Preparation of Chitosan-Composite-Film-Supported Copper Nanoparticles and Their Application in 1,6-Hydroboration Reactions of p-Quinone Methides
Abstract
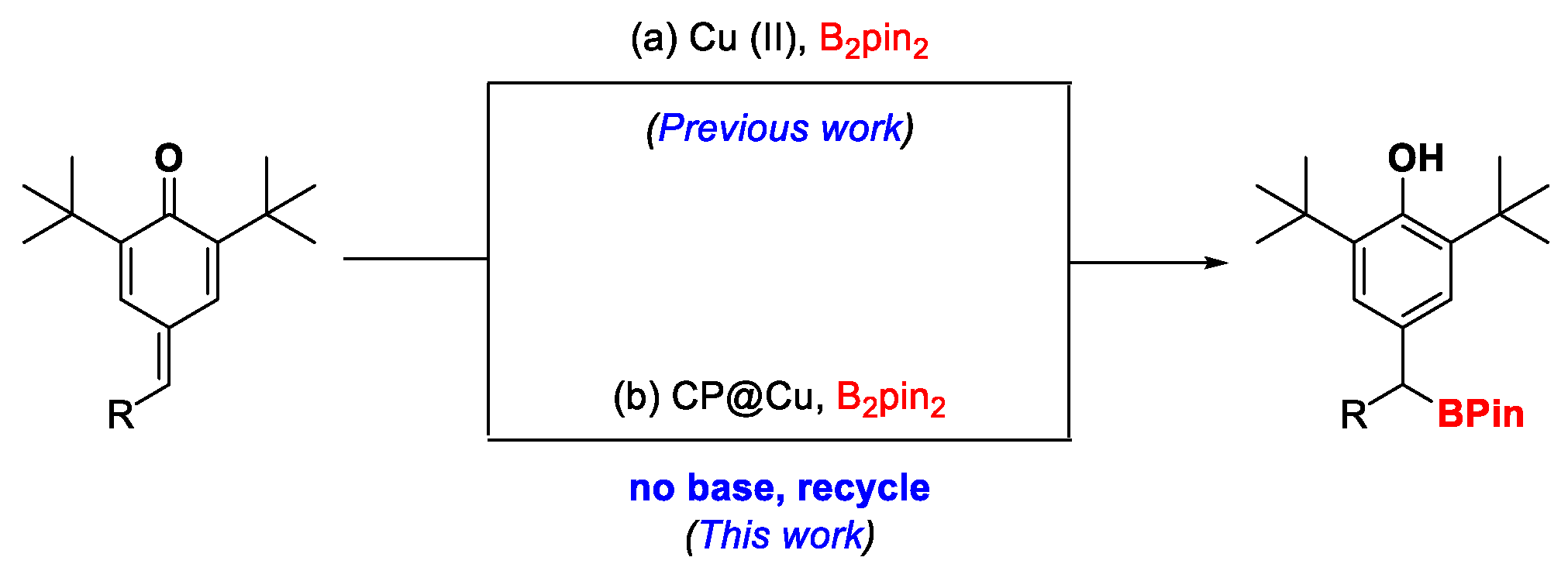
1. Introduction
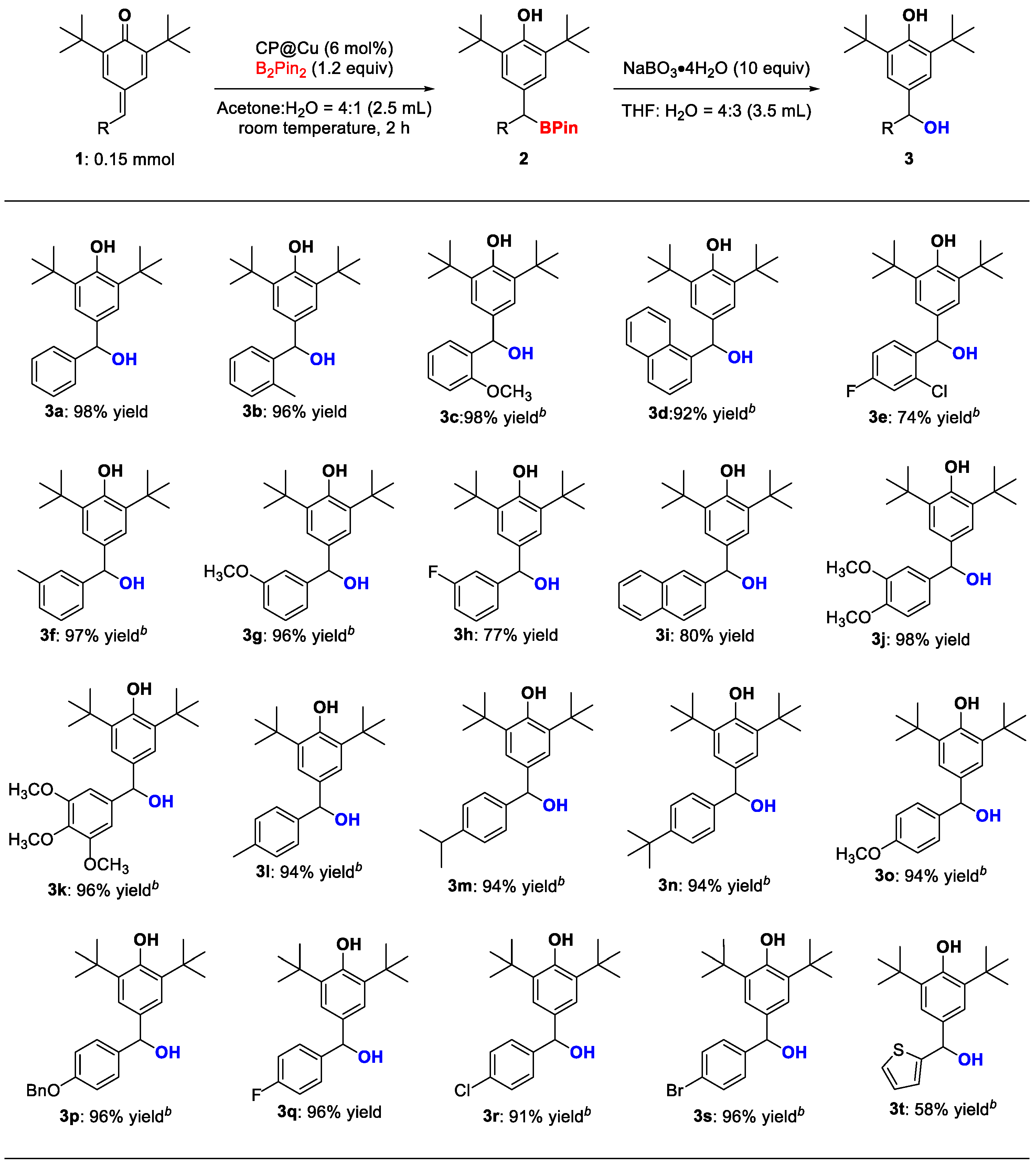
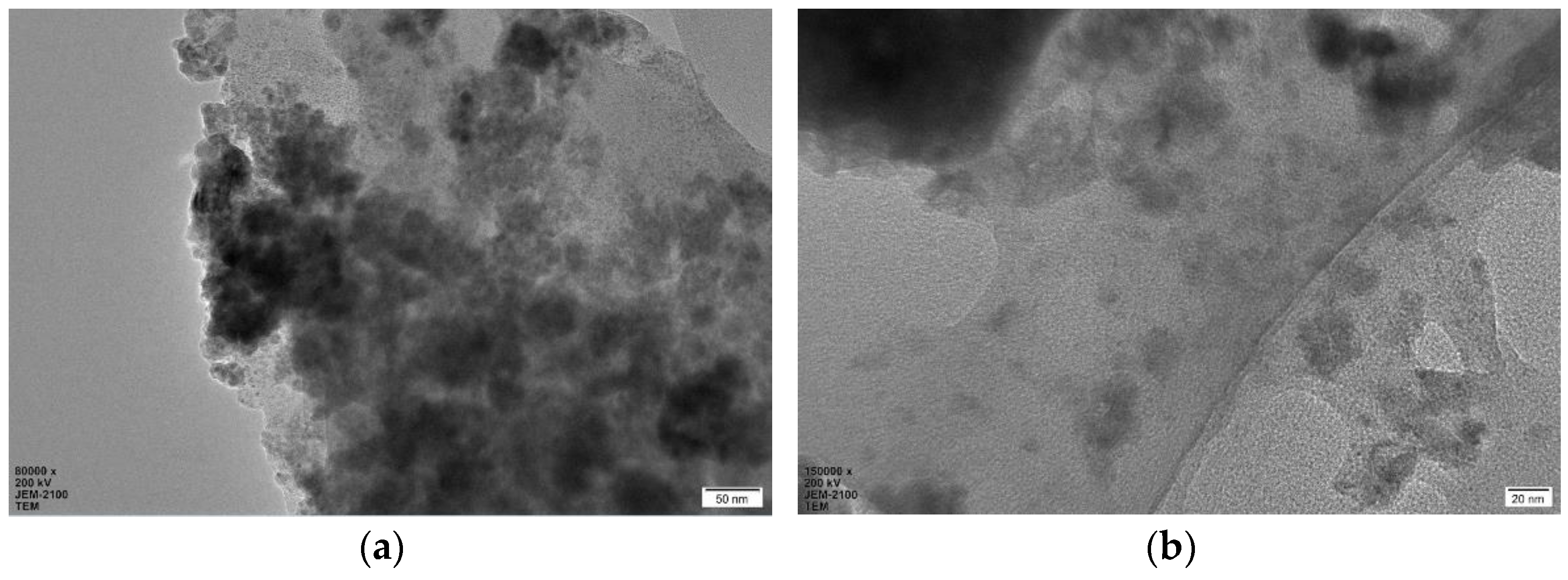
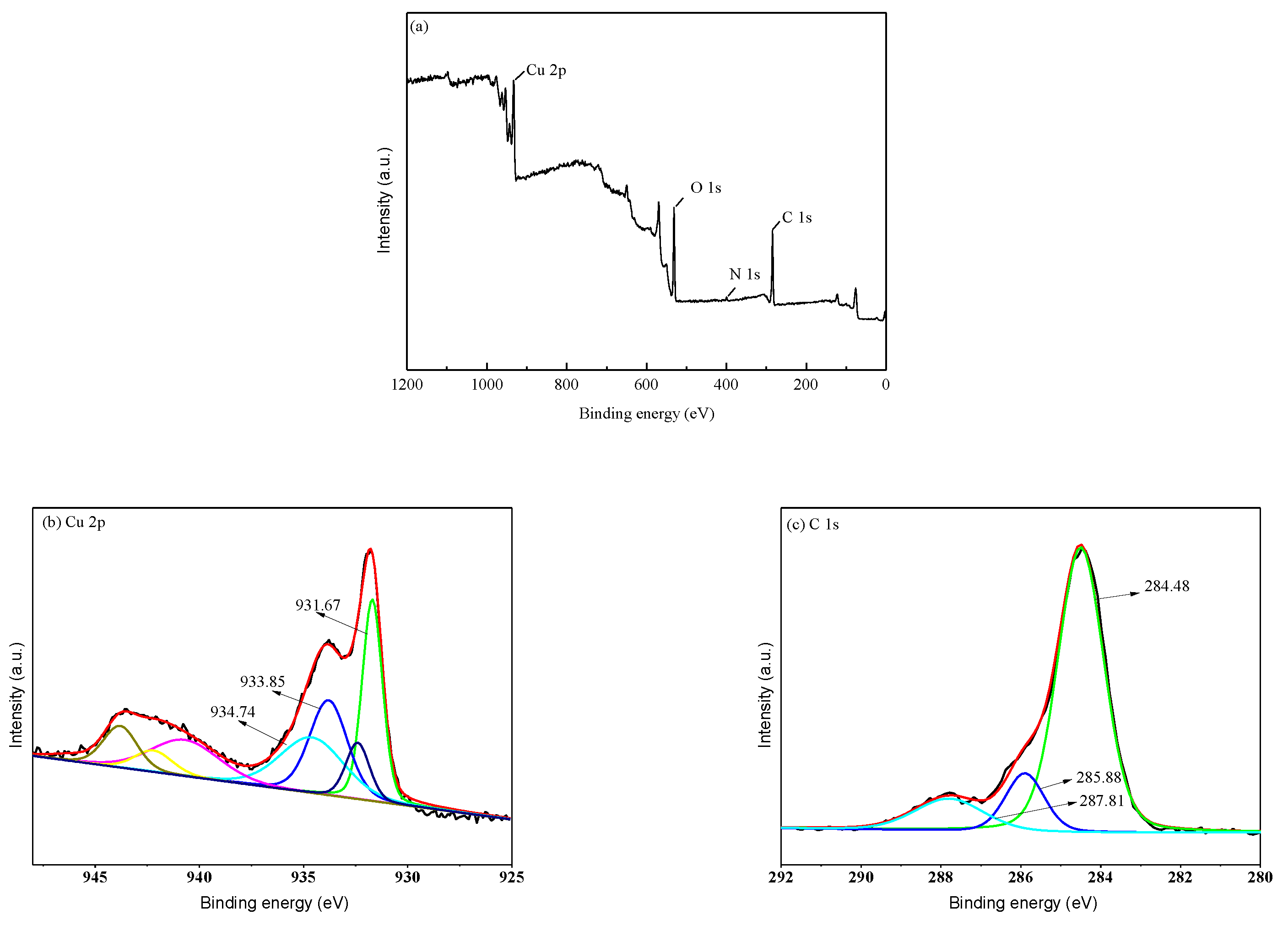
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. General Procedure for the Preparation of CP@Cu NPs
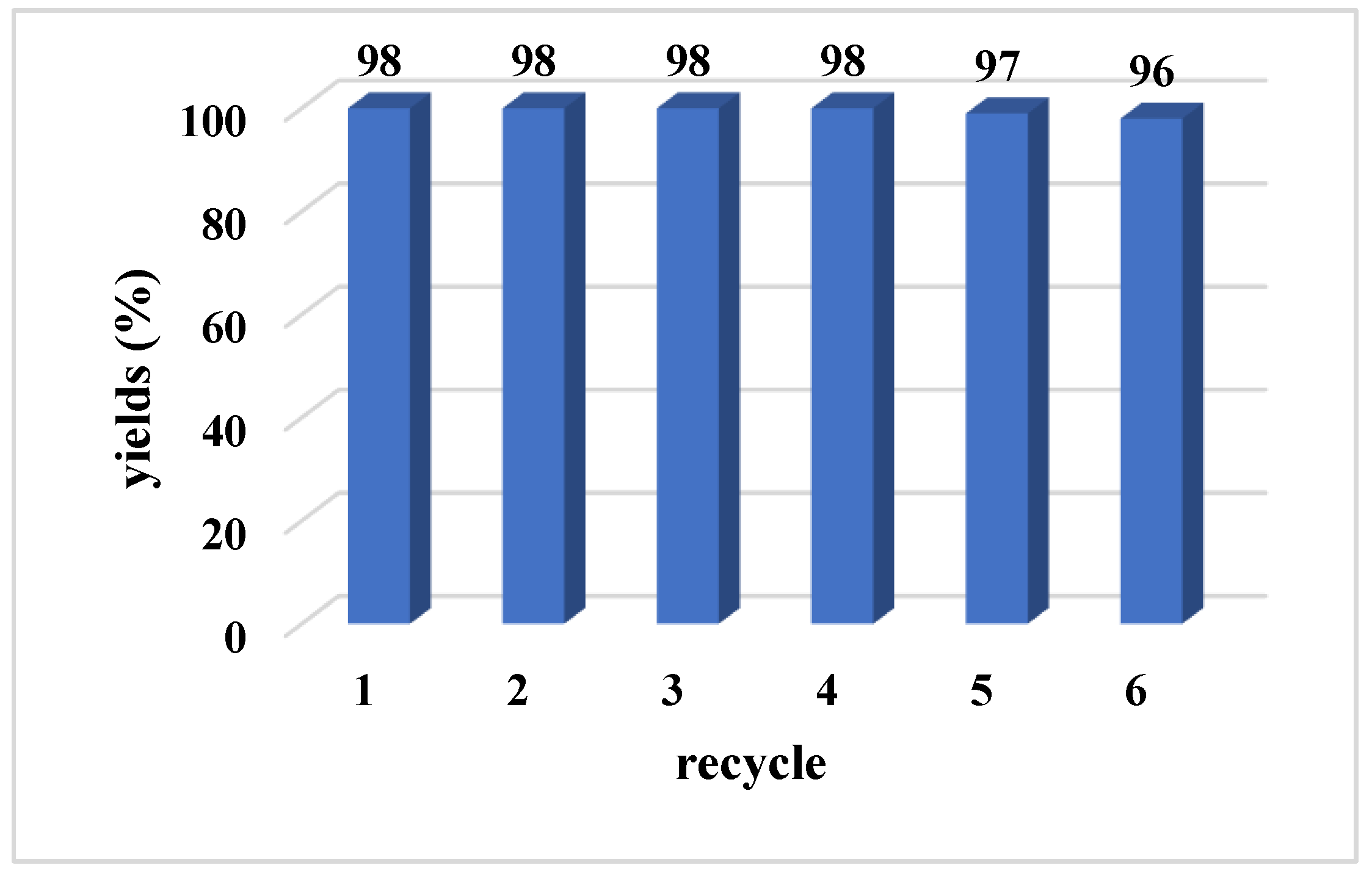
3.4. Recycling and Reuse of CP@Cu NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Coghi, P.S.; Zhu, Y.; Xie, H.; Hosmane, N.S.; Zhang, Y. Organoboron compounds: Effective antibacterial and antiparasitic agents. Molecules 2021, 26, 3309. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Vacca, A.; Ribatti, D. Bortezomib in the treatment of cancer. Recent Pat. Anti-cancer Drug Discov. 2006, 1, 397. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Wang, Y.; Liu, J.; Wang, L. Organoboron molecules and polymers for organic solar cell applications. Chem. Soc. Rev. 2022, 51, 153. [Google Scholar] [CrossRef]
- Sacramento, M.; Costa, G.P.; Barcellos, A.M.; Perin, G.; Lenardão, E.J.; Alves, D. Transition-metal-free C-S, C-Se, and C-Te bond formation from organoboron compounds. Chem. Rec. 2021, 21, 2855. [Google Scholar] [CrossRef]
- McClary, C.A.; Taylor, M.S. Applications of organoboron compounds in carbohydrate chemistry and glycobiology: Analysis, separation, protection, and activation. Carbohydr. Res. 2013, 381, 112. [Google Scholar] [CrossRef]
- Yang, T.; Tang, N.; Wan, Q.; Yin, S.-F.; Qiu, R. Recent progress on synthesis of N,N′-chelate organoboron derivatives. Molecules 2021, 26, 1401. [Google Scholar] [CrossRef]
- Tian, Y.-M.; Guo, X.-N.; Braunschweig, H.; Radius, U.; Marder, T.B. Photoinduced borylation for the synthesis of organoboron compounds. Chem. Rev. 2021, 121, 3561. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Deng, C.; Xie, J.; Kang, X. Recent synthesis developments of organoboron compounds via metal-free catalytic borylation of alkynes and alkenes. Molecules 2018, 24, 101. [Google Scholar] [CrossRef]
- Whyte, A.; Torelli, A.; Mirabi, B.; Zhang, A.; Lautens, M. Copper-catalyzed borylative difunctionalization of π-systems. ACS Catal. 2020, 10, 11578. [Google Scholar] [CrossRef]
- Hu, J.; Ferger, M.; Shi, Z.; Marder, T.B. Recent advances in asymmetric borylation by transition metal catalysis. Chem. Soc. Rev. 2021, 50, 13129. [Google Scholar] [CrossRef]
- Ishiyama, T.; Miyaura, N. Metal-catalyzed reactions of diborons for synthesis of organoboron compounds. Chem. Rec. 2004, 3, 271. [Google Scholar] [CrossRef]
- Bisht, R.; Haldar, C.; Hassan, M.M.M.; Hoque, M.E.; Chaturvedi, J.; Chattopadhyay, B. Metal-catalysed C-H bond activation and borylation. Chem. Soc. Rev. 2022, 51, 5042. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Li, J.; Liu, K.; Ding, W.Y.; Wang, S.; Huang, X.; Li, X.; Yu, P.; Song, Q. Enantioselective Cu-catalyzed double hydroboration of alkynes to access chiral gem-diborylalkanes. Nat. Commun. 2022, 13, 3524. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-H.; Kanai, M.; Shibasaki, M. Copper(I)-secondary diamine complex-catalyzed enantioselective conjugate boration of linear β,β-disubstituted enones. Org. Lett. 2010, 12, 4098. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Hartwig, J.F. Mechanistic studies of copper catalyzed asymmetric hydroboration of alkenes. J. Am. Chem. Soc. 2017, 139, 12758. [Google Scholar] [CrossRef]
- Hoang, G.L.; Takacs, J.M. Enantioselective γ-borylation of unsaturated amides and stereoretentive Suzuki–Miyaura cross-coupling. Chem. Sci. 2017, 8, 4511. [Google Scholar] [CrossRef]
- Jang, W.J.; Song, S.M.; Moon, J.H.; Lee, J.Y.; Yun, J. Copper-catalyzed enantioselective hydroboration of unactivated 1,1-disubstituted alkenes. J. Am. Chem. Soc. 2017, 139, 13660. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.Y.; Yun, J. Copper(I)-taniaphos catalyzed enantiodivergent hydroboration of bicyclic alkenes. Org. Lett. 2015, 17, 764. [Google Scholar] [CrossRef]
- Narayanan, R. Recent advances in noble metal nanocatalysts for Suzuki and Heck cross-coupling reactions. Molecules 2010, 15, 2124. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Razmi, Z. Palladium supported on zinc oxide nanoparticles as efficient heterogeneous catalyst for Suzuki-Miyaura and Hiyama reactions under normal laboratory conditions. Helv. Chim. Acta. 2015, 98, 805. [Google Scholar] [CrossRef]
- Li, W.; Yan, F.; Cai, S.; Ding, L.; Li, B.; Zhang, B.; Zhang, Y.; Zhu, L. Platinum nanoparticles as recyclable heterogeneous catalyst for selective methylation of amines and imines with formic acid: Indirect utilization of CO2. J. Saudi. Chem. Soc. 2022, 26, 101421. [Google Scholar] [CrossRef]
- Rai, R.K.; Gupta, K.; Tyagi, D.; Mahata, A.; Behrens, S.; Yang, X.; Xu, Q.; Pathak, B.; Singh, S.K. Access to highly active Ni-Pd bimetallic nanoparticle catalysts for C-C coupling reactions. Catal. Sci. Technol. 2016, 6, 5567. [Google Scholar] [CrossRef]
- Wen, W.; Han, B.; Yan, F.; Ding, L.; Li, B.; Wang, L.; Zhu, L. Borylation of α,β-unsaturated acceptors by chitosan composite film supported copper nanoparticles. Nanomaterials 2018, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-F.; Sun, Y.-Y.; Wu, Y.-D.; Dai, J.-J.; Xu, J.; Huang, Y.; Xu, H.-J. Borylation and selective reduction of α,β-unsaturated ketones under mild conditions catalyzed by Cu nanoparticles. Tetrahedron 2016, 72, 5691. [Google Scholar] [CrossRef]
- Singh, G.; Pandey, R.; Pankhade, Y.A.; Fatma, S.; Anand, R.V. Construction of oxygen- and nitrogen-based heterocycles from p-quinone methides. Chem. Rec. 2021, 21, 4150. [Google Scholar] [CrossRef]
- Mane, B.B.; Waghmode, S.B. Iron-catalyzed ring opening of cyclopropanols and their 1,6-conjugate addition to p-quinone methides. J. Org. Chem. 2021, 86, 17774. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Chandgude, S.M.; Muthukrishnan, M. Iron catalyzed tandem ring opening/1,6-conjugate addition of cyclopropanols with p-quinone methides: New access to γ,γ-diaryl ketones. Chem. Commun. 2021, 57, 13582. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, B.; Kumagai, N.; Shibasaki, M. Direct catalytic asymmetric 1,6-conjugate addition of amides to p-quinone methides. Org. Lett. 2018, 20, 3070. [Google Scholar] [CrossRef]
- Francesco, B.; Gabriella, R.; Fabio, C.; Marialorena, C.; Roberta, G.; Serafinella, P.C. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: An intra-patient randomized study. Photodiagnosis Photodyn. Ther. 2020, 31, 101803. [Google Scholar]
- Nanna, K.; Vibeke, B.; Sebastian, R.; Peter, H.; Morten, H. Pharmacokinetics of nebulized and oral procaterol in asthmatic and non-asthmatic subjects in relation to doping analysis. Drug Test. Anal. 2016, 8, 1056. [Google Scholar]
- Francesco, L.G.; Colin, G.E. Use of isoxsuprine hydrochloride as a tocolytic agent in the treatment of preterm labour: A systematic review of previous literature. Arzneimittelforschung 2010, 60, 415. [Google Scholar]
- Chiyohiko, S.; Yoshie, M.; Rie, S.; Katsuyuki, S.; Taichi, N.; Masahito, M. Tulobuterol patch maintains diaphragm muscle contractility for over twenty-four hours in a mouse model of sepsis. Tohoku J. Exp. Med. 2009, 218, 271. [Google Scholar]
- Fabiola, O.; Jesus, G.-R.; Julia, C.; Alvaro, C.; Aranzazu, H. Spectro- electrochemical determination of isoprenaline in a pharmaceutical sample. Sensors 2020, 20, 5179. [Google Scholar]
- Lou, Y.; Cao, P.; Jia, T.; Zhang, Y.; Wang, M.; Liao, J. Copper-catalyzed enantioselective 1,6-boration of para-quinone methides and efficient transformation of gem-diarylmethine boronates to triarylmethanes. Angew. Chem. Int. Ed. 2015, 54, 12134. [Google Scholar] [CrossRef] [PubMed]
- JaravaBarrera, C.; Parra, A.; López, A.; Cruz-Acosta, F.; Collado-Sanz, D.; Cárdenas, D.J.; Tortosa, M. Copper-catalyzed borylative aromatization of p-quinone methides: Enantioselective synthesis of dibenzylic boronates. ACS Catal. 2016, 6, 442. [Google Scholar] [CrossRef]
- Li, W.; Wen, W.; Chen, S.; Ding, L.; He, B.; Zhang, Y.; Li, B.; Zhu, L. Copper(II)-catalyzed 1,6-hydroboration reactions of p-quinone methides under ligand-Free conditions: A sequential methodology to gem-disubstituted methanols. Cat. Lett. 2022. [Google Scholar] [CrossRef]
- Xu, P.; Li, B.; Wang, L.; Qin, C.; Zhu, L. A green and recyclable chitosan supported catalyst for the borylation of α,β-unsaturated acceptors in water. Catal. Commun. 2016, 86, 23. [Google Scholar] [CrossRef]
- Zhu, L.; Li, B.; Wang, S.; Wang, W.; Wang, L.; Ding, L.; Qin, C. Recyclable heterogeneous chitosan supported copper catalyst for silyl conjugate addition to α,β-unsaturated acceptors in water. Polymers 2018, 10, 385. [Google Scholar] [CrossRef]
- De Souza, J.F.; da Silva, G.T.; Fajardo, A.R. Chitosan-based film supported copper nanoparticles: A potential and reusable catalyst for the reduction of aromatic nitro compounds. Carbohydr. Polym. 2017, 161, 187. [Google Scholar] [CrossRef]
- Wu, R.X.; Zheng, F.G.; Li, W.W.; Zhong, L.B.; Zheng, Y.M. Electrospun chitosan nanofiber membrane for adsorption of Cu(II) from aqueous solution: Fabrication, characterization and performance. J. Nanosci. Nanotechnol. 2018, 18, 5624. [Google Scholar] [CrossRef]
- Kaiser, M.; Mudasir, A.; Suhail, A.; Saiqa, I. Synthesis, characterization, kinetics, and thermodynamics of EDTA-modified chitosan-carboxymethyl cellulose as Cu(II) ion adsorbent. ACS Omega 2019, 17, 17425. [Google Scholar]
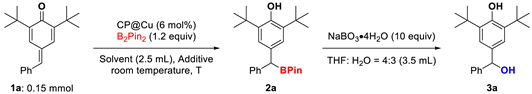 | |||||
|---|---|---|---|---|---|
| Entries | CP@Cu | Solvent | Additive | T (h) | Yields (%) b |
| 1 | 6 (mol%) | EtOH | - | 2 | N.R. |
| 2 | 6 (mol%) | DCM | MeOH (2 equiv.) | 2 | N.R. |
| 3 | 6 (mol%) | THF | MeOH (2 equiv.) | 2 | N.R. |
| 4 | 6 (mol%) | MeCN | MeOH (2 equiv.) | 2 | 34 |
| 5 | 6 (mol%) | Acetone | MeOH (2 equiv.) | 2 | 70 |
| 6 | 6 (mol%) | Acetone | H2O (2 equiv.) | 2 | N.R. |
| 7 | 6 (mol%) | Acetone:H2O (4:1) | - | 2 | 98 |
| 8 | 6 (mol%) | Acetone:H2O (2:1) | - | 2 | 84 |
| 9 | 6 (mol%) | Acetone:H2O (1:2) | - | 2 | 59 |
| 10 | 6 (mol%) | Acetone:H2O (1:4) | - | 2 | <5 |
| 11 | - | Acetone:H2O (4:1) | - | 2 | N.R. |
| 12 | 6 (mol%) | Acetone:H2O (4:1) | - | 1 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wen, W.; Zhao, X.; Zhang, Z.; Li, W.; Zhang, Y.; Li, B.; Zhu, L. Preparation of Chitosan-Composite-Film-Supported Copper Nanoparticles and Their Application in 1,6-Hydroboration Reactions of p-Quinone Methides. Molecules 2022, 27, 7962. https://doi.org/10.3390/molecules27227962
Chen S, Wen W, Zhao X, Zhang Z, Li W, Zhang Y, Li B, Zhu L. Preparation of Chitosan-Composite-Film-Supported Copper Nanoparticles and Their Application in 1,6-Hydroboration Reactions of p-Quinone Methides. Molecules. 2022; 27(22):7962. https://doi.org/10.3390/molecules27227962
Chicago/Turabian StyleChen, Shuhan, Wei Wen, Xue Zhao, Zelang Zhang, Weishuang Li, Yaoyao Zhang, Bojie Li, and Lei Zhu. 2022. "Preparation of Chitosan-Composite-Film-Supported Copper Nanoparticles and Their Application in 1,6-Hydroboration Reactions of p-Quinone Methides" Molecules 27, no. 22: 7962. https://doi.org/10.3390/molecules27227962
APA StyleChen, S., Wen, W., Zhao, X., Zhang, Z., Li, W., Zhang, Y., Li, B., & Zhu, L. (2022). Preparation of Chitosan-Composite-Film-Supported Copper Nanoparticles and Their Application in 1,6-Hydroboration Reactions of p-Quinone Methides. Molecules, 27(22), 7962. https://doi.org/10.3390/molecules27227962








