Pentapeptide-Zinc Chelate from Sweet Almond Expeller Amandin Hydrolysates: Structural and Physicochemical Characteristics, Stability and Zinc Transport Ability In Vitro
Abstract
1. Introduction
2. Results and Discussion
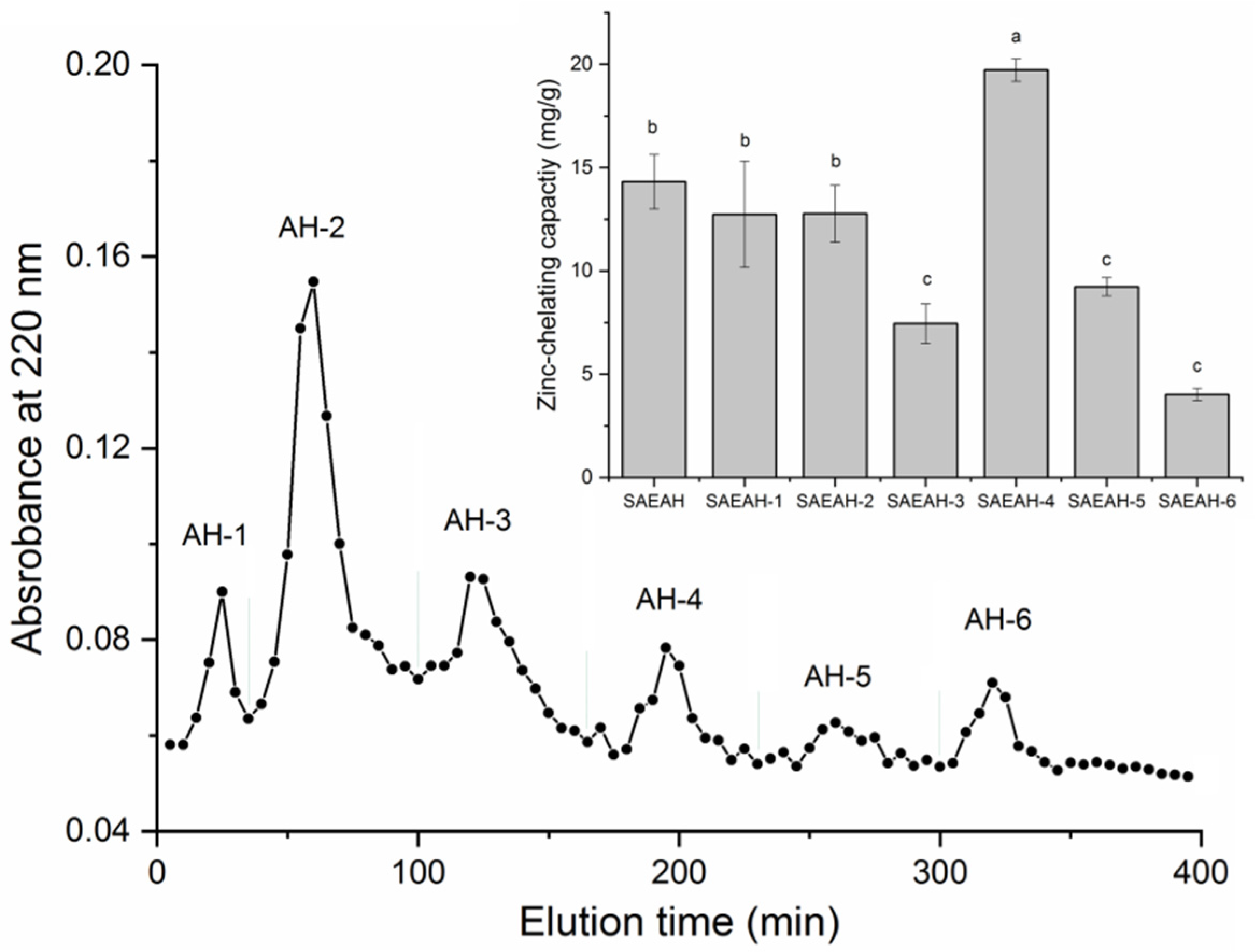
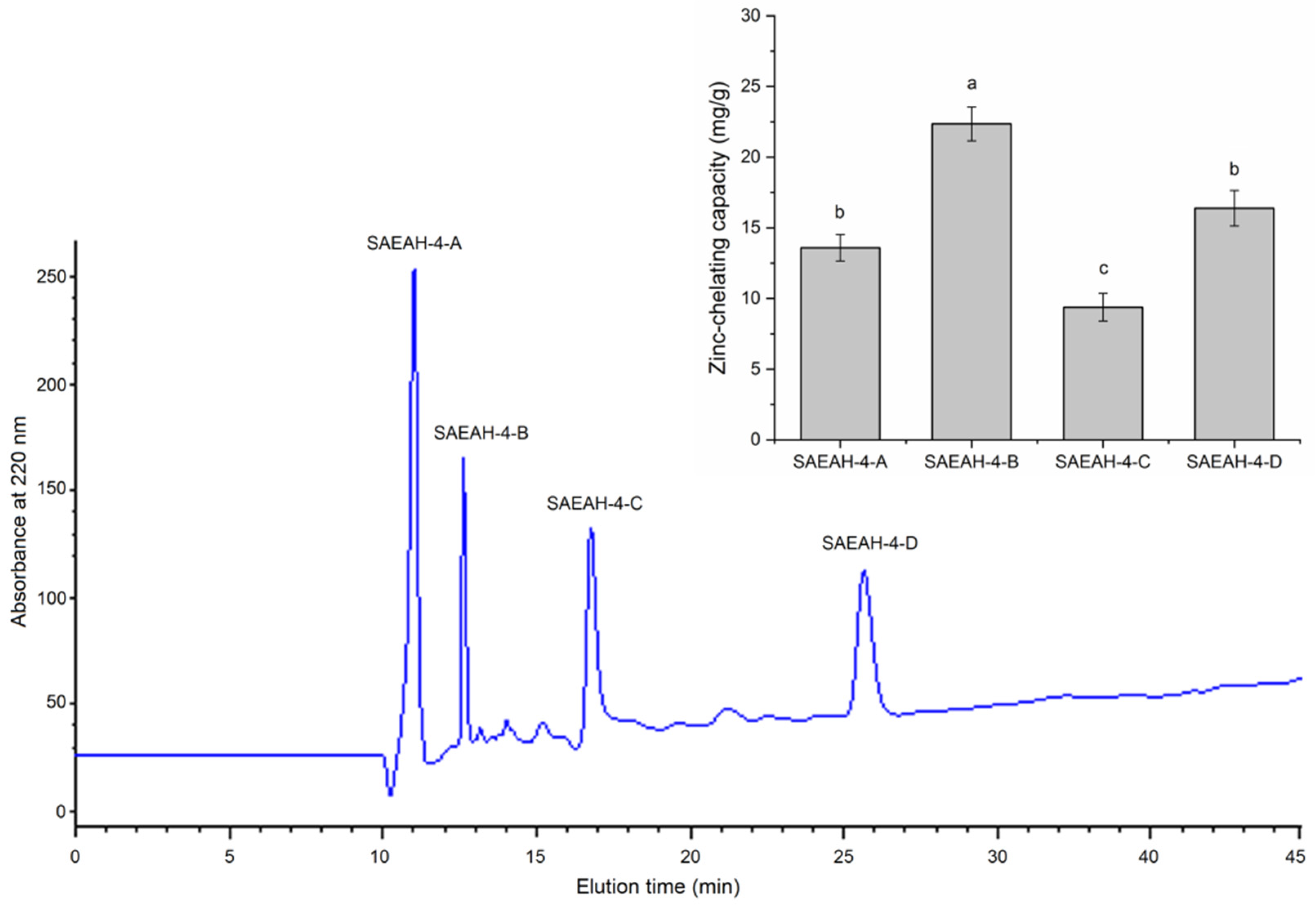
2.1. Isolation and Purification of Peptides of Excellent Zinc-Chelating Ability
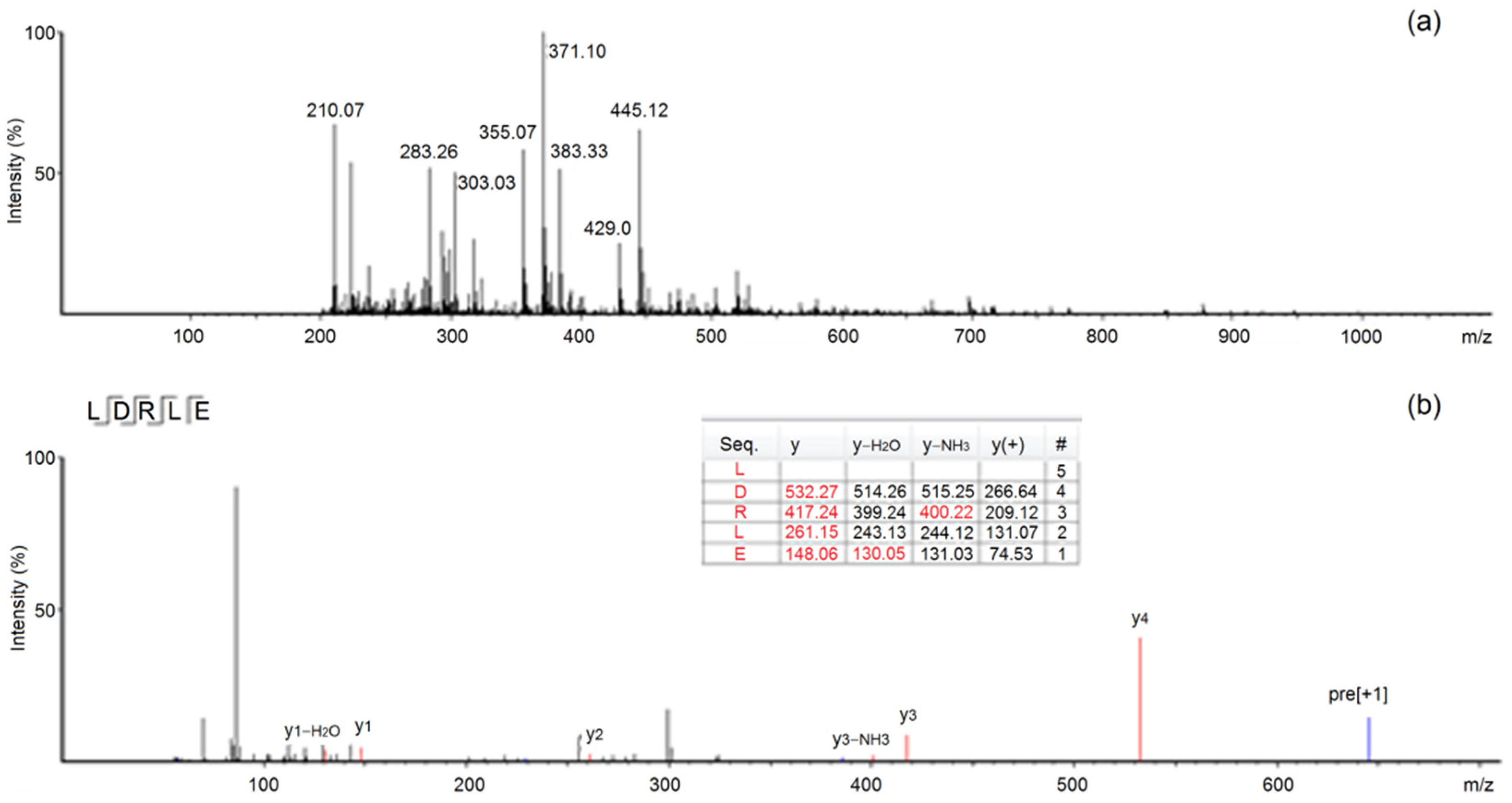
2.2. Characteristics of Peptide Sequence
2.3. Physicochemical Characteristics and Toxicity Analysis of SAEAH Peptides
2.4. Structural Characteristics
2.4.1. Scanning Analysis with Ultraviolet Wavelength
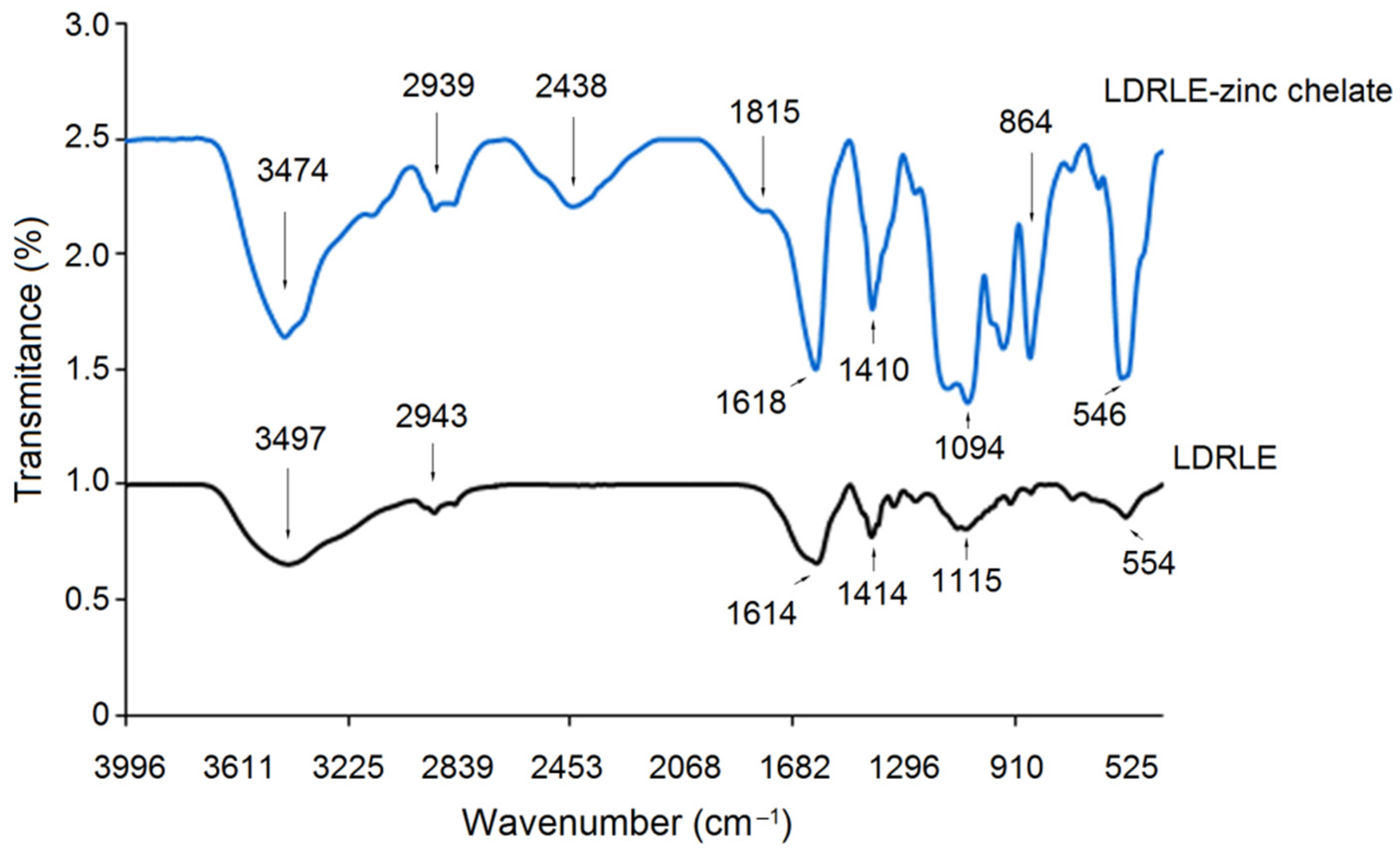
2.4.2. FT-IR Analysis
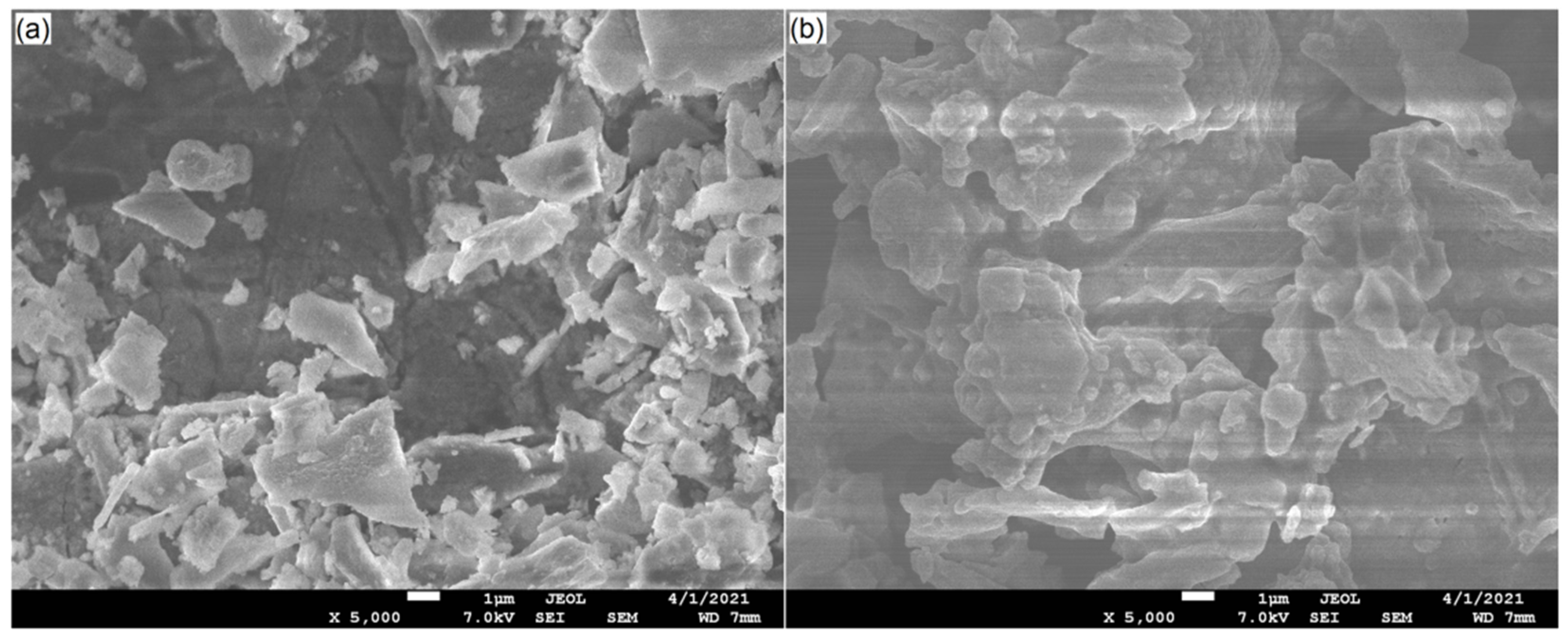
2.4.3. Microstructure
2.5. Stability Profiles of LDRLE-Zinc Chelate
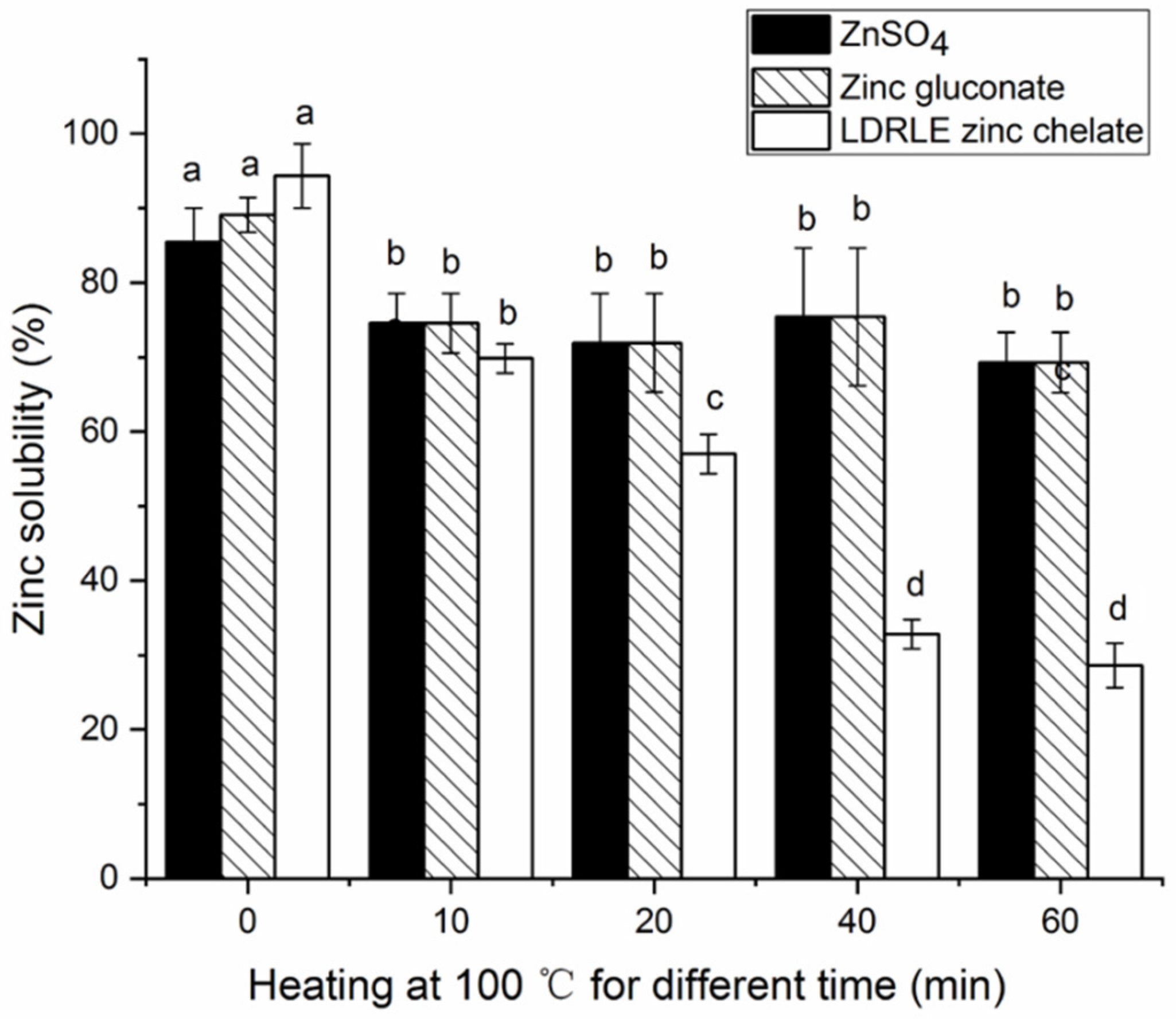
2.5.1. Effects of Thermal Treatment on Zinc-Solubility
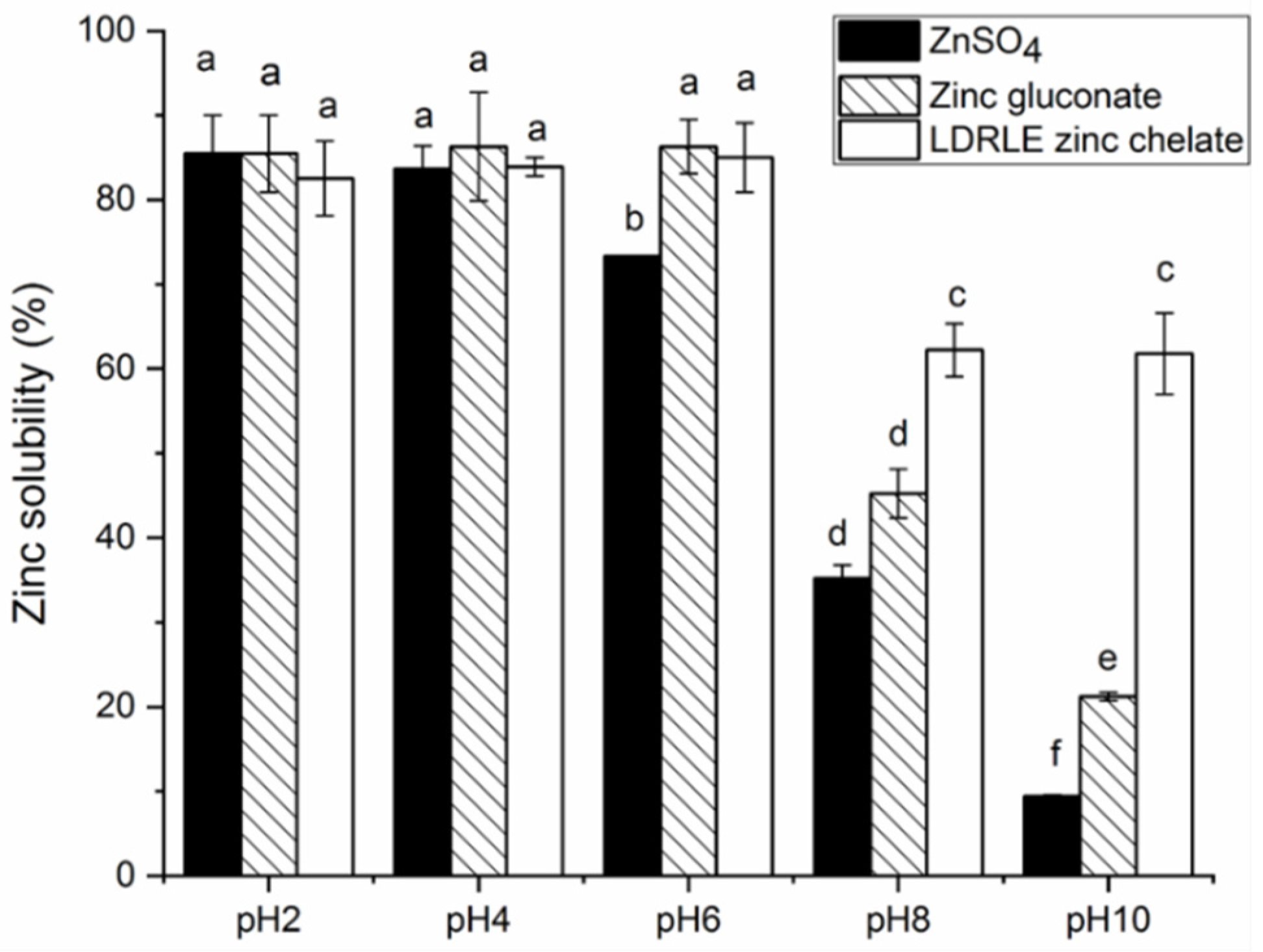
2.5.2. Effect of Various pH Values
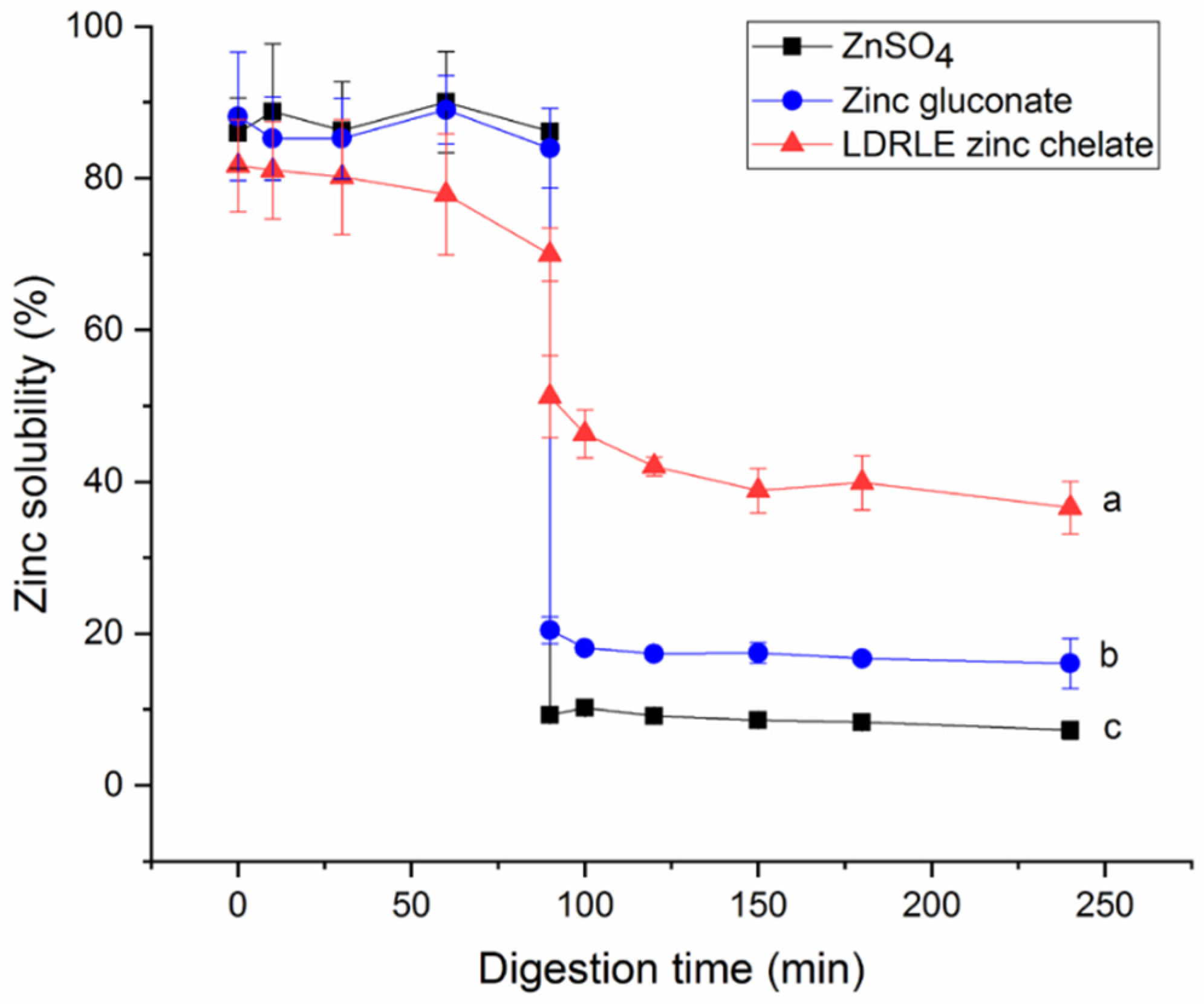
2.5.3. Effect of Gastrointestinal Digestion
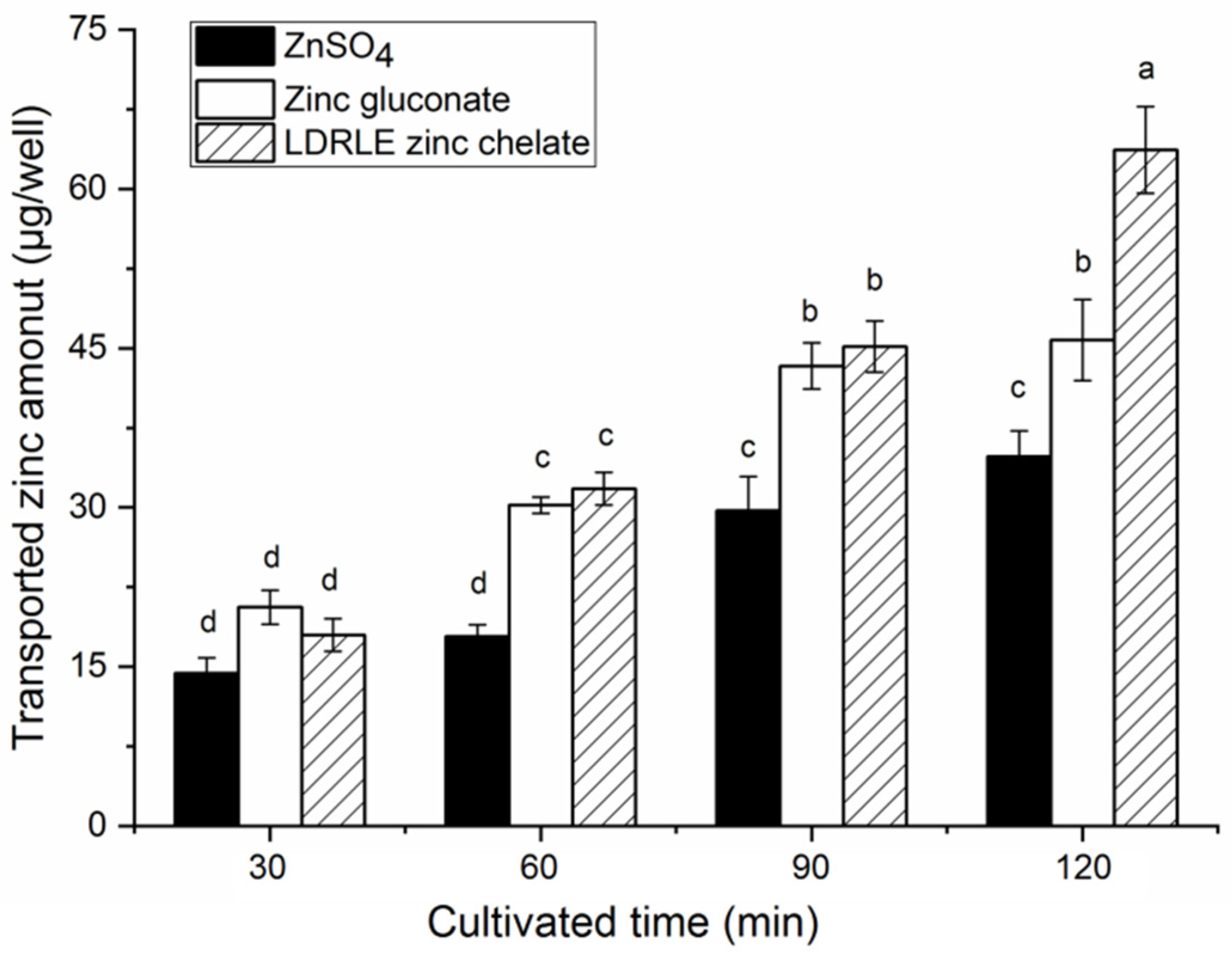
2.6. Zinc Transportation across Caco-2 Cells
3. Materials and Methods
3.1. Materials
3.2. Preparation of Sweet Almond Cake Amandin Hydrolysates
3.3. Purification of SAEAH
3.4. Zinc-Chelating Ability Assay
3.5. Identification, Synthesize and Physicochemical Characteristics of Peptide Sequence
3.6. Toxicity Evaluation
3.7. Preparation of Peptide-Zinc Chelate
3.8. Structural Characteristics
3.8.1. Scanning Analysis with Ultraviolet Wavelength
3.8.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.8.3. Surface Microstructure Analysis
3.9. Stabilities
3.9.1. Thermal Stability
3.9.2. Zinc Solubility at Different pH Values
3.9.3. Effect of the Gastrointestinal Digestion
3.10. Zinc Transport across Caco-2 Cells
3.11. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The enormity of the zinc deficiency problem and available solutions; an overview. Arab. J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Wang, D.; Liu, K.; Cui, P.; Bao, Z.; Wang, T.; Lin, S.; Sun, N. Egg-White-Derived Antioxidant Peptide as an Efficient Nanocarrier for Zinc Delivery through the Gastrointestinal System. J. Agric. Food Chem. 2020, 68, 2232–2239. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Liu, X.; Zhao, M. Particulate nanocomposite from oyster (Crassostrea rivularis) hydrolysates via zinc chelation improves zinc solubility and peptide activity. Food Chem. 2018, 258, 269–277. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, L.; Lin, Y.; Cai, X.; Wang, S. The preservative potential of Octopus scraps peptides-Zinc chelate against Staphylococcus aureus: Its fabrication, antibacterial activity and action mode. Food Control. 2019, 98, 24–33. [Google Scholar]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Chiozzi, R.Z.; Laganà, A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444. [Google Scholar] [CrossRef]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate. Food Chem. 2021, 340, 128056. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Q.; Hong, H.; Liu, H.; Luo, Y. Novel antioxidant and ACE inhibitory peptide identified from Arthrospira platensis protein and stability against thermal/pH treatments and simulated gastrointestinal digestion. Food Res. Inter. 2021, 139, 109908. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S. In vitro stability of bioactive peptides derived from fermented soy milk against heat treatment, pH and gastrointestinal enzymes. LWT-Food Sci. Technol. 2018, 91, 303–307. [Google Scholar] [CrossRef]
- Wong, F.-C.; Xiao, J.; Ong, M.G.L.; Pang, M.-J.; Wong, S.-J.; Teh, L.-K.; Chai, T.-T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622. [Google Scholar] [CrossRef]
- Sahni, P.; Sharma, S.; Surasani, V.K.R. Influence of processing and pH on amino acid profile, morphology, electrophoretic pattern, bioactive potential and functional characteristics of alfalfa protein isolates. Food Chem. 2020, 333, 127503. [Google Scholar] [CrossRef]
- Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; Bell, J.M.L.N.M. Effects of enzymatic extraction of oil and protein from almond cake on the physicochemical and func-tional properties of protein extracts. Food Bioprod. Process. 2020, 122, 280–290. [Google Scholar] [CrossRef]
- Dias, F.F.; de Moura Bell, J.M. Understanding the impact of enzyme-assisted aqueous extraction on the structural, physicochemical, and functional properties of protein extracts from full-fat almond flour. Food Hydrocolloid 2020, 127, 107534. [Google Scholar] [CrossRef]
- Li, S.G.; Chu, S.; Lu, J.K.; Wang, P.; Ma, M.H. Molecular and structural properties of three major protein components from almond kernel. J. Food Process. Preserv. 2018, 42, e13536. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, M.; Dong, S.; Liu, M. Analysis of nutritional composition of bitter almond from different growing areas. Sci. Technol. Food Ind. 2019, 40, 300–305. [Google Scholar]
- Ganesh, S.; Ningtyas, D.W.; Prakash, S. Investigating the functionality of enzymatically (transglutaminase and alcalase) treated almond protein isolate. Food Biosci. 2020, 49, 101914. [Google Scholar] [CrossRef]
- Mirzapour, M.; Rezaei, K.; Sentandreu, M.A. Identification of Potent ACE Inhibitory Peptides from Wild Almond Proteins. J. Food Sci. 2017, 82, 2421–2431. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, J.; Zhao, H.; Zhao, X.; Xue, H.; Sun, Y.; Xue, W. Isolation and structural characterization of antioxidant peptides from degreased apricot seed kernels. J. AOAC Int. 2018, 101, 1661–1663. [Google Scholar] [CrossRef]
- Gu, X.; Gao, T.; Hou, Y.; Li, D.; Fu, L. Identification and characterization of two novel α-glucosidase inhibitory peptides from almond (Armeniaca sibirica) oil manufacture residue. LWT-Food Sci. Technol. 2020, 134, 110215. [Google Scholar] [CrossRef]
- Civera, A.; Galan-Malo, P.; Segura-Gil, I.; Mata, L.; Tobajas, A.P.; Sánchez, L.; Pérez, M.D. Development of sandwich ELISA and lateral flow immunoassay to detect almond in processed food. Food Chem. 2022, 371, 131338. [Google Scholar] [CrossRef]
- Devnani, B.; Ong, L.; Kentish, S.; Gras, S.L. Structure and functionality of almond proteins as a function of pH. Food Struct. 2021, 30, 100229. [Google Scholar] [CrossRef]
- Lihi, N.; Lukács, M.; Szűcs, D.; Várnagy, K.; Sóvágó, I. Nickel(II), zinc(II) and cadmium(II) complexes of peptides containing separate aspartyl and cysteinyl residues. Polyhedron 2017, 133, 364–373. [Google Scholar]
- Liu, R.L.; Ge, X.L.; Gao, X.Y.; Zhan, H.Y.; Shi, T.; Su, N.; Zhang, Z.Q. Two angiotensin-converting enzyme-inhibitory peptides from almond protein and the protective action on vascular endothelial function. Food Funct. 2016, 7, 3733–3739. [Google Scholar]
- Arichaya, M.; Pornlert, A.; Suwimon, K. Chicken foot broth byproduct: A new source for highly effective peptide-calcium chelate. Food Chem. 2020, 345, 128713. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, S.; Sheng, Y.; Feng, Y.; Wang, C. Isolation and characterization of zinc-binding peptides from mung bean protein hydrolysates. Eur. Food Res. Technol. 2020, 246, 113–124. [Google Scholar]
- Hou, H.; Wang, S.; Zhu, X.; Li, Q.; Fan, Y.; Cheng, D.; Li, B. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef]
- Ke, X.; Hu, X.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Xue, C. A novel zinc-binding peptide identified from tilapia (Oreochromis niloticus) skin collagen and transport pathway across Caco-2 monolayers. Food Biosci. 2022, 42, 101127. [Google Scholar] [CrossRef]
- La Rocca, R.; Tsvetkov, P.O.; Golovin, A.V.; Allegro, D.; Barbier, P.; Malesinski, S.; Guerlesquin, F.; Devred, F. Identification of the three zinc-binding sites on tau protein. Int. J. Biol. Macromol. 2022, 209, 779–784. [Google Scholar] [CrossRef]
- Da Teixeira, J.L.P.; Pallone, J.A.L.; Andrade, C.D.; Mesías, M.; Seiquer, I. Bioavailability evaluation of calcium, magnesium and zinc in Brazilian cheese through a combined model of in vitro digestion and Caco-2 cells. J. Food Compos. Anal. 2022, 107, 104365. [Google Scholar] [CrossRef]
- Zhu, S.; Zheng, Y.; He, S.; Su, D.; Nag, A.; Zeng, Q.; Yuan, Y. Novel Zn-Binding Peptide Isolated from Soy Protein Hydrolysates: Purification, Structure, and Digestion. J. Agric. Food Chem. 2021, 69, 483–490. [Google Scholar] [CrossRef]
- Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; Bell, J.M.L.N.M. Aqueous and Enzymatic Extraction of Oil and Protein from Almond Cake: A Comparative Study. Processes 2019, 7, 472. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agr. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Downey, B.; Udenigwe, C. Influence of structural and surface properties of whey-derived peptides on zinc-chelating capacity, and in vitro gastric stability and bioaccessibility of the zinc-peptide complexes. Food Chem. 2018, 240, 1227–1232. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Zheng, Y.; Shi, P.; Zhang, Y.; Liu, Y.; Long, N. Millet bran globulin hydrolysate derived tetrapeptide-ferrous chelate: Preparation, structural characterization, security prediction in silico, and stability against different food processing conditions. LWT-Food Sci. Tech. 2022, 165, 113673. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, K.; Sharma, M.; Nagpal, G.; Chauhan, J.S.; Singh, S.; Gautam, A.; Raghava, P.S. AHTPDB: A comprehensive platform for analysis and presentation of antihypertensive peptides. Nucleic Acids Res. 2015, 43, D956–D962. [Google Scholar] [CrossRef]
- Sudheer, G.; Pallavi, K.; Kumardeep, C.; Ankur, G.; Rahul, K.; Raghava, G.P.S.; Lee, P.R. In silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 2013, 8, 73957. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Ma, X.; Liu, X.; Yin, F.; Li, D.; Nakamura, Y.; Yu, C.; Zhou, D. Characterization of a synthetic zinc-chelating peptide from sea cucumber (Stichopus japonicus) and its gastrointestinal digestion and absorption in vitro. J. Sci. Food Agri. 2022, 102, 4542–4550. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Shi, P.Q.; Li, Y.; Zhuang, Y.L.; Zhang, Y.L.; Liu, L.; Wang, W. A novel ACE-inhibitory hexapeptide from camellia glutelin-2 hydrolysates: Identification, characterization and stability profiles under different food processing conditions. LWT-Food Sci. Technol. 2021, 147, 111682. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

| Peptide Sequence | LDRLE | VDLVAEVPRGL | LDRLE-Zinc Chelate | EDTA a |
|---|---|---|---|---|
| Molecular weight (Da) | 644.77 | 1164.45 | ND | |
| Matched sequence in Prunus armeniaca b | T.LDRLE.V | T.VDLVAEVPRGL.G | ND | |
| Zinc chelating capacity (mg/g) | 24.73 ± 2.45 f | 4.33 ± 0.33 e | 57.17 ± 2.63 d | |
| Hydrophilic amino acid content (%) | 60.00 | 36.36 | 60.00 | ND |
| Hydrophobicity c | −0.41 | −0.08 | ND | |
| Amphiphilicity | 0.74 | 0.75 | ND | |
| Hydrophilicity | 1.08 | 0.40 | ND | |
| Isoelectric point | 4.38 | 4.26 | ND | |
| Toxicity | Non-Toxin | Non-Toxin | Non-Toxin | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ye, Z. Pentapeptide-Zinc Chelate from Sweet Almond Expeller Amandin Hydrolysates: Structural and Physicochemical Characteristics, Stability and Zinc Transport Ability In Vitro. Molecules 2022, 27, 7936. https://doi.org/10.3390/molecules27227936
Zhang J, Ye Z. Pentapeptide-Zinc Chelate from Sweet Almond Expeller Amandin Hydrolysates: Structural and Physicochemical Characteristics, Stability and Zinc Transport Ability In Vitro. Molecules. 2022; 27(22):7936. https://doi.org/10.3390/molecules27227936
Chicago/Turabian StyleZhang, Jiangning, and Zheng Ye. 2022. "Pentapeptide-Zinc Chelate from Sweet Almond Expeller Amandin Hydrolysates: Structural and Physicochemical Characteristics, Stability and Zinc Transport Ability In Vitro" Molecules 27, no. 22: 7936. https://doi.org/10.3390/molecules27227936
APA StyleZhang, J., & Ye, Z. (2022). Pentapeptide-Zinc Chelate from Sweet Almond Expeller Amandin Hydrolysates: Structural and Physicochemical Characteristics, Stability and Zinc Transport Ability In Vitro. Molecules, 27(22), 7936. https://doi.org/10.3390/molecules27227936







