Facile Synthesis of Stable Cerium Dioxide Sols in Nonpolar Solvents
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of CeO2 Nanoparticles
2.3. Surface Modification of CeO2 Nanoparticles
2.4. Characterization Methods
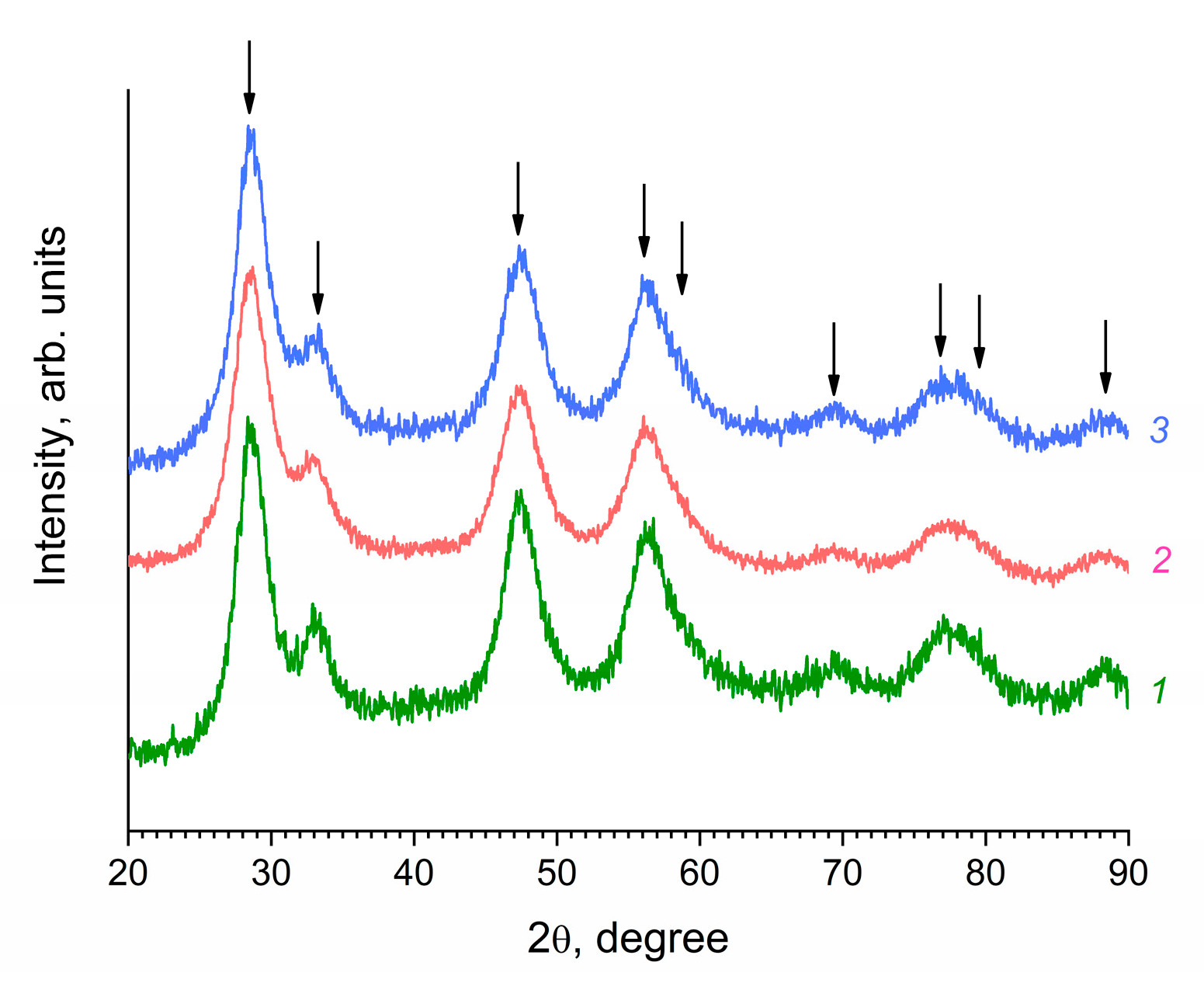
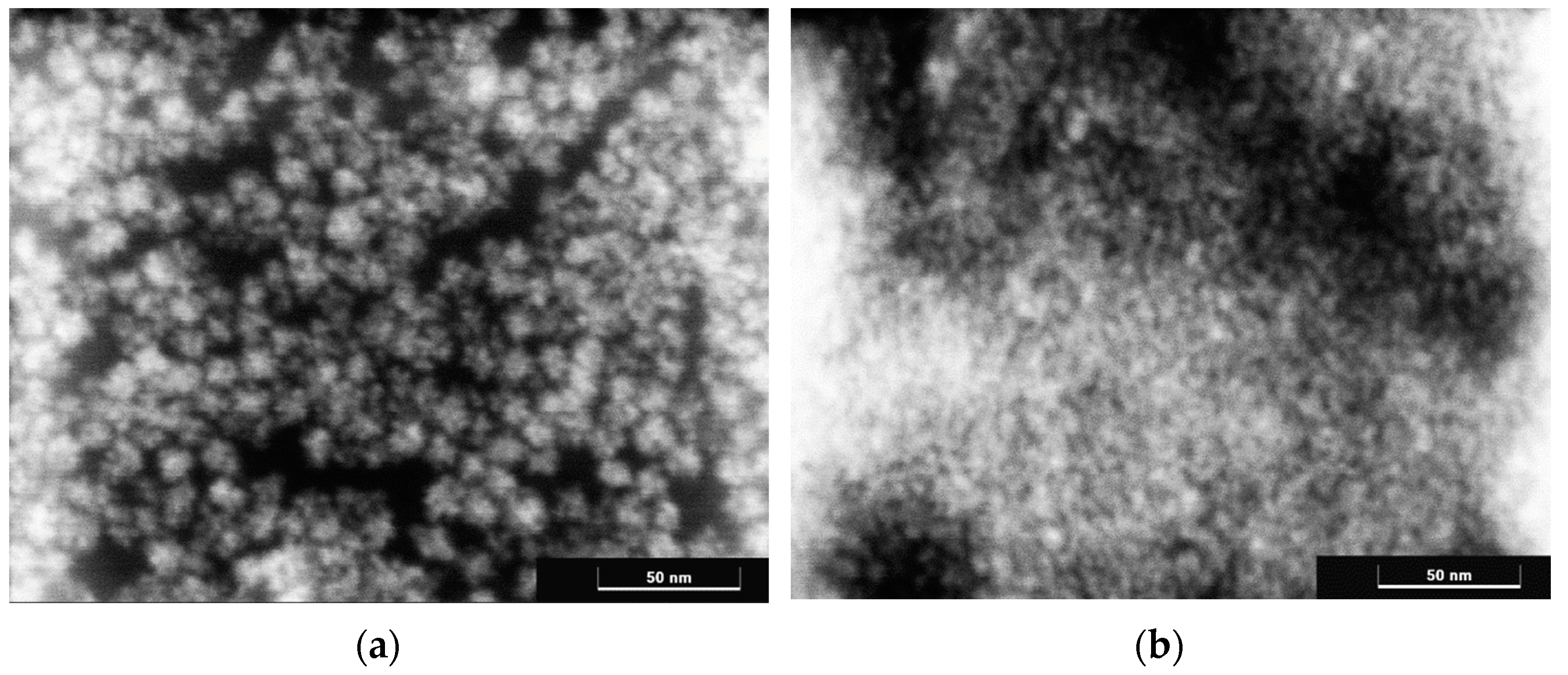
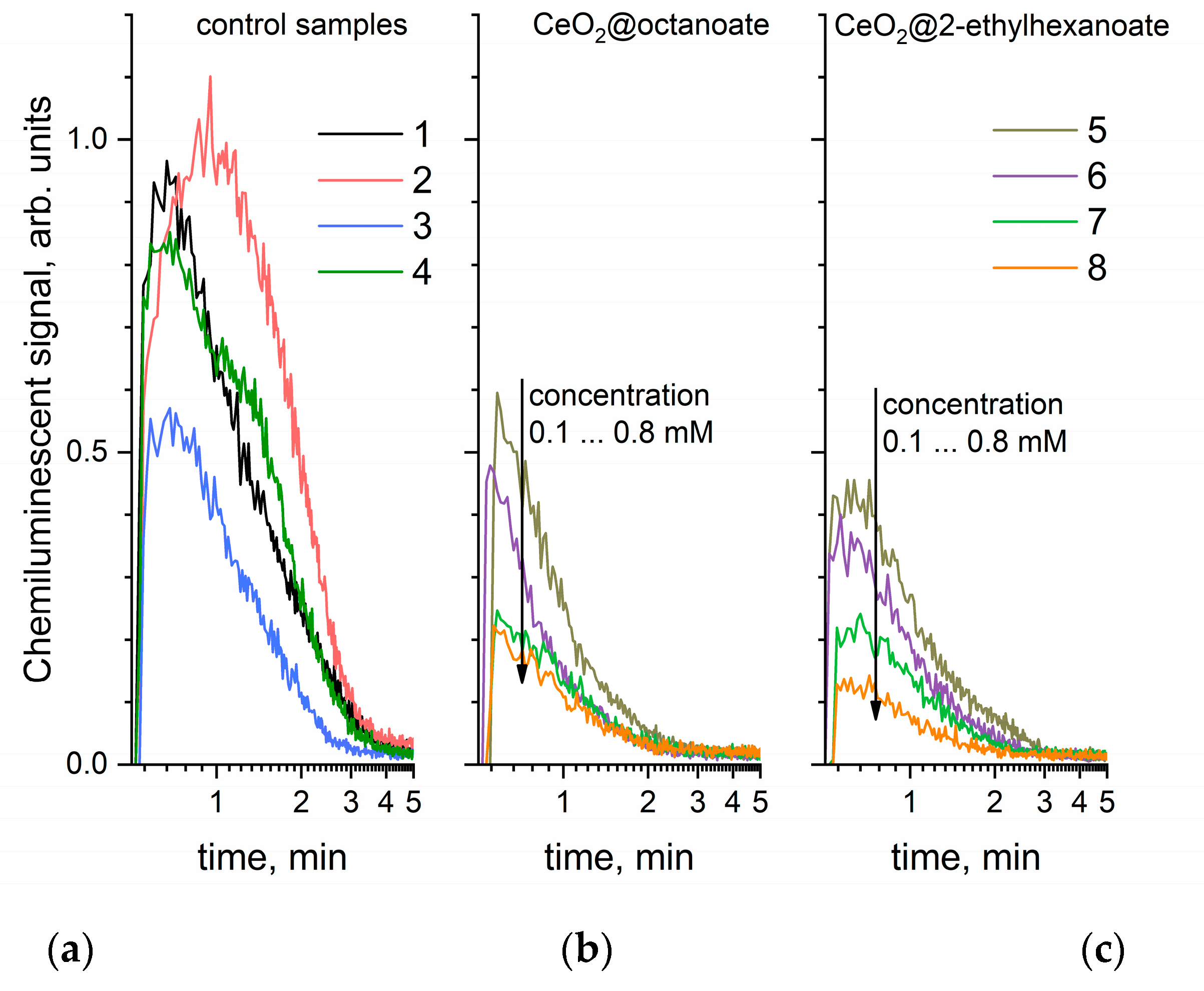
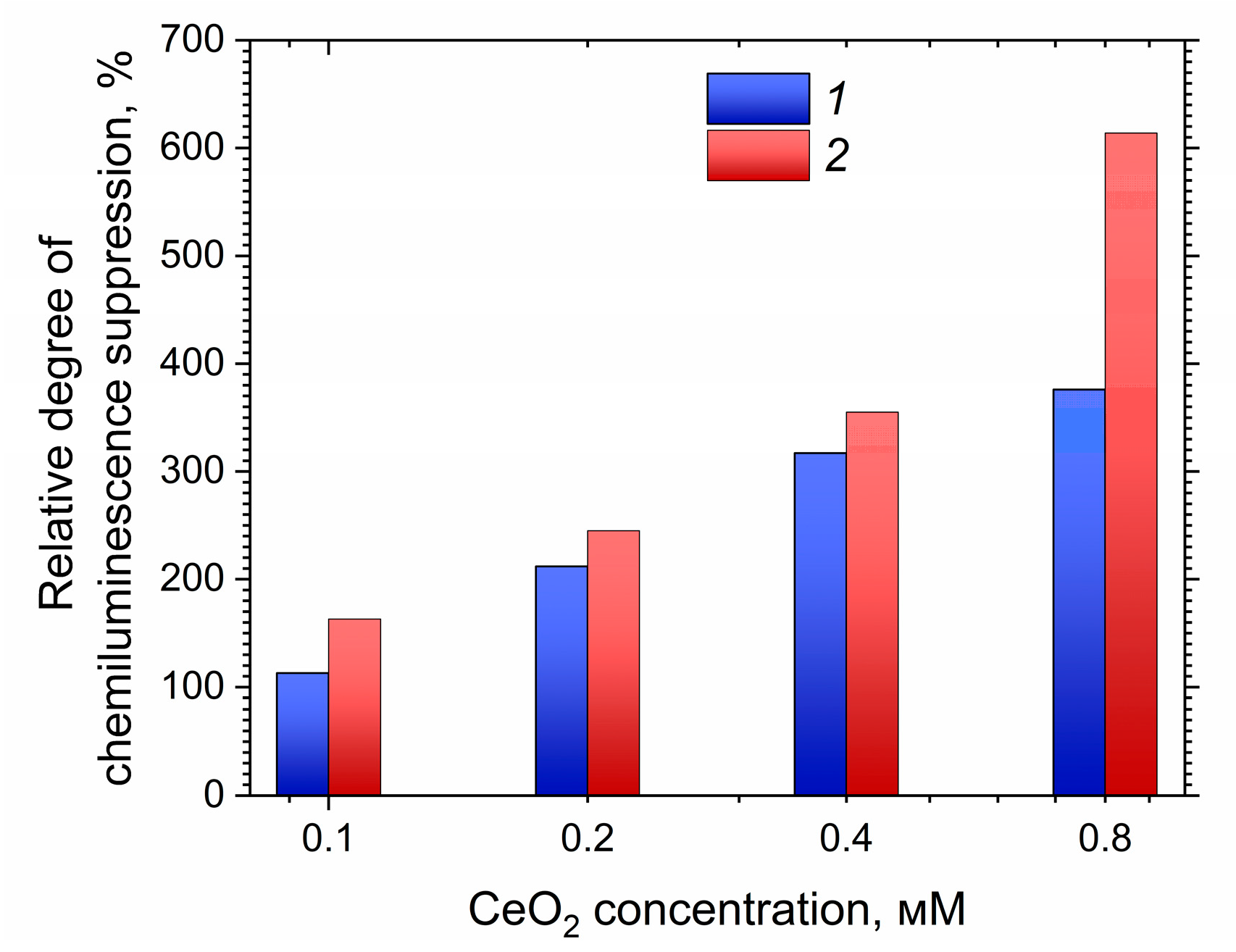
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Karakoti, A.S.; Monteiro-Riviere, N.A.; Aggarwal, R.; Davis, J.P.; Narayan, R.J.; Seif, W.T.; McGinnis, J.; Seal, S. Nanoceria as antioxidant: Synthesis and biomedical applications. JOM 2008, 60, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Banavar, S.; Deshpande, A.; Sur, S.; Andreescu, S. Ceria nanoparticle theranostics: Harnessing antioxidant properties in biomedicine and beyond. J. Phys. Mater. 2021, 4, 042003. [Google Scholar] [CrossRef]
- Walther, R.; Huynh, T.H.; Monge, P.; Fruergaard, A.S.; Mamakhel, A.; Zelikin, A.N. Ceria nanozyme and phosphate prodrugs: Drug synthesis through enzyme mimicry. ACS Appl. Mater. Interfaces 2021, 13, 25685–25693. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, C.; Gao, N.; Ren, J.; Qu, X. Stereoselective nanozyme based on ceria nanoparticles engineered with amino acids. Chem. A Eur. J. 2017, 23, 18146–18150. [Google Scholar] [CrossRef] [PubMed]
- Plakhova, T.V.; Romanchuk, A.Y.; Yakunin, S.N.; Dumas, T.; Demir, S.; Wang, S.; Minasian, S.G.; Shuh, D.K.; Tyliszczak, T.; Shiryaev, A.A.; et al. Solubility of nanocrystalline cerium dioxide: Experimental data and thermodynamic modeling. J. Phys. Chem. C 2016, 120, 22615–22626. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Wang, Z.; Cheng, F.; Zhang, P.; Mai, W.; Tong, Y. Ceria and ceria-based nanostructured materials for photoenergy applications. Nano Energy 2017, 34, 313–337. [Google Scholar] [CrossRef]
- Song, G.; Cheng, N.; Zhang, J.; Huang, H.; Yuan, Y.; He, X.; Luo, Y.; Huang, K. Nanoscale cerium oxide: Synthesis, biocatalytic mechanism, and applications. Catalysts 2021, 11, 1123. [Google Scholar] [CrossRef]
- Karakoti, A.; Singh, S.; Dowding, J.M.; Seal, S.; Self, W.T. Redox-active radical scavenging nanomaterials. Chem. Soc. Rev. 2010, 39, 4422. [Google Scholar] [CrossRef]
- Reed, K.; Cormack, A.; Kulkarni, A.; Mayton, M.; Sayle, D.; Klaessig, F.; Stadler, B. Exploring the properties and applications of nanoceria: Is there still plenty of room at the bottom? Environ. Sci. Nano 2014, 1, 390–405. [Google Scholar] [CrossRef] [Green Version]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef] [Green Version]
- López, J.M.; Gilbank, A.L.; García, T.; Solsona, B.; Agouram, S.; Torrente-Murciano, L. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation. Appl. Catal. B Environ. 2015, 174–175, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Mamontov, E.; Egami, T. Structural defects in a nano-scale powder of CeO2 studied by pulsed neutron diffraction. J. Phys. Chem. Solids 2000, 61, 1345–1356. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475. [Google Scholar] [CrossRef]
- Plakhova, T.V.; Romanchuk, A.Y.; Butorin, S.M.; Konyukhova, A.D.; Egorov, A.V.; Shiryaev, A.A.; Baranchikov, A.E.; Dorovatovskii, P.V.; Huthwelker, T.; Gerber, E.; et al. Towards the surface hydroxyl species in CeO2 nanoparticles. Nanoscale 2019, 11, 18142–18149. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K. Biological, biomedical and pharmaceutical applications of cerium oxide. In Cerium Oxide (CeO2): Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–358. [Google Scholar]
- Popov, A.L.; Popova, N.R.; Selezneva, I.I.; Akkizov, A.Y.; Ivanov, V.K. Cerium oxide nanoparticles stimulate proliferation of primary mouse embryonic fibroblasts in vitro. Mater. Sci. Eng. C 2016, 68, 406–413. [Google Scholar] [CrossRef]
- Özkan, E.; Badaczewski, F.; Cop, P.; Werner, S.; Hofmann, A.; Votsmeier, M.; Amenitsch, H.; Smarsly, B.M. Peering into the formation of cerium oxide colloidal particles in solution by In Situ small-angle X-ray scattering. Langmuir 2020, 36, 9175–9190. [Google Scholar] [CrossRef]
- Dippon, U.; Pabst, S.; Klitzke, S. Colloidal stabilization of CeO2 nanomaterials with polyacrylic acid, polyvinyl alcohol or natural organic matter. Sci. Total Environ. 2018, 645, 1153–1158. [Google Scholar] [CrossRef]
- Ivanova, O.S.; Shekunova, T.O.; Ivanov, V.K.; Shcherbakov, A.B.; Popov, A.L.; Davydova, G.A.; Selezneva, I.I.; Kopitsa, G.P.; Tret’yakov, Y.D. One-stage synthesis of ceria colloid solutions for biomedical use. Dokl. Chem. 2011, 437, 103–106. [Google Scholar] [CrossRef]
- Liu, Y.; He, S.; Uehara, M.; Maeda, H. Wet chemical preparation of well-dispersed colloidal cerium oxide nanocrystals. Chem. Lett. 2007, 36, 764–765. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Teplonogova, M.A.; Ivanova, O.S.; Shekunova, T.O.; Ivonin, I.V.; Baranchikov, A.Y.; Ivanov, V.K. Facile method for fabrication of surfactant-free concentrated CeO2 sols. Mater. Res. Express 2017, 4, 055008. [Google Scholar] [CrossRef]
- Ivanov, V.K.; Polezhaeva, O.S.; Shaporev, A.S.; Baranchikov, A.E.; Shcherbakov, A.B.; Usatenko, A.V. Synthesis and thermal stability of nanocrystalline ceria sols stabilized by citric and polyacrylic acids. Russ. J. Inorg. Chem. 2010, 55, 328–332. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Zholobak, N.M.; Spivak, N.Y.; Ivanov, V.K. Advances and prospects of using nanocrystalline ceria in cancer theranostics. Russ. J. Inorg. Chem. 2014, 59, 1556–1575. [Google Scholar] [CrossRef]
- Yabe, S.; Sato, T. Cerium oxide for sunscreen cosmetics. J. Solid State Chem. 2003, 171, 7–11. [Google Scholar] [CrossRef]
- Parwaiz, S.; Khan, M.M.; Pradhan, D. CeO2-based nanocomposites: An advanced alternative to TiO2 and ZnO in sunscreens. Mater. Express 2019, 9, 185–202. [Google Scholar] [CrossRef]
- Kolesnik, I.V.; Shcherbakov, A.B.; Kozlova, T.O.; Kozlov, D.A.; Ivanov, V.K. Comparative analysis of sun protection characteristics of nanocrystalline cerium dioxide. Russ. J. Inorg. Chem. 2020, 65, 960–966. [Google Scholar] [CrossRef]
- Singh, S.; Dosani, T.; Karakoti, A.S.; Kumar, A.; Seal, S.; Self, W.T. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials 2011, 32, 6745–6753. [Google Scholar] [CrossRef] [Green Version]
- Nyoka, M.; Choonara, Y.E.; Kumar, P.; Kondiah, P.P.D.; Pillay, V. Synthesis of cerium oxide nanoparticles using various methods: Implications for biomedical applications. Nanomaterials 2020, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Tapeinos, C.; Battaglini, M.; Prato, M.; La Rosa, G.; Scarpellini, A.; Ciofani, G. CeO2 nanoparticles-loaded pH-responsive microparticles with antitumoral properties as therapeutic modulators for osteosarcoma. ACS Omega 2018, 3, 8952–8962. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, H.; Wei, D.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration 2021, 1, 20210115. [Google Scholar] [CrossRef]
- Desai, S.A.; Manjappa, A.; Khulbe, P. Drug delivery nanocarriers and recent advances ventured to improve therapeutic efficacy against osteosarcoma: An overview. J. Egypt. Natl. Canc. Inst. 2021, 33, 4. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalysis by Ceria and Related Materials; Catalytic Science Series; Imperial College Press: London, UK, 2002; Volume 2, ISBN 978-1-86094-299-0. [Google Scholar]
- Dale, J.G.; Cox, S.S.; Vance, M.E.; Marr, L.C.; Hochella, M.F. Transformation of cerium oxide nanoparticles from a diesel fuel additive during combustion in a diesel engine. Environ. Sci. Technol. 2017, 51, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, N.; Shaafi, T. Enhancement of diesel fuel properties: Impact of cerium oxide nano additives on diesel engine performance and emissions. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Pandey, A.K.; Nandgaonkar, M.; Pandey, U.; Suresh, S.; Varghese, A. The Effect of Cerium Oxide Nano Particles Fuel Additive on Performance and Emission of Karanja Biodiesel Fueled Compression Ignition Military 585kW Heavy Duty Diesel Engine. SAE Technical Paper. 2018, 2018-01-1818. [Google Scholar] [CrossRef]
- Rao, T.S.; Babu Rao, H.S.; Jilani, S.A.K.; Mutluri, A. Effect of cerium oxide nanoparticles additive blended in palm oil biodiesel as alternative fuel used in diesel engine. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1112, 012012. [Google Scholar] [CrossRef]
- Prabu, A.; Premkumar, I.J.I.; Pradeep, A. An investigation on the performance, combustion and emission characteristics of C.I engine on the addition of antioxidants, oxygenates and nanoparticles as additives in Jatropha biodiesel. Int. J. Ambient Energy 2018, 41, 121–128. [Google Scholar] [CrossRef]
- Subramani, K.; Karuppusamy, M. Performance, combustion and emission characteristics of variable compression ratio engine using waste cooking oil biodiesel with added nanoparticles and diesel blends. Environ. Sci. Pollut. Res. 2021, 28, 63706–63722. [Google Scholar] [CrossRef]
- Wakefield, G.; Wu, X.; Gardener, M.; Park, B.; Anderson, S. EnviroxTM fuel-borne catalyst: Developing and launching a nano-fuel additive. Technol. Anal. Strateg. Manag. 2008, 20, 127–136. [Google Scholar] [CrossRef]
- Auffan, M.; Tella, M.; Liu, W.; Pariat, A.; Cabié, M.; Borschneck, D.; Angeletti, B.; Landrot, G.; Mouneyrac, C.; Giambérini, L.; et al. Structural and physical–chemical behavior of a CeO2 nanoparticle based diesel additive during combustion and environmental release. Environ. Sci. Nano 2017, 4, 1974–1980. [Google Scholar] [CrossRef]
- Park, B.; Martin, P.; Harris, C.; Guest, R.; Whittingham, A.; Jenkinson, P.; Handley, J. Initial in vitro screening approach to investigate the potential health and environmental hazards of EnviroxTM—A nanoparticulate cerium oxide diesel fuel additive. Part. Fibre Toxicol. 2007, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Donaldson, K.; Duffin, R.; Tran, L.; Kelly, F.; Mudway, I.; Morin, J.-P.; Guest, R.; Jenkinson, P.; Samaras, Z.; et al. Hazard and risk assessment of a nanoparticulate cerium oxide-based diesel fuel additive—A case study. Inhal. Toxicol. 2008, 20, 547–566. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.S.; Chai, S.Y.; Cha, D.H.; Choi, Y.S.; Lee, W.I. Syntheses of monodispersed SnO2 and CeO2 nanoparticles through the self-capping role of 2-ethylhexanoate ligands. New J. Chem. 2007, 31, 260–264. [Google Scholar] [CrossRef]
- Kobayashi, K.; Haneda, M.; Ozawa, M. Dispersion of oleate-modified CeO2 nanocrystals in non-polar solvent and aqueous solution. ECS Trans. 2013, 50, 39–49. [Google Scholar] [CrossRef]
- Pettinger, N.W.; Williams, R.E.A.; Chen, J.; Kohler, B. Crystallization kinetics of cerium oxide nanoparticles formed by spontaneous, room-temperature hydrolysis of cerium(IV) ammonium nitrate in light and heavy water. Phys. Chem. Chem. Phys. 2017, 19, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gurau, G.; Kelley, S.P.; Myerson, A.S.; Rogers, R.D. Hydrophobic vs. hydrophilic ionic liquid separations strategies in support of continuous pharmaceutical manufacturing. RSC Adv. 2013, 3, 10019. [Google Scholar] [CrossRef]
- Alley, W.M.; Girard, C.W.; Özkar, S.; Finke, R.G. Model Ziegler-type hydrogenation catalyst precursors, [(1,5-COD)M(μ-O2C8H15)]2 (M = Ir and Rh): Synthesis, characterization, and demonstration of catalytic activity en route to identifying the true industrial hydrogenation catalysts. Inorg. Chem. 2009, 48, 1114–1121. [Google Scholar] [CrossRef]
- Hakim, S.S.; Olsson, M.H.M.; Sørensen, H.O.; Bovet, N.; Bohr, J.; Feidenhans’l, R.; Stipp, S.L.S. Interactions of the calcite {10.4} surface with organic compounds: Structure and behaviour at mineral—Organic interfaces. Sci. Rep. 2017, 7, 7592. [Google Scholar] [CrossRef] [Green Version]
- Bond, A.D. On the crystal structures and melting point alternation of the n-alkyl carboxylic acids. New J. Chem. 2004, 28, 104–114. [Google Scholar] [CrossRef]
- Lah, N.; Koller, J.; Giester, G.; Segedin, P.; Leban, I. Copper(II) carboxylates with 4-aminopyridine: Neutral mononuclear structures, isomerism of aceto compounds and a novel tetranuclear structure. New J. Chem. 2002, 26, 933–938. [Google Scholar] [CrossRef]
- Tackett, J.E. FT-IR Characterization of metal acetates in aqueous solution. Appl. Spectrosc. 1989, 43, 483–489. [Google Scholar] [CrossRef]
- Zhu, H.; Hill, R. The photochemical metal organic deposition of manganese oxide films from films of manganese(II) 2-ethylhexanoate: A mechanistic study. J. Non. Cryst. Solids 2002, 311, 174–184. [Google Scholar] [CrossRef]
- Nakamoto, K. Applications in Coordination Chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–273. ISBN 9780470405888. [Google Scholar]
- Gulicovski, J.J.; Bračko, I.; Milonjić, S.K. Morphology and the isoelectric point of nanosized aqueous ceria sols. Mater. Chem. Phys. 2014, 148, 868–873. [Google Scholar] [CrossRef]
- Zholobak, N.M.; Shcherbakov, A.B.; Bogorad-Kobelska, A.S.; Ivanova, O.S.; Baranchikov, A.Y.; Spivak, N.Y.; Ivanov, V.K. Panthenol-stabilized cerium dioxide nanoparticles for cosmeceutic formulations against ROS-induced and UV-induced damage. J. Photochem. Photobiol. B Biol. 2014, 130, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B.; Shaporev, A.S.; Polezhaeva, O.S.; Baranchikov, A.Y.; Spivak, N.Y.; Tretyakov, Y.D. UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions. J. Photochem. Photobiol. B Biol. 2011, 102, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Fajzulin, I.; Zhu, X.; Möller, M. Nanoparticulate inorganic UV absorbers: A review. J. Coatings Technol. Res. 2015, 12, 617–632. [Google Scholar] [CrossRef]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; DiMento, B.P.; McNeill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A.; et al. Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [Green Version]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [Green Version]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranchikov, A.E.; Razumov, M.I.; Kameneva, S.V.; Sozarukova, M.M.; Beshkareva, T.S.; Filippova, A.D.; Kozlov, D.A.; Ivanova, O.S.; Shcherbakov, A.B.; Ivanov, V.K. Facile Synthesis of Stable Cerium Dioxide Sols in Nonpolar Solvents. Molecules 2022, 27, 5028. https://doi.org/10.3390/molecules27155028
Baranchikov AE, Razumov MI, Kameneva SV, Sozarukova MM, Beshkareva TS, Filippova AD, Kozlov DA, Ivanova OS, Shcherbakov AB, Ivanov VK. Facile Synthesis of Stable Cerium Dioxide Sols in Nonpolar Solvents. Molecules. 2022; 27(15):5028. https://doi.org/10.3390/molecules27155028
Chicago/Turabian StyleBaranchikov, Alexander E., Mikhail I. Razumov, Svetlana V. Kameneva, Madina M. Sozarukova, Tatiana S. Beshkareva, Arina D. Filippova, Daniil A. Kozlov, Olga S. Ivanova, Alexander B. Shcherbakov, and Vladimir K. Ivanov. 2022. "Facile Synthesis of Stable Cerium Dioxide Sols in Nonpolar Solvents" Molecules 27, no. 15: 5028. https://doi.org/10.3390/molecules27155028
APA StyleBaranchikov, A. E., Razumov, M. I., Kameneva, S. V., Sozarukova, M. M., Beshkareva, T. S., Filippova, A. D., Kozlov, D. A., Ivanova, O. S., Shcherbakov, A. B., & Ivanov, V. K. (2022). Facile Synthesis of Stable Cerium Dioxide Sols in Nonpolar Solvents. Molecules, 27(15), 5028. https://doi.org/10.3390/molecules27155028







