Abstract
The synthesis of quinazoline 3-oxides and their derivatives has attracted considerable attention due to their reactivity as intermediates in the synthesis of quinazoline analogues and their ring-expanded derivatives. Despite this, there is no comprehensive review dedicated to the synthesis and chemical transformation of these biologically relevant azaaromatic oxides. This review aims to provide an up-to-date record of the synthesis of quinazoline 3-oxides and their chemical transformation. It is hoped that this information will help medicinal chemistry researchers to design and synthesize new derivatives or analogues to treat various diseases.
1. Introduction
Pyrimidine 1, shown in Figure 1, is a six-membered heterocyclic aromatic organic compound containing two nitrogen atoms at positions 1 and 3. This scaffold forms nuclei of several pharmacologically relevant compounds with a wide spectrum of biological activities, including anti-tubercular, anti-bacterial, anti-fungal, anti-viral, and anti-inflammatory properties [1]. The pyrimidine ring readily undergoes N-oxidation using hydrogen peroxide, m-chloroperbenzoic acid (MCPBA), monopermaleic acid, monoperphtalic acid, or p-methylperbenzoic acid to afford pyrimidine N-oxides [2]. However, this nucleus is susceptible to hydrolysis, ring opening, and decomposition during oxidation resulting in reduced yields of the N-oxide [3]. Benzo-fused pyrimidine derivatives such as quinazolines (1,3-diazanaphthalenes) 2 are also associated with a wide range of biological and pharmacological activities, including anti-cancer, anti-tuberculosis, anti-hypertensive, anti-bacterial, anti-inflammatory, and anti-malarial properties [4]. Considerable effort has been devoted to the synthesis, transformation, and biological properties of these benzo-fused pyrimidine derivatives [4,5,6,7]. Both nitrogen atoms of the pyrimidine nucleus of quinazolines can be oxidised to afford either the 1-oxide or 3-oxide derivatives. Among this class of nitrogen-based heterocycles, quinazoline 3-oxides represent valuable intermediates in the synthesis of benzodiazepine analogues [8] and other polycyclic compounds of biological importance [9]. Many valuable benzodiazepine-based drugs for the treatment of seizures and anxiety, such as chlordiazepoxide and diazepam, were first prepared from the corresponding quinazoline 3-oxides [8]. Despite this, the synthesis, transformation, and applications of the quinazoline-3-oxides have received less attention when compared to the other classes of N-oxides, such as 5-membered heteroaromatic N-oxides, pyridine N-oxides, and diazine N-oxides [8]. Interestingly, quinazoline 3-oxides do not feature in any of the reviews dedicated to the synthesis, biological activity, and chemical transformation of quinazolinones and/or quinazoline derivatives. In view of the considerable interest in quinazoline 3-oxides as bronchodilators, cardiotonics, and fungicides [10], it was decided to provide an up-to-date record of their synthesis and chemical transformation.

Figure 1.
Structures of pyrimidine 1 and quinazoline 2.
2. Methods for the Synthesis of Quinazoline Oxides
2.1. Direct Oxidation of Quinazolines
Although there are several reagents that can be used for the direct oxidation of quinazoline nuclei to N-oxides, this approach is complicated by the lack of selectivity. Moreover, the pyrimidine nucleus is susceptible to hydrolysis, ring opening, and decomposition resulting in reduced yields of the N-oxides. Treatment of 4-alkylsubstituted quinazoline 3a (R = -CH3) and 3b (R = -CH2CH3) with monoperphthalic acid (1.2–1.3 equiv.) in ether at room temperature (RT) for 5 h, for example, afforded mixtures of the corresponding N-1 (4a, b) and N-3 oxides (5a, b) as well as the quinazolinone derivative 6 (Scheme 1) [11]. The preference for N-1 oxidation over the N-3 centre resulted in significantly reduced yields of the biologically relevant quinazoline 3-oxides. Moreover, this reaction produced quinazolin-4(3H)-one as the main product and quinazoline N-oxides as by-products. Recourse to the literature revealed a method that made use of a recombinant soluble di-iron monooxygenase (SDIMO) PmlABCDEF overexpressed in Escherichia coli which was used as a whole-cell biocatalyst to oxidize pyridines, pyrazines, pyrimidines, and their benzo-fused derivatives into the corresponding N-oxides [12]. Quinazoline 2 was among the benzo-fused heterocycles with two nitrogen atoms, which was transformed into quinazoline 3-oxide in 67% yield without any side oxidation products.
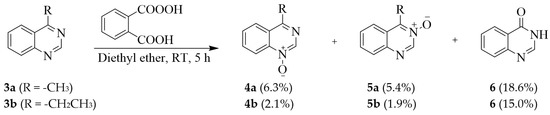
Scheme 1.
Oxidation of quinazoline scaffold.
The drawback associated with the direct N-oxidation of quinazoline scaffold using strong oxidizing agents led to the development of alternative methods for the synthesis of quinazoline 3-oxides, and these strategies are described in detail below.
2.2. Synthesis of Quinazoline 3-Oxides
The most common strategy for the synthesis of quinazoline 3-oxides is based on intramolecular cyclocondensation of the intermediate N-acyl-2-aminoaryl ketone oximes using various reagents.
2.2.1. Intramolecular Cyclocondensation of the N-Acyl-2-aminoaryl Ketone Oximes
The 2-aminoaryl ketone oximes were previously cyclized with triethyl orthoformate to afford quinazoline 3-oxides albeit in low yields [13]. Improved yields of the 2,4-dicarbo substituted quinazoline 3-oxides were achieved via initial acylation of 2-aminoacetophenone followed by intramolecular cyclocondensation of the intermediate N-acyl-2-aminoaryl ketone oximes with hydroxylamine hydrochloride [14,15,16]. The N-oxide of 2,4-dimethylquinazoline 8, for example, was obtained in 75% yield by treatment of (E)-N-(2-(1-(hydroxyimino)ethyl)phenyl)acetamide 7 with hydroxylamine hydrochloride under reflux for 3 h (Scheme 2) [15]. Hydroxylamine hydrochloride serves as a proton source to protonate an oxygen atom of the amide moiety, followed by cyclocondensation of the incipient intermediate A to afford B. The latter then undergoes dehydrogenation to afford the fully aromatic derivative 8. Hitherto, the analogous 2-alkyl/cycloalkyl substituted 4-methylquinazoline 3-oxides were evaluated for biological activity as pulmonary-selective inhibitors of ovalbumin-induced, leukotriene-mediated bronchoconstriction [17]. The most active and selective compounds contained a methyl group at the 4-position, a medium-sized branched alkyl group at the 2-position, and a small electron donating group on the phenyl ring.
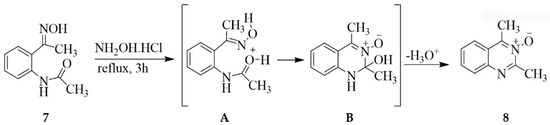
Scheme 2.
Preparation of quinazoline 3-oxides from an N-acyl 2-aminoaryl ketone 7.
Series of quinazoline 3-oxides 10 substituted with various groups on the fused benzo ring were prepared in 12–95% yield by subjecting the 2-aminoacetophenone oxime derivatives 9 to hydroxylamine hydrochloride and pyridine-ethanol mixture under reflux (Scheme 3) [18]. The 2-carbo substituted derivatives of 11, on the other hand, were prepared by treatment of substrates 9 with triethyl orthopropionate or triethyl orthoacetate under reflux for 1–3 h (Scheme 4). The mechanism of this reaction involves the formation of an ethoxymethyleneamino derivative or Schiff base followed by cyclocondensation to afford quinazoline 3-oxide. Analogues of compound 11 were used as cardiotonic and bronchodilating agents [18].
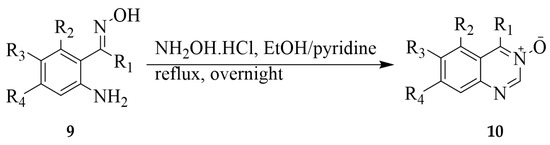
Scheme 3.
Synthesis of polysubstituted quinazoline 3-oxides.
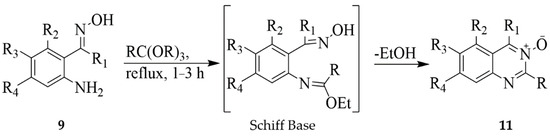
Scheme 4.
Cyclocondensation of 9 with triethyl orthopropionate or triethyl orthoacetate.
The 2-aminobenzaldoxime 12 was subjected to a one-pot reaction with benzaldehyde derivatives in the presence of H2O2-sodium tungstate in THF to afford after 24 h the corresponding quinazoline 3-oxides 13 in good overall yields (69–81%) [19]. The mechanism of this reaction is envisaged to involve the initial nucleophilic addition of the aniline derivative to the carbaldehyde group followed by cyclocondensation of the intermediate Schiff base A to afford a dihydroquinazoline 3-oxide derivative B (Scheme 5). H2O2-sodium tungstate then serves as an oxidizing system on B to afford 13.
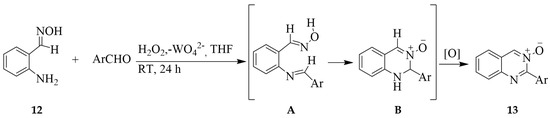
Scheme 5.
One-pot synthesis of quinazoline 3-oxides from 2-aminobenzaldoxime and benzaldehyde derivatives.
Series of N-[2-(1-hydroxyiminoethyl)phenyl]amides and N-(4-halo-2-(1-(hydroxyimino)ethyl)phenyl)amide derivatives 14 (R = alkyl or aryl) were subjected to acid promoted intramolecular cyclization with trifluoroacetic acid (TFA) under reflux for 2 h to afford upon aqueous workup and purification through silica gel column chromatography the corresponding 2,4-dicarbo substituted quinazoline 3-oxides 15 in 72–89% yield (Scheme 6) [20]. These compounds resulted from initial protonation of the amide oxygen by TFA followed by an attack of the activated amide carbon of A to form intermediate B. The heterocyclic ring of the latter underwent spontaneous dehydrogenation to afford a fully aromatic derivative. These compounds were, in turn, evaluated through enzymatic assays (in vitro and in silico) for potential inhibitory effect against cyclooxygenase-1/2 (COX-1/2) and lipoxygenase-5 (LOX-5) activities as well as for free radical scavenging potential and cytotoxicity. Structure–activity relationship analysis suggested that the presence of a halogen atom at the C-6 position and a 2-aryl group enhanced the inhibitory effect against COX-2, and this observation was well supported by molecular docking studies. The presence of a π-electron delocalizing group on the fused benzo ring, on the other hand, enhanced the free radical scavenging effect of the quinazoline 3-oxides.
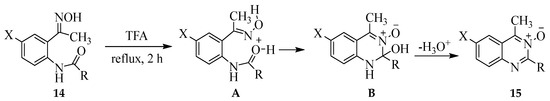
Scheme 6.
TFA-mediated intramolecular cyclization of oximes 14.
Methods that employ aryloximes and isothiocyanate for the construction of quinazoline 3-oxides derivatives in the presence of iodine have also been developed. The (2-aminophenyl)(phenyl)methanone oximes 16 (X = H or Cl) and arylisothiocyanates 17, for example, were reacted with iodine in dimethylsulphoxide (DMSO) at RT to afford the corresponding 2-(arylamino)-4-phenylquinazoline 3-oxide derivatives 18 in 94–98% yield (Scheme 7) [21]. This reaction proceeded via the initial condensation of the oxime 16 with arylisothiocyanate 17 in DMSO to afford the thiourea intermediate A. Iodine-mediated cyclization of A afforded intermediate B via N–C and S–I bond formation. Aromatization of the latter intermediate occurred with the generation of HI and S to afford the cyclized aromatic products 18. The analogous oximes derived from 2-aminoacetophenone [22] or 2-amino-5-bromo-3-iodoacetophenones [23], on the other hand, have previously been found to undergo methanesulfonyl chloride-mediated cyclization in the presence of triethylamine in dichloromethane at RT to afford the corresponding 1H-indazoles. Under similar reaction conditions, the N-aryl o-aminoacetophenone oximes afforded a variety of N-aryl-1H-indazoles and the analogous benzimidazoles when 2-aminopyridine and trimethylamine were used as bases, respectively [24].
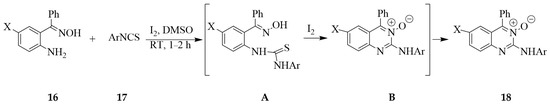
Scheme 7.
Iodine-mediated synthesis of the N,4-disubstituted quinazoline 3-oxide.
The oxime derivatives 19 were reacted with ethoxycarbonyl isothiocyanate in ethyl acetate to form the intermediate thioureas 20, which spontaneously yclized in refluxing ethanol to afford the desired substituted ethyl (3-oxido-2-quinazolinyl)carbamates 21 in good yields (Scheme 8) [25].
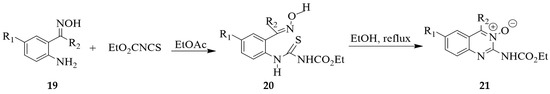
Scheme 8.
Synthesis of quinazoline oxide carbamates.
Madabhushi et al. previously employed Zinc(II) triflate (Zn(OTf)2) as a Lewis acid catalyst in anhydrous toluene under reflux to affect the cyclocondensation of 2-aminoaryl ketones 22 with acetohydroxamic acid derivatives 23 to afford the corresponding 2,4-disubstituted quinazoline 3-oxides 24 (Scheme 9) [26]. The mechanism of this reaction involves an initial attack of the electrophilic carbonyl carbon of 2-aminoacetophenone by the acetohydroxamic acid derivative to generate intermediate A. The latter would then undergo rapid intramolecular cyclization through the reaction of N-acetyl carbonyl with adjacent amine moiety followed by dehydration of B with the assistance of zinc species as a Lewis acid to produce a quinazoline 3-oxide with the elimination of two molecules of water [26].
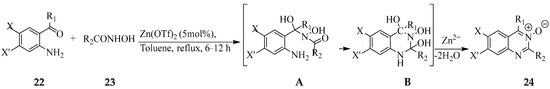
Scheme 9.
One-step synthesis of 2,4-disubstituted quinazoline 3-oxides 24.
Methods involving the use of transition metals for the synthesis of quinazoline 3-oxides have also been developed, and examples are discussed in the next section.
2.2.2. Transition Metal-Mediated Reactions to Afford Quinazoline N-Oxides
The N-(2-(1-(hydroxyimino)ethyl)phenyl)benzamide 27 was prepared as a sole product by subjecting acetophenone oxime 25 and 1,4,2-dioxazol-5-one 26 to dichloro(pentamethylcyclopentadienyl)rhodium(II) dimer ([Cp*RhCl2]2) as a catalyst in methanol under reflux for 12 h (Scheme 10) [27]. Attempted cyclization of this N-(2-(1-(hydroxyimino)ethyl)phenyl)benzamide in acetic acid by these authors resulted in the recovery of the starting material with no quinazoline 3-oxide detected in the reaction mixture. The keto oxime 25 was found to undergo Zn(II)-catalyzed cyclocondensation-dehydration in tetrafluoroethylene (TFE) under a nitrogen atmosphere at 80 °C in a pressure tube to afford the quinazoline 3-oxide 28 in yield of 93% [27].
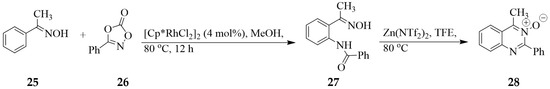
Scheme 10.
Amidation of keto oxime with 1,4,2-dioxazol-5-ones and Zn(II) catalyzed cyclization.
A one-pot Rh(III)-catalyzed C−H activation-amidation of the ketoximes 29 and 1,4,2-dioxazol-5-ones 30, and subsequent Zn(II) catalyzed cyclocondensation-dehydration of the incipient N-(2-(1-(hydroxyimino)ethyl)phenyl)benzamide afforded the 2,4-dicarbo substituted quinazoline 3-oxides 31 (Scheme 11) [27]. The active RhCp*X2 (X = NTf2 or OAc) species is envisaged to be generated from the anion exchange between [RhCp*Cl2]2 and Zn(NTf)2 or HOAc.
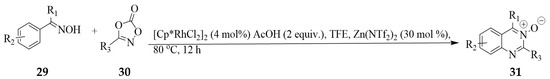
Scheme 11.
One-pot synthesis of quinazoline 3-oxides from ketoximes and 1,4,2-dioxazol-5-ones.
Sawant et al. developed a direct one-pot, three-component reaction of 2-azidobenzaldehyde, isocyanide, and hydroxylamine hydrochloride to afford quinazoline-3-oxides [10]. Palladium acetate catalyzed reaction of 2-azidobenzaldehyde 32, isocyanides, and hydroxylamine hydrochloride in toluene in the presence of 4 Å molecular sieves under reflux afforded the quinazoline-3-oxides 33 in a single-pot operation (Scheme 12). The mechanistic study revealed that the reaction proceeds via initial palladium-catalyzed azide–isocyanide denitrogenative coupling to afford intermediate A. Oximation of the carbaldehyde moiety of this intermediate and subsequent 6-exo-dig cyclization afforded the quinazoline 3-oxide derivative. The addition of 4 Å molecular sieves improved the overall yield of the desired product by removing water produced in situ during the formation of hydrazine. Although several substituted isocyanides reacted well under these conditions, the aromatic and secondary isocyanides failed to react, and the starting materials were recovered unchanged.
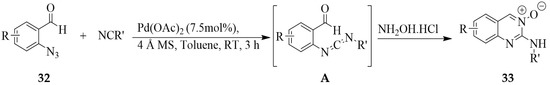
Scheme 12.
Palladium-catalyzed synthesis of substituted quinazoline 3-oxides 33.
Another conventional approach for the synthesis of quinazoline 3-oxides involves the dehydrogenation of the corresponding readily accessible 1,2-dihydroquinazoline 3-oxides [26], as described below.
2.3. Dehydrogenation of the 1,2-Dihydroquinazoline 3-Oxides
2-Aminobenzaldehyde or 2-aminoacetophenone derivatives readily undergo oximation with hydroxylamine hydrochloride in the presence of an amine base to afford the corresponding 2-aminobenzaldoximes or 2-aminoacetophenone oxime derivatives, respectively. Nucleophilic addition of the o-aminobenzaldoximes [28] or 2-aminoacetophenone oxime derivatives [29,30] to benzaldehyde derivatives and subsequent in situ cyclocondensation of the resultant intermediate afforded the corresponding 1,2-dihydroquinazoline 3-oxides. The latter were, in turn, evaluated for cytotoxicity against the human promyelocytic leukaemia HL-60 and lymphoblastic leukaemia NALM-6 cell lines [30]. The oxime derived from 2-aminoacetophenone 34 (R = H), for example, has previously been reacted with a series of aryl aldehydes in the presence of p-toluene sulfonic acid as a catalyst in ethanol at RT for 5–15 min. to afford the corresponding 1,2-dihydroquinazoline 3-oxides 35 (Scheme 13) [29]. Under similar reaction conditions, the oxime derived from 2-(methylamino)acetophenone (R = CH3) afforded after 1 h, the corresponding 1,2-dihydroquinazoline 3-oxides [31]. Samandran et al. also synthesised a series of the 1,2-dihydroquinazoline 3-oxides from the reaction of equimolar amounts of amino oximes with the corresponding aldehydes in ethanol at RT for 24 h [19].
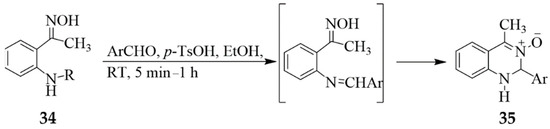
Scheme 13.
Synthesis of the 1,2-dihydroquinazoline 3-oxides 35.
2-Aminoacetophenone oxime analogue 36 was previously reacted with butanedione monooxime 37 in acetic acid under reflux for 24 h to afford ketoximes 38 (Scheme 14) [30]. The latter were, in turn, cyclized in ethanol–acetic acid mixture under reflux for 24 h to afford the corresponding quinazoline 3-oxides 39 in 60–75% yield (Table 1). These quinazoline 3-oxides were evaluated for cytotoxic activities against the human leukaemia HL-60 cells under hypoxic and aerobic conditions using tirapazamine as the reference standard.
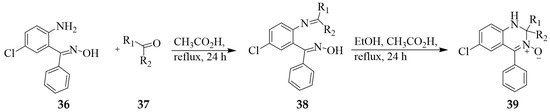
Scheme 14.
Synthesis of quinazoline 3-oxides 39.

Table 1.
Substitution pattern and percentage yields of quinazoline 3-oxides 39a–c.
Chen and Yang previously exposed 4-methyl-2-(4-nitrophenyl)-1,2-dihydroquinazoline-3-oxide 40 to visible light in the presence of 0.5 mol % tris(bipyridine)ruthenium(II) chloride (Ru(bpy)3Cl2) as a photocatalyst in acetonitrile under aerobic conditions and isolated 4-methyl-2-(4-nitrophenyl)quinazoline 3-oxide 41 in 63% yield (Scheme 15) [29]. No product was obtained when the photooxidation of 40 was conducted under argon atmosphere prompting the authors to suggest the importance of molecular oxygen as the oxidant for this photoreaction. It is envisaged that visible light excited the Ru(bpy)32+ to accept one electron from NH of 40 to yield the cation radical 40′ and the Ru(bpy)3+ (see ref [29] for fragmentation pattern). Electron transfer from the latter to molecular oxygen yielded the superoxide anion radical and regenerated the ground-state photocatalyst Ru(bpy)32+. It is envisaged that the cation radical 40′ underwent proton and hydrogen transfers to the superoxide anion radical to furnish the quinazoline 3-oxide 41 extruding hydrogen peroxide as a by-product.
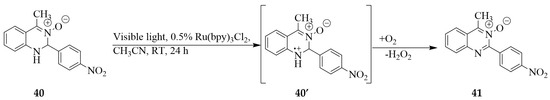
Scheme 15.
Light-induced dehydrogenation of a 1,2-dihydroquinazolin 3-oxide derivative.
Oxidizing agents such as 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) [32], active manganese oxide (MnO2) [8], and hydrogen peroxide (H2O2)-tungstate [19] were used before to transform the dihydroquinazoline 3-oxides into the corresponding quinazoline 3-oxides. A series of quinazoline 3-oxides 42 (X = H, 6-Cl/Br or 7-Me) substituted at the 2-position with an alkyl or benzyl group and an electron donating or withdrawing group at the 4-position (alkyl, aryl, or heteroaryl) were synthesized in good to excellent yields (54–88%) by oxidation of the corresponding 1,2-dihydroquinazoline 3-oxides 43 using 3 equiv. of activated MnO2 in dichloromethane at 50 °C (Scheme 16) [8]. The advantage of the use of MnO2 as an oxidant is the ease of its removal from the reaction mixture which involves simple filtration.
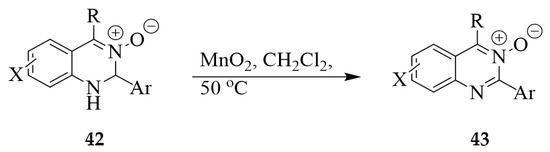
Scheme 16.
Active manganese oxide-mediated dehydrogenation of 43.
Coșkun et al. have also dehydrogenated the dihydroquinazoline 3-oxides 44 using H2O2-sodium tungstate oxidant system in THF to afford after 24 h at RT the corresponding quinazoline 3-oxides 45 (Scheme 17) [33]. However, the one-pot synthesis of these quinazoline 3-oxides from the 2-aminobenzaldoximes (refer to Scheme 5) proceeded in a relatively short time resulting in improved overall yields [19].
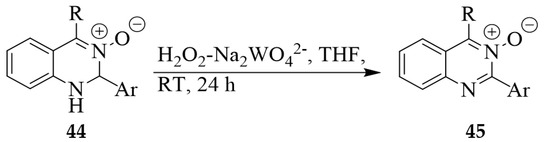
Scheme 17.
H2O2-sodium tungstate mediated dehydrogenation of dihydroquinazoline 3-oxides.
2.4. Chemical Transformation of Quinazoline 3-Oxides
Quinazolines N-oxides can undergo deoxygenation into quinazolines [34], acetoxylation [29] and ring expansion to benzodiazepines [8,25].
2.4.1. Deoxygenation of Quinazoline N-Oxides
The N–O bond in pyrimidine N-oxides is cleaved by catalytic reduction, low-valent phosphorus (PCl3 or POCl3) or titanium (TiCl3) reagents, as well as by the more common metals used for hydrogenolysis. Deoxygenation of 4-methyl-2-phenylquinazoline 3-oxide 46 using Zn in the presence of aqueous NH4Cl in THF afforded 4-methyl-2-phenylquinazoline 47 in 71% yield (Scheme 18) [26]. Deoxygenation of the analogous quinazoline 1-oxides 4a (R = CH3) and 4b (R = -CH2CH3), on the other hand, was achieved through catalytic hydrogenation (Raney Ni catalyst in MeOH under hydrogen (H2) stream) to afford 4-substituted quinazoline 48 in 33–43% yield (Scheme 19) [11].
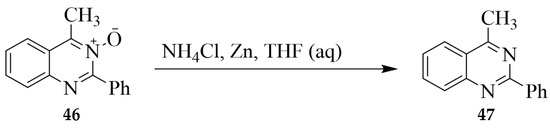
Scheme 18.
Zn-catalyzed deoxygenation of 4-methyl-2-phenylquinazoline 3-oxide 46.
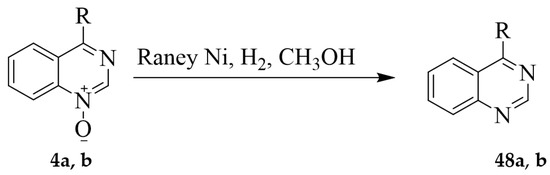
Scheme 19.
Raney nickel catalysed deoxygenation of 4a and 4b.
A mixture of N-oxide 46 and phosphorus oxychloride in chloroform was heated at reflux for 15 min. followed by aqueous work-up and purification through silica gel column chromatography to afford 49 in 18% yield (Scheme 20) [15]. Improved yield (70%) of this quinazoline derivative was observed when this quinazoline 3-oxide was treated with PCl5 in dichloromethane at RT for 15 min. [30].
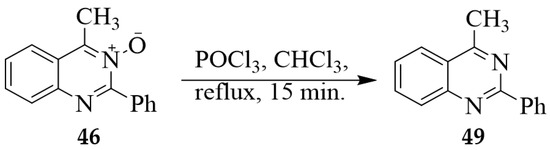
Scheme 20.
POCl3-mediated reduction of quinazoline 3-oxide 46.
2.4.2. Alkoxylation of Quinazoline N-Oxides
The highly acidic proton of the methyl group at the C-4 position of quinazoline-3-oxide scaffold has been found to promote acetoxylation to ester derivatives. 4-Methyl-7-methoxy-2-phenyl substituted quinazoline 3-oxide 50, for example, was subjected to acetic anhydride under reflux for 0.5 h to afford the ester derivative 51 in 82% yield (Scheme 21) [27].
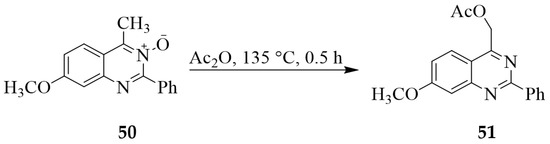
Scheme 21.
Alkoxylation of 50 with acetic anhydride under reflux.
2.4.3. Alkylation of Quinazoline N-Oxides
N-Oxide moiety in aza-heteroarene represents an efficient and removable directing group for ortho C–H bond activation. Zhao et al., for example, effected a copper-catalyzed oxidative coupling reaction between Csp2–H of quinazoline 3-oxide 52 and Csp2–H of benzaldehyde derivatives in the presence of tert-butyl hydroperoxide (TBHP) in dichloromethane at 40 °C under nitrogen atmosphere to furnish the quinazolinone derivatives 53 (Scheme 22) [34]. Both aliphatic and aromatic substituents at the 2-position of the quinazoline 3-oxide scaffold were tolerated though the yields decreased with the increase of the chain length from the methyl to the propyl group. α,β-Unsaturated aldehydes, heteroaryl aldehydes, and aliphatic aldehydes were also found to be suitable acyl donors to afford cyclic hydroxamic esters in good to excellent yields.
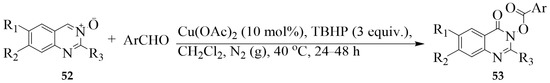
Scheme 22.
Copper-catalyzed oxidative coupling of quinazoline 3-oxide and benzaldehydes.
The authors observed the formation of quinazoline aryl ketone derivatives 54 from 52 in the presence of Cu(OAc)2, albeit in low yields when the reaction was quenched prematurely. The quinazoline aryl ketones 54 were isolated as sole products in the presence of trimethylsilyl azide (TMSN3) and copper carbonate (CuCO3) (Scheme 23). Controlled reactions revealed that compounds 53 are the consequence of initial in situ Baeyer–Villiger oxidation of quinazoline aryl ketones 54 followed by intramolecular acyl transfer to afford 53 [34].

Scheme 23.
Formation of quinazoline aryl ketones in the presence of CuCO3–TMSN3 mixture.
In a subsequent study, these authors employed this strategy on benzylic Csp3–H bonds with quinazoline 3-oxides 52 in the presence of CuSO4 (3 mol %), TBHP (2 equiv.), 20 mol % of tetrabutylammonium iodide (TBAI), and NaI (70 mol %) in dichloromethane at 70 °C in sealed tubes to afford after 12 h the corresponding quinazolinone derivatives 55 (Scheme 24) [35].

Scheme 24.
Oxidative coupling of quinazoline 3-oxides 52 and benzylic derivatives.
A copper-catalyzed oxidative coupling reaction between Csp2–H of quinazoline 3-oxide 56 and Csp2–H of formamides in the presence of copper hydroxide and TBHP in dichloroethane (DCE) also afforded the analogous O-quinazolinone carbamates 57 (Scheme 25) [36]. The latter are envisaged to be formed through a reaction sequence involving radical addition, Baeyer–Villiger oxidation, and intramolecular acyl transfer [36].
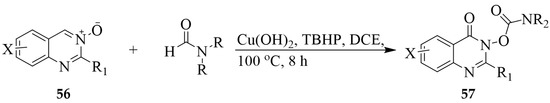
Scheme 25.
Copper-catalyzed oxidative coupling of quinazoline 3-oxides and formamides.
Quinazoline 3-oxides 58 reacted with primary amines in the presence of TBHP as the oxidant in dioxane under reflux for 24–44 h to afford the quinazolin-4(3H)-one derivatives 59 (Scheme 26) [37]. The mechanism of this reaction was investigated using control reactions, and ESI-MS analysis revealed a complex reaction involving multiple bond dissociation/recombination steps. These mild reactions and metal-free conditions were found to be compatible with a broad range of primary amines, producing a series of quinoxaline-4(3H)-ones. Moreover, this methodology also afforded 3-(2-(1H-indol-3-yl) ethyl)quinazolin-4(3H)-one 60 in 70% yield, which is a precursor for the synthesis of bioactive rutaempine and (±)-evodiamine (Scheme 27) [37].
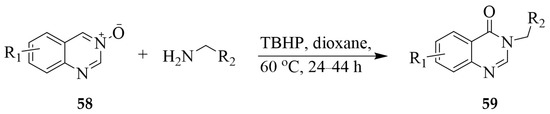
Scheme 26.
TBHP oxidative coupling of quinazoline 3-oxides and primary amines.
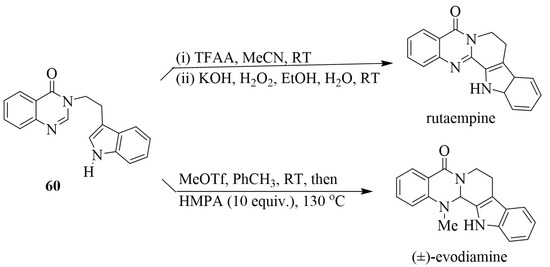
Scheme 27.
Synthesis of rutaempine and evodiamine from 60.
Direct C-4 alkylation of the 2-unsubstituted and 2-aryl substituted quinazoline-3-oxides was previously achieved with open chain (1,2-dimethoxyethane, diethoxymethane or diethyl ether) or cyclic ethers (1,3-dioxolane or 1,3-benzodioxole) in the presence of tert-butyl peroxybenzoate (TBPB) afforded series of oxidative cross-coupling products in moderate to good yields [38]. Scheme 28 shows the reactions of 1,4-dioxane with 61a (R = H) and 61b (R = Ar) as representative models for the radical oxidative cross-coupling reaction of the sp3 C–H bond in ethers with the sp2 C–H bond in quinazoline-3-oxide to afford 62a and 62b, respectively. The mechanism of this radical oxidative cross-coupling reaction is envisaged to involve the initial decomposition of TBPB to generate a tert-butoxyl radical and a benzoate radical. The most reactive tert-butoxyl radical then abstracted hydrogen from 1,4-dioxane, and the resultant dioxane radical added to quinazoline 3-oxide 61 to generate a quinazoline-3-oxide radical. Abstraction of a hydrogen atom from the quinazoline-3-oxide radical by less sterically hindered benzoate radical (versus tert-butoxyl radical) afforded quinazoline-3-oxide 62 and benzoic acid as a by-product [38].
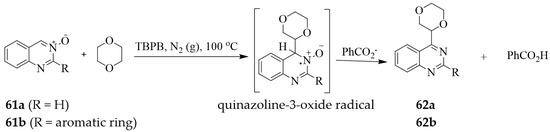
Scheme 28.
Cross-coupling of quinazoline-3-oxides 61a and 61b with various ethers.
Copper(II) chloride has been employed to catalyze the Csp2–H bond (C-4) of the 2-aryl substituted quinazoline-3-oxide 63 and the Csp2–H bond (C-3) of various indoles 64 to facilitate the cross-dehydrogenative-coupling reaction between these two chromophores to afford the quinazoline 3-oxide appended indole hybrids 65 (Scheme 29) [39]. The N-methylindoles substituted with alkyl, halide, or alkoxy group at the 5-position afforded the expected quinazoline 3-oxide–indole hybrids in moderate to good yields. However, indoles substituted on nitrogen with an electron-withdrawing group, such as the tosyl group, failed to react. Subsequent dehydrogenation of these molecular hybrids with PCl5 (1.2 equiv.) in toluene at RT afforded quinazoline-indole hybrids 66.
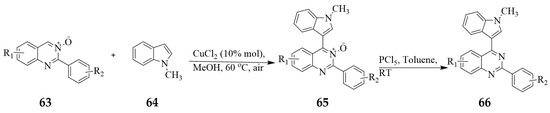
Scheme 29.
Copper-catalyzed cross-dehydrogenative coupling of quinazoline 3-oxides and indoles.
Quinazoline 3-oxides are valuable intermediates in the synthesis of benzodiazepine analogues [8] and other polycyclic compounds of biological importance [9], and examples of these reactions are described in the next sections. The analogous 4-(1-benzyl-1H-indol-3-yl)-6,7-dimethoxyquinazoline has previously been found to exhibit moderate activity against protein tyrosine kinase ErbB-2, with little or no activity against the epidermal growth factor receptor tyrosine kinase (EGFR-TK) [40]. The 4-(indole-3-yl)quinazolines, on the other hand, were found to be highly potent EGFR-TK inhibitors with excellent cytotoxic properties against several cancer cell lines [41].
2.5. Synthesis of Polycyclic Quinazoline Derivatives and Benzodiazepine Analogues
The oxygen atom of quinazoline 3-oxides can also participate in ring-closure reactions to yield polycyclic derivatives. The 3-oxidoquinazoline-2-carbamates 67, for example, were found to undergo reductive ring closure to afford the 3,9-dihydro-2H-[1,2,4]oxadiazolo[3,2-b]quinazolin-2-ones 68 (Scheme 30) [26].
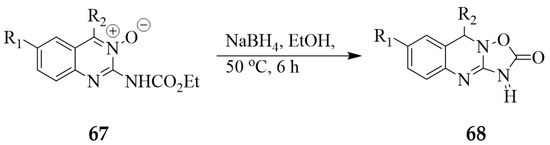
Scheme 30.
Synthesis of polycyclic quinazolines.
Wu et al. reported a copper-catalyzed [3 + 2] cycloaddition of quinazoline 3-oxides 69 with alkylidenecyclopropane derivatives to afford the angular polycyclic quinazoline derivatives 70 (Scheme 31) [42]. Methyl, methoxy, fluoro and chloro functionalities were all tolerated, leading to the formation of the corresponding N-(2-(5-oxa-6-azaspiro[2.4]hept-6-en7-yl)phenyl) 69 in high yield.
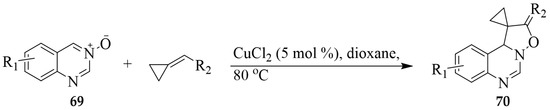
Scheme 31.
[3 + 2] cycloaddition of quinazoline 3-oxide with alkylidenecyclopropane derivatives.
Yin et al. studied the [3 + 2] cycloaddition reaction between the quinazoline 3-oxides 71 (R2 = H, alkyl, aryl) and various alkene derivatives 72 such as methyl 3-methoxyacrylate (R3 = -CO2Me, R4 = -OMe), ethyl-3-ethoxyacrylate (R3 = -CO2Et, R4 = -OEt), dimethyl maleate (R3, R4 = -CO2Me), acrylonitrile (R3 = -CN, R4 = H) and 5-methyl-hex-2-enoic acid methyl ester (R3 = -CO2Me, R4 = -CH2CH(CH3)2) to afford a series of isoxazolo[2,3-c]quinazoline 73 in good to excellent yield with total regio- and stereoselectivities (Scheme 32) [9]. A density functional theory (DFT) method using the B3LYP/6-31G(d) basis set further predicted the reaction to be under thermodynamic control and to favour exclusive formation of the ortho-exo cycloadduct in agreement with experimental finding [43]. Hitherto, Heaney et al. reacted 2-styrylquinazoline 3-oxide 71 (R2 = -CH=CHPh) with phenyl vinyl sulfone or N-methyl maleimide in THF under reflux and isolated the corresponding isoxazolo[2,3-c]quinazoline derivatives in very low yields [44]. These tricyclic compounds were found to be unstable in solution at RT and to rearrange to other complex products.
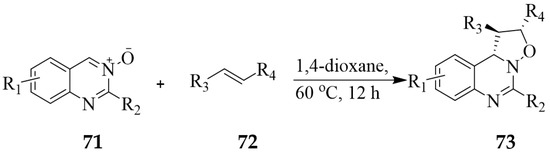
Scheme 32.
[3 + 2] Cycloaddition between quinazoline 3-oxide and methyl 3-methoxyacrylate.
The use of acetylene derivatives as dipolarophiles on the 4-carbo substituted quinazoline 3-oxides, on the other hand, resulted in the isolation of benzodiazepine analogues instead of the polycyclic quinazoline derivatives. The 2-aryl-4-methylquinazoline 3-oxides 74, for example, were reacted with dimethyl acetylenedicarboxylate (DMAD) 75 in THF at 70° for 4 to afford the methyl 5-(2-methoxy-2-oxoacetyl)-4-methyl-2-phenylsubstituted-5H-benzo[d][1,3]diazepine-5-carboxylates 76 in 67–74% yield (Scheme 33) [8]. The formation of these benzodiazepine derivatives is envisaged to proceed via the rearrangement of the incipient tricyclic quinazoline intermediate A as represented in the Scheme.
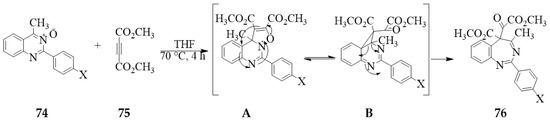
Scheme 33.
DMAD assisted ring expansion of 74 into benzodiazepine analogues 76.
DMAD, on the other hand, reacted with the isomeric 2-methyl-4-phenylquinazoline 3-oxide 77 in benzene-(m)ethanol followed by purification on basic alumina to afford the phenyl acrylates 78 (13–21%) and the potentially tautomeric benzodiazepines 79 in 5–14% yield together with smaller amounts of other products (Scheme 34) [28].
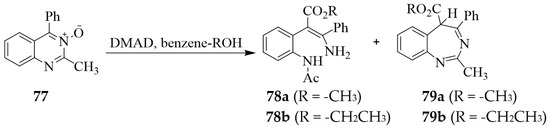
Scheme 34.
Reaction of 2-methyl-4-phenylquinazoline 3-oxide 77 with DMAD.
The analogous 4-methyl-2-styrylquinazoline 3-oxides 80a–d were also reacted with dimethyl acetylenedicarboxylate (DMAD) 75a (R2 = -CO2CH3) or methyl propiolate 75b (R2 = H) as dipolarophiles (2 equiv.) in dry THF under reflux for 16 h followed through purification by column chromatography on either silica gel (SiO2) or neutral alumina (Al2O) to afford the corresponding benzodiazepine analogues 81a–d (Scheme 35) [44]. The presence of the 2-styryl group resulted in significantly reduced yields of the corresponding benzodiazepine derivatives compared to the products of the reaction of analogous 2-phenyl-4-methylquinazoline 3-oxides 74 with DMAD (refer to Scheme 33 above). The heterocyclic ring of these compounds was hydrolysed during purification by column chromatography on either or both SiO2 or Al2O (Table 2) and the extend of hydrolysis depended on the nature of the C-4 and C-5 substituents on the diazepine ring. The presence of the carbon-carbon double bond in the five-membered ring of the tricyclic quinazoline intermediate implicated in the reaction of the 4-carbo substituted quinazoline 3-oxides 74, 77 or 80 with acetylene derivative 75a or 75b facilitated the rearrangement and subsequent ring enlargement to afford benzodiazepine analogues.
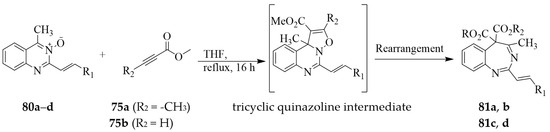
Scheme 35.
Cyclization of 80a–d with DMAD 75a or methyl propiolate 75b.

Table 2.
Substitution pattern and percentage yields of benzodiazepines 81a–d.
2.6. Ring Expansion of Quinazoline 3-Oxides to Afford Benzodiazepine Analogues
Nucleophilic attack on C-2 of 2-chloromethyl quinazoline 3-oxide 82 by methylamine followed sequentially by ring opening of intermediate A and intramolecular displacement of chlorine atom by nitrogen atom of the oxime moiety afforded chlordiazepoxide 83 as shown in Scheme 36 [26]. Acid hydrolysis of chlordiazepoxide and in situ hydrolysis of the benzodiazepin-2-one 4-oxide intermediate 84 followed by its PCl3 mediated deoxygenation afforded 1,4-benzodiazepine (diazepam) 85. Benzodiazepines enhance the effect of the neurotransmitter gamma-amino butyric acid (GABA-A), resulting in sedative, hypnotic, anxiolytic, anticonvulsant and muscle relaxant properties [45]. These properties make benzodiazepines and their analogues useful drugs in the treatment of anxiety, insomnia, agitation, seizures, muscle spasms, alcohol withdrawal and as a premedication for medical or dental procedures. Chlordiazepoxide and diazepam, for example, are the central nervous system (CNS) agents used for the treatment of muscle spasms, seizures, trauma, and anxiety disorders [8].
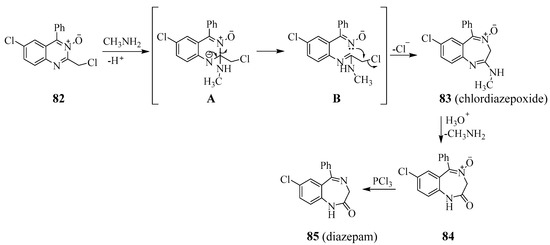
Scheme 36.
Reaction of methylamine with 2-chloromethyl quinazoline 3-oxide.
3. Conclusions
Aromatic N-oxides are desirable biologically active compounds with a potential for application in pharmaceutical and agrochemical industries. It is imperative for medicinal chemists to continue to develop environmentally friendly and mild methods for the production of quinazoline 3-oxides. This scaffold is capable of undergoing various chemical transformations into biologically-relevant polysubstituted quinazolines and their polynuclear derivatives, as well as ring expansion to afford the benzodiazepine analogues with CNS activity. The potential for the quinazoline 3-oxide scaffold to undergo transition metal catalyzed cross-dehydrogenative-coupling, on the other hand, makes them suitable candidates for the design and synthesis of other novel biologically-relevant molecular hybrids. Moreover, the presence of a halogen atom on the fused benzo ring of the quinazoline 3-oxide framework would facilitate further chemical transformation via transition metal catalyzed cross-coupling reactions to afford polysubstituted derivatives. It is envisaged that this review will help medicinal chemistry researchers to design and synthesize new quinazoline 3-oxides and their derivatives and investigate their biological properties to treat various diseases.
Funding
This project was funded by the University of South Africa and the National Research Foundation (NRF) in South Africa (NRF GUN: 118554).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author thanks Marole M. Maluleka of the University of Limpopo for helpful suggestions.
Conflicts of Interest
The author declares no conflict of interest.
References
- Yerragunta, V.; Patil, P.; Anusha, V.; KumaraSwamy, T.; Suman, S.; Samhitha, T. Pyrimidine and its biological activity: A review. PharmaTutor 2013, 1, 39–44. [Google Scholar]
- Kočevar, M.; Kokvar, W.; Mlakar, B.; Perdič, M.; Petrie, A.; Polaac, B.; Verček, B. The synthesis of pyrimidine l-oxides: A new transformation of amide oxides. Tetrahedron Lett. 1992, 33, 2195–2198. [Google Scholar] [CrossRef]
- Ashburn, S.P.; Coates, R.M. Preparation of oxazoline N-oxides and imidate N-oxides by amide acetal condensation and their [3+2] cycloaddition reaction. J. Org. Chem. 1985, 50, 3073–3076. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 95. [Google Scholar] [CrossRef]
- Faheem, M.; Tiwan, A.K.; Singh, V.K. A review on modern synthetic route for the construction of 1,3-diazanaphthalene moiety. Curr. Org. Chem. 2020, 24, 1108–1138. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A. Chemical insights into the synthetic chemistry of quinazolines: Recent advances. Front. Chem. 2021, 8, 594717. [Google Scholar] [CrossRef]
- Ye, X.; Chen, Z.; Zhang, Z.; Fu, Y.; Deng, Z.; Peng, Y. A convenient approach to 2,4-disubstituted quinazoline-3-oxides using active MnO2 as the oxidant. Can. J. Chem. 2019, 97, 682–689. [Google Scholar] [CrossRef]
- Yin, Z.; Li, X.; Deng, Z.; Yang, Q.; Peng, Y. The synthesis of isoxazolo[2,3-c]quinazolines via a cycloaddition of quinazoline-3-oxides and acrylates. Tetrahedron Lett. 2020, 61, 151818. [Google Scholar] [CrossRef]
- Pathare, R.S.; Maurya, A.K.; Kumari, A.; Agnihotri, V.K.; Vermac, V.P.; Sawant, D.M. Synthesis of quinazoline-3-oxides via a Pd(II) catalyzed azide–isocyanide coupling/cyclocondensation reaction. Org. Biomol. Chem. 2019, 17, 363–368. [Google Scholar] [CrossRef]
- Higashino, T.; Amano, T.; Tamura, Y.; Katsumata, N.; Washizu, Y.; Takeshi, O.; Hayashi, E. On N-oxidation of 4-alkyl-, 4-phenyl-quinazoline and reaction of 4-methylquinazoline-1-oxide. Chem. Pharm. Bull. 1972, 20, 1874–1882. [Google Scholar] [CrossRef]
- Petkevičius, V.; Vaitekũnas, J.; Tauraite, D.; Stankevičiũtė, J.; Šarlauskas, J.; Čėnas, J.; Meškys, R. A biocatalytic synthesis of heteroaromaticn-oxides by whole cells of Escherichia coli expressing the multicomponent, soluble di-iron monooxygenase (SDIMO) PmlABCDEF. Adv. Synth. Catal. 2019, 361, 2456–2465. [Google Scholar]
- Armarego, W.L.F. Quinazolines. Part V. Covalent hydration in quinazoline 3-oxides. J. Chem. Soc. 1962, 5030–5036. [Google Scholar] [CrossRef]
- Kovendi, A.; Kircz, M. New synthesis for quinazoline N3-oxides and 1,2-dihydroquinazoline N3-oxides. Chem. Ber. 1965, 9, 1049–1059. [Google Scholar]
- Alzogaray, R.A.; Fontán, A.; Camps, F.; Masuh, H.; Orihuela, P.S.; Fernández, D.; Cork, A.; Zerba, E. Behavioural response of Triatoma infestans (Klug) (Hemiptera: Reduviidae) to quinazolines. Molecules 2005, 10, 1190–1196. [Google Scholar] [CrossRef]
- Heaney, F.; Lawless, E. 2-Vinyl quinazoline 3-oxide; preparation from acid induced cyclocondensation of 2-acylaminoaryloximes. J. Heterocycl. Chem. 2007, 44, 569–574. [Google Scholar] [CrossRef]
- Combs, D.W.; Rampulla, M.S.; Russell, R.K.; Rampulla, R.; Klaubert, D.H.; Ritchie, D.; Meeks, A.S.; Kirchner, T. Design, synthesis and bronchodilatory activity of a series of quinazoline-3-oxides. Drug Des. Del. 1990, 6, 241–254. [Google Scholar]
- Combs, D.W.; Fallotico, R. Substituted Quinazoline-3-Oxides Providing Pharmacological Activity. U.S. Patent 4,745,118, 17 May 1988. [Google Scholar]
- Samandram, R.; Korukçu, M.Ç.; Coşkun, N. Eco-friendly H2O2 oxidation of 1,2-dihydroquinazoline-3-oxides to quinazoline-3-oxides. Synth. Commun. 2021, 51, 2349–2356. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Onwu, E.E.; Agbo, E.N.; Maluleka, M.M.; More, G.K.; Choong, Y.S. Synthesis, in vitro and in silico enzyme (COX-1/2 & LOX-5), free radical scavenging profiling of the 2,4-dicarbo substituted quinazoline 3-oxides. Med. Chem. Res. 2022, 31, 146–164. [Google Scholar]
- Jatangi, N.; Palakodety, R.K. I2-Catalyzed oxidative synthesis of N,4-disubstituted quinazolines and quinazoline oxides. Org. Biomol. Chem. 2019, 17, 3714–3717. [Google Scholar] [CrossRef]
- Counceller, C.M.; Eichman, C.C.; Wray, B.C.; Stambuli, J.P. A practical, metal-free synthesis of 1H-indazoles. Org. Lett. 2008, 10, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, M.J.; Magwaza, N.M.; Gildenhuys, S.; Setshedi, I.B. Synthesis, α-glucosidase inhibition and antioxidant activity of the 7-carbo–substituted 5-bromo-3-methylindazoles. Bioorg. Chem. 2020, 97, 103702. [Google Scholar] [CrossRef] [PubMed]
- Wray, B.C.; Stambuli, J.P. Synthesis of N-arylindazoles and benzimidazoles from a common intermediate. Org. Lett. 2010, 12, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Renaut, P.P.; Durand, P.; Ratel, P. 3,9-Dihydro-2H-[1,2,4]-oxadiazolo[3,2-b]quinazolin-2-ones: First synthesis of the parent heterocycle, 7- and 9-substituted derivatives. Synthesis 2000, 14, 2009–2012. [Google Scholar] [CrossRef]
- Madabhushi, S.; Mallu, K.K.R.; Jillella, R.; Kurva, S.; Singh, R. One-step method for synthesis of 2,4-disubstituted quinazoline 3-oxides by reaction of a 2-aminoaryl ketone with a hydroxamic acid using Zn(OTf)2 as the catalyst. Tetrahedron Lett. 2014, 55, 1979–1982. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Yang, X.; Zhou, X.; Li, X. Rh(III)- and Zn(II)-catalyzed synthesis of quinazoline N-oxides via C–H amidation–cyclization of oximes. Org. Lett. 2016, 18, 6144–6147. [Google Scholar] [CrossRef]
- Heaney, F.; McCarthy, T.; Mahon, M.; Nacereddine, V. Bridgehead nitrogen heterocycles which contain the quinazoline moiety–synthesis and cycloaddition of 1,2-dihydroquinazoline 3-oxides. Org. Biomol. Chem. 2005, 3, 4351–4361. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yang, D.-Y. Visible light-mediated synthesis of quinazolines from 1,2-dihydroquinazoline-3-oxides. Tetrahedron 2013, 69, 10438–10444. [Google Scholar] [CrossRef]
- Mikiciuk-Olasik, E.; Baszczak-Światkiewiz, K.; Żurek, E.; Krajewska, U.; Różalski, M.; Kruszyński, R.; Bartczak, T.J. New derivatives of quinazoline and 1,2-dihydroquinazoline N3-oxide with expected antitumor activity. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 239–246. [Google Scholar] [CrossRef]
- Wu, C.-K.; Yang, D.-Y. Visible-light-mediated reaction: Synthesis of quinazolinones from 1,2-dihydroquinazoline 3-oxides. RSC Adv. 2016, 6, 65988–65994. [Google Scholar] [CrossRef]
- Eynde, J.J.V.; Godin, J.; Mayence, A.; Maquestiau, A.; Anders, E. A new and convenient method for the preparation of 2-substituted quinazolines. Synthesis 1993, 9, 867–869. [Google Scholar] [CrossRef]
- Coșkun, N.; Cetin, M. A new regioselective synthesis and ambient light photochemistry of quinazoline 1-oxides. Tetrahedron 2007, 63, 2966–2972. [Google Scholar] [CrossRef]
- Fan, L.; Wang, T.; Tian, Y.; Xiong, F.; Wu, S.; Liang, Q.; Zhao, J. Copper-catalyzed oxidative coupling between quinazoline 3-oxides and unactivated aldehydes: An efficient approach to functionalized quinazolines. Chem. Commun. 2016, 52, 5375–5378. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, Z.; Wang, T.; Liang, Q.; Zhao, J. Copper-catalyzed oxidative functionalization of benzylic C–H bonds with quinazoline 3-oxides. Org. Chem. Front. 2018, 5, 2492–2495. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Yu, W.; Fan, L.; Wang, T.; Fu, J. Copper-catalyzed oxidative coupling of quinazolin-3-oxides: Synthesis of O-quinazolinic carbamates. J. Org. Chem. 2022, 87, 5136–5148. [Google Scholar] [CrossRef]
- Luo, J.; Wan, J.; Wu, L.; Yang, L.; Wang, T. tert-Butyl hydroperoxide promoted the reaction of quinazoline-3-oxides with primary amines affording quinazolin-4(3H)-ones. J. Org. Chem. 2022, 87, 9864–9874. [Google Scholar] [CrossRef]
- Yang, Q.; Lou, M.; Yin, Z.; Deng, Z.; Ding, Q.; Peng, Y. Direct C-4 alkylation of quinazoline N-oxides with ethers via an oxidative cross-coupling reaction under metal-free conditions. Org. Biomol. Chem. 2018, 45, 8724–8731. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, Z.; Zheng, L.; Yuan, J.; Wei, S.; Ding, Q.; Peng, Y. Copper-catalyzed cross-dehydrogenative coupling between quinazoline 3-oxides and indoles. RSC Adv. 2019, 9, 5870–5877. [Google Scholar] [CrossRef]
- Lüth, A.; Lowe, W. Syntheses of 4-(indole-3-yl)quinazolines—A new class of epidermal growth factor receptor tyrosine kinase (EGFR-TK) inhibitors. Eur. J. Med. Chem. 2008, 43, 1478–1488. [Google Scholar] [CrossRef]
- Lüth, A.; Lowe, W. A novel synthesis of EGFR-tyrosine-kinase inhibitors with 4-(indol-3-yl)quinazoline structure. J. Heterocycl. Chem. 2008, 45, 703–708. [Google Scholar] [CrossRef]
- An, Y.; Zheng, D.; Wu, J. An unexpected copper(ii)-catalyzed three-component reaction of quinazoline 3-oxide, alkylidenecyclopropane, and water. Chem. Commun. 2014, 50, 9165–9167. [Google Scholar] [CrossRef] [PubMed]
- Nacereddine, A.K. A MEDT computational study of the mechanism, reactivity and selectivity of non-polar [3+2] cycloaddition between quinazoline-3-oxide and methyl 3-methoxyacrylate. J. Mol. Model. 2020, 26, 328. [Google Scholar] [CrossRef] [PubMed]
- Heaney, J.F.; Lawless, E.; Mahon, M.; McArdle, P.; Cunningham, D. 1,3-Dipolar character of 2-vinyl quinazoline 3-oxides; first and second generation cycloaddition products. Org. Biomol. Chem. 2006, 4, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Kumar, N.; Anshu, A.; Sharma, P.; Kishore, D. 1,5-Benzodiazepines: Overview of properties and synthetic aspects. Res. J. Chem. Sci. 2013, 3, 90–103. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).