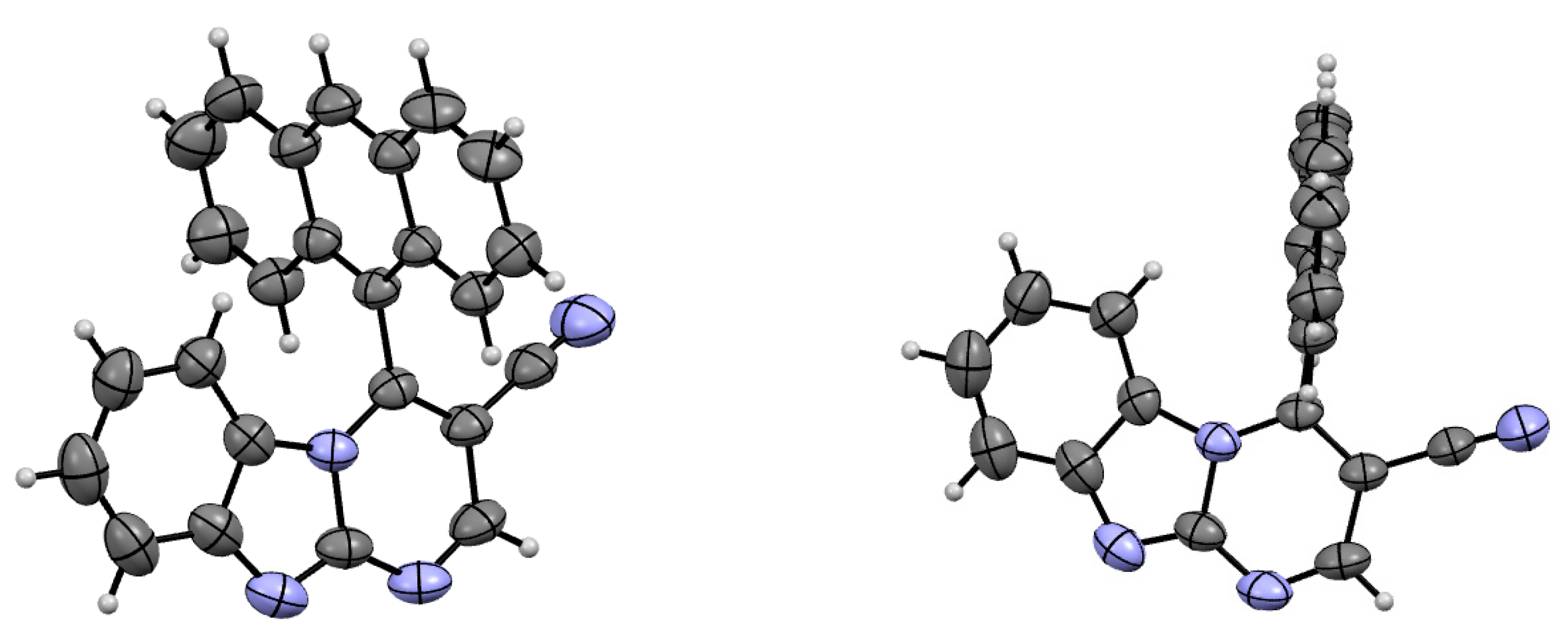
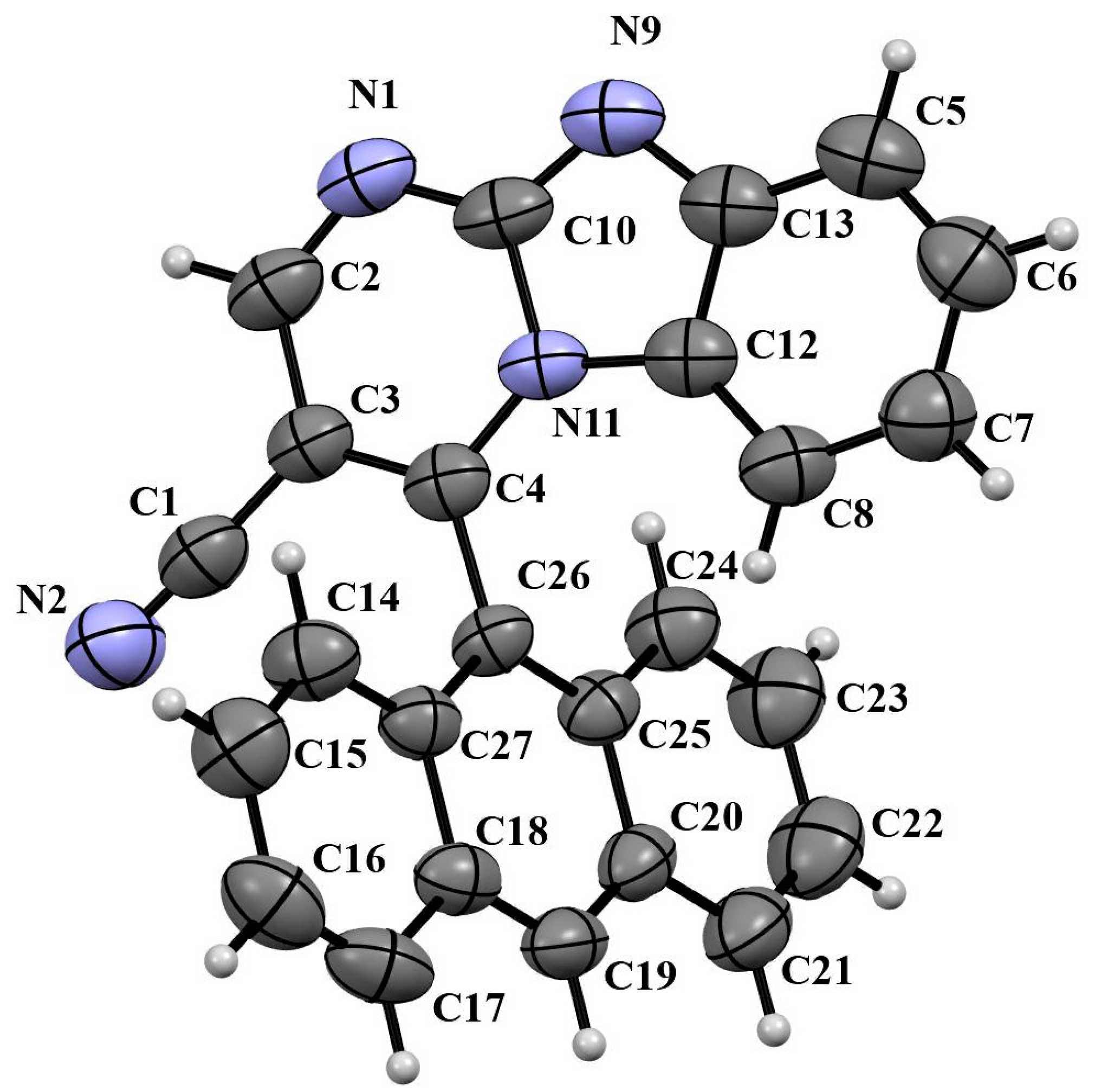
2.3. Crystallography
3. Materials and Methods
3.1. Chemical Experiment
3.2. Crystallography Experiment
3.3. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alqarni, S.; Cooper, L.; Galvan Achi, J.; Bott, R.; Sali, V.K.; Brown, A.; Santarsiero, B.D.; Krunic, A.; Manicassamy, B.; Peet, N.P.; et al. Synthesis, Optimization, and Structure–Activity Relationships of Imidazo[1,2-a]Pyrimidines as Inhibitors of Group 2 Influenza A Viruses. J. Med. Chem. 2022, 65, 14104–14120. [Google Scholar] [CrossRef] [PubMed]
- Massari, S.; Bertagnin, C.; Pismataro, M.C.; Donnadio, A.; Nannetti, G.; Felicetti, T.; Di Bona, S.; Nizi, M.G.; Tensi, L.; Manfroni, G.; et al. Synthesis and Characterization of 1,2,4-Triazolo[1,5-a]Pyrimidine-2-Carboxamide-Based Compounds Targeting the PA-PB1 Interface of Influenza A Virus Polymerase. Eur. J. Med. Chem. 2021, 209, 112944. [Google Scholar] [CrossRef] [PubMed]
- Perlíková, P.; Hocek, M. Pyrrolo[2,3-d]Pyrimidine (7-Deazapurine) as a Privileged Scaffold in Design of Antitumor and Antiviral Nucleosides. Med. Res. Rev. 2017, 37, 1429–1460. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Soto-Acosta, R.; Geraghty, R.J.; Chen, L. Zika Virus Inhibitors Based on a 1,3-Disubstituted 1H-Pyrazolo[3,4-d]Pyrimidine-Amine Scaffold. Molecules 2022, 27, 6109. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Oliva, P.; Prencipe, F.; Manfredini, S.; Budassi, F.; Brancale, A.; Ferla, S.; Hamel, E.; Corallo, D.; Aveic, S.; et al. Design, Synthesis and Biological Investigation of 2-Anilino Triazolopyrimidines as Tubulin Polymerization Inhibitors with Anticancer Activities. Pharmaceuticals 2022, 15, 1031. [Google Scholar] [CrossRef]
- Yu, G.-X.; Hu, Y.; Zhang, W.-X.; Tian, X.-Y.; Zhang, S.-Y.; Zhang, Y.; Yuan, S.; Song, J. Design, Synthesis and Biological Evaluation of [1,2,4]Triazolo[1,5-a]Pyrimidine Indole Derivatives against Gastric Cancer Cells MGC-803 via the Suppression of ERK Signaling Pathway. Molecules 2022, 27, 4996. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Quan, Y.; Wang, Y.; Wang, Y.; Li, Y. Design, Synthesis, and Biological Evaluation of 2,6,7-Substituted Pyrrolo[2,3-d]Pyrimidines as Cyclin Dependent Kinase Inhibitor in Pancreatic Cancer Cells. Bioorganic Med. Chem. Lett. 2021, 33, 127725. [Google Scholar] [CrossRef]
- Ding, R.; Wang, X.; Fu, J.; Chang, Y.; Li, Y.; Liu, Y.; Liu, Y.; Ma, J.; Hu, J. Design, Synthesis and Antibacterial Activity of Novel Pleuromutilin Derivatives with Thieno[2,3-d]Pyrimidine Substitution. Eur. J. Med. Chem. 2022, 237, 114398. [Google Scholar] [CrossRef]
- Sutherland, H.S.; Choi, P.J.; Lu, G.-L.; Giddens, A.C.; Tong, A.S.T.; Franzblau, S.G.; Cooper, C.B.; Palmer, B.D.; Denny, W.A. Synthesis and Structure–Activity Relationships for the Anti-Mycobacterial Activity of 3-Phenyl-N-(Pyridin-2-Ylmethyl)Pyrazolo[1,5-a]Pyrimidin-7-Amines. Pharmaceuticals 2022, 15, 1125. [Google Scholar] [CrossRef]
- Shen, J.; Deng, X.; Sun, R.; Tavallaie, M.S.; Wang, J.; Cai, Q.; Lam, C.; Lei, S.; Fu, L.; Jiang, F. Structural Optimization of Pyrazolo[1,5-a]Pyrimidine Derivatives as Potent and Highly Selective DPP-4 Inhibitors. Eur. J. Med. Chem. 2020, 208, 112850. [Google Scholar] [CrossRef]
- Peytam, F.; Adib, M.; Shourgeshty, R.; Firoozpour, L.; Rahmanian-Jazi, M.; Jahani, M.; Moghimi, S.; Divsalar, K.; Faramarzi, M.A.; Mojtabavi, S.; et al. An Efficient and Targeted Synthetic Approach towards New Highly Substituted 6-Amino-Pyrazolo[1,5-a]Pyrimidines with α-Glucosidase Inhibitory Activity. Sci. Rep. 2020, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Tigreros, A.; Macías, M.; Portilla, J. Expeditious Ethanol Quantification Present in Hydrocarbons and Distilled Spirits: Extending Photophysical Usages of the Pyrazolo[1,5-a]Pyrimidines. Dye. Pigment. 2022, 202, 110299. [Google Scholar] [CrossRef]
- Fedotov, V.V.; Ulomsky, E.N.; Belskaya, N.P.; Eltyshev, A.K.; Savateev, K.V.; Voinkov, E.K.; Lyapustin, D.N.; Rusinov, V.L. Benzimidazoazapurines: Design, Synthesis, and Photophysical Study. J. Org. Chem. 2021, 86, 8319–8332. [Google Scholar] [CrossRef]
- Tigreros, A.; Castillo, J.C.; Portilla, J. Cyanide Chemosensors Based on 3-Dicyanovinylpyrazolo[1,5-a]Pyrimidines: Effects of Peripheral 4-Anisyl Group Substitution on the Photophysical Properties. Talanta 2020, 215, 120905. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, R.; Lan, J.; Zhang, H.; Yan, L.; Pu, X.; Huang, Z.; Wu, D.; You, J. Oxidative C-H/C-H Cross-Coupling of [1,2,4]Triazolo[1,5-a]Pyrimidines with Indoles and Pyrroles: Discovering Excited-State Intramolecular Proton Transfer (ESIPT) Fluorophores. Org. Lett. 2019, 21, 4058–4062. [Google Scholar] [CrossRef]
- Ooyama, Y.; Uenaka, K.; Ohshita, J. Development of a Functionally Separated D–π-A Fluorescent Dye with a Pyrazyl Group as an Electron-Accepting Group for Dye-Sensitized Solar Cells. Org. Chem. Front. 2015, 2, 552–559. [Google Scholar] [CrossRef]
- Dinastiya, E.M.; Verbitskiy, E.V.; Gadirov, R.M.; Samsonova, L.G.; Degtyarenko, K.M.; Grigoryev, D.V.; Kurtcevich, A.E.; Solodova, T.A.; Tel’minov, E.N.; Rusinov, G.L.; et al. Investigation of 4,6-Di(Hetero)Aryl-Substituted Pyrimidines as Emitters for Non-Doped OLED and Laser Dyes. J. Photochem. Photobiol. A Chem. 2021, 408, 113089. [Google Scholar] [CrossRef]
- Fecková, M.; le Poul, P.; Bureš, F.; Robin-le Guen, F.; Achelle, S. Nonlinear Optical Properties of Pyrimidine Chromophores. Dye. Pigment. 2020, 182, 108659. [Google Scholar] [CrossRef]
- Debnath, S.; Parveen, S.; Pradhan, P.; Das, I.; Das, T. Benzo[4,5]Imidazo[1,2-a]Pyridines and Benzo[4,5]Imidazo[1,2-a]Pyrimidines: Recent Advancements in Synthesis of Two Diversely Important Heterocyclic Motifs and Their Derivatives. New J. Chem. 2022, 46, 10504–10534. [Google Scholar] [CrossRef]
- Fedotov, V.V.; Rusinov, V.L.; Ulomsky, E.N.; Mukhin, E.M.; Gorbunov, E.B.; Chupakhin, O.N. Pyrimido[1,2-a]Benzimidazoles: Synthesis and Perspective of Their Pharmacological Use. Chem. Heterocycl. Comp. 2021, 57, 383–409. [Google Scholar] [CrossRef]
- Fedotov, V.V.; Ulomskiy, E.N.; Gorbunov, E.B.; Eltsov, O.S.; Voinkov, E.K.; Savateev, K.V.; Drokin, R.A.; Kotovskaya, S.K.; Rusinov, V.L. 3-Nitropyrimido[1,2-a]Benzimidazol-4-Ones: Synthesis and Study of Alkylation Reaction. Chem. Heterocycl. Comp. 2017, 53, 582–588. [Google Scholar] [CrossRef]
- Manna, S.K.; Das, T.; Samanta, S. Polycyclic Benzimidazole: Synthesis and Photophysical Properties. ChemistrySelect 2019, 4, 8781–8790. [Google Scholar] [CrossRef]
- Vil’, V.A.; Grishin, S.S.; Baberkina, E.P.; Alekseenko, A.L.; Glinushkin, A.P.; Kovalenko, A.E.; Terent’ev, A.O. Electrochemical Synthesis of Tetrahydroquinolines from Imines and Cyclic Ethers via Oxidation/Aza-Diels-Alder Cycloaddition. Adv. Synth. Catal. 2022, 364, 1098–1108. [Google Scholar] [CrossRef]
- Steinke, T.; Wonner, P.; Gauld, R.M.; Heinrich, S.; Huber, S.M. Catalytic Activation of Imines by Chalcogen Bond Donors in a Povarov [4+2] Cycloaddition Reaction. Chem. A Eur. J. 2022, 28, e202200917. [Google Scholar] [CrossRef] [PubMed]
- Clerigué, J.; Ramos, M.T.; Menéndez, J.C. Mechanochemical Aza-Vinylogous Povarov Reactions for the Synthesis of Highly Functionalized 1,2,3,4-Tetrahydroquinolines and 1,2,3,4-Tetrahydro-1,5-Naphthyridines. Molecules 2021, 26, 1330. [Google Scholar] [CrossRef]
- Cores, Á.; Clerigué, J.; Orocio-Rodríguez, E.; Menéndez, J.C. Multicomponent Reactions for the Synthesis of Active Pharmaceutical Ingredients. Pharmaceuticals 2022, 15, 1009. [Google Scholar] [CrossRef]
- Jiménez-Aberásturi, X.; Palacios, F.; de los Santos, J.M. Sc(OTf)3-Mediated [4 + 2] Annulations of N-Carbonyl Aryldiazenes with Cyclopentadiene to Construct Cinnoline Derivatives: Azo-Povarov Reaction. J. Org. Chem. 2022, 87, 11583–11592. [Google Scholar] [CrossRef]
- Ghashghaei, O.; Masdeu, C.; Alonso, C.; Palacios, F.; Lavilla, R. Recent Advances of the Povarov Reaction in Medicinal Chemistry. Drug Discov. Today Technol. 2018, 29, 71–79. [Google Scholar] [CrossRef]
- Sun, Y.; Lei, Z.; Ma, H. Twisted Aggregation-Induced Emission Luminogens (AIEgens) Contribute to Mechanochromism Materials: A Review. J. Mater. Chem. C 2022, 10, 14834–14867. [Google Scholar] [CrossRef]
- Cooper, M.W.; Zhang, X.; Zhang, Y.; Ashokan, A.; Fuentes-Hernandez, C.; Salman, S.; Kippelen, B.; Barlow, S.; Marder, S.R. Delayed Luminescence in 2-Methyl-5-(Penta(9-Carbazolyl)Phenyl)-1,3,4-Oxadiazole Derivatives. J. Phys. Chem. A 2022, 126, 7480–7490. [Google Scholar] [CrossRef]
- Gong, X.; Xiang, Y.; Ning, W.; Zhan, L.; Gong, S.; Xie, G.; Yang, C. A Heterocycle Fusing Strategy for Simple Construction of Efficient Solution-Processable Pure-Red Thermally Activated Delayed Fluorescence Emitters. J. Mater. Chem. C 2022, 10, 15981–15988. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wu, Y.; Qin, K.; Xu, D.; Wang, D.; Ma, H.; Ning, S.; Wu, Z. A 2-Phenylfuro[2,3-b]Quinoxaline-Triphenylamine-Based Emitter: Photophysical Properties and Application in TADF-Sensitized Fluorescence OLEDs. New J. Chem. 2022, 46, 18854–18864. [Google Scholar] [CrossRef]
- Hojo, R.; Mayder, D.M.; Hudson, Z.M. Donor–Acceptor Materials Exhibiting Deep Blue Emission and Thermally Activated Delayed Fluorescence with Tris(Triazolo)Triazine. J. Mater. Chem. C 2021, 9, 14342–14350. [Google Scholar] [CrossRef]
- Rodella, F.; Saxena, R.; Bagnich, S.; Banevičius, D.; Kreiza, G.; Athanasopoulos, S.; Juršėnas, S.; Kazlauskas, K.; Köhler, A.; Strohriegl, P. Low Efficiency Roll-off Blue TADF OLEDs Employing a Novel Acridine–Pyrimidine Based High Triplet Energy Host. J. Mater. Chem. C 2021, 9, 17471–17482. [Google Scholar] [CrossRef]
- Devesing Girase, J.; Rani Nayak, S.; Tagare, J.; Shahnawaz; Ram Nagar, M.; Jou, J.-H.; Vaidyanathan, S. Solution-Processed Deep-Blue (Y∼0.06) Fluorophores Based on Triphenylamine-Imidazole (Donor-Acceptor) for OLEDs: Computational and Experimental Exploration. J. Inf. Disp. 2022, 23, 53–67. [Google Scholar] [CrossRef]
- Anupriya; Justin Thomas, K.R.; Nagar, M.R.; Jou, J.-H. Effect of Cyano Substituent on the Functional Properties of Blue Emitting Imidazo[1,2-a]Pyridine Derivatives. Dye. Pigment. 2022, 206, 110658. [Google Scholar] [CrossRef]
- Ohsawa, T.; Sasabe, H.; Watanabe, T.; Nakao, K.; Komatsu, R.; Hayashi, Y.; Hayasaka, Y.; Kido, J. A Series of Imidazo[1,2-f]Phenanthridine-Based Sky-Blue TADF Emitters Realizing EQE of over 20%. Adv. Opt. Mater. 2019, 7, 1801282. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Zhao, J.-W.; Li, P.; Feng, T.; Wang, W.-J.; Tao, S.-L.; Tong, Q.-X. Novel Phenanthroimidazole-Based Blue AIEgens: Reversible Mechanochromism, Bipolar Transporting Properties, and Electroluminescence. New J. Chem. 2018, 42, 8924–8932. [Google Scholar] [CrossRef]
- Godumala, M.; Choi, S.; Cho, M.J.; Choi, D.H. Thermally Activated Delayed Fluorescence Blue Dopants and Hosts: From the Design Strategy to Organic Light-Emitting Diode Applications. J. Mater. Chem. C 2016, 4, 11355–11381. [Google Scholar] [CrossRef]
- Kothavale, S.; Lee, K.H.; Lee, J.Y. Molecular Design Strategy of Thermally Activated Delayed Fluorescent Emitters Using CN-Substituted Imidazopyrazine as a New Electron-Accepting Unit. Chem. Asian J. 2020, 15, 122–128. [Google Scholar] [CrossRef]
- Anupriya; Thomas, K.R.J.; Nagar, M.R.; Shahnawaz; Jou, J.-H. Phenanthroimidazole Substituted Imidazo[1,2-a]Pyridine Derivatives for Deep-Blue Electroluminescence with CIEy ∼ 0.08. J. Photochem. Photobiol. A Chem. 2022, 423, 113600. [Google Scholar] [CrossRef]
- Taniya, O.S.; Fedotov, V.V.; Novikov, A.S.; Sadieva, L.K.; Krinochkin, A.P.; Kovalev, I.S.; Kopchuk, D.S.; Zyryanov, G.V.; Liu, Y.; Ulomsky, E.N.; et al. Abnormal Push-Pull Benzo[4,5]Imidazo[1,2-a][1,2,3]Triazolo[4,5-e]Pyrimidine Fluorophores in Planarized Intramolecular Charge Transfer (PLICT) State: Synthesis, Photophysical Studies and Theoretical Calculations. Dye. Pigment. 2022, 204, 110405. [Google Scholar] [CrossRef]
- Barik, S.; Skene, W.G. Turning-on the Quenched Fluorescence of Azomethines through Structural Modifications: Turning-on the Quenched Fluorescence of Azomethines. Eur. J. Org. Chem. 2013, 2013, 2563–2572. [Google Scholar] [CrossRef]
- Barluenga, J.; Aznar, F.; Valdes, C.; Cabal, M.P. Stereoselective Synthesis of 4-Piperidone and 4-Aminotetrahydropyridine Derivatives by the Imino Diels-Alder Reaction of 2-Amino-1,3-Butadienes. J. Org. Chem. 1993, 58, 3391–3396. [Google Scholar] [CrossRef]
- Montalvo-González, R.; Ariza-Castolo, A. Molecular Structure of Di-Aryl-Aldimines by Multinuclear Magnetic Resonance and X-Ray Diffraction. J. Mol. Struct. 2003, 655, 375–389. [Google Scholar] [CrossRef]
- Nowicka, A.; Liszkiewicz, H.; Nawrocka, W.; Wietrzyk, J.; Kempińska, K.; Dryś, A. Synthesis and Antiproliferative Activity in Vitro of New 2-Aminobenzimidazole Derivatives. Reaction of 2-Arylideneaminobenzimidazole with Selected Nitriles Containing Active Methylene Group. Open Chem. 2014, 12, 1047–1055. [Google Scholar] [CrossRef]
- Bogolubsky, A.V.; Moroz, Y.S.; Mykhailiuk, P.K.; Panov, D.M.; Pipko, S.E.; Konovets, A.I.; Tolmachev, A. A One-Pot Parallel Reductive Amination of Aldehydes with Heteroaromatic Amines. ACS Comb. Sci. 2014, 16, 375–380. [Google Scholar] [CrossRef]
- Yu, J.; Hu, P.; Zhou, T.; Xu, Y. Synthesis of Benzimidazole Thiazolinone Derivatives under Microwave Irradiation. J. Chem. Res. 2011, 35, 672–673. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yellol, G.S.; Lin, P.-T.; Sun, C.-M. Base-Catalyzed Povarov Reaction: An Unusual[1,3]Sigmatropic Rearrangement to Dihydropyrimidobenzimidazoles. Org. Lett. 2011, 13, 5120–5123. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Narhe, B.D.; Chang, Y.-S.; Sun, C.-M. One-Pot, Two-Step Synthesis of Imidazo[1,2-a]Benzimidazoles via A Multicomponent [4 + 1] Cycloaddition Reaction. ACS Comb. Sci. 2013, 15, 551–555. [Google Scholar] [CrossRef]
- Shah, A.P.; Hura, N.; Kishore Babu, N.; Roy, N.; Krishna Rao, V.; Paul, A.; Kumar Roy, P.; Singh, S.; Guchhait, S.K. A Core-Linker-Polyamine (CLP) Strategy Enables Rapid Discovery of Antileishmanial Aminoalkylquinolinecarboxamides That Target Oxidative Stress Mechanism. ChemMedChem 2022, 17, e202200109. [Google Scholar] [CrossRef]
- Kuznetsova, E.A.; Smolobochkin, A.V.; Rizbayeva, T.S.; Gazizov, A.S.; Voronina, J.K.; Lodochnikova, O.A.; Gerasimova, D.P.; Dobrynin, A.B.; Syakaev, V.V.; Shurpik, D.N.; et al. Diastereoselective Intramolecular Cyclization/Povarov Reaction Cascade for the One-Pot Synthesis of Polycyclic Quinolines. Org. Biomol. Chem. 2022, 20, 5515–5519. [Google Scholar] [CrossRef] [PubMed]
- Vicente-García, E.; Catti, F.; Ramón, R.; Lavilla, R. Unsaturated Lactams: New Inputs for Povarov-Type Multicomponent Reactions. Org. Lett. 2010, 12, 860–863. [Google Scholar] [CrossRef]
- Thakur, D.; Nagar, M.R.; Tomar, A.; Dubey, D.K.; Kumar, S.; Swayamprabha, S.S.; Banik, S.; Jou, J.-H.; Ghosh, S. Through Positional Isomerism: Impact of Molecular Composition on Enhanced Triplet Harvest for Solution-Processed OLED Efficiency Improvement. ACS Appl. Electron. Mater. 2021, 3, 2317–2332. [Google Scholar] [CrossRef]
- Anupriya; Thomas, K.R.J.; Nagar, M.R.; Shahnawaz; Jou, J.-H. Imidazo[1,2-a]Pyridine Based Deep-Blue Emitter: Effect of Donor on the Optoelectronic Properties. J. Mater. Sci. Mater. Electron. 2021, 32, 26838–26850. [Google Scholar] [CrossRef]
- Leung, C.W.T.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. A Photostable AIE Luminogen for Specific Mitochondrial Imaging and Tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef]
- Suman, G.R.; Pandey, M.; Chakravarthy, A.S.J. Review on New Horizons of Aggregation Induced Emission: From Design to Development. Mater. Chem. Front. 2021, 5, 1541–1584. [Google Scholar] [CrossRef]
- Kwon, M.S.; Gierschner, J.; Yoon, S.-J.; Park, S.Y. Unique Piezochromic Fluorescence Behavior of Dicyanodistyrylbenzene Based Donor-Acceptor-Donor Triad: Mechanically Controlled Photo-Induced Electron Transfer (eT) in Molecular Assemblies. Adv. Mater. 2012, 24, 5487–5492. [Google Scholar] [CrossRef]
- Le Bahers, T.; Adamo, C.; Ciofini, I. A Qualitative Index of Spatial Extent in Charge-Transfer Excitations. J. Chem. Theory Comput. 2011, 7, 2498–2506. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Peach, M.J.G.; Benfield, P.; Helgaker, T.; Tozer, D.J. Excitation Energies in Density Functional Theory: An Evaluation and a Diagnostic Test. J. Chem. Phys. 2008, 128, 044118. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, W.; Sztuba, B.; Kowalska, M.W.; Liszkiewicz, H.; Wietrzyk, J.; Nasulewicz, A.; Pełczyńska, M.; Opolski, A. Synthesis and Antiproliferative Activity in Vitro of 2-Aminobenzimidazole Derivatives. Il Farmaco 2004, 59, 83–91. [Google Scholar] [CrossRef]
- Rene, L.; Poncet, J.; Auzou, G. A One Pot Synthesis of β-Cyanoenamines. Synthesis 1986, 1986, 419–420. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]




 | |||||
|---|---|---|---|---|---|
| No. | Solvent 2 | Activating Agent (Catalysts) | X, Equiv | Reaction Condition 3 | Yield, % 4 |
| entry 1 | EtOH | BF3∙Et2O | 0.5 | reflux, 5 h | 35 |
| entry 2 | i-PrOH | BF3∙Et2O | 0.5 | reflux, 5 h | 46 |
| entry 3 | n-BuOH | BF3∙Et2O | 0.5 | reflux, 5 h | 50 |
| entry 4 | Toluene | BF3∙Et2O | 0.5 | reflux, 5 h | - |
| entry 5 | n-BuOH | BF3∙Et2O | 0.5 | reflux, 6 h | 51 |
| entry 6 | AcOH | - | - | reflux, 5 h | - |
| entry 7 | n-BuOH | Et3N | 0.5 | reflux, 5 h | - |
| entry 8 | n-BuOH | BF3∙Et2O | 1.0 | reflux, 5 h | 63 |
| entry 9 | n-BuOH | BF3∙Et2O | 1.5 | reflux, 5 h | 74 |
| entry 10 | n-BuOH | BF3∙Et2O | 2.0 | reflux, 5 h | 76 |
 | ||||||
|---|---|---|---|---|---|---|
| No. | Solvent 2 | Oxidant 3 | X, Equiv 3 | Reaction Condition 4 | Conversion, % 5 | Yield, % 6 |
| entry 1 | DMF | - | - | Heating 100 °C, 1 h | 10 | 5 |
| entry 2 | DMF | - | - | Heating 140 °C, 1 h | 15 | 6 |
| entry 3 | DMF | - | - | Heating 140 °C, 4 h | 30 | 17 |
| entry 4 | DMF | - | - | Heating 140 °C, 12 h | 40 | 30 |
| entry 5 | DMF | MnO2 | 1.0 | Heating 120 °C, 6 h | 100 | 83 |
| entry 6 | DMF | MnO2 | 2.0 | Heating 120 °C, 4 h | 100 | 84 |
| entry 7 | DMF | MnO2 | 3.0 | Heating 120 °C, 1.5 h | 100 | 86 |
| entry 8 | DMF | MnO2 | 4.0 | Heating 120 °C, 1.0 h | 100 | 85 |
| No. | λabsmax, nm (εM, 104 M−1 cm−1) 1 | λemmax, nm 2 | Stokes Shift, nm/cm−1 | τav, ns 3 | Φf, % 4 |
|---|---|---|---|---|---|
| 6a | 268 (4.8) 413 (0.74) | 554 | 141/6162 | 2.43 | 7.5 |
| 6b | 271 (3.53) 312 (0.52) 320 (0.52) 387 (0.11) | 540 | 153/7321 | 5.12 | 1.9 |
| 6c | 254 (14.5) 327 (0.45) 342 (0.45) 371 (0.49) 391 (0.48) | 550 | 159/7394 | 8.78 | <0.1 |
| 6d | 264 (4.12) 421 (0.76) | 567 | 146/6116 | 1.59 | 2.9 |
| 6e | 266 (5.65) 280 (5.30) 377 (0.14) | 520 | 143/7294 | 6.24 | 1.1 |
| 6f | 255 (13.21) 323 (0.43) 336 (0.47) 372 (0.48) 392 (0.45) | 524 | 132/6426 | 2.34 | 4.8 |
| No. | In PVA Film | In Powder | ||
|---|---|---|---|---|
| λemmax, nm | Φf, (%) 1 | λemmax, nm | Φf, (%) 1 | |
| 6a | 546 | 4.8 | 572 | 20.5 |
| 6b | 545 | 49.6 | 517 | 17.8 |
| 6c | 546 | 25.6 | 511 | 3.9 |
| 6d | 545 | 13.9 | 626 | 8.3 |
| 6e | 542 | 12.0 | 509 | 19.3 |
| 6f | 540 | 34.5 | 525 | 3.4 |
| Compound | HOMO, eV | LUMO, eV | ΔE, eV |
|---|---|---|---|
| 6a | −5.43 | −2.04 | 3.39 |
| 6b | −5.96 | −2.13 | 3.83 |
| 6c | −5.56 | −2.16 | 3.40 |
| 6d | −5.38 | −2.14 | 3.24 |
| 6e | −6.04 | −2.23 | 3.81 |
| 6f | −5.59 | −2.21 | 3.38 |
| Compound | Dipole Moment in Ground Multiplicity State (Debye) | Dipole Moment in Excited Multiplicity State (Debye) | D(Å) | Sr (a.u.) | t (Å) |
|---|---|---|---|---|---|
| 6b | 3.1676 | 4.3108 | 0.978 | 0.62822 | −0.326 |
| 6e | 4.0313 | 1.9340 | 0.927 | 0.62287 | −0.550 |
| 6a | 3.1463 | 9.2304 | 3.722 | 0.50976 | 0.617 |
| 6d | 6.5432 | 13.7974 | 3.832 | 0.50975 | 0.678 |
| 6c | 3.8859 | 1.8867 | 4.406 | 0.26547 | 2.599 |
| 6f | 1.7563 | 3.5274 | 4.523 | 0.24338 | 2.707 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotov, V.V.; Valieva, M.I.; Taniya, O.S.; Aminov, S.V.; Kharitonov, M.A.; Novikov, A.S.; Kopchuk, D.S.; Slepukhin, P.A.; Zyryanov, G.V.; Ulomsky, E.N.; et al. 4-(Aryl)-Benzo[4,5]imidazo[1,2-a]pyrimidine-3-Carbonitrile-Based Fluorophores: Povarov Reaction-Based Synthesis, Photophysical Studies, and DFT Calculations. Molecules 2022, 27, 8029. https://doi.org/10.3390/molecules27228029
Fedotov VV, Valieva MI, Taniya OS, Aminov SV, Kharitonov MA, Novikov AS, Kopchuk DS, Slepukhin PA, Zyryanov GV, Ulomsky EN, et al. 4-(Aryl)-Benzo[4,5]imidazo[1,2-a]pyrimidine-3-Carbonitrile-Based Fluorophores: Povarov Reaction-Based Synthesis, Photophysical Studies, and DFT Calculations. Molecules. 2022; 27(22):8029. https://doi.org/10.3390/molecules27228029
Chicago/Turabian StyleFedotov, Victor V., Maria I. Valieva, Olga S. Taniya, Semen V. Aminov, Mikhail A. Kharitonov, Alexander S. Novikov, Dmitry S. Kopchuk, Pavel A. Slepukhin, Grigory V. Zyryanov, Evgeny N. Ulomsky, and et al. 2022. "4-(Aryl)-Benzo[4,5]imidazo[1,2-a]pyrimidine-3-Carbonitrile-Based Fluorophores: Povarov Reaction-Based Synthesis, Photophysical Studies, and DFT Calculations" Molecules 27, no. 22: 8029. https://doi.org/10.3390/molecules27228029
APA StyleFedotov, V. V., Valieva, M. I., Taniya, O. S., Aminov, S. V., Kharitonov, M. A., Novikov, A. S., Kopchuk, D. S., Slepukhin, P. A., Zyryanov, G. V., Ulomsky, E. N., Rusinov, V. L., & Charushin, V. N. (2022). 4-(Aryl)-Benzo[4,5]imidazo[1,2-a]pyrimidine-3-Carbonitrile-Based Fluorophores: Povarov Reaction-Based Synthesis, Photophysical Studies, and DFT Calculations. Molecules, 27(22), 8029. https://doi.org/10.3390/molecules27228029







