Abstract
2-deoxythiosugars are more stable than 2-deoxysugars occurring broadly in bioactive natural products and pharmaceutical agents. An effective and direct methodology to stereoselectively synthesize α-2-deoxythioglycosides catalyzed by AgOTf has been developed. Various alkyl thiols and thiophenols were explored and the desired products were formed in good yields with excellent α-selectivity. This method was further applied to the syntheses of S-linked disaccharides and late-stage 2-deoxyglycosylation of estrogen, L-menthol, and zingerone thiols successfully.
1. Introduction
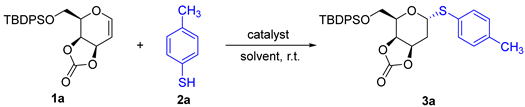
2-deoxysugars occur broadly in bioactive natural products and pharmaceutical agents [1,2,3]. They have been applied as clinical drugs in treating various diseases, including heart failure, cancers, and bacterial and viral infections [4,5,6,7]. However, the glycosidic bond of 2-deoxysugar is easily hydrolyzed by enzymes or acids, resulting in a short half-life in vivo, which limits its application in drug development [8,9]. As sulfur is in the same group as oxygen, it is often used as a bioisostere to replace oxygen atoms in medicinal chemistry, indicating a longer half-life and better biological activity [10,11,12,13]. Stereoselective synthesis of 2-deoxythiosugar is quite challenging because there is no C2-group to direct the anomeric selectivity through the neighboring participation effect. Many efforts have been made to develop the strategies of 2-deoxythioglycosylation. Conventionally, 2-deoxysugar synthesis studies could be performed with saturated glycosyl donors, the high stereoselectivity of which relied on a specific structure of a well-assembled glycosyl donor [14,15]. On the other hand, unsaturated glycosyl donors (glycals) could be applied directly to construct 2-deoxysugars stereoselectively [16,17]. Toste and coworkers developed an effective synthesis of 2-deoxyglycosides from glycal donors mediated by a catalytic Re(V)-oxo complex [18]. Recently, Wan′s group successfully achieved access to α-1,1′-2-deoxy thioglycosides stereoselectively catalyzed by ReOCl3(SMe2)(OPPh3) as shown in Scheme 1a [19]. With the development of organocatalysis, Kancharla’s group utilized a bulky pyridinium salt 2,4,6-tri-tert-butylpyridine-hydrochloric acid (TTBPy·HCl) to catalyze the reaction between glycals and thiols giving 2-deoxy-β-galactosides with an α/β ratio from 3.8:1 to β only (Scheme 1b) [20]. However, rhenium catalysts are quite expensive and the synthesis of organocatalysts is tedious. Therefore, the stereoselective synthesis of 2-deoxythioglycosides is still highly challenging. In view of our long-standing interest in exploring 3,4-O-carbonate-glycal donors [21,22,23,24,25,26,27], herein, we report an effective and stereoselective 2-deoxythioglycosylation catalyzed by the commercially available catalyst silver trifluoromethanesulfonate (AgOTf) as shown in Scheme 1c. The target α-2-deoxythiosugars were able to be obtained in good yields with high stereoselectivity under mild conditions.
Scheme 1.
Synthesis of 2-deoxythioglycosides from glycal donors.
2. Results
First, 3,4-O-carbonate galactal 1a was adopted as a glycosyl donor and p-methylthiophenol 2a was adopted as a glycosyl acceptor to optimize the conditions for 2-deoxythioglycosylation (Table 1). Initially, Cu(OTf)2 was examined as the catalyst, giving 2-deoxysugar 3a in a yield of 32% with high α-selectivity (α/β > 20:1) in dichloromethane (entry 1). Then, various Lewis acids were screened (entries 2–8). For example, Hg(OTf)2 could increase the yield to 59% (entry 2), while Yb(OTf)3 decreased the yield to 30% (entry 3). Fe(OTf)3 was also able to catalyze this reaction to give the desired product in a 64% yield (entry 4). There was no reaction when Ni(OTf)2 and Dy(OTf)3 were examined (entries 5, 7). AgOTf could increase the yield to 80% (entry 8), which was the best catalyst for this reaction. Then various solvents were examined, including toluene, THF, MeCN, dimethyl carbonate (DMC), and diethyl carbonate (DEC) (entries 9–15). The yield was lower (43%) in toluene (entry 9), while no reaction was observed in THF (entry 10), acetonitrile (entry 11), DMF (entry 14), and DMSO (entry 15). The green solvent (DMC, entry 12) was applied, and the yield decreased to 57%, while DEC (entry 13) was also attempted to proceed with the reaction, but only a 33% yield was observed. Therefore, the condition was finalized as Ag(OTf) as the catalyst in DCM at room temperature.

Table 1.
Condition optimization for the 2-deoxythioglycosylation a.
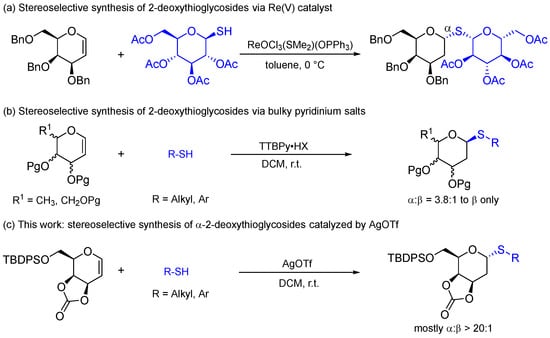
With the optimized condition in hand, the substrate scope was first explored using thiophenols (Scheme 2). The aryl 2-deoxythioglycosides (3a-3c) were obtained using p-, m- and o-thiocresols as acceptors in yields of 80–82% and with an α/β ratio from 15:1 to > 20:1 determined by 1H NMR. Fortunately, the crystal of 3c was obtained and the configuration was confirmed as α-2-deoxy-D-galactoside by single-crystal X-ray diffraction. 3,4-Dimethylthiophenol also worked well giving 3d in an 82% yield and with a ratio of α/β = 20:1. Besides methyl substitution thiophenols, 4-methoxythiophenol reacted with 3,4-O-carbonate-D-galactal 1a to form 3e in a 73% yield with a ratio of α/β > 20:1. The bulkier substitute group on the phenyl ring also tolerated this method well. For example, 4-tert-butylthiophenol was applied to generate 3f in an 81% yield and with an α/β ratio of 18:1. Thiophenol 2-deoxysugar 3g was obtained stereoselectively (α/β > 20:1) in an 80% yield. When thiophenols were substituted by electron-withdrawing groups, the desired products (3h-3j) could still be formed in good yields but stereoselectivity decreased slightly. The 2-deoxythiosugar 3h was formed in a yield of 75% with a ratio of α:β = 11:1 using 4-bromothiophenol as the acceptor. 2-Bromothiophenol and 4-fluorothiophenol were examined and gave target products in a 71–74% yield at the ratios of α:β = 15:1 and α:β = 12:1, respectively.
Scheme 2.
Substrate scope of aryl 2-deoxythioglycosides.
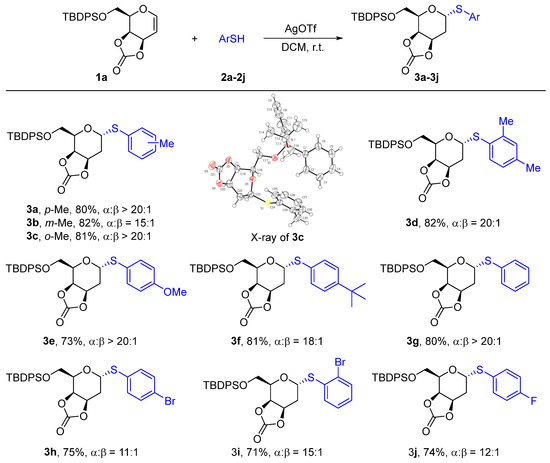
Besides the substrate study of the thiophenol acceptors, the alkyl thiols were also employed to synthesize 2-deoxythioglycosides, including S-linked disaccharides. As shown in Scheme 3, two primary thiols ethyl and n-octyl mercaptan were utilized to react with glycal 1a to form 2-deoxythiosugars 5a and 5b in high yields (78–86%) with excellent stereoselectivity (α/β > 20:1). When n-butyl, iso-butyl, and sec-butyl mercaptan were employed, the corresponding α-2-deoxythioglycosides 5c-5e were all able to be generated in high yields (>80%). It is worth noting that the bulky tert-butyl mercaptan could also be well compatible with this method and gave 5f in an 80% yield with exclusive α-selectivity, indicating that the strategy was less affected by steric hindrance. Benzyl mercaptan was applied to form the 2-deoxythiogalactoside 5g in a yield of 82% with a ratio of α/β > 20:1. Methyl thioglycolate was also applied as a glycosyl acceptor and the desired product 5h was obtained in an 80% yield. Encouraged by these observations, we continued to study the synthesis of S-linked α-disaccharides. For example, 5i was achieved successfully from 3,4-O-carbonate-galactal 1 and 1,2:5,6-di-O-isopropylidene-α-D-allofuranose in a yield of 67% with exclusive α-selectivity. The α,β-1,1′-2-deoxythioglycosides 5j was obtained from glycal 1 and 1-thio-β-D-glucose tetraacetate in a yield of 69% with excellent stereoselectivity (α/β > 20:1).
Scheme 3.
Substrate scope of alkyl 2-deoxythioglycosides.
These successes stimulated us to further apply this 2-deoxythioglycosylation methodology to the modification of bioactive natural products (In the Supplementary Materials). Estrogen is a female sex hormone, one of the three main endogenous estrogens, which plays a vital role in human life and is also used as a medicine in clinical treatment [28,29,30]. S-Linked estrone 2-deoxygalactoside 7a was successfully achieved by AgOTf at room temperature with a 78% yield and excellent α-selectivity (Scheme 4). L-Menthol is the main component of peppermint and also demonstrates analgesic, antibacterial, and anti-inflammatory effects [31]. L-Menthol-2-deoxythioglycoside 7b was generated in a 60% yield with high α-selectivity. Zingerone extracted from ginger indicates antioxidant, anti-inflammatory and anti-cancer, and antibacterial bioactivity [32,33,34], and it was also converted to be a thiol to react with glycal 1a giving 2-deoxythiosugar 7c in a 72% yield with a ratio of α/β > 20:1.
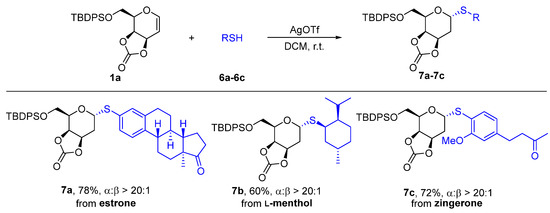
Scheme 4.
Late-stage 2-deoxythioglycosylation of natural product thiols.
Based on the results and literature research, a possible mechanism of AgOTf-catalyzed 2-deoxythioglycosylation was proposed as shown in Scheme 5. Silver triflate coordinated with the double bond of glycal from the bottom face to form intermediate A because of the steric effect [35,36]. After the pronation of the reactive olefin, the oxocarbenium ion B would be generated [1,4,37]. Alkylthio anion (RS-) would attack the anomeric position from the bottom face to yield α-2-deoxythioglycosides because the 3,4-O-carbonate ring would block the upper face.
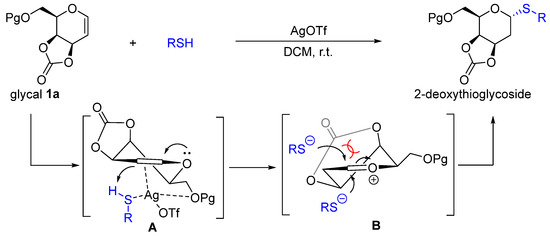
Scheme 5.
Proposed mechanism.
3. Conclusions
In conclusion, we have developed an effective strategy to synthesize 2-deoxythioglycosides using 3,4-O-carbonate glycal donors catalyzed by silver triflate in mild conditions. This reaction could tolerate various alkyl thiols and thiophenols, all the target products were obtained in moderate to good yields with excellent α-selectivity. S-Linked disaccharides and late-stage functionalization of natural product thiols were achieved successfully as well. The results of this study suggest that this method may be a promising alternative way to access the 2-deoxythioglycosides applied in natural product synthesis and drug development.
4. Materials and Methods
General Procedure. The 3,4-O-carbonate glycal donor (0.100 mmol) and thiol reagent (0.110 mmol) were added to anhydrous dichloromethane (2.00 mL) in a Schlenk tube, followed by adding silver triflate (0.01 mmol) under N2 atmosphere. The reaction mixture was stirred at room temperature and monitored by TLC. Then, aqueous sodium bicarbonate was added to quench the reaction, extracted with dichloromethane, washed by aqueous sodium bicarbonate and dried by sodium sulfate. The organic layer was collected and removed under reduced pressure to afford a crude product which was purified by silica gel flash chromatography with a gradient solvent system (petroleum ether/ethyl acetate as eluent) to yield 2-deoxythioglycosides.
4-Methylphenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside(3a). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 42.7 mg, yield 80%; 1H NMR (400 MHz, CDCl3) δ 7.73–7.63 (m, 4H, Ar-H), 7.47–7.39 (m, 6H, Ar-H), 7.33–7.27 (m, 2H, Ar-H), 7.01–7.03 (m, 2H, Ar-H), 5.45 (dd, J = 9.8, 6.5 Hz, 1H, H-1), 5.05 (ddd, J = 8.8, 4.4, 2.0 Hz, 1H, H-3), 4.90 (dd, J = 8.6, 1.6 Hz, 1H, H-4), 4.16 (ddd, J = 7.7, 6.0, 1.6 Hz, 1H, H-5), 3.84 (dd, J = 10.2, 7.6 Hz, 1H, H-6), 3.77 (dd, J = 10.2, 6.2 Hz, 1H, H-6’), 2.64 (ddd, J = 15.8, 6.5, 3.5 Hz, 1H, H-2), 2.29 (s, 3H, PhCH3), 1.88 (ddd, J = 15.9, 9.8, 3.0 Hz, 1H, H-2’), 1.06 (s, 9H, Si-tBu). 13C NMR (100 MHz, CDCl3) δ 154.2, 138.4, 135.7, 135.6, 133.2, 133.1, 132.8, 130.1, 130.0, 129.9, 129.4, 128.0, 80.8, 73.5, 72.4, 68.5, 61.6, 28.9, 26.9, 21.3, 19.4. HRMS (ESI) m/z: calcd. for C30H34O5SSiNa+ (M + Na)+ 557.1788, found 557.1786; = +45.7 (c = 1.0, CHCl3).
3-Methylphenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside(3b). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 43.8 mg, yield 82%; 1H NMR (400 MHz, CDCl3) δ 7.69 (ddd, J = 8.0, 5.2, 1.6 Hz, 4H, Ar-H), 7.58–7.34 (m, 6H, Ar-H), 7.27–7.20 (m, 2H, Ar-H), 7.19–7.03 (m, 2H, Ar-H), 5.56 (dd, J = 9.8, 6.5 Hz, 1H, H-1), 5.08 (ddd, J = 8.4, 4.0, 2.0 Hz, 1H, H-3), 4.93 (dd, J = 8.6, 1.6 Hz, 1H, H-4), 4.19 (ddd, J = 7.7, 6.0, 1.6 Hz, 1H, H-5), 3.87 (dd, J = 10.2, 7.7 Hz, 1H, H-6), 3.80 (dd, J = 10.2, 6.1 Hz, 1H, H-6’), 2.68 (ddd, J = 15.8, 6.5, 3.5 Hz, 1H, H-2), 2.27 (s, 3H, PhCH3), 1.92 (ddd, J = 15.9, 9.8, 3.0 Hz, 1H, H-2’), 1.08 (s, 9H, Si-tBu). 13C NMR (100 MHz, CDCl3) δ 154.2, 138.9, 135.7, 135.6, 133.2, 133.1, 132.9, 132.8, 130.1, 130.0, 129.3, 128.9, 128.9, 128.0, 80.5, 73.4, 72.4, 68.6, 61.5, 29.0, 26.9, 21.4, 19.4. HRMS (ESI) m/z: calcd. for C30H34O5SSiNa+ (M + Na)+ 557.1788, found 557.1790; = +62.2 (c = 1.0, CHCl3).
2-Methylphenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside(3c). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving white solid 43.2 mg, yield 81%; m. p.: 155.7–157.1 °C. 1H NMR (400 MHz, CDCl3) δ 7.64–7.67 (m, 4H, Ar-H), 7.73–7.45 (m, 7H, Ar-H), 7.20–7.09 (m, 2H, Ar-H), 7.03–7.07 (m, 1H, Ar-H), 5.53 (dd, J = 9.8, 6.4 Hz, 1H, H-1), 5.07 (ddd, J = 8.6, 4.2, 2.0 Hz, 1H, H-3), 4.93 (dd, J = 8.7, 1.6 Hz, 1H, H-4), 4.17 (ddd, J = 8.0, 6.0, 1.7 Hz, 1H, H-5), 3.83 (dd, J = 10.1, 8.0 Hz, 1H, H-6), 3.75 (dd, J = 10.1, 6.0 Hz, 1H, H-6’), 2.67 (ddd, J = 15.8, 6.5, 3.5 Hz, 1H, H-2), 2.33(s, 3H, PhCH3), 1.96 (ddd, J = 15.9, 9.8, 2.9 Hz, 1H, H-2’), 1.04 (s, 9H, Si-tBu). 13C NMR (100 MHz, CDCl3) δ 154.2, 139.4, 135.7, 135.6, 133.2, 132.8, 132.8, 130.3, 130.1, 130.0, 128.0, 128.0, 126.8, 79.8, 73.4, 72.4, 68.6, 61.4, 29.2, 26.9, 20.9, 19.4. HRMS (ESI) m/z: calcd. for C30H34O5SSiNa+ (M + Na)+ 557.1788, found 557.1793; = +43.2 (c = 1.0, CHCl3)
2,4-Methoxylphenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-D-galactopyranoside (3d). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 44.9 mg, yield 82%; 1H NMR (400 MHz, CDCl3) δ 7.77–7.64 (m, 4H, Ar-H), 7.52–7.40 (m, 6H, Ar-H), 7.31–7.33 (m, 1H, Ar-H), 7.03–6.99 (m, 1H, Ar-H), 6.85–6.88 (m, 1H, Ar-H), 5.45 (dd, J = 9.8, 6.4 Hz, 1H, H-1), 5.13–5.03 (m, 1H, H-3), 4.96 (dd, J = 8.6, 1.6 Hz, 1H, H-4), 4.18 (ddd, J = 7.8, 5.8, 1.6 Hz, 1H, H-5), 3.86 (dd, J = 10.1, 8.2 Hz, 1H, H-6), 3.76 (dd, J = 10.0, 5.9 Hz, 1H, H-6’), 2.68 (ddd, J = 15.8, 6.4, 3.5 Hz, 1H, H-2), 2.33 (s, 3H, PhCH3), 2.28 (s, 3H, PhCH3), 1.96 (ddd, J = 15.9, 9.9, 2.9 Hz, 1H, H-2’), 1.08 (s, 9H, Si-tBu). 13C NMR (100 MHz, CDCl3) δ 154.3, 140.0, 138.5, 135.7, 135.6, 134.0, 133.2, 132.8, 131.3, 130.1, 130.0, 128.7, 128.0, 127.5, 80.2, 73.4, 72.4, 68.4, 61.4, 29.1, 26.9, 21.2, 21.0, 19.4. HRMS (ESI) m/z: calcd. for C31H36O5SSiNa+ (M + Na)+ 571.1945, found 571.1941; = +68.4 (c = 1.0, CHCl3).
4-Methoxylphenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranside (3e). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 40.1 mg, yield 73%; 1H NMR (400 MHz, CDCl3) δ 7.69–7.72 (m, 4H, Ar-H), 7.41–7.48 (m, 6H, Ar-H), 7.35–7.38 (m, 2H, Ar-H), 6.74–6.76 (m, 2H, Ar-H), 5.38 (dd, J = 9.8, 6.4 Hz, 1H, H-1), 5.06 (ddd J = 8.6, 4.4, 2.2Hz, 1H, H-3), 4.91 (dd, J = 8.6, 1.6 Hz, 1H, H-4), 4.18 (ddd, J = 7.7, 6.1, 1.6 Hz, 1H, H-5), 3.87 (dd, J = 10.3, 7.5 Hz, 1H, H-6), 3.80 (dd, J = 9.4, 5.3 Hz, 1H, H-6’), 3.77 (s, 3H, PhOCH3), 2.65 (ddd, J = 15.9, 6.5, 3.5 Hz, 1H, H-2), 1.89 (ddd, J = 15.8, 9.8, 3.0 Hz, 1H, H-2’), 1.10 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 160.2, 154.2, 135.7, 135.7, 135.6, 133.2, 132.8, 130.1, 130.0, 128.0, 123.3, 114.7, 81.2, 73.5, 72.5, 68.5, 61.7, 55.4, 28.9, 27.0, 19.4. HRMS (ESI) m/z: calcd. for C30H34O6SSiNa+ (M + Na)+ 573.1738, found 573.1739; = +90.6 (c = 1.0, CHCl3).
4-tert-Butylphenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (3f). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 46.7 mg, yield 81%; 1H NMR (400 MHz, CDCl3) δ 7.69–7.72 (m, 4H, Ar-H), 7.52–7.38 (m, 6H, Ar-H), 7.41–7.34 (m, 2H, Ar-H), 7.30–7.22 (m, 2H, Ar-H), 5.51 (dd, J = 9.7, 6.5 Hz, 1H, H-1), 5.07 (ddd, J = 8.6, 4.2, 2.2 Hz, 1H, H-3), 4.92 (dd, J = 8.7, 1.7 Hz, 1H, H-4), 4.22 (ddd, J = 7.6, 6.2, 1.6 Hz, 1H, H-5), 3.88 (dd, J = 10.2, 7.5 Hz, 1H, H-6), 3.82 (dd, J = 10.2, 6.2 Hz, 1H, H-6’), 2.67 (ddd, J = 15.9, 6.5, 3.5 Hz, 1H, H-2), 1.91 (ddd, J = 15.9, 9.8, 3.0 Hz, 1H, H-2’), 1.29 (s, 9H, C-tBu), 1.09 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 154.2, 151.4, 135.7, 135.6, 133.2, 132.8, 132.6, 130.1, 130.0, 129.6, 128.0, 126.2, 80.7, 73.5, 72.4, 68.5, 61.6, 34.7, 31.3, 29.0, 26.9, 19.4. HRMS (ESI) m/z: calcd. for C33H40O5SSiNa+ (M + Na)+ 599.2258, found 599.2259; = +104.8 (c = 1.0, CHCl3).
Phenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (3g). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 41.6 mg, yield 80%; 1H NMR (400 MHz, CDCl3) δ 7.70 (ddd, J = 8.0, 4.0, 1.7 Hz, 4H, Ar-H), 7.53–7.34 (m, 8H, Ar-H), 7.27–7.21 (m, 3H, Ar-H), 5.57 (dd, J = 9.7, 6.5 Hz, 1H, H-1), 5.08 (ddd, J = 8.4, 4.0, 2.4 Hz, 1H, H-3), 4.92 (dd, J = 8.6, 1.6 Hz, 1H, H-4), 4.20 (ddd, J = 7.6, 6.2, 1.7 Hz, 1H, H-5), 3.87 (dd, J = 10.2, 7.5 Hz, 1H, H-6), 3.81 (dd, J = 10.3, 6.2 Hz, 1H, H-6’), 2.69 (ddd, J = 15.9, 6.5, 3.5 Hz, 1H, H-2), 1.92 (ddd, J = 15.8, 9.8, 3.0 Hz, 1H, H-2’), 1.08 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 154.2, 135.7, 135.6, 133.4, 133.2, 132.8, 132.3, 130.1, 130.0, 129.1, 128.0, 80.5, 73.4, 72.4, 68.6, 61.6, 29.0, 26.9, 19.4. HRMS (ESI) m/z: calcd. for C29H32O5SSiNa+ (M + Na)+ 543.1632, found 543.1642; = +67.9 (c = 1.0, CHCl3).
4-Bromophenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (3h). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 46.0 mg, yield 75%; 1H NMR (400 MHz, CDCl3) δ 7.66–7.70 (m, 4H, Ar-H), 7.51–7.39 (m, 6H, Ar-H), 7.36–7.20 (m, 3H, Ar-H), 7.29 (d, J = 2.4 Hz, 1H, Ar-H), 5.54 (dd, J = 9.8, 6.5 Hz, 1H, H-1), 5.07 (ddd, J = 8.6, 4.4, 2.2 Hz, 1H, H-3), 4.90 (dd, J = 8.6, 1.7 Hz, 1H, H-4), 4.15–4.19 (m, 1H, H-5), 3.87 (dd, J = 10.4, 7.1 Hz, 1H, H-6), 3.79 (dd, J = 10.3, 6.3 Hz, 1H, H-6’), 2.69 (ddd, J = 15.9, 6.5, 3.5 Hz, 1H, H-2), 1.89 (ddd, J = 15.9, 9.9, 3.0 Hz, 1H, H-2’), 1.07 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 154.1, 135.7, 135.6, 133.7, 133.6, 133.1, 132.8, 132.6, 132.3, 132.2, 130.1, 130.0, 128.0, 122.3, 80.5, 73.4, 72.3, 68.8, 61.6, 28.8, 26.9, 19.4. HRMS (ESI) m/z: calcd. for C29H31BrO5SSiNa+ (M + Na)+ 621.0737, found 621.0734; = +45.6 (c = 1.0, CHCl3).
2-Bromophenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (3i). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 43.5 mg, yield 71%; 1H NMR (400 MHz, CDCl3) δ 7.64–7.69 (m, 4H, Ar-H), 7.55 (ddd, J = 7.9, 2.5, 1.5 Hz, 2H, Ar-H), 7.50–7.35 (m, 6H, Ar-H), 7.17–7.20 (m,1H, Ar-H), 7.06–7.11 (m, 1H, Ar-H), 5.71 (dd, J = 9.8, 6.5 Hz, 1H, H-1), 5.12 (ddd, J = 8.6, 4.0, 2.2 Hz, 1H, H-3), 4.95 (dd, J = 8.6, 1.6 Hz, 1H, H-4), 4.21 (ddd, J = 7.8, 6.3, 1.7 Hz, 1H, H-5), 3.85 (dd, J = 10.2, 7.5 Hz, 1H, H-6), 3.79 (dd, J = 10.2, 6.3 Hz, 1H, H-6’), 2.74 (ddd, J = 15.9, 6.6, 3.5 Hz, 1H, H-2), 1.98 (ddd, J = 15.8, 9.8, 2.9 Hz, 1H, H-2’), 1.04 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 154.2, 135.7, 135.6, 135.2, 133.1, 132.8, 132.0, 130.1, 130.0, 128.6, 128.20, 128.0, 125.2, 79.3, 76.8, 72.3, 68.9, 61.3, 28.6, 26.9, 19.3. HRMS (ESI) m/z: calcd. for C29H31BrO5SSiNa+ (M + Na)+ 621.0737, found 621.0735; = +82.4 (c = 1.0, CHCl3).
4-Fluorophenyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (3j). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 39.8 mg, yield 74%; 1H NMR (400 MHz, CDCl3) δ 7.79–7.67 (m, 4H, Ar-H), 7.56–7.40 (m, 8H, Ar-H), 6.90–6.93 (m, 2H, Ar-H), 5.46 (dd, J = 9.9, 6.5 Hz, 1H, H-1), 5.07 (ddd, J = 8.6, 4.2, 2.0 Hz, 1H, H-3), 4.90 (dd, J = 8.7, 1.6 Hz, 1H, H-4), 4.16–4.20 (m, 1H, H-5), 3.87 (dd, J = 10.4, 7.2 Hz, 1H, H-6), 3.80 (dd, J = 10.3, 6.3 Hz, 1H, H-6’), 2.68 (ddd, J = 15.9, 6.5, 3.4 Hz, 1H, H-2), 1.89 (ddd, J = 15.8, 9.8, 2.9 Hz, 1H, H-2’), 1.09 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 163.0 (d, J = 246.7 Hz), 154.1, 135.7, 135.6, 135.1 (d, J = 8.2 Hz), 133.1, 132.8, 130.1, 130.0, 128.0, 116.2 (d, J = 21.6 Hz), 81.0, 73.4, 72.4, 68.7, 61.7, 28.8, 26.9, 19.4. HRMS (ESI) m/z: calcd. for C29H31FO5SSiNa+ (M + Na)+ 561.1538, found 561.1541; = +86.3 (c = 1.0, CHCl3).
Ethyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (5a). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 40.6 mg, yield 86%; 1H NMR (400 MHz, CDCl3) δ 7.67–7.71 (m, 4H, Ar-H), 7.60–7.36 (m, 6H, Ar-H), 5.39 (dd, J = 9.3, 6.5 Hz, 1H, H-1), 5.02 (ddd, J = 8.4, 4.8, 2.4 Hz, 1H, H-3), 4.86 (dd, J = 8.5, 1.7 Hz, 1H, H-4), 4.23–4.01 (m, 1H, H-5), 3.85 (dd, J = 10.3, 7.3 Hz, 1H, H-6), 3.78 (dd, J = 10.3, 6.4 Hz, 1H, H-6’), 2.67–2.75 (m, 1H, H-2), 2.63–2.49 (m, 2H, CH2CH3), 1.79 (ddd, J = 15.8, 9.3, 3.2 Hz, 1H, H-2’), 1.24 (dd, J = 13.4, 6.7 Hz, 3H, CH2CH3), 1.08 (s, 9H, Si-tBu). 13C NMR (100 MHz, CDCl3) δ 154.4, 135.7, 135.6, 133.2, 132.9, 130.1, 130.0, 128.0, 127.9, 76.7, 73.7, 72.5, 68.0, 61.7, 29.2, 26.9, 24.7, 19.3, 15.0. HRMS (ESI) m/z: calcd. For C25H32O5SsiNa+ (M + Na)+ 495.1632, found 495.1634; = +54.2 (c = 1.0, CHCl3).
Octyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (5b). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 43.3 mg, yield 78%; 1H NMR (400 MHz, CDCl3) δ 7.68–7.72 (m, 4H, Ar-H), 7.40–7.50 (m, 6H, Ar-H), 5.35 (dd, J = 9.3, 6.5 Hz, 1H, H-1), 5.02 (ddd, J = 8.4, 4.5, 2.3 Hz, 1H, H-3), 4.87 (dd, J = 8.5, 1.7 Hz, 1H, H-4), 4.13 (ddd, J = 7.4, 6.9, 1.6 Hz, 1H, H-5), 3.85 (dd, J = 10.2, 7.3 Hz, 1H, H-6), 3.78 (dd, J = 10.2, 6.4 Hz, 1H, H-6’), 2.68 (ddd, J = 12.9, 8.1, 6.6 Hz, 1H, H-2), 2.57–2.41 (m, 2H), 1.79 (ddd, J = 15.8, 9.3, 3.1 Hz, 1H, H-2’), 1.59–1.51 (m, 2H, CH2), 1.41–1.12 (m, 10H, CH2), 1.08 (s, 9H, Si-tBu), 0.89 (dd, J = 7.6, 5.7 Hz, 3H, CH3). 13C NMR (100 MHz, CDCl3) δ 154.4, 135.7, 135.6, 133.2, 132.9, 130.1, 130.0, 128.0, 127.9, 77.1, 73.7, 72.5, 67.9, 61.7, 31.9, 30.7, 29.9, 29.3, 29.2, 29.2, 29.0, 26.9, 22.8, 19.3, 14.2. HRMS (ESI) m/z: calcd. for C31H44O5SSiNa+ (M + Na)+ 579.2571, found 579.2556; = +51.4 (c = 1.0, CHCl3).
n-Buty-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (5c). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 41.5 mg, yield 83%; 1H NMR (400 MHz, CDCl3) δ 7.48–7.71 (m, 4H, Ar-H), 7.55–7.36 (m, 6H, Ar-H), 5.35 (dd, J = 9.3, 6.5 Hz, 1H, H-1), 5.02 (ddd, J = 8.5, 4.4, 2.4 Hz, 1H, H-3), 4.87 (dd, J = 8.5, 1.7 Hz, 1H, H-4), 4.13 (ddd, J = 8.3, 6.4, 1.6 Hz, 1H, H-5), 3.85 (dd, J = 10.2, 7.4 Hz, 1H, H-6), 3.77 (dd, J = 10.2, 6.3 Hz, 1H, H-6’), 2.69 (ddd, J = 14.7, 8.1, 6.7 Hz, 1H, H-2), 2.60–2.46 (m, 2H, CH2), 1.78 (ddd, J = 15.7, 9.3, 3.1 Hz, 1H, H-2’), 1.60–1.49 (m, 2H, CH2), 1.41–1.32 (m, 2H, CH2), 1.08 (s, 9H, Si-tBu), 0.87 (dd, J = 14.6, 6.9 Hz, 3H, CH3). 13C NMR (100 MHz, CDCl3) δ 154.4, 135.7, 135.6, 133.2, 132.9, 130.1, 130.0, 128.0, 127.9, 77.1, 73.7, 72.5, 67.9, 61.6, 31.9, 30.4, 29.2, 26.9, 22.0, 19.4, 13.7. HRMS (ESI) m/z: calcd. for C27H36O5SSiNa+ (M + Na)+ 523.1945, found 523.1945; = +36.4 (c = 1.0, CHCl3).
Isobutyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (5d). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 41.5 mg, yield 83%; 1H NMR (400 MHz, CDCl3) δ 7.67–7.71(m, 4H, Ar-H), 7.54–7.34 (m, 6H, Ar-H), 5.31 (dd, J = 9.3, 6.5 Hz, 1H, H-1), 5.02 (ddd, 8.6, 4.6, 2.4, 1H, H-3), 4.88 (dd, J = 8.5, 1.6 Hz, 1H, H-4), 4.19–4.04 (m, 1H, H-5), 3.85 (dd, J = 10.2, 7.5 Hz, 1H, H-6), 3.77 (dd, J = 10.2, 6.3 Hz, 1H, H-6’), 2.79–2.50 (m, 2H, H-2, CHHCH(CH3)2), 2.42 (dd, J = 12.9, 7.2 Hz, 1H, CHHCH(CH3)2), 1.84–1.74 (m, 2H, H-2’, CH2CH(CH3)2), 1.08 (s, 9H, Si-tBu), 0.94 (dd, J = 6.6, 2.7 Hz, 6H, CH3CHCH3). 13C NMR (100 MHz, CDCl3) δ 154.4, 135.7, 135.6, 135.6, 133.2, 133.0, 130.1, 130.0, 128.0, 127.9, 77.7, 73.7, 72.5, 67.9, 61.7, 39.7, 29.4, 28.8, 26.9, 22.1, 22.0, 19.4. HRMS (ESI) m/z: calcd. for C27H36O5SSiNa+ (M + Na)+ 523.1945, found 523.1942; = +63.3 (c = 1.0, CHCl3).
sec-Butyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (5e). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 41.0 mg, yield 82%; 1H NMR (400 MHz, CDCl3) δ 7.67–7.71 (m, 4H, Ar-H), 7.53–7.37 (m, 6H, Ar-H), 5.43 (dd, J = 9.3, 6.5 Hz, 1H, H-1), 5.00–5.05 (m, 1H, H-3), 4.89 (ddd, J = 8.3, 6.1, 1.7 Hz, 1H, H-4), 4.13–4.17 (m, 1H, H-5), 3.92–3.81 (m, 1H, H-6), 3.75 (ddd, J = 10.1, 6.1, 4.0 Hz, 1H, H-6’), 2.86–2.95 (m, 1H, CHCH2CH3), 2.53 (dddd, J = 15.7, 6.2, 3.8, 2.1 Hz, 1H, H-2), 1.81 (dddd, J = 15.8, 9.3, 4.5, 3.2 Hz, 1H, H-2’), 1.57–1.41 (m, 2H, CHCH2CH3), 1.29 (d, J = 6.9 Hz, 2H, CHCH2H), 1.20 (d, J = 7.0 Hz, 1H, CHCH2H), 1.08 (s, 9H, Si-tBu), 0.91 (dt, J = 23.3, 7.4 Hz, 3H, CHCH2CH3). 13C NMR (100 MHz, CDCl3) δ 154.4, 135.7, 135.7, 135.6, 135.6, 133.2, 130.1, 130.0, 128.0, 127.9, 76.6, 73.6, 72.5, 67.8, 61.6, 41.8, 40.0, 30.2, 26.9, 21.3, 19.4, 11.4. 13C NMR (100 MHz, CDCl3) δ.154.4, 135.7, 135.7, 135.6, 135.6, 133.2, 130.1, 130.0, 128.0, 127.9, 75.8, 73.6, 72.5, 67.8, 61.6, 41.2, 39.3, 29.4, 26.9, 21.3, 19.4, 11.3. HRMS (ESI) m/z: calcd. for C27H36O5SSiNa+ (M + Na)+ 523.1945, found 523.1946; = +35.6 (c = 1.0, CHCl3).
tert-Butyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (5f). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 40.0 mg, yield 80%; 1H NMR (400 MHz, CDCl3) δ 7.69–7.72 (m, 4H, Ar-H), 7.56–7.37 (m, 6H, Ar-H), 5.56 (dd, J = 9.4, 6.5 Hz, 1H, H-1), 5.03 (ddd, J = 8.4, 4.8, 2.4 Hz, 1H, H-3), 4.91 (dd, J = 8.5, 1.6 Hz, 1H, H-4), 4.13 (ddd, J = 7.9, 5.7, 1.6 Hz, 1H, H-5), 3.85 (dd, J = 10.0, 8.3 Hz, 1H, H-6), 3.72 (dd, J = 10.0, 5.8 Hz, 1H, H-6’), 2.49 (ddd, J = 15.7, 6.6, 3.8 Hz, 1H, H-2), 1.81 (ddd, J = 15.8, 9.4, 3.1 Hz, 1H, H-2’), 1.31 (s, 9H, C-tBu), 1.07 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 154.6, 135.7, 135.6, 133.2, 132.8, 130.1, 130.0, 128.0, 127.9, 75.4, 73.5, 72.6, 67.6, 61.2, 44.5, 31.8, 29.4, 26.9, 19.4. HRMS (ESI) m/z: calcd. for C27H36O5SSiNa+ (M + Na)+ 523.1945, found 523.1949; = +40.2 (c = 1.0, CHCl3).
Benzyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (5g). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 43.8 mg, yield 82%; 1H NMR (400 MHz, CDCl3) δ 7.70–7.73 (m, 4H, Ar-H), 7.55–7.33 (m, 6H, Ar-H), 7.30–7.10 (m, 5H, Ar-H), 5.21 (dd, J = 9.2, 6.6 Hz, 1H, H-1), 5.00 (ddd, J = 8.4, 4.6, 2,4 Hz, 1H, H-3), 4.86 (dd, J = 8.5, 1.7 Hz, 1H, H-4), 4.14–4.18(m, 1H, H-5), 3.92–3.83 (m, 2H, H-6, PhCHH), 3.76 (dd, J = 10.3, 6.4 Hz, 1H, H-6’), 3.66 (d, J = 13.6 Hz, 1H, PhCHH), 2.46 (ddd, J = 15.7, 6.6, 3.8 Hz, 1H, H-2), 1.77 (ddd, J = 15.8, 9.2, 3.2 Hz, 1H, H-2’), 1.11 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 154.3, 137.9, 135.7, 135.6, 133.2, 132.9, 130.1, 130.0, 129.0, 128.7, 128.0, 127.3, 76.0, 73.6, 72.5, 68.2, 61.7, 34.5, 28.8, 27.0, 19.4. HRMS (ESI) m/z: calcd. for C30H34O5SSiNa+ (M + Na)+ 557.1788, found 557.1777; = +140.3 (c = 1.0, CHCl3).
2-Methoxy-2-oxoethyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranooside (5h). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 41.2 mg, yield 80%; 1H NMR (400 MHz, CDCl3) δ 7.67–7.70 (m, 4H, Ar-H), 7.54–7.37 (m, 6H, Ar-H), 5.50 (dd, J = 9.5, 6.6 Hz, 1H, H-1), 5.05 (ddd, J = 8.6, 4.0, 2.2 Hz, 1H, H-3), 4.88 (dd, J = 8.6, 1.7 Hz, 1H, H-4), 4.14–4.03 (m, 1H, H-5), 3.84 (dd, J = 10.2, 7.2 Hz, 1H, H-6), 3.77 (dd, J = 10.2, 6.4 Hz, 1H, H-6’), 3.67 (s, 3H, CH2COOCH3), 3.52 (d, J = 15.5 Hz, 1H, CHHCOOCH3), 3.17 (d, J = 15.5 Hz, 1H, CHHCOOCH3), 2.61 (ddd, J = 15.8, 6.5, 3.5 Hz, 1H, H-2), 1.76 (ddd, J = 15.8, 9.5, 3.1 Hz, 1H, H-2’), 1.08 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 170.7, 154.2, 135.7, 135.6, 133.1, 132.8, 130.1, 130.0, 128.0, 77.4, 73.5, 72.3, 68.4, 61.5, 52.7, 31.5, 28.6, 26.9, 19.4. HRMS (ESI) m/z: calcd. for C26H32O7SSiNa+ (M + Na)+ 539.1530, found 539.1519; = +28.0 (c = 1.0, CHCl3).
3-S-(6-O-(tert-Butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-a-D-galactopyranosyl)-1,2,3,4-di-O-isopropylidene-β-D-glucofuranoside (5i). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column giving colorless oil 45.9 mg, yield 67%; 1H NMR (400 MHz, CDCl3) δ 7.66 (ddd, J = 7.8, 3.6, 1.7 Hz, 4H, Ar-H), 7.57–7.35 (m, 6H, Ar-H), 5.93 (d, J = 3.6 Hz, 1H, H-1b), 5.05 (dd, J = 9.6, 6.0 Hz, 1H, H-1a), 4.97–5.00 (m, 1H, H-3a), 4.85 (dd, J = 8.3, 1.7 Hz, 1H, H-4a), 4.45 (dd, J = 3.5, 1.8 Hz, 1H, H-2b), 4.30–4.33 (m, 1H, H-5b), 4.04–4.08 (m, 1H, H-5a), 3.92–3.65 (m, 5H, H-6a, 6’a, H-4b, H-7b, 7’b), 3.60 (dd, J = 4.5, 2.0 Hz, 1H, H-3b), 2.41 (ddd, J = 15.7, 5.6, 4.2 Hz, 1H, H-2a), 1.89 (ddd, J = 15.7, 6.4, 3.7 Hz, 1H, H-2’a), 1.57 (s, 3H, CH3), 1.49 (s, 3H, CH3), 1.45 (s, 3H, CH3), 1.31 (s, 3H, CH3), 1.06 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 154.4, 135.7, 135.6, 133.2, 132.9, 130.1, 130.0, 128.0, 112.3, 106.4, 95.21, 83.6, 83.2, 77.9, 73.8, 73.5, 72.1, 67.9, 67.7, 61.7, 47.5, 31.6, 29.7, 27.5, 27.1, 27.0, 26.6, 19.4. HRMS (ESI) m/z: calcd. for C35H47O10SSi+ (M + H)+ 687.2654, found 687.2670; = +27.2 (c = 1.0, CHCl3).
6-S-(6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-a-D-galactopyranosyl)-,2,3,4,6-tetra-O-acetyl-β-D-glucopyranoside (5j). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving white solid 53.0 mg, yield 69%; m.p.: 144.6–146.3 °C. 1H NMR (400 MHz, CDCl3) δ 7.84–7.56 (m, 4H, Ar-H), 7.65–7.35 (m, 6H, Ar-H), 5.56 (dd, J = 9.8, 6.4 Hz, 1H, H-1a), 5.12–5.17(m, 1H, H-3b), 5.09–5.02 (m, 2H, H-3a, H-2b), 5.02–4.97 (m, 2H, H-4a, H-4b), 4.61 (d, J = 10.1 Hz, 1H, H-1b), 4.11 (ddd, J = 8.0, 6.8, 1.5 Hz, 1H, H-5), 4.00 (dd, J = 12.5, 4.8 HZ, 1H, H-6a), 3.90 (dd, J = 12.5, 2.2 Hz, 1H, H-6’a), 3.90–3.79 (m, 2H, H-6, 6’b), 3.60 (ddd, J = 10.1, 4.7, 2.2 Hz, 1H, H-5b), 2.56 (ddd, J = 15.8, 6.4, 3.3 Hz, 1H, H-2a), 2.20–1.97 (m, 9H, OAc), 1.89–1.82 (m, 4H, H-2’a, OAc), 1.07 (s, 9H, Si-tBu). 13C NMR (100 MHz, CDCl3) δ 170.6, 170.3, 169.5, 169.2, 154.1, 135.7, 135.6, 133.1, 132.7, 130.1, 130.0, 128.0, 83.3, 77.6, 76.4, 73.9, 73.1, 72.02, 71.1, 68.2, 67.9, 61.8, 60.7, 29.0, 26.9, 20.8, 20.7, 19.4. HRMS (ESI) m/z: calcd. for C37H46O14SSiNa+ (M + Na)+ 797.2270, found 797.2265; = +21.1 (c = 1.0, CHCl3).
Estronyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (7a). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 54.3 mg, yield 78%; 1H NMR (400 MHz, CDCl3) δ 7.80–7.64 (m, 4H, Ar-H), 7.52–7.36 (m, 6H, Ar-H), 7.26–7.10 (m, 3H, Ar-H), 5.51 (dd, J = 9.8, 6.5 Hz, 1H, H-1), 5.08 (ddd, J = 8.6, 4.1, 2.4 Hz, 1H, H-3), 4.94 (dd, J = 8.6, 1.6 Hz, 1H, H-4), 4.20 (ddd, J = 7.6, 5.9, 1.6 Hz, 1H, H-5), 3.88 (dd, J = 10.2, 7.8 Hz, 1H, H-6), 3.79 (dd, J = 10.2, 6.0 Hz, 1H, H-6’), 2.83–2.74 (m, 2H, PhCH2), 2.68 (ddd, J = 15.9, 6.5, 3.4 Hz, 1H, H-2), 2.52 (dd, J = 18.9, 8.6 Hz, 1H, PhCH), 2.34–2.38 (m, 1H, COCHH), 2.22–2.23 (m, 1H, COCHH), 2.23–2.04 (m, 3H, CH2, CHH), 2.04–1.86 (m, 3H, H-2’, CH2), 1.71–1.53 (m, 3H, CH2, CHH), 1.50–1.37 (m, 2H, CHCH), 1.09 (s, 9H, Si-tBu), 0.91 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3) δ 220.9, 154.2, 140.1, 137.5, 135.7, 135.6, 133.3, 133.2, 132.8, 130.3, 130.1, 130.0, 129.9, 128.0, 126.2, 80.7, 73.4, 72.4, 68.5, 61.5, 50.6, 48.1, 44.4, 38.0, 36.0, 31.7, 29.3, 28.9, 26.9, 26.4, 25.7, 21.7, 19.4, 13.9. HRMS (ESI) m/z: calcd. For C41H48O5SsiNa+ (M + Na)+ 719.2833, found 719.2830; = +114.6 (c = 1.0, CHCl3).
L-Menthyl-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside(7b). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 34.9 mg, yield 60%; 1H NMR (400 MHz, CDCl3) δ 7.83–7.55 (m, 4H, Ar-H), 7.55–7.34 (m, 6H, Ar-H), 5.32 (dd, J = 9.7, 6.4 Hz, 1H, H-1), 5.06 (ddd, J = 8.7, 3.1 Hz, 1H, H-3), 5.01–5.08 (m, 1H, H-4), 4.14 (ddd, J = 9.5, 5.5, 1.5 Hz, 1H, H-5), 3.84–3.88 (m, 1H, H-6), 3.74 (dd, J = 9.6, 5.5 Hz, 1H, H-6’), 3.36–3,37 (m, 1H, SCH), 2.55 (ddd, J = 15.8, 6.4, 3.5 Hz, 1H, H-2), 1.88–1.95 (m, 2H, CH, CH), 1.83 (ddd, J = 15.8, 9.7, 2.8 Hz, 1H, H-2’), 1.77–1.69 (m, 2H, CH2), 1.52–1.43 (m, 1H, CHH), 1.25–1.13 (m, 1H, CHH), 1.08 (s, 9H, Si-tBu), 1.05–0.99 (m, 2H, CH, CHH), 0.88–0.92 (m, 4H, CH3, CHH), 0.83 (d, J = 6.6 Hz, 3H, CH3), 0.67 (d, J = 6.5 Hz, 3H, CH3).13C NMR (100 MHz, CDCl3) δ 154.5, 135.6, 135.5, 133.2, 132.8, 130.1, 130.0, 128.0, 127.9, 75.4, 73.5, 72.6, 67.5, 61.0, 48.3, 44.5, 40.3, 35.4, 29.9, 29.5, 26.9, 26.6, 26.4, 22.2, 21.0, 20.4, 19.4. HRMS (ESI) m/z: calcd. for C33H46O5SSiNa+ (M + Na)+ 605.2727, found 605.2728; = +56.7 (c = 1.0, CHCl3).
Zingerone-1-thio-6-O-(tert-butyldiphenylsilyl)-3,4-O-carbonate-2-deoxy-α-D-galactopyranoside (7c). The title compound was prepared according to the general procedure of 2-deoxythioglycosides synthesis and purified by flash column chromatography giving colorless oil 41.2 mg, yield 72%; 1H NMR (400 MHz, CDCl3) δ 7.81–7.63 (m, 4H), 7.52–7.34 (m, 6H, Ar-H), 7.33–7.14 (m, 1H, Ar-H), 6.69 (d, J = 1.6 Hz, 1H, Ar-H), 6.60 (dd, J = 7.8, 1.7 Hz, 1H, Ar-H), 5.61 (dd, J = 9.6, 6.5 Hz, 1H, H-1), 5.08–5.10 (m, 1H, H-3), 4.95 (dd, J = 8.7, 1.6 Hz, 1H, H-4), 4.22 (ddd, J = 7.9, 5.9, 1.6 Hz, 1H, H-5), 3.94–3.78 (m, 4H, H-6, OMe), 3.70 (dd, J = 10.0, 6.0 Hz, 1H, H-6’), 2.82–2.86 (m, 2H, CH2CH2), 2.77–2.63 (m, 3H, H-2, CH2CH2), 2.14 (s, 3H, CH2COCH3), 1.92 (ddd, J = 15.8, 9.6, 3.0 Hz, 1H, H-2’), 1.07 (s, 9H, Si-tBu).13C NMR (100 MHz, CDCl3) δ 207.7, 158.4, 154.3, 143.3, 135.7, 135.6, 134.5, 133.2, 132.8, 130.0, 129.9, 128.0, 121.0, 118.1, 111.3, 78.6, 73.5, 72.5, 68.23, 61.4, 55.9, 45.0, 30.2, 29.8, 28.9, 26.9, 19.3. HRMS (ESI) m/z: calcd. for C34H40O7SSiNa+ (M + Na)+ 643.2165, found 643.2154; = +99.8 (c = 1.0, CHCl3).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27227979/s1, general information, NMR spectra and X-ray crystal structure and data, Figure S1: Synthesis of estrone thiol, Figure S2: Synthesis of L-menthol thiol, Figure S3: Synthesis of zingerone thiol, Figure S4: ORTEP drawing of compound 3c showing thermal ellipsoids at the 50% probability level (CCDC: 2212881), Table S1: Crystal data and structure refinement for 3c. The data for known compounds were checked in comparison with literature for consistency [38,39,40,41].
Author Contributions
Conceptualization, H.Y. and L.C.; methodology, X.Y.; validation, Y.C., C.X. and L.M.; formal analysis, Y.W.; investigation, T.X.; data curation, X.Y.; writing—original draft preparation, X.Y and H.Y.; writing—review and editing, H.Y.; supervision, H.Y.; project administration, H.Y and L.C.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 111 Project (D20015) and the Educational Commission of Hubei Province of China (D20221204).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are included within the manuscript or the supplementary data file.
Acknowledgments
The authors acknowledged the NMR analysis support of Nianyu Huang from Hubei Key Laboratory of Natural Products Research and Development, China Three Gorges University.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Bennett, C.S.; Galan, M.C. Methods for 2-deoxyglycoside synthesis. Chem. Rev. 2018, 118, 7931–7985. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, K.M.; Xiong, D.C.; Zhang, H.; Li, T.; Li, B.; Qin, X.; Bai, J.; Ye, X.-S. Stereoselective electro-2-deoxyglycosylation from glycals. Angew. Chem. Int. Ed. 2020, 59, 15204–15208. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Du, X.; Jiao, H.; An, Q.; Chen, R.; Fang, P.; Wang, J.; Yu, B. Carbohydrate-based drugs launched during 2000−2021. Acta Pharm. Sin. B 2022, 12, 3783–3821. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xu, Y.; Wang, H.; Meng, L.; Wan, Q. Recent progress on the synthesis of 2-deoxy glycosides. Sci. China Chem. 2017, 60, 1162–1179. [Google Scholar] [CrossRef]
- Wan, L.Q.; Zhang, X.; Zou, Y.; Shi, R.; Cao, J.G.; Xu, S.Y.; Deng, L.F.; Zhou, L.; Gong, Y.; Shu, X.; et al. Nonenzymatic stereoselective S-glycosylation of polypeptides and proteins. J. Am. Chem. Soc. 2021, 143, 11919–11926. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Dao, Y.; Mo, J.; Dong, S.; Shoda, S.-I.; Ye, X.-S. Protection-free site-directed peptide or protein S-glycosylation and its application in the glycosylation of glucagon-like peptide 1. CCS Chem. 2021, 4, 2316–2323. [Google Scholar] [CrossRef]
- Pal, K.B.; Guo, A.; Das, M.; Lee, J.; Báti, G.; Yip, B.R.P.; Loh, T.-P.; Liu, X.-W. Iridium-promoted deoxyglycoside synthesis: Stereoselectivity and mechanistic insight. Chem. Sci. 2021, 12, 2209–2216. [Google Scholar] [CrossRef]
- Adhikari, S.; Baryal, K.N.; Zhu, D.; Li, X.; Zhu, J. Gold-catalyzed synthesis of 2-deoxy glycosides using S-but-3-ynyl thioglycoside donors. ACS Catal. 2013, 3, 57–60. [Google Scholar] [CrossRef]
- Zhu, J.; Baryal, K. Stereoselective synthesis of S-linked 2-deoxy sugars. Synlett 2013, 25, 308–312. [Google Scholar] [CrossRef]
- Zhu, F.; Miller, E.; Zhang, S.Q.; Yi, D.; O’Neill, S.; Hong, X.; Walczak, M.A. Stereoretentive C(sp3)-S cross-coupling. J. Am. Chem. Soc. 2018, 140, 18140–18150. [Google Scholar] [CrossRef]
- Tamburrini, A.; Colombo, C.; Bernardi, A. Design and synthesis of glycomimetics: Recent advances. Med. Res. Rev. 2020, 40, 495–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiao, Y.; Luo, H.; Huang, N.; Lai, M.; Zou, K.; Yao, H. Catalyst-controlled regiodivergent synthesis of 1- and 3-thiosugars with high stereoselectivity and chemoselectivity. ACS Catal. 2021, 11, 5287–5293. [Google Scholar] [CrossRef]
- Hua, M.; Sun, Y.; Zhang, X.; Yao, H.; Huang, N. Convenient cobalt-catalyzed stereoselective synthesis of β-D-thioglucosides. Chin. J. Org. Chem 2022, 42, 2140–2154. [Google Scholar] [CrossRef]
- Hoang, K.M.; Lees, N.R.; Herzon, S.B. Programmable synthesis of 2-deoxyglycosides. J. Am. Chem. Soc. 2019, 141, 8098–8103. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Baryal, K.N.; Adhikari, S.; Zhu, J.L. Direct synthesis of 2-deoxy-β-glycosides via anomeric O-alkylation with secondary electrophiles. J. Am. Chem. Soc. 2014, 136, 3172–3175. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Jayaraman, N. Catalytic ceric ammonium nitrate mediated synthesis of 2-deoxy-1-thioglycosides. Carbohydr. Res. 2004, 339, 2197–2204. [Google Scholar] [CrossRef]
- Meng, S.; Li, X.; Zhu, J. Recent advances in direct synthesis of 2-deoxy glycosides and thioglycosides. Tetrahedron 2021, 88, 132140. [Google Scholar] [CrossRef]
- Sherry, B.D.; Loy, R.N.; Toste, F.D. Rhenium(V)-catalyzed synthesis of 2-deoxy-α-glycosides. J. Am. Chem. Soc. 2004, 126, 4510–4511. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, B.; Shu, P.; Meng, L.; Zeng, J.; Wan, Q. Rhenium(V)-catalyzed synthesis of 1,1’-2-deoxy thioglycosides. Carbohydr. Res. 2021, 508, 108415. [Google Scholar] [CrossRef]
- Mukherji, A.; Addanki, R.B.; Halder, S.; Kancharla, P.K. Sterically strained Brønsted pair catalysis by bulky pyridinium salts: Direct stereoselective synthesis of 2-deoxy and 2,6-dideoxy-β-thioglycosides from glycals. J. Org. Chem. 2021, 86, 17226–17243. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, S.; Leng, W.-L.; Leow, M.-L.; Xiang, S.; He, J.-X.; Liao, H.; Le Mai Hoang, K.; Liu, X.-W. Catalyst-controlled stereoselective O-glycosylation: Pd(0) vs Pd(II). ACS Catal. 2017, 7, 5456–5460. [Google Scholar] [CrossRef]
- Lai, M.; Othman, K.A.; Yao, H.; Wang, Q.; Feng, Y.; Huang, N.; Liu, M.; Zou, K. Open-air stereoselective construction of C-aryl glycosides. Org. Lett. 2020, 22, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, H.; Hua, M.; Jiao, Y.; He, H.; Liu, M.; Huang, N.; Zou, K. Direct N-glycosylation of amides/amines with glycal donors. J. Org. Chem. 2020, 85, 7485–7493. [Google Scholar] [CrossRef] [PubMed]
- Abdulmajeed Othman, K.; Cai, J.; Xie, R.; Zhou, X.; Yao, H.; Huang, N. Synthesis and absolute configuration of (2R,3S,4Z,6Z)-1,3-bis(benzyloxy)-8-chloro-7-((E)-(2-(2,4-dinitro-phenyl)hydrazono)methyl)octa-4,6-dien-2-ol. Chin. J. Struct. Chem. 2020, 39, 1781–1787. [Google Scholar]
- Feng, Y.; Su, H.; Mukula Otukol, B.J.; Zhang, X.; Yao, H.; Huang, N. Synthesis and crystal sructure of tert-butyl(((2R,3R,6R)-3-hydroxy-6-(nitromethyl)-3,6-dihydro-2H-pyran-2-yl)methyl)carbonate. Chin. J. Struct. Chem. 2021, 40, 1205–1212. [Google Scholar]
- Wang, Q.; Lai, M.; Luo, H.; Ren, K.; Wang, J.; Huang, N.; Deng, Z.; Zou, K.; Yao, H. Stereoselective O-glycosylation of glycals with arylboronic acids using air as the oxygen source. Org. Lett. 2022, 24, 1587–1592. [Google Scholar] [CrossRef]
- Ding, W.-Y.; Liu, H.-H.; Cheng, J.K.; Yao, H.; Xiang, S.-H.; Tan, B. Palladium catalyzed decarboxylative β-C-glycosylation of glycals with oxazol-5-(4H)-ones as acceptors. Org. Chem. Front. 2022, 9, 6149–6155. [Google Scholar] [CrossRef]
- Chernikova, N.; Frantsiyants, E.; Molseyenko, T.; Komarova, E.; Adamyan, M.; Nikitin, I. Content of estrone and estrogen metabolites in tissues of hysterocarcinoma, adenomyosis, and hysteromyoma tissue. J. Clin. Oncol. 2014, 32, e22214. [Google Scholar] [CrossRef]
- Escandon, P.; Nicholas, S.E.; Cunningham, R.L.; Murphy, D.A.; Riaz, K.M.; Karamichos, D. The role of estriol and estrone in keratoconic stromal sex hormone receptors. Int. J. Mol. Sci. 2022, 23, 916. [Google Scholar] [CrossRef]
- Canario, C.; Matias, M.; Brito, V.; Santos, A.O.; Falcao, A.; Silvestre, S.; Alves, G. New estrone oxime derivatives: Synthesis, cytotoxic evaluation and docking studies. Molecules 2021, 26, 2687. [Google Scholar] [CrossRef]
- Zhao, R.L.; He, Y.M. Network pharmacology analysis of the anti-cancer pharmacological mechanisms of Ganoderma lucidum extract with experimental support using Hepa1-6-bearing C57 BL/6 mice. J. Ethnopharmacol. 2018, 210, 287–295. [Google Scholar] [CrossRef]
- Kung, M.L.; Lin, P.Y.; Huang, S.T.; Tai, M.H.; Hsieh, S.L.; Wu, C.C.; Yeh, B.W.; Wu, W.J.; Hsieh, S. Zingerone nanotetramer strengthened the polypharmacological efficacy of zingerone on human hepatoma cell lines. ACS Appl. Mater. Interfaces 2019, 11, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.F.; Rehman, M.U.; Raish, M.; Kazi, M.; Rao, P.G.M.; Alnemer, O.; Ahmad, P.; Ahmad, A. Zingerone [4-(3-methoxy-4-hydroxyphenyl)-butan-2] attenuates lipopolysaccharide-induced inflammation and protects rats from sepsis associated multi organ damage. Molecules 2020, 25, 5127. [Google Scholar] [CrossRef]
- Heo, K.T.; Park, K.W.; Won, J.; Lee, B.; Jang, J.H.; Ahn, J.O.; Hwang, B.Y.; Hong, Y.S. Construction of an artificial biosynthetic pathway for Zingerone production in Escherichia coli using benzalacetone synthase from Piper methysticum. J. Agric. Food. Chem. 2021, 69, 14620–14629. [Google Scholar] [CrossRef] [PubMed]
- Sau, A.; Williams, R.; Palo-Nieto, C.; Franconetti, A.; Medina, S.; Galan, M.C. Palladium-catalyzed direct stereoselective synthesis of deoxyglycosides from glycals. Angew. Chem. Int. Ed. 2017, 56, 3640–3644. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Reddy, T.R.; Gurawa, A.; Kashyap, S. Copper(II)-catalyzed stereoselective 1,2-addition vs. Ferrier glycosylation of “armed” and “disarmed” glycal donors. Org. Biomol. Chem. 2020, 18, 4848–4862. [Google Scholar] [CrossRef]
- Kumar, M.; Gurawa, A.; Kumar, N.; Kashyap, S. Bismuth-catalyzed stereoselective 2-deoxyglycosylation of disarmed/armed glycal donors. Org. Lett. 2022, 24, 575–580. [Google Scholar] [CrossRef]
- Jin, M.; Ren, W.; Qian, D.W.; Yang, S.D. Direct allylic C(sp3)-H alkylation with 2-naphthols via cooperative palladium and copper catalysis: Construction of cyclohexadienones with quaternary carbon centers. Org. Lett. 2018, 20, 7015–7019. [Google Scholar] [CrossRef]
- van den Hoogenband, A.; Lange, J.H.M.; Bronger, R.P.J.; Stoit, A.R.; Terpstra, J.W. A simple, base-free preparation of S-aryl thioacetates as surrogates for aryl thiols. Tetrahedron Lett. 2010, 51, 6877–6881. [Google Scholar] [CrossRef]
- Li, P.-K.; Pillai, R.; Young, B.L.; Bender, W.H.; Martino, D.M.; Lint, F.-T. Synthesis and biochemical studies of estrone sulfatase inhibitors. Steroids 1993, 58, 106–111. [Google Scholar]
- Hsu, J.-L.; Fang, J.-M. Stereoselective synthesis of δ-lactones from 5-oxoalkanals via one-pot sequential acetalization, tishchenko reaction, and lactonization by cooperative catalysis of samarium ion and mercaptan. J. Org. Chem. 2001, 66, 8573–8584. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).