Fluorescence and Nonlinear Optical Response of Graphene Quantum Dots Produced by Pulsed Laser Irradiation in Toluene
Abstract
1. Introduction
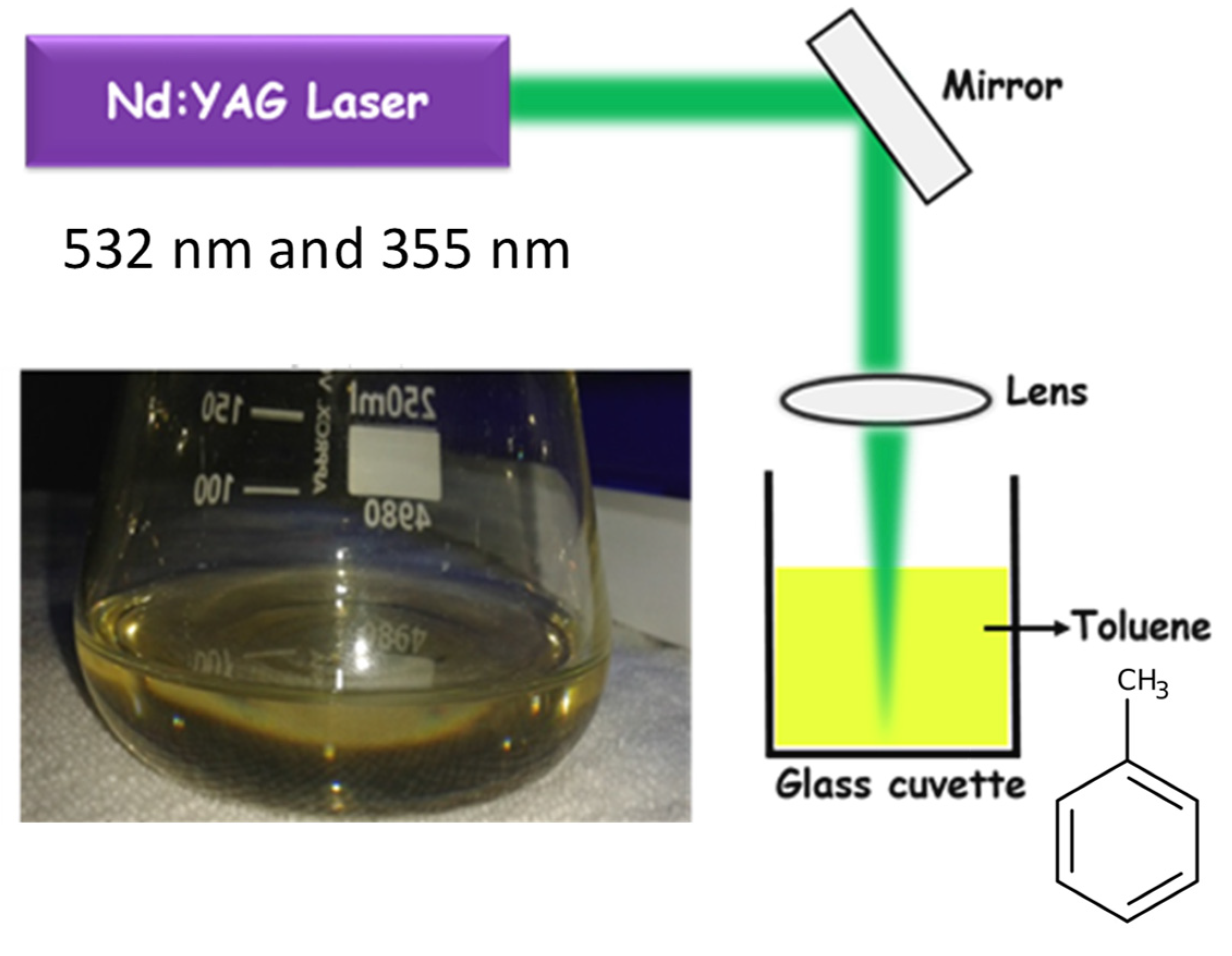
2. Experimental Details
2.1. Synthesis
2.2. Characterization
3. Results and Discussion
3.1. Morphological Analysis
3.2. XPS, FT-IR and Raman Analyses
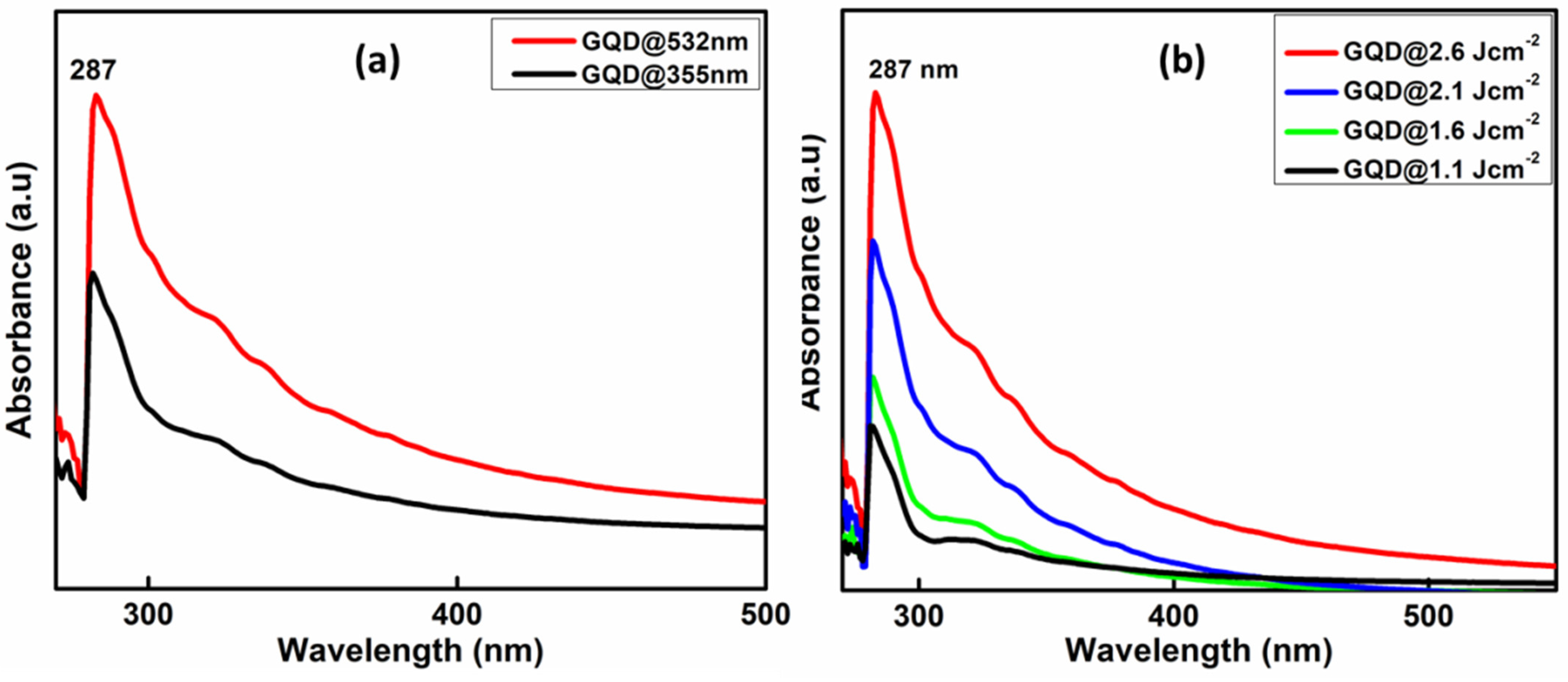
3.3. UV-Visible Absorption Spectroscopy
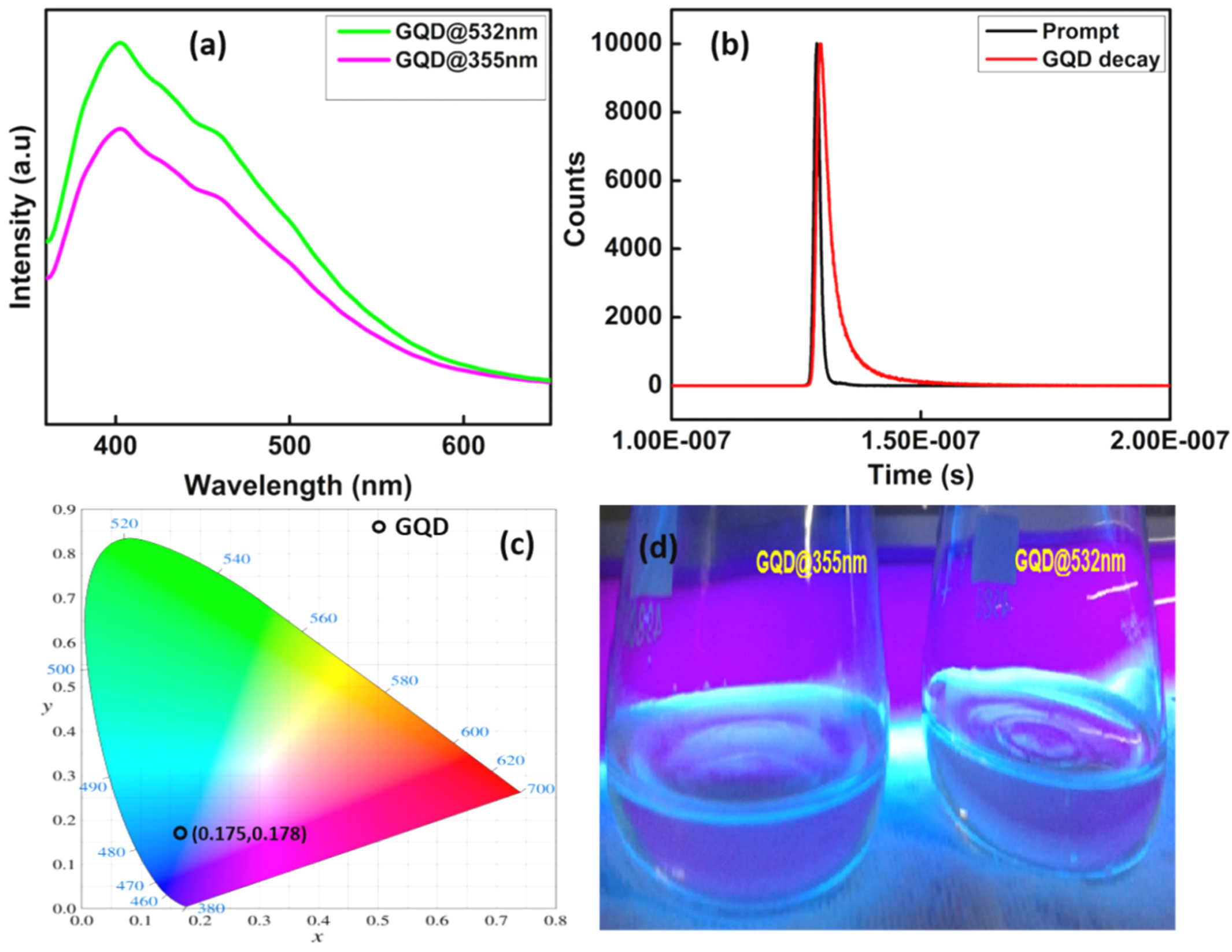
3.4. Photoluminescence Spectra
3.5. Nonlinear Optical Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene quantum dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Zheng, J.; Barton, R.A.; Englund, D. Broadband Coherent Absorption in Chirped-Planar-Dielectric Cavities for 2D-Material-Based Photovoltaics and Photodetectors. ACS Photon. 2014, 1, 768–774. [Google Scholar] [CrossRef]
- Urich, A.; Unterrainer, K.; Mueller, T. Intrinsic Response Time of Graphene Photodetectors. Nano Lett. 2011, 11, 2804–2808. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Yu, Y.-J.; Choi, H.; Choi, C.-G. Graphene-based plasmonic photodetector for photonic integrated circuits. Opt. Express 2014, 22, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Sobon, G.; Sotor, J.; Pasternak, I.; Strupiński, W.; Krzempek, K.; Kaczmarek, P.; Abramski, K.M. Chirped pulse amplification of a femtosecond Er-doped fiber laser mode-locked by a graphene saturable absorber. Laser Phys. Lett. 2013, 10, 35104. [Google Scholar] [CrossRef]
- Sun, Z.; Hasan, T.; Torrisi, F.; Popa, D.; Privitera, G.; Wang, F.; Bonaccorso, F.; Basko, D.M.; Ferrari, A.C. Graphene Mode-Locked Ultrafast Laser. ACS Nano 2010, 4, 803–810. [Google Scholar] [CrossRef]
- Sun, H.; Autschbach, J. Electronic Energy Gaps for π-Conjugated Oligomers and Polymers Calculated with Density Functional Theory. J. Chem. Theory Comput. 2014, 10, 1035–1047. [Google Scholar] [CrossRef]
- Hong, S.Y.; Dadap, J.I.; Petrone, N.; Yeh, P.C.; Hone, J.; Osgood, R.M., Jr. Optical third-harmonic generation in graphene. Phys. Rev. X 2013, 3, 021014. [Google Scholar] [CrossRef]
- Sørngård, S.A.; Simonsen, S.I.; Hansen, J.P. High-order harmonic generation from graphene: Strong attosecond pulses with arbitrary polarization. Phys. Rev. A 2013, 87, 053803. [Google Scholar] [CrossRef]
- Shen, T.-Z.; Hong, S.-H.; Song, J.-K. Electro-optical switching of graphene oxide liquid crystals with an extremely large Kerr coefficient. Nat. Mater. 2014, 13, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, X.; Wang, Q.; Huang, H.; Chen, W.; Wee, A.T.S.; Ji, W. Giant Two-Photon Absorption in Bilayer Graphene. Nano Lett. 2011, 11, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, R.; Zhao, Y.; Peng, Y.; Hong, X.; Xu, Q.; Zhao, S. Synthesis of CdTe/CdS/ZnS quantum dots and their application in imaging of hepatocellular carcinoma cells and immunoassay for alpha fetoprotein. Nanotechnology 2010, 21, 305101. [Google Scholar] [CrossRef] [PubMed]
- Mashford, B.S.; Stevenson, M.; Popovic, Z.; Hamilton, C.; Zhou, Z.; Breen, C.; Steckel, J.; Bulovic, V.; Bawendi, M.; Coe-Sullivan, S.; et al. High-efficiency quantum-dot light-emitting devices with enhanced charge injection. Nat. Photon. 2013, 7, 407–412. [Google Scholar] [CrossRef]
- Choi, H.; Radich, E.J.; Kamat, P.V. Sequentially Layered CdSe/CdS Nanowire Architecture for Improved Nanowire Solar Cell Performance. J. Phys. Chem. C 2013, 118, 206–213. [Google Scholar] [CrossRef]
- Lad, A.D.; Prem Kiran, P.; Ravindra Kumar, G.; Mahamuni, S. Three-photon absorption in ZnSe and Zn Se/Zn S quantum dots. Appl. Phys. Lett. 2007, 90, 133113. [Google Scholar] [CrossRef]
- Somers, R.C.; Bawendi, M.G.; Nocera, D.G. CdSe nanocrystal based chem-/bio- sensors. Chem. Soc. Rev. 2007, 36, 579–591. [Google Scholar] [CrossRef]
- Dennis, A.M.; Mangum, B.D.; Piryatinski, A.; Park, Y.-S.; Hannah, D.C.; Casson, J.L.; Williams, D.J.; Schaller, R.D.; Htoon, H.; Hollingsworth, J.A. Suppressed Blinking and Auger Recombination in Near-Infrared Type-II InP/CdS Nanocrystal Quantum Dots. Nano Lett. 2012, 12, 5545–5551. [Google Scholar] [CrossRef]
- Shavel, A.; Gaponik, A.N.; Eychmüller, A. Efficient UV-Blue Photoluminescing Thiol-Stabilized Water-Soluble Alloyed ZnSe(S) Nanocrystals. J. Phys. Chem. B 2004, 108, 5905–5908. [Google Scholar] [CrossRef]
- Rieger, R.; Müllen, K. Forever young: Polycyclic aromatic hydrocarbons as model cases for structural and optical studies. J. Phys. Org. Chem. 2010, 23, 315–325. [Google Scholar] [CrossRef]
- Güçlü, A.D.; Potasz, P.; Hawrylak, P. Excitonic absorption in gate-controlled graphene quantum dots. Phys. Rev. B 2010, 82, 155445. [Google Scholar] [CrossRef]
- Yamijala, S.S.; Bandyopadhyay, A.; Pati, S.K. Structural Stability, Electronic, Magnetic, and Optical Properties of Rectangular Graphene and Boron Nitride Quantum Dots: Effects of Size, Substitution, and Electric Field. J. Phys. Chem. C 2013, 117, 23295–23304. [Google Scholar] [CrossRef]
- Lin, L.; Rong, M.; Luo, F.; Chen, D.; Wang, Y.; Chen, X. Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. TrAC Trends Anal. Chem. 2014, 54, 83–102. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Bai, G.; Liu, C.P.; Hao, J.; Lin, J.; Jiang, H.; Teng, K.S.; Yang, Z.; et al. Deep Ultraviolet to Near-Infrared Emission and Photoresponse in Layered N-Doped Graphene Quantum Dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, B.; Li, L.-S. Colloidal Graphene Quantum Dots with Well-Defined Structures. Accounts Chem. Res. 2012, 46, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.J.; Liu, Z.B.; Li, Z.R.; Huang, X.R.; Sun, C.C. Shape effect of graphene quantum dots on enhancing second-order nonlinear optical response and spin multiplicity in NH2–GQD–NO2 systems. J. Phys. Chem. C 2011, 115, 16282–16286. [Google Scholar] [CrossRef]
- Yoneda, K.; Nakano, M.; Fukuda, K.; Champagne, B. The Odd Electron Density Is the Guide toward Achieving Organic Molecules with Gigantic Third-Order Nonlinear Optical Responses. J. Phys. Chem. Lett. 2012, 3, 3338–3342. [Google Scholar] [CrossRef]
- Deb, J.; Paul, D.; Sarkar, U. Density Functional Theory Investigation of Nonlinear Optical Properties of T-Graphene Quantum Dots. J. Phys. Chem. A 2020, 124, 1312–1320. [Google Scholar] [CrossRef]
- Tepliakov, N.V.; Orlov, A.V.; Kundelev, E.V.; Rukhlenko, I.D. Twisted Bilayer Graphene Quantum Dots for Chiral Nanophotonics. J. Phys. Chem. C 2020, 124, 22704–22710. [Google Scholar] [CrossRef]
- Ma, L.; Xiang, W.; Gao, H.; Wang, J.; Ni, Y.; Liang, X. Facile synthesis of tunable fluorescent carbon dots and their third-order nonlinear optical properties. Dyes Pigment. 2016, 128, 1–7. [Google Scholar] [CrossRef]
- Tetsuka, H.; Nagoya, A.; Tamura, S.-I. Graphene/nitrogen-functionalized graphene quantum dot hybrid broadband photodetectors with a buffer layer of boron nitride nanosheets. Nanoscale 2016, 8, 19677–19683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Wang, L.; Zhang, H.-C.; Liu, Y.; Wang, H.-Y.; Kang, Z.-H.; Lee, S.-T. Graphitic carbon quantum dots as a fluorescent sensing platform for highly efficient detection of Fe3+ ions. RSC Adv. 2013, 3, 3733–3738. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Lan, C.; Zhao, S. Graphene quantum dots as effective probes for label-free fluorescence detection of dopamine. Sensors Actuators B: Chem. 2016, 223, 246–251. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Y.; Na, W.; Zhang, X.; Li, Y.; Su, X. Graphene quantum dots as selective fluorescence sensor for the detection of ascorbic acid and acid phosphatase via Cr (VI)/Cr (III)-modulated redox reaction. J. Mater. Chem. B 2016, 4, 3278–3285. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, A.; Shan, F.; Yang, W.; Zhang, W.; Li, D.; Liu, J. One-step synthesis of graphene quantum dots from defective CVD graphene and their application in IGZO UV thin film phototransistor. Carbon 2016, 100, 201–207. [Google Scholar] [CrossRef]
- Qian, Z.; Shan, X.; Chai, L.; Ma, J.; Chen, J.; Feng, H. Si-Doped Carbon Quantum Dots: A Facile and General Preparation Strategy, Bioimaging Application, and Multifunctional Sensor. ACS Appl. Mater. Interfaces 2014, 6, 6797–6805. [Google Scholar] [CrossRef]
- Shen, C.; Ge, S.; Pang, Y.; Xi, F.; Liu, J.; Dong, X.; Chen, P. Facile and scalable preparation of highly luminescent N,S co-doped graphene quantum dots and their application for parallel detection of multiple metal ions. J. Mater. Chem. B 2017, 5, 6593–6600. [Google Scholar] [CrossRef]
- Li, M.; Wu, W.; Ren, W.; Cheng, H.-M.; Tang, N.; Zhong, W.; Du, Y. Synthesis and upconversion luminescence of N-doped graphene quantum dots. Appl. Phys. Lett. 2012, 101, 103107. [Google Scholar] [CrossRef]
- Ogale, S.B.; Patil, P.P.; Phase, D.M.; Bhandarkar, Y.V.; Kulkarni, S.K.; Ghaisas, S.V.; Kanetkar, S.M.; Bhide, V.G.; Guha, S. Synthesis of metastable phases via pulsed-laser-induced reactive quenching at liquid-solid interfaces. Phys. Rev. B 1987, 36, 8237–8250. [Google Scholar] [CrossRef]
- Gonçalves, H.; Jorge, P.A.; Fernandes, J.R.A.; da Silva, J.C.E. Hg(II) sensing based on functionalized carbon dots obtained by direct laser ablation. Sens. Actuators B Chem. 2010, 145, 702–707. [Google Scholar] [CrossRef]
- Yang, G.W. Laser ablation in liquids: Applications in the synthesis of nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [Google Scholar] [CrossRef]
- Yan, Z.; Chrisey, D.B. Pulsed laser ablation in liquid for micro-/nanostructure generation. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 204–223. [Google Scholar] [CrossRef]
- Xin, Y.; Kitasako, T.; Maeda, M.; Saitow, K.-I. Solvent dependence of laser-synthesized blue-emitting Si nanoparticles: Size, quantum yield, and aging performance. Chem. Phys. Lett. 2017, 674, 90–97. [Google Scholar] [CrossRef]
- Tarasenka, N.; Stupak, A.; Tarasenko, N.; Chakrabarti, S.; Mariotti, D. Structure and Optical Properties of Carbon Nanoparticles Generated by Laser Treatment of Graphite in Liquids. ChemPhysChem 2017, 18, 1074–1083. [Google Scholar] [CrossRef]
- Castro, H.P.; Souza, V.S.; Scholten, J.D.; Dias, J.H.; Fernandes, J.A.; Rodembusch, F.S.; Dos Reis, R.; Dupont, J.; Teixeira, S.R.; Correia, R.R. Synthesis and characterisation of fluorescent carbon nanodots produced in ionic liquids by laser ablation. Chem.–A Eur. J. 2016, 22, 138–143. [Google Scholar] [CrossRef]
- Thongpool, V.; Phunpueok, A.; Piriyawong, V.; Limsuwan, S.; Limsuwan, P. Pulsed Laser Ablation of Graphite Target in Dimethyformamide. Energy Procedia 2013, 34, 610–616. [Google Scholar] [CrossRef]
- Narasimhan, A.K.; Santra, T.S.; Rao, M.S.R.; Krishnamurthi, G. Oxygenated graphene quantum dots (GQDs) synthesized using laser ablation for long-term real-time tracking and imaging. RSC Adv. 2017, 7, 53822–53829. [Google Scholar] [CrossRef]
- Santiago, S.R.; Lin, T.N.; Chang, C.H.; Wong, Y.A.; Lin, C.A.; Yuan, C.T.; Shen, J.L. Synthesis of N-doped graphene quantum dots by pulsed laser ablation with diethylenetriamine (DETA) and their photoluminescence. Phys. Chem. Chem. Phys. 2017, 19, 22395–22400. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.; Zeng, X.; Lu, Y. Preparation of carbon dots by non-focusing pulsed laser irradiation in toluene. Chem. Commun. 2015, 52, 819–822. [Google Scholar] [CrossRef]
- Gokhale, R.R.; Thakare, V.; Warule, S.S.; Lefez, B.; Hannoyer, B.; Jog, J.P.; Ogale, S. From small aromatic molecules to functional nanostructured carbon by pulsed laser-induced photochemical stitching. AIP Adv. 2012, 2, 022130. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, S.; Chang, Y.; Yu, D.; Jiang, Y. Direct photodissociation of toluene molecules to photoluminescent carbon dots under pulsed laser irradiation. Carbon 2016, 105, 416–423. [Google Scholar] [CrossRef]
- Nancy, P.; Jose, J.; Joy, N.; Valluvadasan, S.; Philip, R.; Antoine, R.; Thomas, S.; Kalarikkal, N. Fabrication of Silver-Decorated Graphene Oxide Nanohybrids via Pulsed Laser Ablation with Excellent Antimicrobial and Optical Limiting Performance. Nanomaterials 2021, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep Ultraviolet Photoluminescence of Water-Soluble Self-Passivated Graphene Quantum Dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Sarkar, S.; Gandla, D.; Venkatesh, Y.; Bangal, P.R.; Ghosh, S.; Yang, Y.; Misra, S. Graphene quantum dots from graphite by liquid exfoliation showing excitation-independent emission, fluorescence upconversion and delayed fluorescence. Phys. Chem. Chem. Phys. 2016, 18, 21278–21287. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Rashid, S.A.; Shafie, S.; Soleimani, H. Laser ablation synthesis of Ag nanoparticles in graphene quantum dots aqueous solution and optical properties of nanocomposite. Appl. Phys. A 2019, 125, 82. [Google Scholar] [CrossRef]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnology 2004, 2, 3. [Google Scholar] [CrossRef]
- Tratsiak, Y.; Trusova, E.; Bokshits, Y.; Korjik, M.; Vaitkevičius, A.; Tamulaitis, G. Garnet-type crystallites, their isomorphism and luminescence properties in glass ceramics. CrystEngComm 2018, 21, 687–693. [Google Scholar] [CrossRef]
- Sakho, E.H.M.; Oluwafemi, O.S.; Perumbilavil, S.; Philip, R.; Kala, M.S.; Thomas, S.; Kalarikkal, N. Rapid and facile synthesis of graphene oxide quantum dots with good linear and nonlinear optical properties. J. Mater. Sci. Mater. Electron. 2016, 27, 10926–10933. [Google Scholar] [CrossRef]
- Nair, A.K.; Bhavitha, K.B.; Perumbilavil, S.; Sankar, P.; Rouxel, D.; Kala, M.S.; Thomas, S.; Kalarikkal, N. Multifunctional nitrogen sulfur co-doped reduced graphene oxide–Ag nano hybrids (sphere, cube and wire) for nonlinear optical and SERS applications. Carbon 2018, 132, 380–393. [Google Scholar] [CrossRef]
- Woldu, T.; Raneesh, B.; Sreekanth, P.; Reddy, M.R.; Philip, R.; Kalarikkal, N. Size dependent nonlinear optical absorption in BaTiO3 nanoparticles. Chem. Phys. Lett. 2015, 625, 58–63. [Google Scholar] [CrossRef]
- Raneesh, B.; Nandakumar, K.; Saha, A.; Das, D.; Soumya, H.; Philip, J.; Sreekanth, P.; Philip, R. Composition-structure–physical property relationship and nonlinear optical properties of multiferroic hexagonal ErMn1−xCrxO3 nanoparticles. RSC Adv. 2015, 5, 12480–12487. [Google Scholar] [CrossRef]
- Chen, K.; Su, W.; Wang, Y.; Ge, H.; Zhang, K.; Wang, Y.; Xie, X.; Gomes, V.G.; Sun, H.; Huang, L. Nanocomposites of carbon nanotubes and photon upconversion nanoparticles for enhanced optical limiting performance. J. Mater. Chem. C 2018, 6, 7311–7316. [Google Scholar] [CrossRef]
- Ma, C.; Wang, C.; Gao, B.; Adams, J.; Wu, G.; Zhang, H. Recent progress in ultrafast lasers based on 2D materials as a saturable absorber. Appl. Phys. Rev. 2019, 6, 041304. [Google Scholar] [CrossRef]
- Sutherland, R.L.; Hopkins, F.K. Handbook of Nonlinear Optics. Opt. Eng. 1997, 36, 964. [Google Scholar] [CrossRef]
- Karatay, A.; Aksoy, Ç.; Yaglioglu, H.G.; Elmali, A.; Kürüm, U.; Ateş, A.; Gasanly, N. The nonlinear and saturable absorption characteristics of Ga0.90In0.10Se and Ga0.85In0.15Se semiconductor crystals and their amorphous thin films. J. Opt. 2011, 13, 075203. [Google Scholar] [CrossRef]


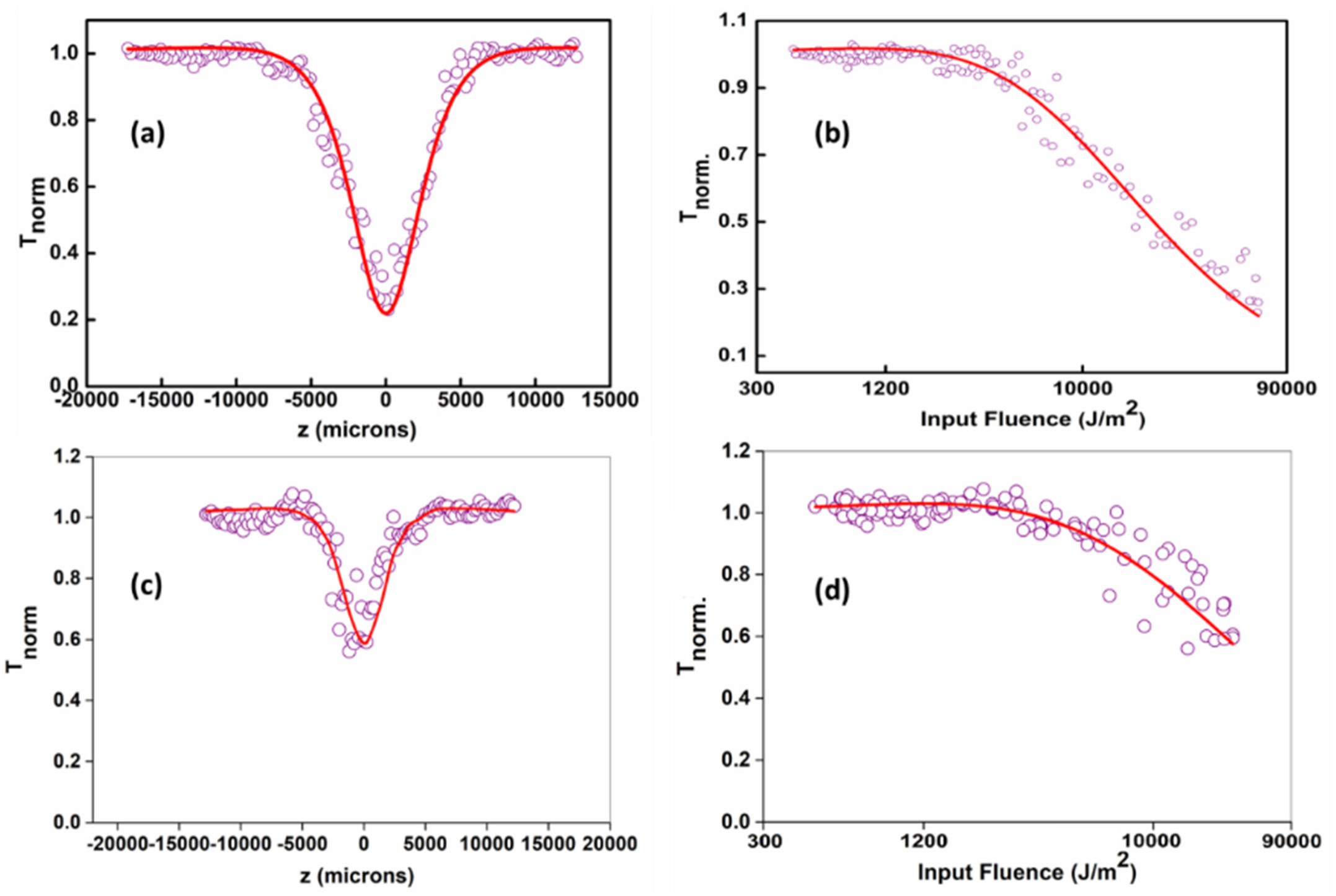
| Sample | Energy (µJ) | βeff (× 10−10 mW−1) | Isat (× 1010 Wm−2) | Optical Limiting Threshold (Jcm−2) |
|---|---|---|---|---|
| GQDs@532 nm | 25 | 4.3 | 57.7 | 0.45 |
| GQD@355 nm | 25 | 0.48 | 139.9 | 6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nancy, P.; Joy, N.; Valluvadasan, S.; Philip, R.; Thomas, S.; Antoine, R.; Kalarikkal, N. Fluorescence and Nonlinear Optical Response of Graphene Quantum Dots Produced by Pulsed Laser Irradiation in Toluene. Molecules 2022, 27, 7988. https://doi.org/10.3390/molecules27227988
Nancy P, Joy N, Valluvadasan S, Philip R, Thomas S, Antoine R, Kalarikkal N. Fluorescence and Nonlinear Optical Response of Graphene Quantum Dots Produced by Pulsed Laser Irradiation in Toluene. Molecules. 2022; 27(22):7988. https://doi.org/10.3390/molecules27227988
Chicago/Turabian StyleNancy, Parvathy, Nithin Joy, Sivakumaran Valluvadasan, Reji Philip, Sabu Thomas, Rodolphe Antoine, and Nandakumar Kalarikkal. 2022. "Fluorescence and Nonlinear Optical Response of Graphene Quantum Dots Produced by Pulsed Laser Irradiation in Toluene" Molecules 27, no. 22: 7988. https://doi.org/10.3390/molecules27227988
APA StyleNancy, P., Joy, N., Valluvadasan, S., Philip, R., Thomas, S., Antoine, R., & Kalarikkal, N. (2022). Fluorescence and Nonlinear Optical Response of Graphene Quantum Dots Produced by Pulsed Laser Irradiation in Toluene. Molecules, 27(22), 7988. https://doi.org/10.3390/molecules27227988









