Synthesis of Tetrasubstituted Phosphorus Analogs of Aspartic Acid as Antiproliferative Agents
Abstract
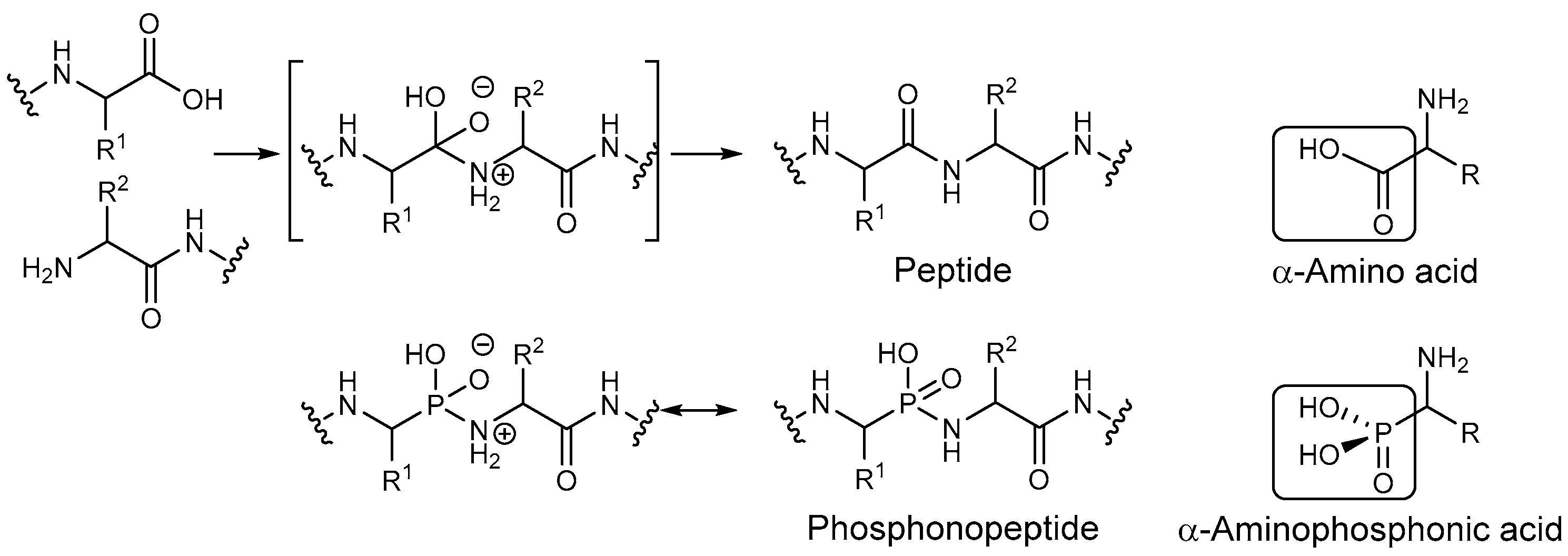
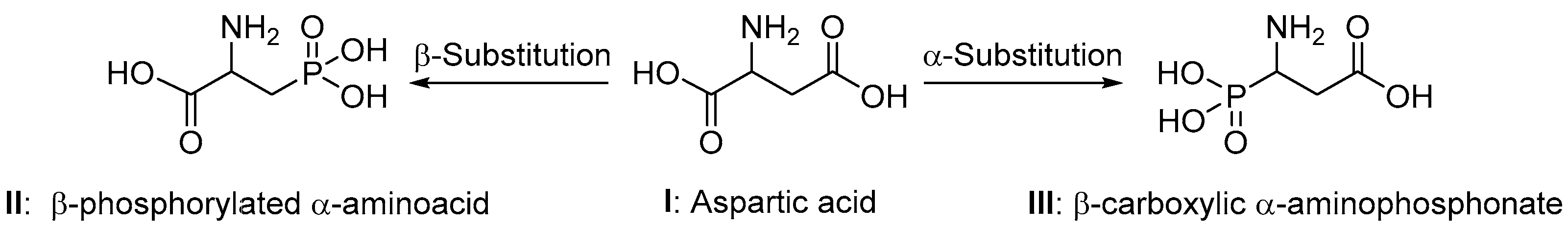
1. Introduction
2. Results and Discussion
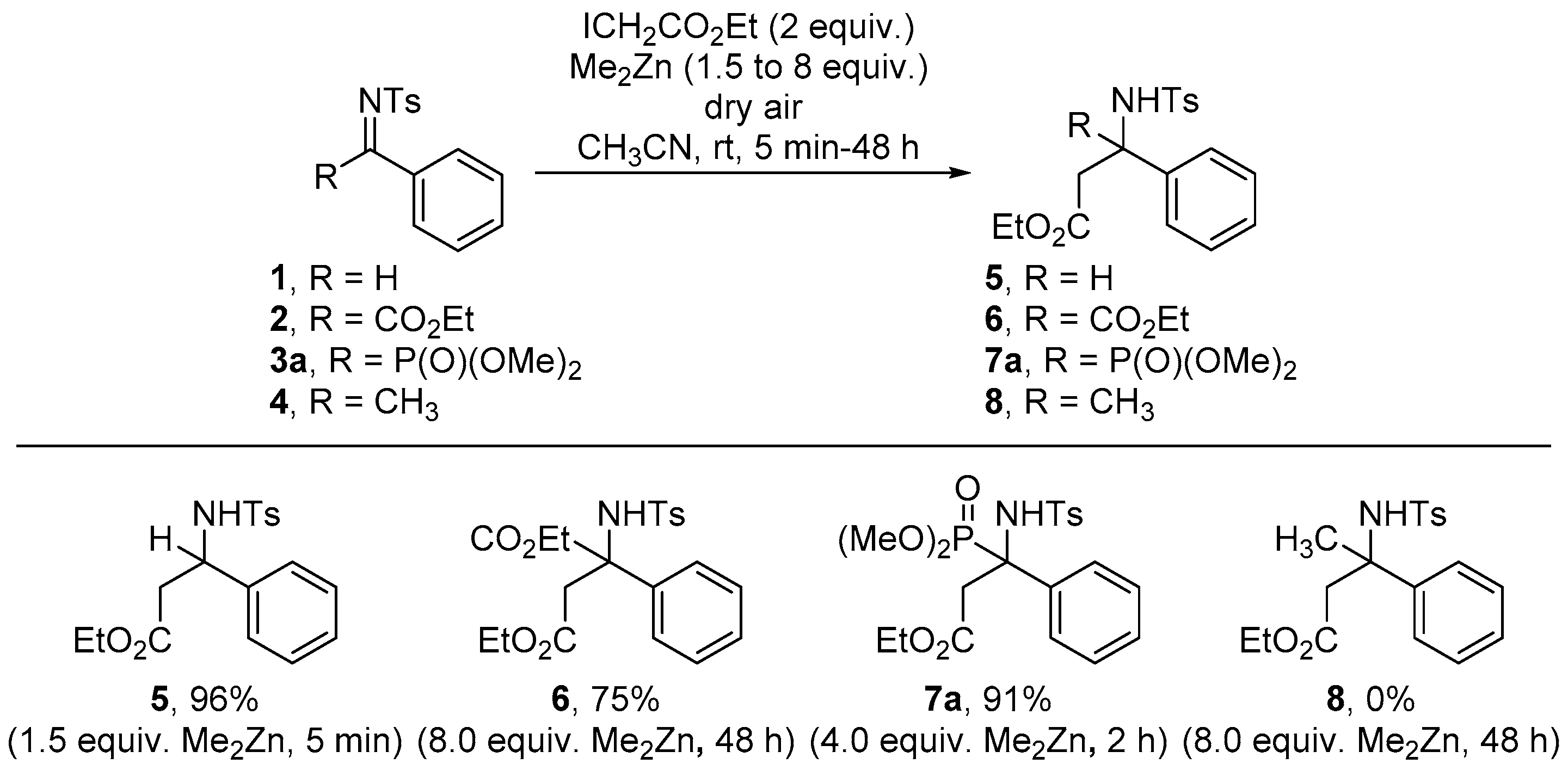
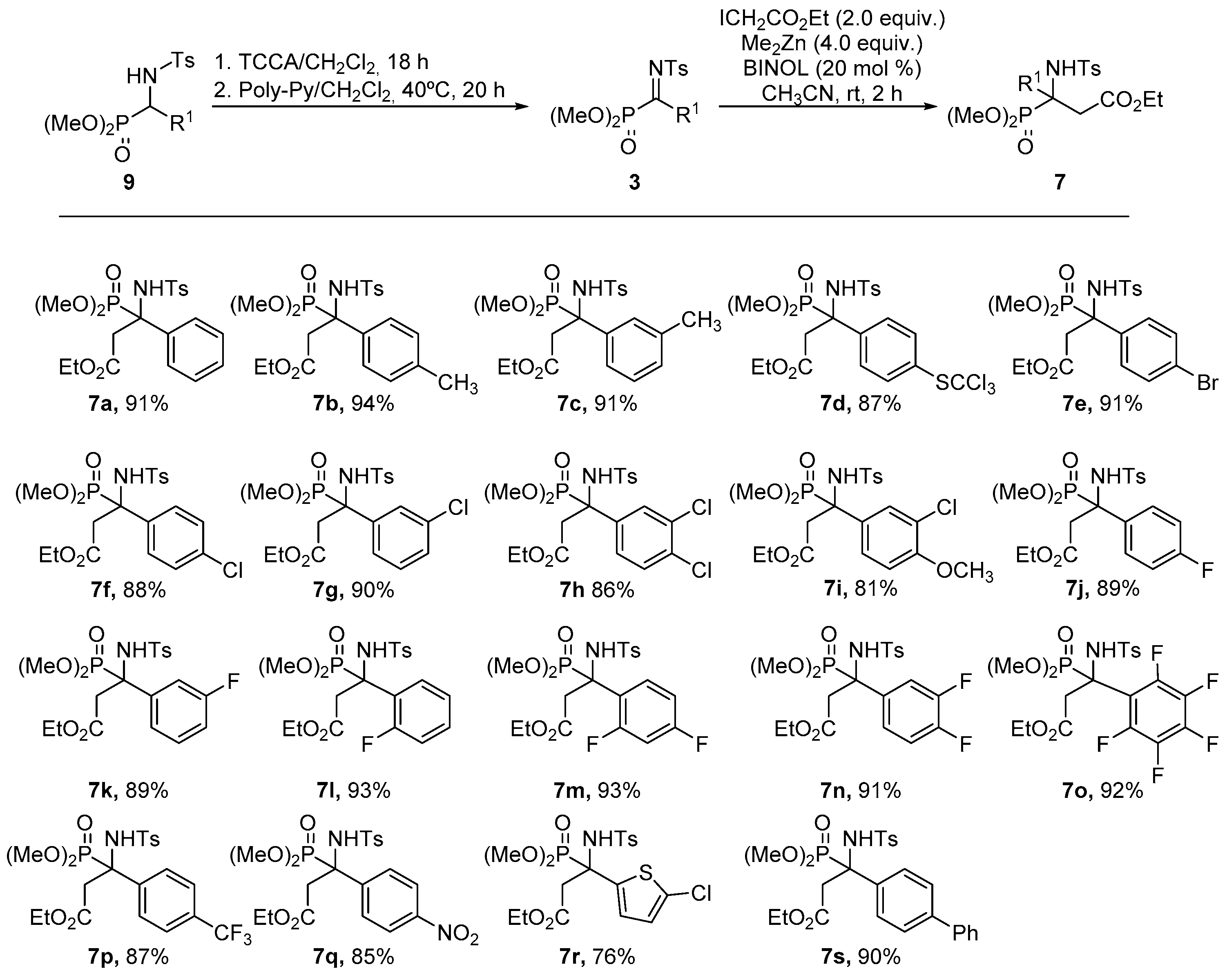
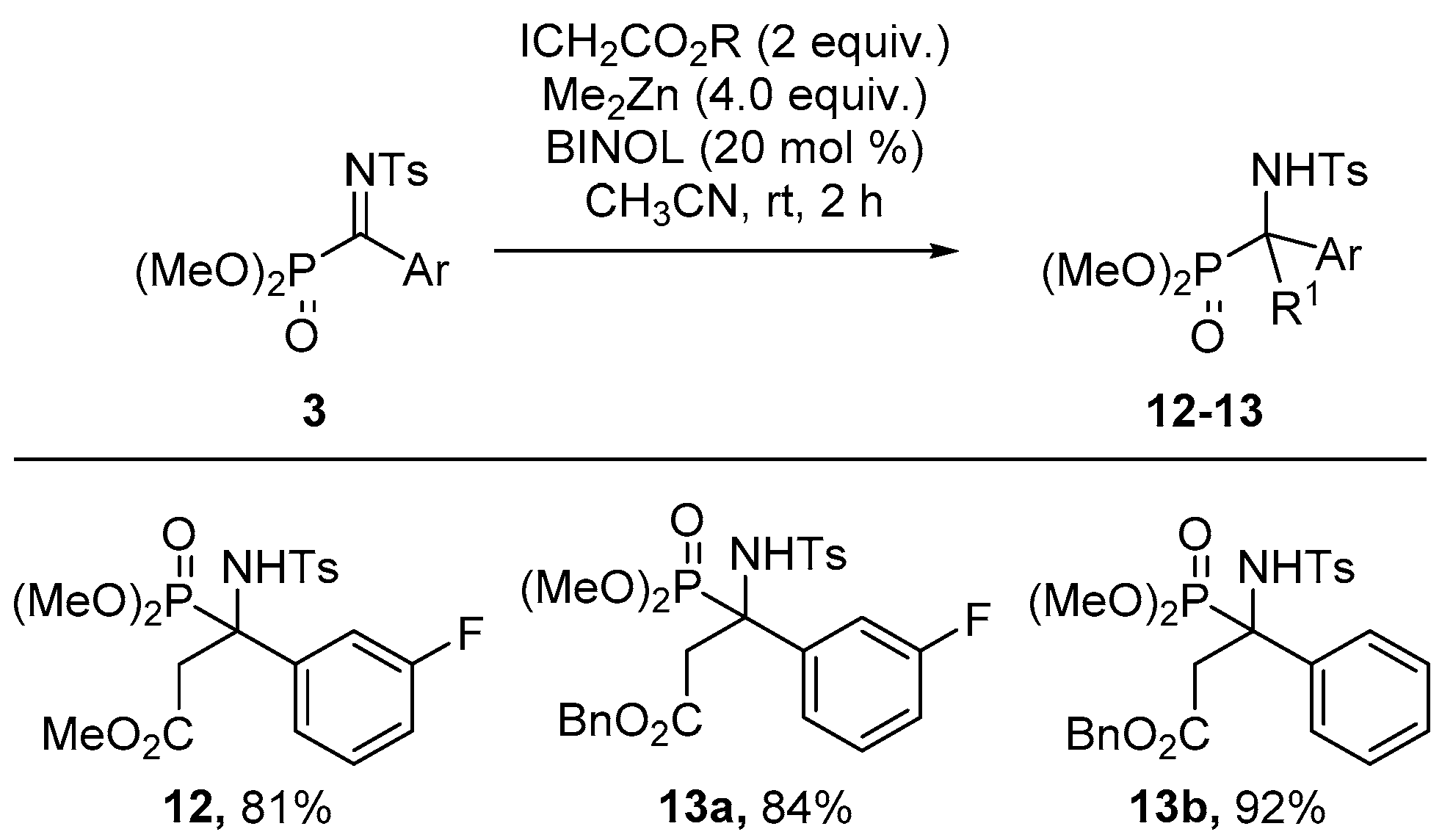
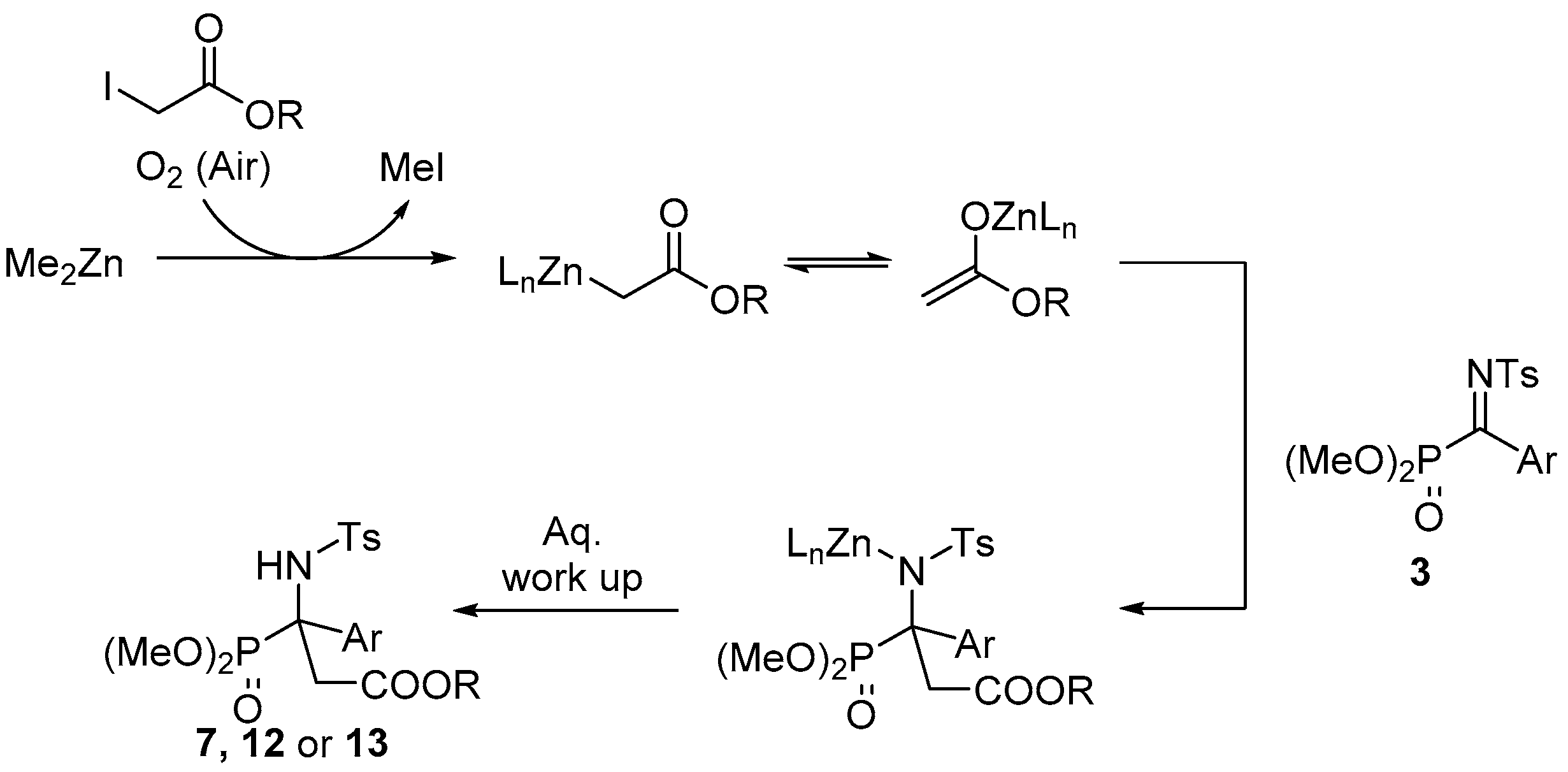
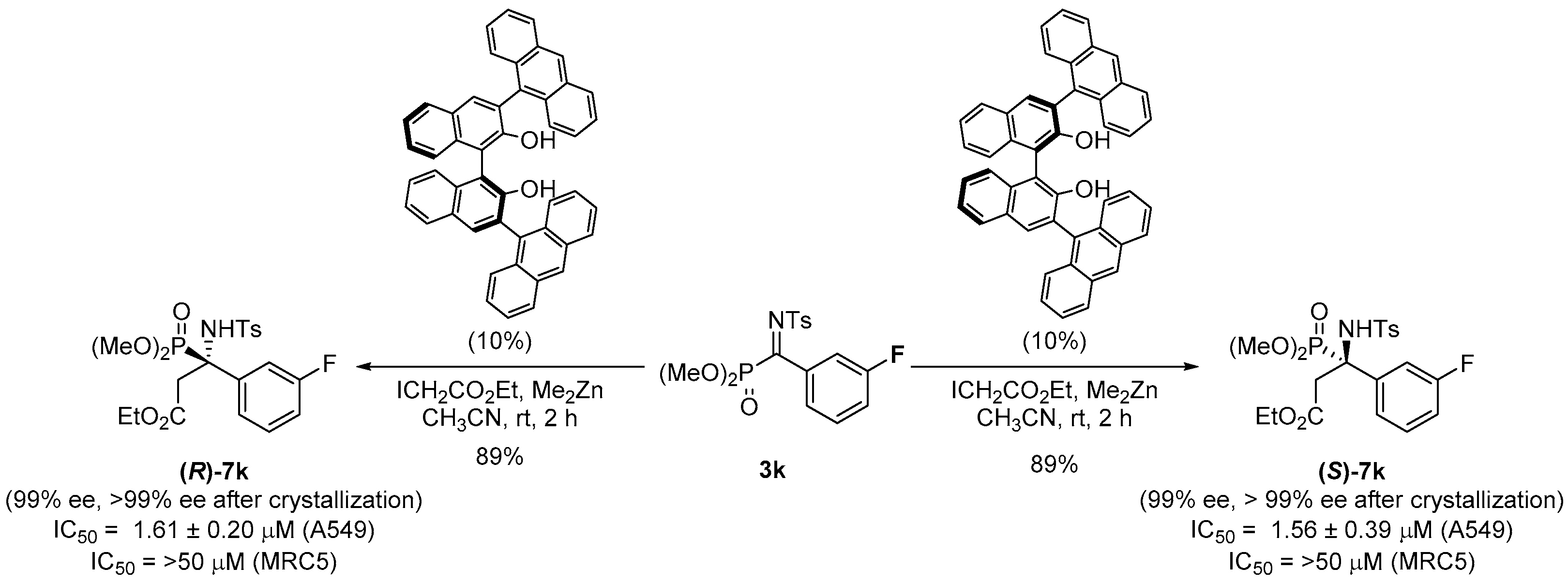
2.1. Chemistry
2.2. Biological Results
3. Materials and Methods
3.1. Chemistry
3.1.1. General Experimental Information
3.1.2. Compounds Purity Analysis
3.1.3. Experimental Procedures and Characterization Data for Compounds 3, 5, 6, 7, 12, 13, 14 and 18
General Procedure for Synthesis of α-Ketiminophosphonates 3
Procedure for the Synthesis of Ethyl 3-((4-methylphenyl)sulfonamido)-3-phenylpropanoate 5
Procedure for the Synthesis of Diethyl 2-((4-methylphenyl)sulfonamido)-2-phenylsuccinate 6
General Procedure for the Aza-Reformatsky Reaction of α-Ketiminophosphonates 3
Procedure for the Obtention of 3-(Dimethoxyphosphoryl)-3-((4-methylphenyl)sulfonamido)-3-phenylpropanoic acid 14
Procedure for the Obtention of Dimethyl (1-((4-methylphenyl)sulfonamido)-1-phenylethyl)phosphonate 18
3.2. Biology
3.2.1. Materials
3.2.2. Cell Culture
3.2.3. Cytotoxicity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Légaré, J. Population Aging: Economic and Social Consequences. In International Encyclopedia of the Social & Behavioral Sciences, 2nd ed.; Elsevier: Oxford, UK, 2015; p. 540. [Google Scholar]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359. [Google Scholar] [CrossRef] [PubMed]
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A. A concise review on cancer treatment methods and delivery systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769. [Google Scholar] [CrossRef]
- Horsman, G.P.; Zechel, D.L. Phosponate biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638. [Google Scholar] [CrossRef]
- Msaouel, P.; Galanis, E.; Koutsilieris, M. Somatostatin and somatostatin receptors: Implications for neoplastic growth and cancer biology. Expert Op. Investig. Drugs 2009, 18, 1297. [Google Scholar] [CrossRef]
- Caraglia, M.; D’Alessandro, A.M.; Marra, M.; Giuberti, G.; Vitale, G.; Viscomi, C.; Colao, A.M.; del Prete, S.; Tagliaferri, P.; Tassone, P.; et al. The farnesyl transferase inhibitor R115777 (Zarnestra®) synergistically enhances growth inhibition and apoptosis induced on epidermoid cancer cells by Zoledronic acid (Zometa®) and Pamidronate. Oncogene 2004, 23, 6900. [Google Scholar] [CrossRef][Green Version]
- Kukhar, V.P.; Hudson, H.R. (Eds.) Aminophosphonic and Aminophosphinic Acids. In Chemistry and Biological Activity; Wiley: Chichester, UK, 2000. [Google Scholar]
- Moonen, K.; Laureyn, I.; Stevens, C.V. Synthetic Methods for Azaheterocyclic Phosphonates and Their Biological Activity. Chem. Rev. 2004, 104, 2177. [Google Scholar] [CrossRef] [PubMed]
- Berlicki, L.; Kafarski, P. Computer-Aided Analysis and Design of Phosphonic and Phosphinic Enzyme Inhibitors as Potential Drugs and Agrochemicals. Curr. Org. Chem. 2005, 9, 1829. [Google Scholar] [CrossRef]
- Kafarski, P.; Lejczak, B. Aminophosphonic Acids of Potential Medical Importance. Curr. Med. Chem. Anti Cancer Ag. 2001, 1, 301. [Google Scholar] [CrossRef]
- Reddy, M.V.N.; Balakrishna, A.; Kumar, M.A.; Reddy, G.C.S.; Sankar, A.U.R.; Reddy, C.S.; Krishna, T.M. One-Step Synthesis and Bioassay of N-Phosphoramidophosphonates. Chem. Pharm. Bull. 2009, 57, 1391. [Google Scholar] [CrossRef]
- Che, J.-Y.; Xu, X.-Y.; Tang, Z.-L.; Gu, Y.-C.; Shi, D.-G. Synthesis and herbicidal activity evaluation of novel α-amino phosphonate derivatives containing a uracil moiety. Bioorg. Med. Chem. Lett. 2016, 26, 1310. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.N.; Kumar, B.S.; Balakrishna, A.; Reddy, C.S.; Nayak, S.K.; Reddy, C.D. One-pot synthesis of novel α-amino phosphonates using tetramethylguanidine as a catalyst. Arkivoc 2007, 15, 246. [Google Scholar] [CrossRef]
- Dake, S.A.; Raut, D.S.; Kharat, K.R.; Mhaske, R.S.; Deshmukh, S.U.; Pawar, R.P. Ionic liquid promoted synthesis, antibacterial and in vitro antiproliferative activity of novel α-aminophosphonate derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 2527. [Google Scholar] [CrossRef]
- El-Sayed, N.; Ewies, E.F.; Boulos, L.S.; Moharam, M.E. Synthesis of Novel Alkyl (dialkoxyphosphoryl)-1H-indole-3-yl)acetate, Dialkoxyphosphoryl[2,3 b]indole-3-carboxylate and Dialkyl methyl phosphonate Derivatives Using Wittig-Horner Reagents and their Antimicrobial Activity. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 926. [Google Scholar]
- Sivala, M.R.; Devineni, S.R.; Golla, M.; Medarametla, V.; Pothuru, G.K.; Chamarthi, N.R. A heterogeneous catalyst, SiO₂-ZnBr₂: An efficient neat access for α-aminophosphonates and antimicrobial activity evaluation. J. Chem. Sci. 2016, 128, 1303. [Google Scholar] [CrossRef]
- Rao, K.U.M.; Swapna, S.; Manidhar, D.M.; Reddy, K.M.K.; Reddy, C.S. Efficient Synthesis of α-Aminophosphonates and Evaluation of Significance of P=O Group towards Antioxidant Activity. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 232. [Google Scholar] [CrossRef]
- Damiche, R.; Chafaa, S. Synthesis of new bioactive aminophosphonates and study of their antioxidant, anti-inflammatory and antibacterial activities as well the assessment of their toxicological activity. J. Mol. Struct. 2017, 1130, 1009. [Google Scholar] [CrossRef]
- Srikant, B.; Parth, S.; Garg, S.K.; Misha, S.; Kaur, P.K.; Singh, S.; Chakrabarti, K.A. α-Aminophosphonates as novel anti-leishmanial chemotypes: Synthesis, biological evaluation, and CoMFA studies. MedChemCom. 2014, 5, 665. [Google Scholar]
- Mulla, S.A.R.; Pathay, M.Y.; Chavan, S.; Gample, S.P.; Sarkar, D. Highly efficient one-pot multi-component synthesis of α-aminophosphonates and bis-α-aminophosphonates catalyzed by heterogeneous reusable silica supported dodecatungstophosphoric acid (DTP/SiO2) at ambient temperature and their antitubercular evaluation against Mycobactrium Tuberculosis. RSC Adv. 2014, 4, 7666. [Google Scholar]
- Bhattacharya, A.K.; Raunt, D.S.; Rana, K.C.; Polanki, I.K.; Khan, M.S.; Tram, S. Diversity-oriented synthesis of α-aminophosphonates: A new class of potential anticancer agents. Eur. J. Med. Chem. 2013, 66, 146. [Google Scholar] [CrossRef]
- Yao, G.-Y.; Ye, M.-Y.; Huang, R.-Z.; Li, Y.-J.; Pan, Y.-J.; Xu, Q.; Liao, Z.-X.; Wang, H.-S. Synthesis and antitumor activities of novel rhein α-aminophosphonates conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 501. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Ding, H.; Chen, X.; Wang, H.; Tang, X. Synthesis, X-ray crystal structure, DNA/protein binding and cytotoxicity studies of five α-aminophosphonate N-derivatives. Bioorg. Chem. 2016, 69, 132. [Google Scholar] [CrossRef]
- Huang, R.-Z.; Wang, C.-Y.; Li, J.-F.; Yao, G.-Y.; Pang, Y.-M.; Ye, M.-Y.; Wang, H.-S.; Zhang, Y. Synthesis, antiproliferative and apoptosis-inducing effects of novel asiatic acid derivatives containing α-aminophosphonates. RSC Adv. 2016, 6, 62890. [Google Scholar] [CrossRef]
- Johnson, E.C. Aspartic acid. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Qian, R.; Kuliszewska, E.; Macoratti, E.; Hammerschmidt, F. Chemoenzymatic Synthesis of Racemic and Enantiomerically Pure Phosphaaspartic Acid and Phosphaarginine. Eur. J. Org. Chem. 2017, 2017, 4836. [Google Scholar] [CrossRef]
- Łyżwa, P.; Błaszczyk, J.; Sieroń, L.; Mikołajczyk, M. Asymmetric Synthesis of Structurally Diverse Aminophosphonic Acids by Using Enantiopure N-(p-Tolylsulfinyl)cinnamaldimines as Reagents. Eur. J. Org. Chem. 2013, 2013, 2106. [Google Scholar] [CrossRef]
- Campbell, M.M.; Carruthers, N.I.; Mickel, S.J. Aminophosphonic and aminophosphinic acid analogues of aspartic acid. Tetrahedron 1982, 38, 2513. [Google Scholar] [CrossRef]
- Vaseila, A.; Voeffray, R. Asymmetric Synthesis of α-Aminophosphonic Acids by Cycloaddition of N-Glycosyl-C-dialkoxyphosphonoylnitrones. Helv. Chim. Acta 1982, 65, 1953. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kiyohara, H.; Nakamura, Y.; Matsubara, R. Catalytic Asymmetric Synthesis of α-Amino Phosphonates Using Enantioselective Carbon−Carbon Bond-Forming Reactions. J. Am. Chem. Soc. 2004, 126, 6558. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Feng, F.-F.; Nie, J.; Zhang, F.-G.; Ma, J.-A. Enantioselective Construction of Amino Carboxylic-Phosphonic Acid Derivatives Enabled by Chiral Amino Thiourea-Catalyzed Decarboxylative Mannich Reaction. Adv. Synth. Catal. 2022, 364, 1908. [Google Scholar] [CrossRef]
- Shao, Q.; Wu, L.; Chen, J.; Gridnev, I.D.; Yang, G.; Xie, F.; Zhang, W. Copper (II)/RuPHOX-Catalyzed Enantioselective Mannich-Type Reaction of Glycine Schiff Bases with Cyclic Ketimines. Adv. Synth. Catal. 2018, 360, 4625. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Jana, U. General Procedure for the Synthesis of α-Amino Phosphonates from Aldehydes and Ketones Using Indium(III) Chloride as a Catalyst. Org. Lett. 1999, 1, 1141. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Nie, J.-S.L.J.; Ma, J.-A. Organocatalytic Asymmetric Decarboxylative Mannich Reaction of β-Keto Acids with Cyclic α-Ketiminophosphonates: Access to Quaternary α-Aminophosphonates. Org. Lett. 2018, 20, 3643. [Google Scholar] [CrossRef]
- Ross, N.A.; MacGregor, R.R.; Bartsch, R.A. Synthesis of β-lactams and β-aminoesters via high intensity ultrasound-promoted Reformatsky reactions. Tetrahedron 2004, 60, 2035. [Google Scholar] [CrossRef]
- Brinner, K.; Doughan, B.; Poon, D.J. Scalable Synthesis of β-Amino Esters via Reformatsky Reaction with N-tert-Butanesulfinyl Imines. Synlett 2009, 2009, 991. [Google Scholar] [CrossRef]
- Su, L.; Xu, M.-H. Asymmetric Reformatsky-Type Reaction of Isatin-Derived N-Sulfinyl Ketimines: Efficient and Practical Synthesis of Enantiopure Chiral 2-Oxoindolinyl-β3,3-Amino Esters. Synthesis 2016, 48, 2595. [Google Scholar]
- Jing, Z.T.; Huang, Y.G.; Qing, F.L. Synthesis of α-fluoro-β-amino acids via the Reformatsky reaction of chiral N-tert-butylsulfinylimines with ethyl bromofluoroacetate. Chin. Chem. Lett. 2011, 22, 919. [Google Scholar] [CrossRef]
- Anan, K.; Iso, Y.; Oguma, T.; Nakahara, K.; Suzuki, S.; Yamamoto, T.; Matsuoka, E.; Ito, H.; Sakaguchi, G.; Ando, S.; et al. Trifluoromethyl Dihydrothiazine-Based β-Secretase (BACE1) Inhibitors with Robust Central β-Amyloid Reduction and Minimal Covalent Binding Burden. ChemMedChem 2019, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- Woltering, T.J.; Wostl, W.; Hilpert, H.; Rogers-Evans, M.; Pinard, E.; Mayweg, A.; Göbel, M.; Banner, D.W.; Benz, J.; Travagli, M.; et al. BACE1 inhibitors: A head group scan on a series of amides. Bioorg. Med. Chem. Lett. 2013, 23, 4239. [Google Scholar] [CrossRef]
- Hilpert, H.; Guba, W.; Woltering, T.J.; Wostl, W.; Pinard, E.; Mauser, H.; Mayweg, A.V.; Rogers-Evans, M.; Humm, R.; Krummenacher, D.; et al. β-Secretase (BACE1) Inhibitors with High in Vivo Efficacy Suitable for Clinical Evaluation in Alzheimer’s Disease. J. Med. Chem. 2013, 56, 3980. [Google Scholar] [CrossRef]
- de Munck, L.; Vila, C.; Muñoz, M.C.; Pedro, J.R. Catalytic Enantioselective Aza-Reformatsky Reaction with Cyclic Imines. Chem. Eur. J. 2016, 22, 17590. [Google Scholar] [CrossRef] [PubMed]
- Ukaji, Y.; Yoshida, Y.; Inomata, K. Asymmetric addition of a Reformatsky-type reagent to 3,4-dihydroisoquinoline N-oxides. Tetrahedron Asymmetry 2000, 11, 733–736. [Google Scholar] [CrossRef]
- Cozzi, P.G. A Catalytic Enantioselective Imino-Reformatsky Reaction. Adv. Synth. Catal. 2006, 348, 2075. [Google Scholar] [CrossRef]
- Yutaka, U.; Shoichi, T.; Yoshie, H.; Katsuhiko, I. Asymmetric Addition of Reformatsky-Type Reagent to Imines Utilizing Diisopropyl Tartrate as a Chiral Auxiliary. Chem. Lett. 2001, 30, 254. [Google Scholar]
- Maestro, A.; de Marigorta, E.M.; Palacios, F.; Vicario, J. Enantioselective Aza-Reformatsky Reaction with Ketimines. Org. Lett. 2019, 21, 9473. [Google Scholar] [CrossRef]
- del Corte, X.; López-Francés, A.; Maestro, A.; Villate-Beitia, I.; Sainz-Ramos, M.; de Marigorta, E.M.; Pedraz, J.L.; Vicario, J. A Multicomponent Protocol for the Synthesis of Highly Functionalized γ-Lactam Derivatives and Their Applications as Antiproliferative Agents. Pharmaceuticals 2021, 14, 782. [Google Scholar] [CrossRef]
- del Corte, X.; López-Francés, A.; de Marigorta, E.M.; Palacios, F.; Vicario, J. Stereo- and Regioselective [3 + 3] Annulation Reaction Catalyzed by Ytterbium: Synthesis of Bicyclic 1,4-Dihydropyridines. Adv. Synth. Catal. 2021, 363, 4761–4771. [Google Scholar] [CrossRef]
- del Corte, X.; López-Francés, A.; Maestro, A.; Villate-Beitia, I.; Sainz-Ramos, M.; de Marigorta, E.M.; Palacios, F.; Alonso, C.; de los Santos, J.M.; Pedraz, J.L.; et al. Multicomponent Synthesis of Unsaturated γ-Lactam Derivatives. Applications as Antiproliferative Agents through the Bioisosterism Approach: Carbonyl vs. Phosphoryl Group. Pharmaceuticals 2022, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Martín-Encinas, E.; de Marigorta, E.M.; Alonso, C.; Rubiales, G.; Vicario, J.; Palacios, F. Synthesis of novel antiproliferative hybrid bis-(3-indolyl)methane phosphonate derivatives. Eur. J. Med. Chem. 2018, 158, 874. [Google Scholar] [CrossRef] [PubMed]
- López-Francés, A.; del Corte, X.; de Marigorta, E.M.; Palacios, F.; Vicario, J. Ugi reaction on α-phosphorated ketimines for the synthesis of tetrasubstituted α-aminophosphonates and their applications as antiproliferative agents. Molecules 2021, 26, 1654. [Google Scholar] [CrossRef] [PubMed]
- López-Francés, A.; del Corte, X.; Serna-Burgos, Z.; de Marigorta, E.M.; Palacios, F.; Vicario, J. Exploring the Synthetic Potential of γ-Lactam Derivatives Obtained from a Multicomponent Reaction. Applications as Antiproliferative Agents. Molecules 2022, 27, 3624. [Google Scholar] [CrossRef]
- Maestro, A.; de Marigorta, E.M.; Palacios, F.; Vicario, J. Enantioselective α-aminophosphonate functionalization of indole ring through an organocatalyzed Friedel-Crafts reaction. J. Org. Chem. 2019, 84, 1094. [Google Scholar] [CrossRef] [PubMed]
- Vicario, J.; Ezpeleta, J.M.; Palacios, F. Asymmetric Cyanation of α-Ketiminophosphonates Catalyzed by Cinchona Alkaloids: Enantioselective Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives from Trisubstituted α-Aminophosphonates. Adv. Synth. Catal. 2012, 354, 2641. [Google Scholar] [CrossRef]
- Vicario, J.; Ortiz, P.; Palacios, F. Synthesis of Tetrasubstituted α-Aminophosphonic Acid Derivatives from Trisubstituted α-Aminophosphonates. Eur. J. Org. Chem. 2013, 2013, 7095. [Google Scholar] [CrossRef]
- Vicario, J.; Ortiz, P.; Ezpeleta, J.M.; Palacios, F. Asymmetric Synthesis of Functionalized Tetrasubstituted α-Aminophosphonates through Enantioselective Aza-Henry Reaction of Phosphorylated Ketimines. J. Org. Chem. 2015, 80, 156. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in the asymmetric Reformatsky-type reaction. Beilstein J. Org. Chem. 2018, 14, 325. [Google Scholar] [CrossRef]
- Fernández-Ibáñez, M.A.; Maciá, B.; Alonso, D.A.; Pastor, I.M. Recent Advances in the Catalytic Enantioselective Reformatsky Reaction. Eur. J. Org. Chem. 2013, 2013, 7028. [Google Scholar] [CrossRef]
- Recio, R.; Vengut-Climent, E.; Mouillac, B.; Orcel, H.; López-Lázaro, M.; Calderón-Montaño, J.M.; Alvarez, E.; Khiar, N.; Design, I.F. synthesis and biological studies of a library of NK1-Receptor Ligands Based on a 5-arylthiosubstituted 2-amino-4,6-diaryl-3-cyano-4H-pyran core: Switch from antagonist to agonist effect by chemical modification. Eur. J. Med. Chem. 2017, 138, 644. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.J.; Kümmerle, A.E.; Fraga, C.A.M. The Methylation Effect in Medicinal Chemistry. Chem. Rev. 2011, 111, 5215. [Google Scholar] [CrossRef]
- Schönherr, H.; Cernak, T. Profound methyl effects in drug discovery and a call for new C-H methylation reactions. Angew. Chem. Int. Ed. 2013, 52, 12256. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Bégué, J.P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; Wiley: Hoboken, NJ, USA, 2007; 365p. [Google Scholar]

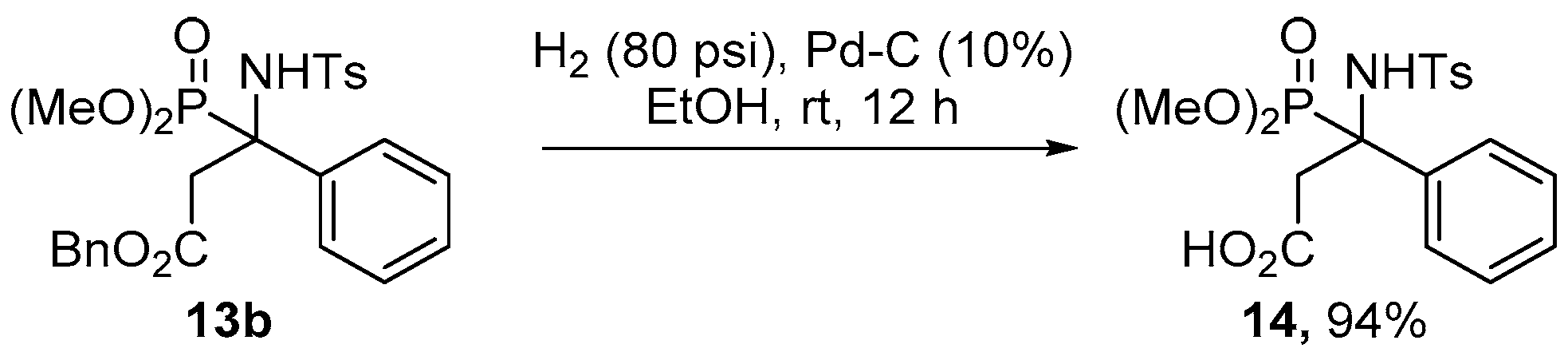

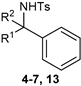 | ||||||
|---|---|---|---|---|---|---|
| Entry | Comp. | R1 | R2 | IC50 (μM) | ||
| SKOV3 | A549 | MRC5 | ||||
| 1 | 5 | CH2CO2Et | H | >50 | 18.68 ± 2.16 | >50 |
| 2 | 6 | CH2CO2Et | CO2Et | >50 | 14.17 ± 0.41 | >50 |
| 3 | 7a | CH2CO2Et | P(O)(OMe)2 | >50 | 2.66 ± 0.26 | >50 |
| 4 | 9a | H | P(O)(OMe)2 | >50 | 17.56 ± 1.3 | >50 |
| 5 | 18 | CH3 | P(O)(OMe)2 | >50 | >50 | n.d. |
| 6 | Doxorubicin | 0.13 ± 0.098 | <0.1 | >50 | ||
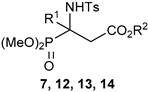 | ||||||
|---|---|---|---|---|---|---|
| Entry | Comp. | R1 | R2 | IC50 (μM) | ||
| SKOV3 | A549 | MRC5 | ||||
| 1 | 7a | Ph | Et | >50 | 2.66 ± 0.26 | >50 |
| 2 | 7b | 4-Me-C6H4 | Et | >50 | 0.34 ± 0.04 | 29.62 ± 2.98 |
| 3 | 7c | 3-Me-C6H4 | Et | >50 | 2.00 ± 0.52 | >50 |
| 4 | 7d | 4-Cl3CS-C6H4 | Et | 6.43 ±0.64 | 4.41 ± 0.29 | 2.47 ± 0.30 |
| 5 | 7e | 4-Br-C6H4 | Et | >50 | 1.08 ± 0.09 | >50 |
| 6 | 7f | 4-Cl-C6H4 | Et | 24.12 ± 1.45 | 3.09 ± 0.14 | >50 |
| 7 | 7g | 3-Cl-C6H4 | Et | >50 | 2.96 ± 0.34 | 38.05 ± 1.61 |
| 8 | 7h | 3,4-Cl2-C6H3 | Et | 6.94 ± 0.63 | 1.05 ± 0.42 | 11.29 ± 1.16 |
| 9 | 7i | 3-Cl-4-MeO-C6H3 | Et | >50 | 1.44 ± 0.15 | >50 |
| 10 | 7j | 4-F-C6H4 | Et | >50 | 7.15 ± 0.24 | >50 |
| 11 | 7k | 3-F-C6H4 | Et | >50 | 0.59 ± 0.09 | >50 |
| 12 | 7l | 2-F-C6H4 | Et | >50 | 0.90 ± 0.12 | >50 |
| 13 | 7m | 2,4-F2-C6H3 | Et | >50 | 2.24 ± 0.31 | >50 |
| 14 | 7n | 3,4-F2-C6H3 | Et | >50 | 5.70 ± 0.70 | >50 |
| 15 | 7o | C6F5 | Et | 20.46 ± 2.75 | 3.65 ± 0.21 | >50 |
| 16 | 7p | 4-CF3-C6H4 | Et | 9.80 ± 0.60 | 20. 30 ± 1.14 | >50 |
| 17 | 7q | 4-NO2-C6H4 | Et | >50 | 0.67 ± 0.06 | >50 |
| 18 | 7r | 5-Cl-2-thienyl | Et | 11.77 ± 0.60 | 1.04 ± 0.28 | >50 |
| 19 | 7s | 4-Ph-C6H4 | Et | 17.01 ± 1.22 | 1.48 ± 0.40 | >50 |
| 20 | 12 | 3-F-C6H4 | Me | >50 | 24.11 ± 4.01 | n.d. |
| 21 | 13a | 3-F-C6H4 | Bn | >50 | 12.75 ± 2.38 | n.d. |
| 22 | 13b | Ph | Bn | >50 | 5.20 ± 0.16 | >50 |
| 23 | 14 | Ph | H | >50 | 20.61 ± 1.05 | >50 |
| 24 | Doxorubicin | 0.13 ± 0.098 | <0.1 | >50 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Corte, X.; Maestro, A.; López-Francés, A.; Palacios, F.; Vicario, J. Synthesis of Tetrasubstituted Phosphorus Analogs of Aspartic Acid as Antiproliferative Agents. Molecules 2022, 27, 8024. https://doi.org/10.3390/molecules27228024
del Corte X, Maestro A, López-Francés A, Palacios F, Vicario J. Synthesis of Tetrasubstituted Phosphorus Analogs of Aspartic Acid as Antiproliferative Agents. Molecules. 2022; 27(22):8024. https://doi.org/10.3390/molecules27228024
Chicago/Turabian Styledel Corte, Xabier, Aitor Maestro, Adrián López-Francés, Francisco Palacios, and Javier Vicario. 2022. "Synthesis of Tetrasubstituted Phosphorus Analogs of Aspartic Acid as Antiproliferative Agents" Molecules 27, no. 22: 8024. https://doi.org/10.3390/molecules27228024
APA Styledel Corte, X., Maestro, A., López-Francés, A., Palacios, F., & Vicario, J. (2022). Synthesis of Tetrasubstituted Phosphorus Analogs of Aspartic Acid as Antiproliferative Agents. Molecules, 27(22), 8024. https://doi.org/10.3390/molecules27228024









