Differences in Chemical Constituents between Dalbergia oliveri Heartwood and Sapwood and Their Effect on Wood Color
Abstract
1. Introduction
2. Results and Discussion
2.1. Main Chemical Components Analysis of D. oliveri Sapwood and Heartwood
2.1.1. Main Chemical Component Contents and PH Value of Sapwood and Heartwood
2.1.2. Characterization of Klason Structure
2.2. UV-Visible Spectroscopy of D. oliveri Extracts
2.3. Identification of Pigment Components in D. oliveri Heartwood and Sapwood Extract
2.3.1. Flavonoids
2.3.2. Isoflavones
2.3.3. Flavonol
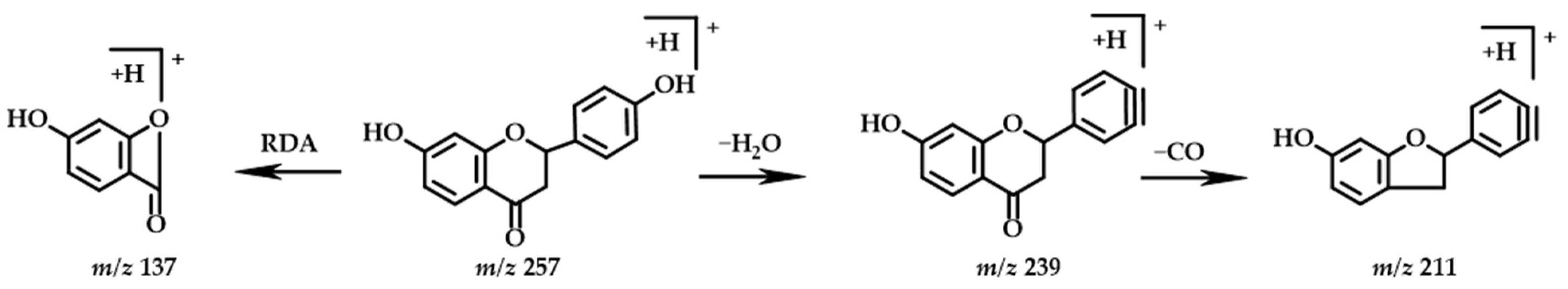
2.3.4. Flavanones
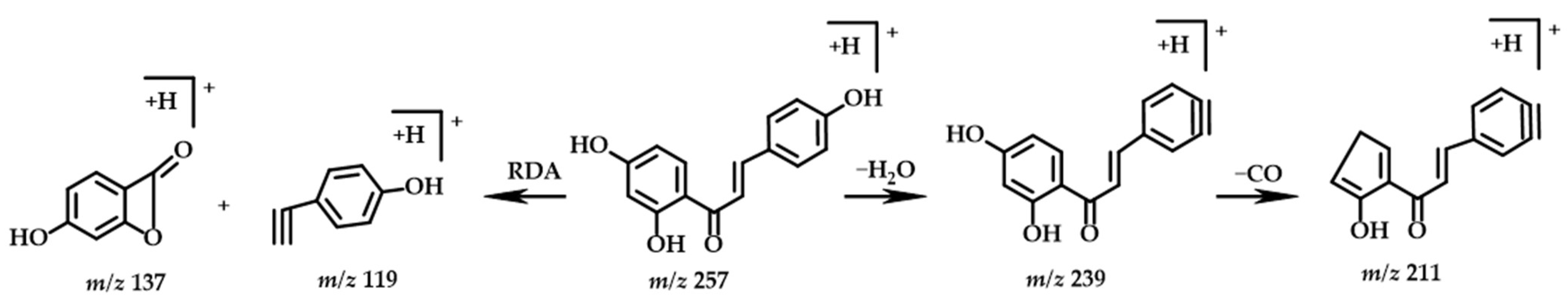
2.3.5. Chalcone
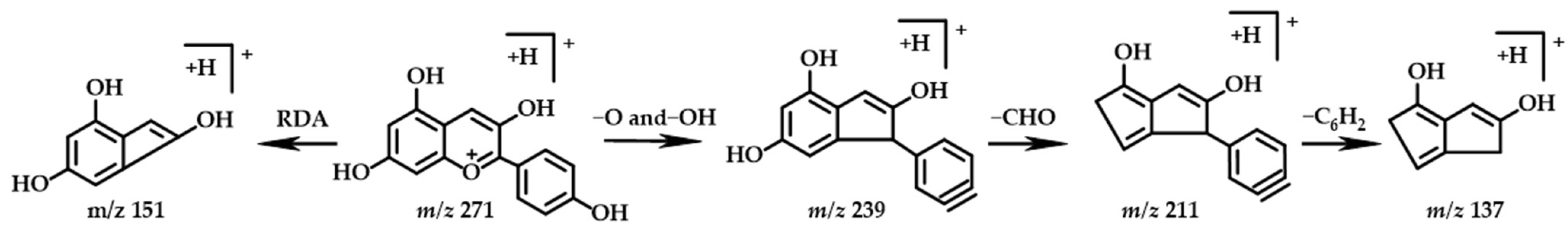
2.3.6. Anthocyanin
2.4. Analysis of the Causes of Color Difference between D. oliveri Heartwood and Sapwood
2.4.1. Different Pigment Components between Heartwood and Sapwood of D. oliveri
2.4.2. Differences in the content of main chemical components between heartwood and sapwood of D. oliveri
3. Materials and Methods
3.1. Materials and Preparation of Extracts
3.2. Reagents and Standard
3.3. Determination of Chemical Composition
3.4. Fourier Infrared Spectroscopy (FTIR) Analysis of Lignin
3.5. UV Spectroscopic Analysis
3.6. Ultra-High Performance Liquid Chromatography-Mass Spectrometry (UHPLC-MS) Analysis
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aerts, R.; Volkaert, H.; Roongruangsree, N.; Roongruangsree, U.; Swennen, R.; Muys, B. Site requirements of the endangered rosewood Dalbergia oliveri in a tropical deciduous forest in northern Thailand. For. Ecol. Manag. 2009, 259, 117–123. [Google Scholar] [CrossRef][Green Version]
- Attakorn, P.; Suphan, S.; Tamaporn, L.; Paweena, P.; Viroj, W. Inhibition of Heinz body induction in an in vitro model and total antioxidant activity of medicinal Thai plants. Asian Pac. J. Cancer Prev. 2005, 6, 458–463. [Google Scholar]
- Nguyen, T.; Doan, P.; Thi, T.; Tran, H.; Pham, L.; Thi, P.; Bach, G.; Matthäus, B.; Tran, T. Fatty acids, tocopherols, and phytosterol composition of seed oil and phenolic compounds and antioxidant activity of fresh seeds from three Dalbergia species grown in vietnam. Processes 2020, 8, 542. [Google Scholar] [CrossRef]
- Pluempanupat, S.; Kumrungsee, N.; Pluempanupat, W.; Ngamkitpinyo, K.; Chavasiri, W.; Bullangpoti, V.; Koul, O. Laboratory evaluation of Dalbergia oliveri (Fabaceae: Fabales) extracts and isolated isoflavonoids on Aedes aegypti (Diptera: Culicidae) mosquitoes. Ind. Crops Prod. 2013, 44, 653–658. [Google Scholar] [CrossRef]
- Nhunga, P.; Chib, M.; Thub, Q.; Thuongc, H.; Banc, V.; Delld, B. Market and policy setting for the trade in Dalbergia tonkinensis, a rare and valuable rosewood, in Vietnam. Trees For. People 2020, 1, 100002. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Wang, K.; Li, J.; Zhang, S.; Xia, C.; Shi, S.Q.; Cai, L. Improvement of water resistance, dimensional stability, and mechanical properties of poplar wood by rosin impregnation. Eur. J. Wood Wood Prod. 2016, 74, 177–184. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yu, Z.; Qi, C. Properties of fast-growing poplar wood simultaneously treated with dye and flame retardant. Eur. J. Wood Wood Prod. 2017, 75, 325–333. [Google Scholar] [CrossRef]
- Brocco, V.F.; Paes, J.B.; Costa, L.G.D.; Kirker, G.T.; Brazolin, S. Wood color changes and termiticidal properties of teak heartwood extract used as a wood preservative. Holzforschung 2020, 74, 233–245. [Google Scholar] [CrossRef]
- Che, N.; Yang, V. A recent (2009–2021) perspective on sustainable color and textile coloration using natural plant resources. Heliyon 2022, 8, 10979. [Google Scholar] [CrossRef]
- Arpana, K.; Surabhi, M. Consumer awareness for natural dyes. Indian J. Health Wellbeing 2017, 8, 1012–1014. [Google Scholar]
- Lee, D.; Cho, D.; Lee, J.; Shin, H.Y. Fabrication of nontoxic natural dye from sappan wood. Korean J. Chem. Eng. 2008, 25, 354–358. [Google Scholar] [CrossRef]
- Zhu, T.; Ren, K.; Sheng, J.; Zhang, Q.; Li, J.; Lin, J. Natural dye extracted from Dalbergia cochinchinensis residue with water fastness, mildew resistance and permeability properties for wood staining. Wood Sci. Technol. 2022, 56, 969–988. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, S.; Ren, K.; Chen, J.; Lin, J.; Li, J. Colorability of dyed wood veneer using natural dye extracted from Dalbergia cochinchinensis with different organic solvents. BioResources 2018, 13, 7197–7211. [Google Scholar] [CrossRef]
- Zhu, T.; Sheng, L.; Chen, B.; Ren, K.; Wu, Z.; Wu, H.; Li, J.; Liu, G. Staining of wood veneers with anti-UV property using the natural dye extracted from Dalbergia cohinchinensis. J. Clean. Prod. 2020, 284, 124770. [Google Scholar] [CrossRef]
- Sa, A.N.; Lee, J.S. Combination dyeing of Juniperus Chinensis heartwood and Alnus japonica heartwood extracts. Fash. Text. Res. J. 2015, 17, 127–136. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Liu, Y.; Shi, Z.; Li, L.; Xie, S.; Zhao, G.; Ping, P. Comparative metabolomics analysis reveals the color variation between heartwood and sapwood of Chinese fir (Cunninghamia lanceolata (Lamb.) Hook. Ind. Crops Prod. 2021, 169, 113656. [Google Scholar] [CrossRef]
- Rger, M.; Alexander, B. Wood colour variation in sapwood and heartwood of young trees of Tectona grandis and its relationship with plantation characteristics, site, and decay resistance. Ann. For. Sci. 2010, 67, 109. [Google Scholar]
- Pâques, E.; García-Casas, C.; Charpentier, J. Distribution of heartwood extractives in hybrid larches and in their related European and Japanese larch parents: Relationship with wood colour parameters. Eur. J. For. Res. 2013, 132, 61–69. [Google Scholar] [CrossRef]
- Liu, X.; Ma, F.; Chen, Y. Study on chemical property of Dalbergia oliveri Gamble. Hubei Agric. Sci. 2017, 56, 331–333. [Google Scholar]
- Aguayo, G.; Quintupill, L.; Castillo, R.; Baeza, J.; Freer, J.; Mendonça, T. Determination of differences in anatomical and chemical characteristics of tension and opposite wood of 8-year old eucalyptus globulus. Maderas Cienc. Tecnol. 2010, 12, 241–251. [Google Scholar] [CrossRef]
- Miranda, I.; Sousa, V.; Ferreira, J.; Pereira, H. Chemical characterization and extractives composition of heartwood and sapwood from Quercus faginea. PLoS ONE 2017, 12, 0179268. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aquino, F.; González-Peña, M.M.; Valdez-Hernández, J.I.; Revilla, U.S.; Romero-Manzanares, A. Chemical characterization and fuel properties of wood and bark of two oaks from Oaxaca, Mexico. Ind. Crops Prod. 2015, 65, 90–95. [Google Scholar] [CrossRef]
- Ma, R.; Liu, H.; Fu, Y.; Li, Y.; Wei, P.; Liu, Z. Variation of chemical components in sapwood, transition zone, and heartwood of Dalbergia odorifera and its relationship with heartwood formation. Forests 2021, 12, 577. [Google Scholar] [CrossRef]
- Kačíková, D.; Kačík, F.; Čabalová, I.; Ďurkovič, J. Effects of thermal treatment on chemical, mechanical and colour traits in Norway spruce wood. Bioresour. Technol. 2013, 144, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Gierlinger, N.; Jacques, D.; Grabner, M.; Wimmer, R.; Schwanninger, M.; Rozenberg, P.; Pâques, L.E. Colour of larch heartwood and relationships to extractives and brown-rot decay resistance. Trees 2003, 18, 102–108. [Google Scholar] [CrossRef]
- Dun, Q.; Peng, H.; Mai, Q.; Deng, Z.; Zhang, B. Qualitative and quantitative analysis of soluble and bound anthocyanins in black soybean seed coat by high performance liquid chromatography-mass spectrometry. Food Sci. 2019, 40, 178–186. [Google Scholar]
- Jiang, S.; Wei, Y.; Liu, Z.; Ni, C.; Gu, H.; Peng, W. Molecules and functions of rosewood: Pterocarpus santalinus. J. King Saud. Univ. Sci. 2020, 32, 1712–1717. [Google Scholar] [CrossRef]
- Shao, F.; Zhang, L.; Guo, J.; Liu, X.; Ma, W.; Wilson, L.W.; Deyou, Q. A comparative metabolomics analysis of the components of heartwood and sapwood in Taxus chinensis (Pilger) Rehd. Sci. Rep. 2019, 9, 17647. [Google Scholar] [CrossRef]
- Kumar, D.M.; Shruti, S.; Shringika, M.; Priyanka, S.; Kumar, S.P. Secondary metabolite profiling and characterization of diterpenes and flavones from the methanolic extract of Andrographis paniculata using HPLC-LC-MS/MS. Future J. Pharm. Sci. 2021, 7, 184. [Google Scholar]
- Pereira, C.; Yariwake, J.H.; Mccullagh, M. Distinction of the C-glycosylflavone isomer pairs orientin/isoorientin and vitexin/isovitexin using HPLC-MS exact mass measurement and in-source CID. Phytochem. Anal. 2010, 16, 295–301. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Fu, Y.; Sun, J. Components, stability and antioxidant activity of pigment from Dalbergia barieasis heartwood. Sci. Silvae Sin. 2021, 57, 126–134. [Google Scholar]
- Zhang, Q.; Wei, L.; Yang, Y.; Luo, C.; Zhu, Z.; Liu, Z.; Fu, Y.; Sun, J. dyeing process and mechanism of Eucalyptus veneer with Pterocarpus macrocarpus Kurz heartwood pigment as natural dye. BioResources 2020, 4, 8925–8943. [Google Scholar] [CrossRef]
- Yazhou, Z.; Hubiao, C. Investigations of the fragmentation behavior of 11 isoflavones with ESI-IT-TOF-MSn. J. Chin. Pharm. Sci. 2014, 23, 631–641. [Google Scholar]
- Zahra, G.; Khadijeh, B.; Ali, S. Fractional analysis of dichloromethane extract of Scutellaria araxensis Grossh root and shoot by HPLC-PDA-ESI-MSn. Nat. Prod. Res. 2021, 15, 4031–4035. [Google Scholar]
- Barnes, S.; Schug, K.A. Structural characterization of cyanidin-3,5-diglucoside and pelargonidin-3,5-diglucoside anthocyanins: Multi-dimensional fragmentation pathways using high performance liquid chromatography-electrospray ionization-ion trap-time of flight mass spectrometry. Int. J. Mass Spectrom. 2011, 308, 71–80. [Google Scholar] [CrossRef]
- Soo-Yun, P.; Sun-Hyung, L.; Sun-Hwa, H.; Yunsoo, Y.; Tae, P.W.; Yeon, K.D.; Un, P.S.; Kwang, K.J. Metabolite profiling approach reveals the interface of primary and secondary metabolism in colored cauliflowers (Brassica oleracea L. Ssp. Botrytis). J. Agric. Food Chem. 2013, 61, 6999–7007. [Google Scholar]
- Wang, N.; Zhang, C.; Bian, S.; Chang, P.; Xuan, L.; Fan, L.; Yu, Q.; Liu, Z.; Gu, C.; Zhang, S.; et al. Flavonoid components of different color magnolia flowers and their relationship to cultivar selections. Hortscience 2019, 54, 404–408. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Lapo, R.; Agata, S.C.V.; Ugo, C.; Marynka, U.; Edgardo, G.; Antonio, P.W.; Francesca, T.; Stefania, N.; Massimo, D.B. Liquid chromatographic quadrupole Time-of-Flight mass spectrometric untargeted profiling of (Poly)phenolic compounds in rubus idaeus l. And rubus occidentalis l. Fruits and their comparative evaluation. Antioxidants 2021, 10, 704. [Google Scholar]
- Lao-Jer, C.; Geza, H. Structural aspects of anthocyanin-flavonoid complex formation and its role in plant color. Phytochemistry 1981, 20, 297–303. [Google Scholar]
- Zheng, Y.; Wu, B.; Deng, J.; Jiang, S. Extraction process optimization of total flavones from Moringa oleifera leaves based on computeraided response surface method. Rev. Ibérica Sist. Tecnol. Inf. 2016, 5, 287–298. [Google Scholar]
- Qiu, H.; Liu, R.; Long, L. Analysis of chemical composition of extractives by acetone and the chromatic aberration of Teak (Tectona Grandis L.F.) from China. Molecules 2019, 24, 1989. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Wilson, I.W.; Shao, F.; Qiu, D. Identification of the genes involved in anthocyanin biosynthesis and accumulation in Taxus Chinensis. Genes 2019, 10, 982. [Google Scholar] [CrossRef]
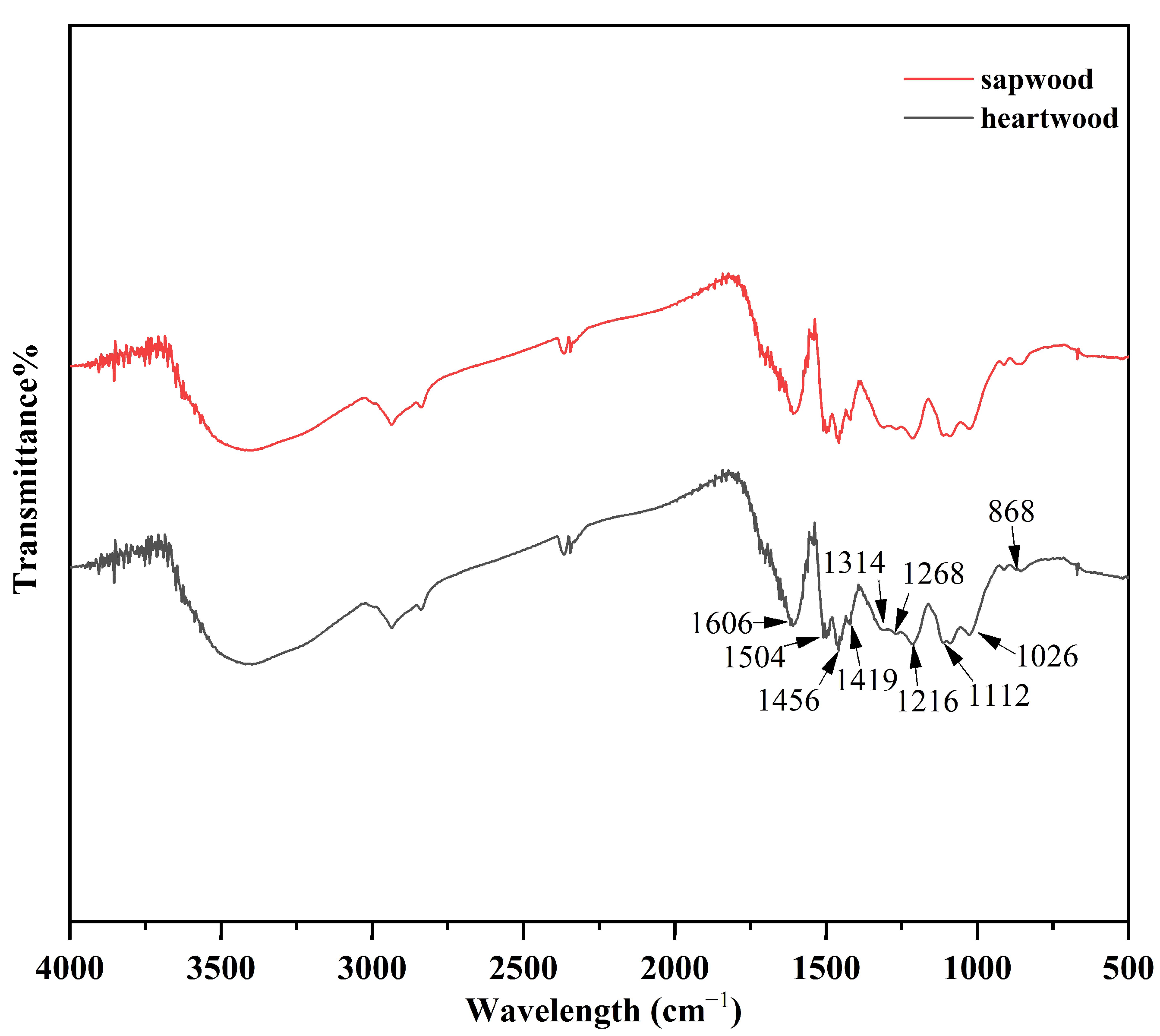
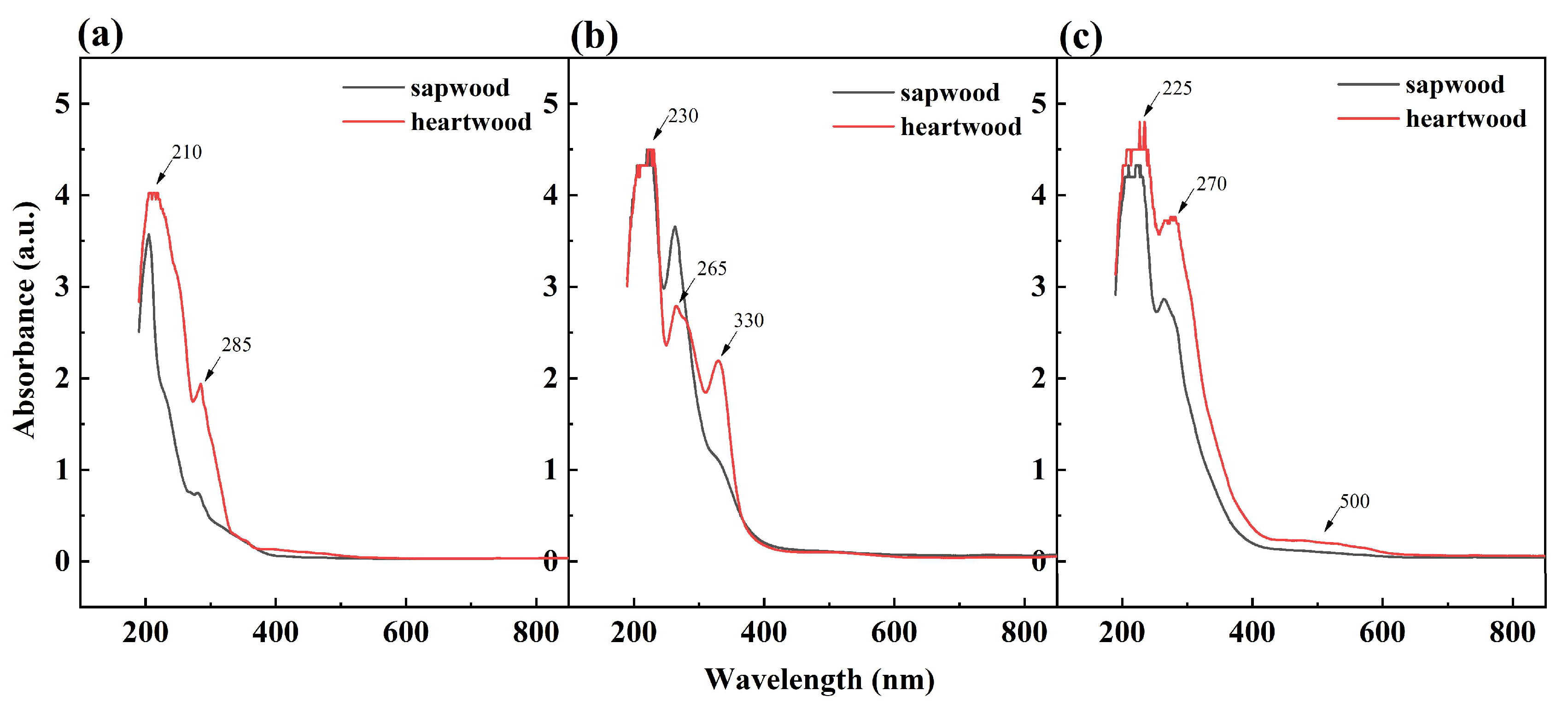
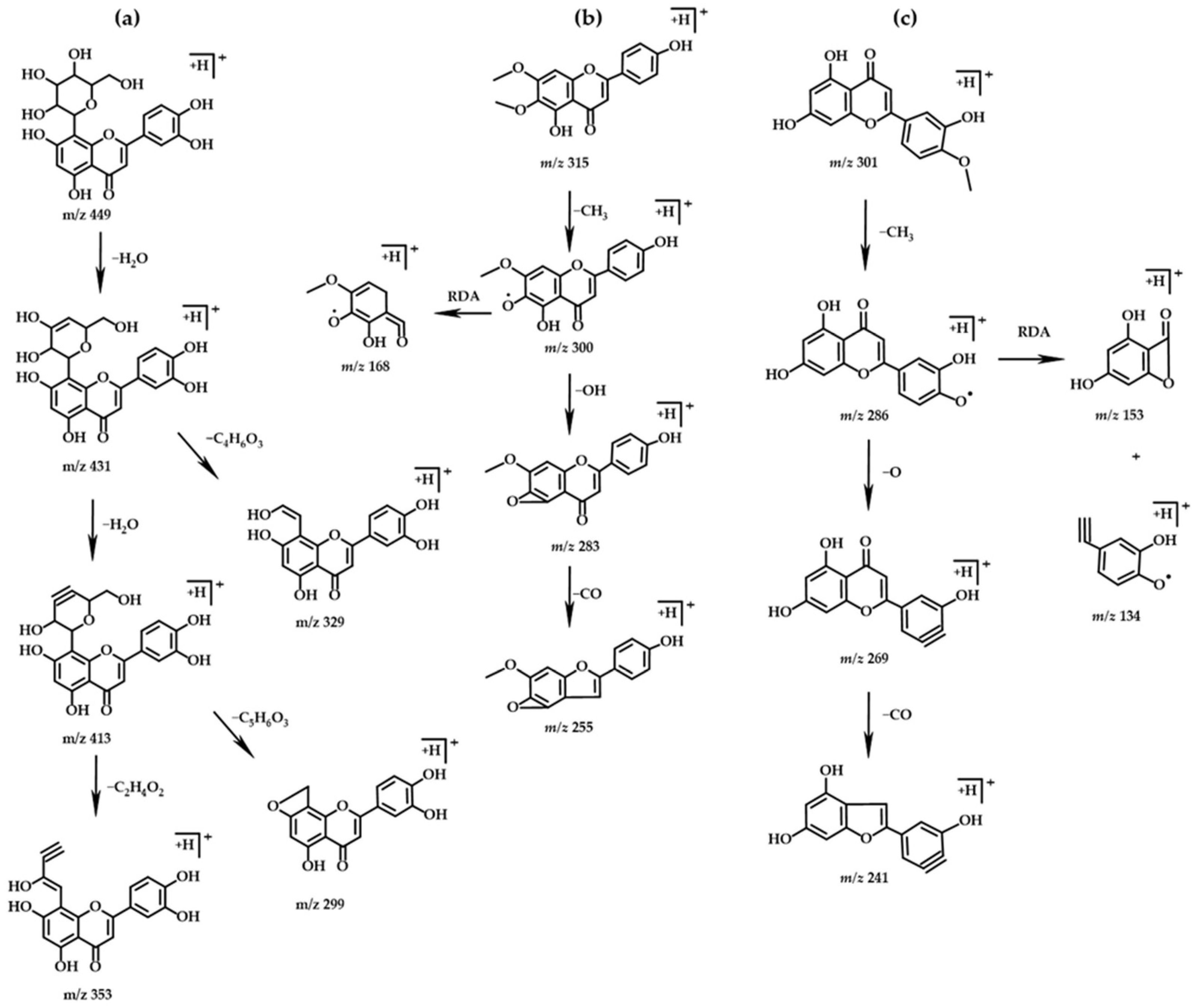
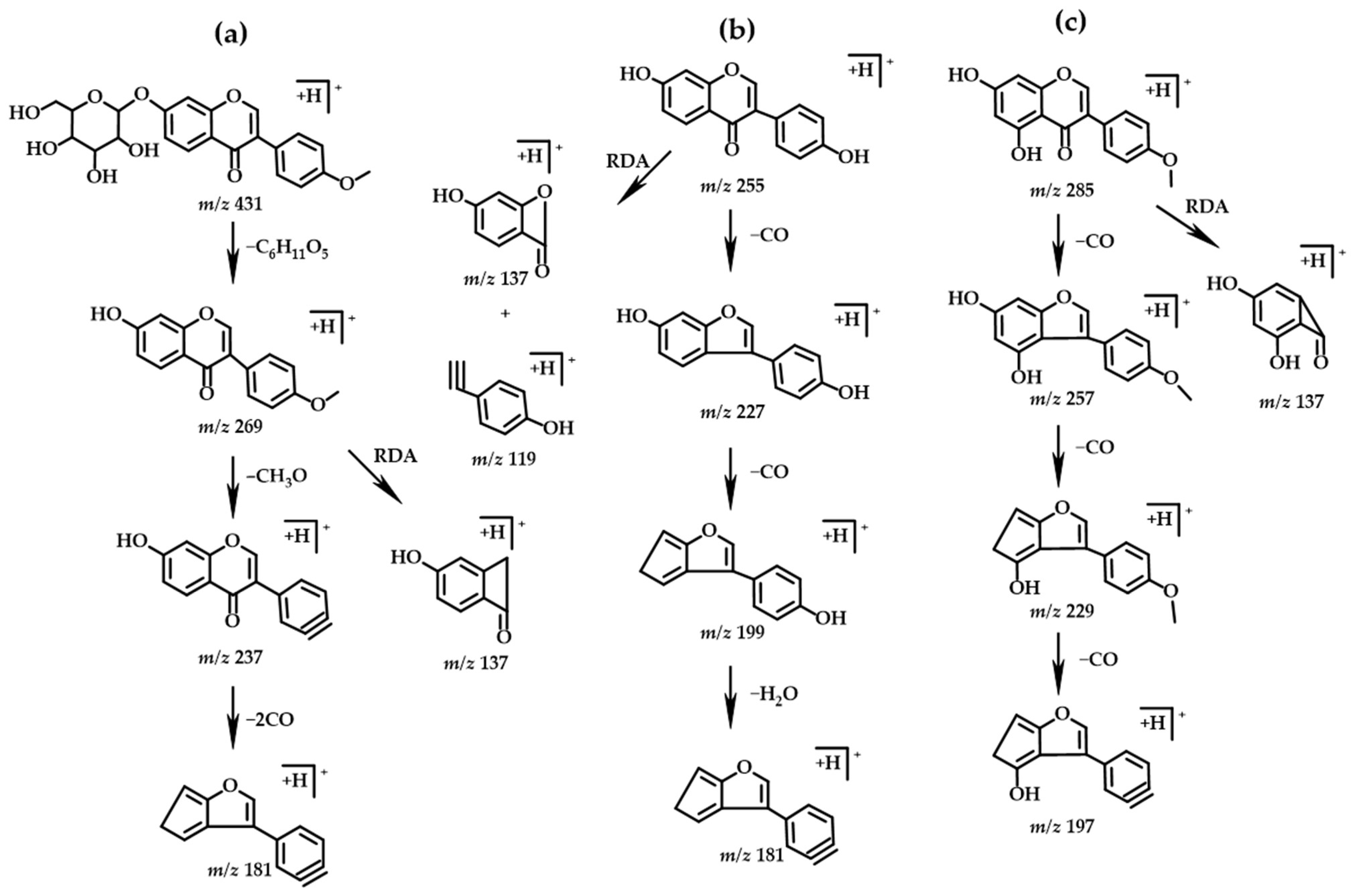

| PH Value | Holocellulose (%) | α-Cellulose (%) | Klason Lignin (%) | |||
|---|---|---|---|---|---|---|
| Acid-Insoluble Lignin | Acid-Soluble Lignin | Total | ||||
| sapwood | 6.28 | 80.35 ± 1.0 a | 63.63 ± 0.3 a | 25.96 ± 0.6 a | 1.94 ± 0.1 a | 27.90 ± 0.6 a |
| heartwood | 5.92 | 71.81 ± 2.2 b | 66.81 ± 0.8 b | 26.79 ± 0.4 a | 1.45 ± 0.1 b | 28.24 ± 0.3 a |
| No. | Structure Type | TR | Molecular Formula | Molecular Mass [M+H]+ | MS2 | Identification | |
|---|---|---|---|---|---|---|---|
| Theoretical | Measured | ||||||
| 1 | Flavonoids | 4.68 | C21H20O11 | 449.1078 | 449.1091 | 431.0955, 413.0857, 353.0653, 329.0649, 299.0543 | Orientin |
| 2 | Flavonoids | 5.38 | C21H20O10 | 433.1129 | 433.1124 | 415.1023, 397.0915, 313.0700, 283.0597, 255.0652 | Vitexin |
| 3 | Isoflavones | 5.52 | C21H20O9 | 417.1180 | 417.1177 | 255.0634, 177.1118, 133.0858 | Daidzin |
| 4 | Flavanones | 7.34 | C15H12O5 | 273.0758 | 273.0755 | 255.0649, 227.0703, 209.0584, 163.0387, 137.0232, 135.0438 | 2-(3,4-dihydroxyphenyl)-7-hydroxy-3,4-dihydro-2H-1-benzopyran-4-one |
| 5 | Flavonoids | 7.46 | C16H12O6 | 301.0707 | 301.0705 | 286.0469, 269.0439, 241.0491, 213.0544 | Hispidulin |
| 6 | Flavonols | 8.14 | C16H12O7 | 317.0656 | 317.0654 | 285.0390, 257.0437, 229.0490, 153.0181 | Isorhamnetin |
| 7 | Isoflavones | 8.46 | C22H22O9 | 431.1337 | 431.1334 | 269.0802, 237.0541, 237.0541 181.0645, 137.0231 | Ononin |
| 8 * | Flavanones | 8.51 | C15H12O4 | 257.0808 | 257.0807 | 239.0697, 211.0750, 137.0232 | Liquiritigenin |
| 9 * | Isoflavones | 9.04 | C15H10O4 | 255.0652 | 255.0651 | 227.0700, 199.0750, 181.0644, 137.0231, 119.0490 | Daidzein |
| 10 | Flavonoids | 9.29 | C17H14O6 | 315.0863 | 315.0860 | 300.0621, 283.0595, 255.0645, 168.0567 | Scrophulein |
| 11 | Flavonoids | 9.47 | C15H10O4 | 255.0652 | 255.0649 | 137.0231, 119.0492 | Chrysin |
| 12 | Anthocyanin | 9.61 | C15H10O5 | 271.0601 | 271.0599 | 239.0700, 211.0755, 151.0389, 137.0232 | Pelargonidin |
| 13 | Flavanones | 9.73 | C15H12O5 | 273.0758 | 273.0756 | 255.0655, 153.0181, 119.0493 | Naringenin |
| 14 | Isoflavones | 9.96 | C22H22O10 | 447.1286 | 447.1285 | 285.0751, 241.0488, 213.0543 | Glycitin |
| 15 * | Isoflavones | 10.12 | C15H10O5 | 271.0601 | 271.0600 | 225.0542, 197.0590, 169.0645, 137.0232 | Genistein |
| 16 | Isoflavones | 10.41 | C16H12O5 | 285.0758 | 285.0758 | 253.0488, 225.0542, 197.0595, 137.0231 | Glycitein |
| 17 | Flavonoids | 10.51 | C17H14O5 | 299.0914 | 299.0916 | 285.0711, 283.0599, 167.0339, 119.0490 | 5-hydroxy-6,7-dimethoxy-2-phenyl-4H-chromen-4-one |
| 18 | Flavonoids | 10.52 | C16H12O6 | 301.0707 | 301.0707 | 286.0466, 269.0438, 241.0488, 153.0179, 134.0360 | Diosmetin |
| 19 | Chalcone | 11.13 | C15H12O4 | 257.0808 | 257.0807 | 239.0699, 211.0752, 137.0232, 119.0492 | Isoliquiritigenin |
| 20 * | Isoflavones | 11.52 | C16H12O4 | 269.0808 | 269.0806 | 254.0565, 237.0544, 137.0234, 118.0414 | Formononetin |
| 21 * | Isoflavones | 12.78 | C16H12O5 | 285.0758 | 285.0757 | 257.0803, 229.0856, 197.0595, 137.0231 | Biochanin A |
| Structure | Compound | Color | |
|---|---|---|---|
| sapwood | isoflavone | daidzin | white |
| isoflavone | ononin | white | |
| isoflavone | glycitin | white | |
| heartwood | flavonoid | vitexin | yellow |
| flavonol | isorhamnetin | yellow | |
| anthocyanin | pelargonidin | red |
| Compound | Calibration Caves | R2 | Heartwood (ug/g) | Sapwood (ug/g) |
|---|---|---|---|---|
| Liquiritigenin | y = 1,779,112.4834x + 16,661,916.1497 | 0.996 | 293.6505 | 31.7227 |
| Genistein | y = 1,371,988.0761x + 14,319,545.4588 | 0.995 | 161.8764 | 58.3365 |
| Isoliquiritigenin | y = 1,779,112.4834x + 16,661,916.1497 | 0.999 | 4787.8213 | 18.9532 |
| Formononetin | y = 3,740,086.0193x + 16,632,919.1163 | 0.999 | 2133.5930 | 57.3800 |
| Biochanin A | y = 2,293,814.2766x + 19,031,906.6542 | 0.997 | 2000.0880 | 3.4303 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Ma, R.; Fu, Y. Differences in Chemical Constituents between Dalbergia oliveri Heartwood and Sapwood and Their Effect on Wood Color. Molecules 2022, 27, 7978. https://doi.org/10.3390/molecules27227978
Wei L, Ma R, Fu Y. Differences in Chemical Constituents between Dalbergia oliveri Heartwood and Sapwood and Their Effect on Wood Color. Molecules. 2022; 27(22):7978. https://doi.org/10.3390/molecules27227978
Chicago/Turabian StyleWei, Liuming, Ruoke Ma, and Yunlin Fu. 2022. "Differences in Chemical Constituents between Dalbergia oliveri Heartwood and Sapwood and Their Effect on Wood Color" Molecules 27, no. 22: 7978. https://doi.org/10.3390/molecules27227978
APA StyleWei, L., Ma, R., & Fu, Y. (2022). Differences in Chemical Constituents between Dalbergia oliveri Heartwood and Sapwood and Their Effect on Wood Color. Molecules, 27(22), 7978. https://doi.org/10.3390/molecules27227978








