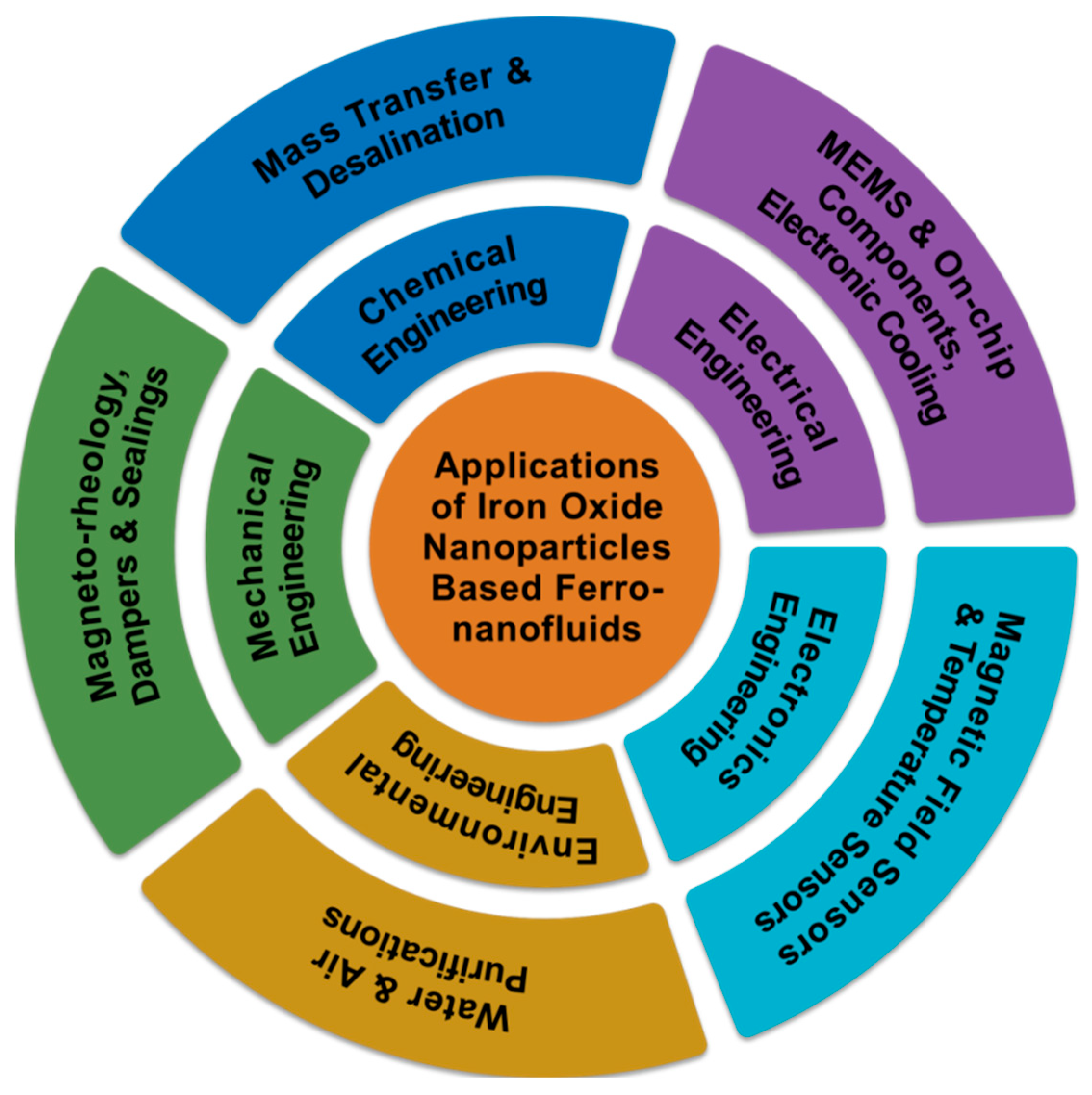
Iron Oxide Nanoparticle-Based Ferro-Nanofluids for Advanced Technological Applications
Abstract
1. Introduction
2. Chemical Engineering Applications
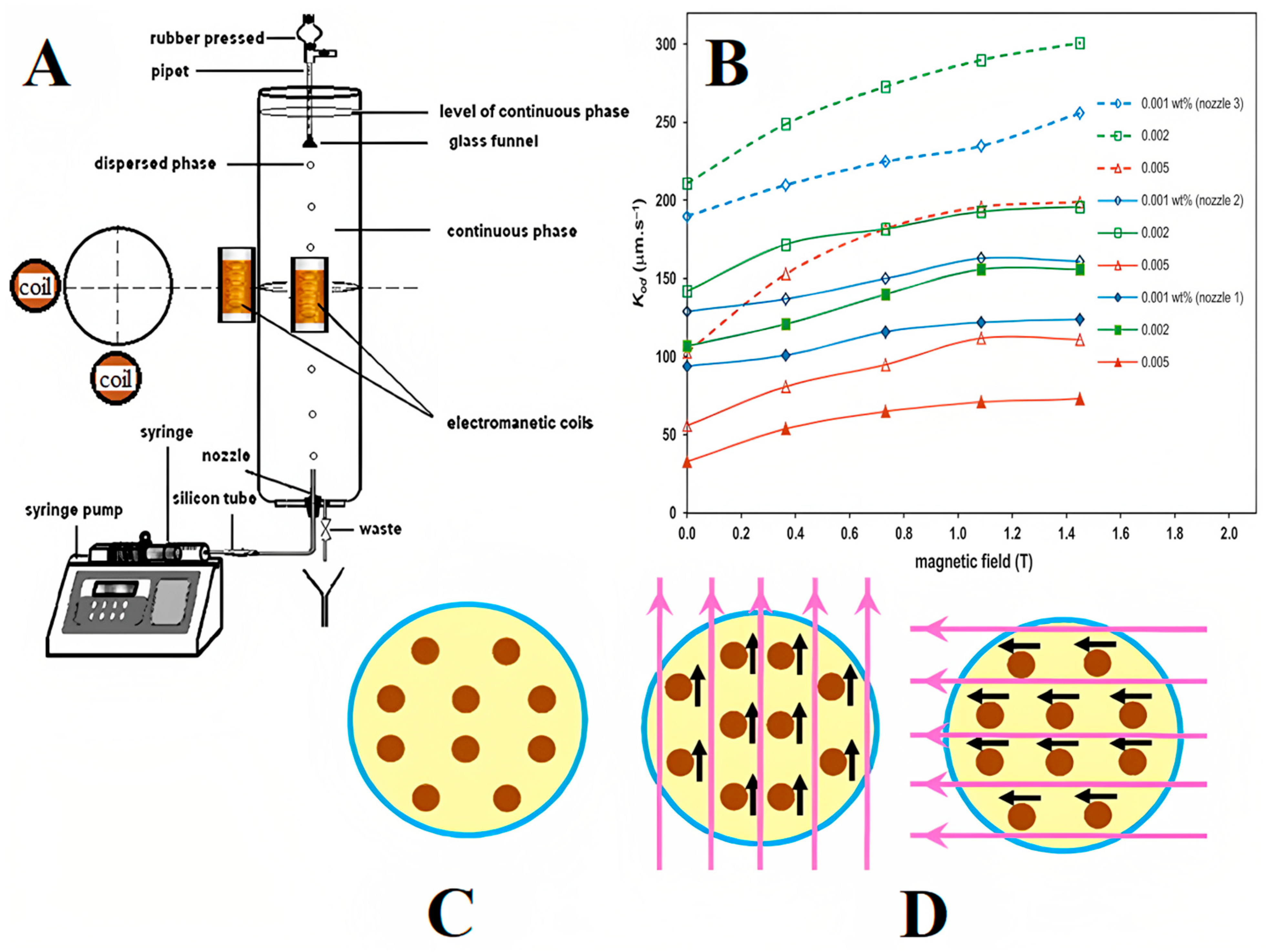
Mass Transfer
3. Electrical and Electronics Applications
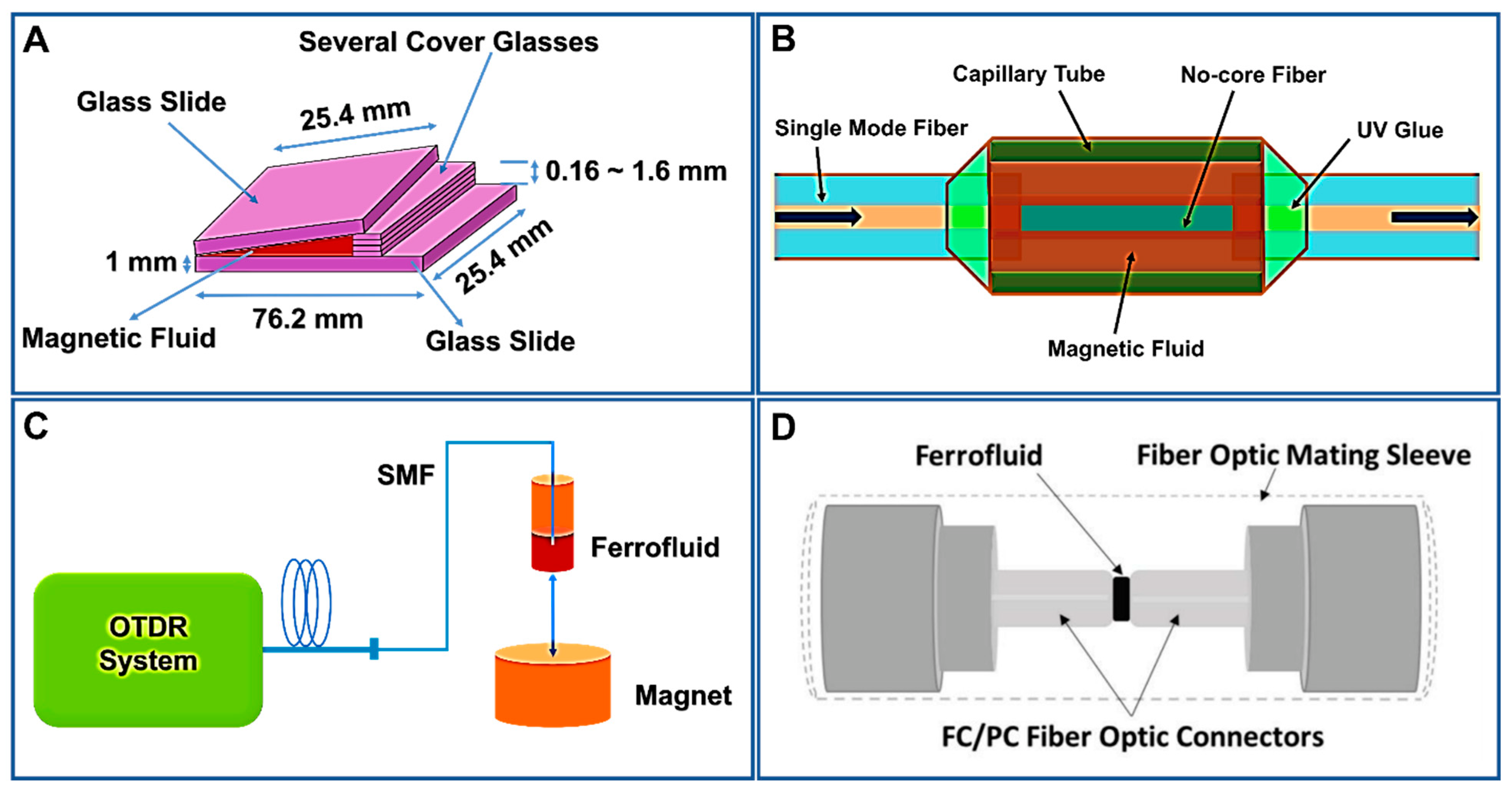
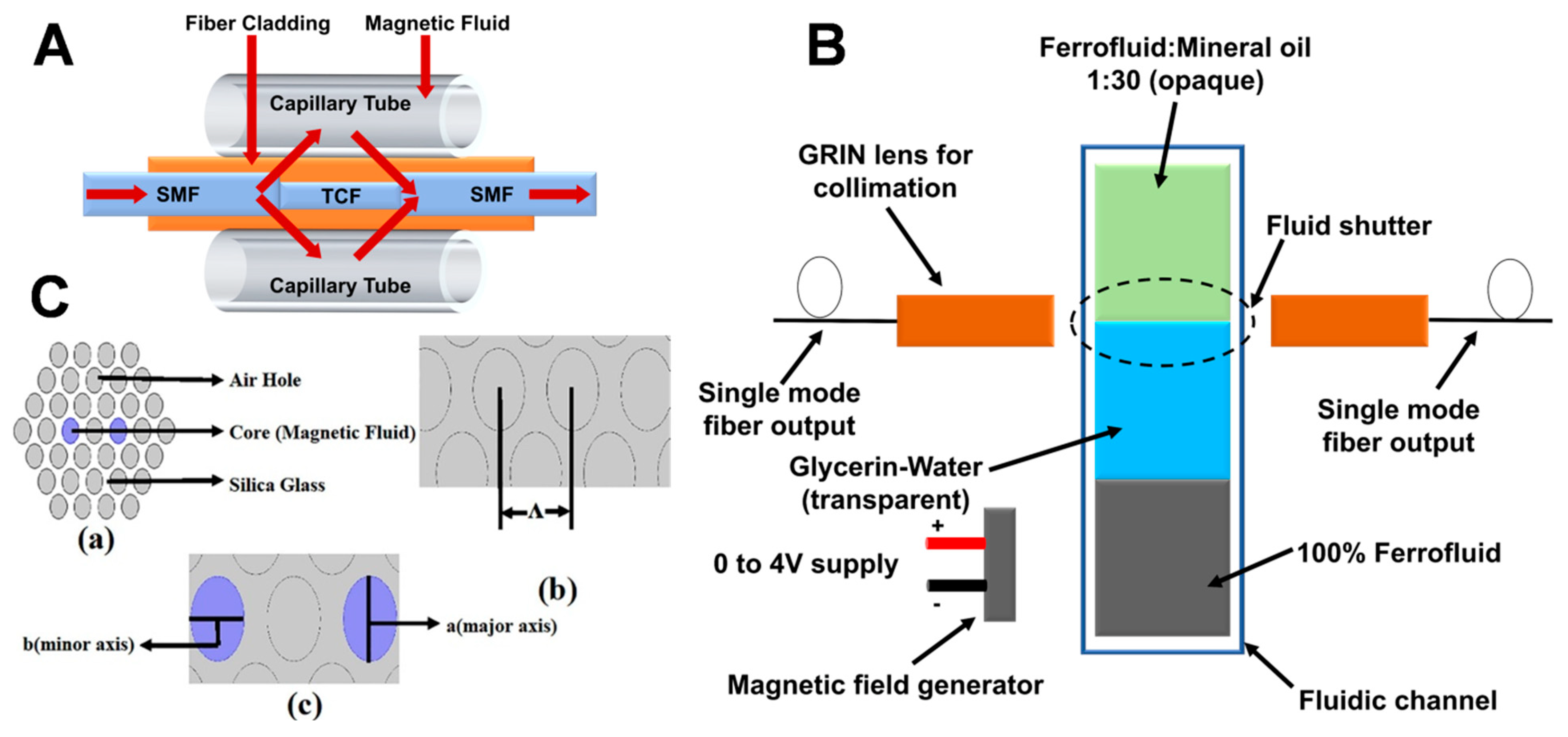
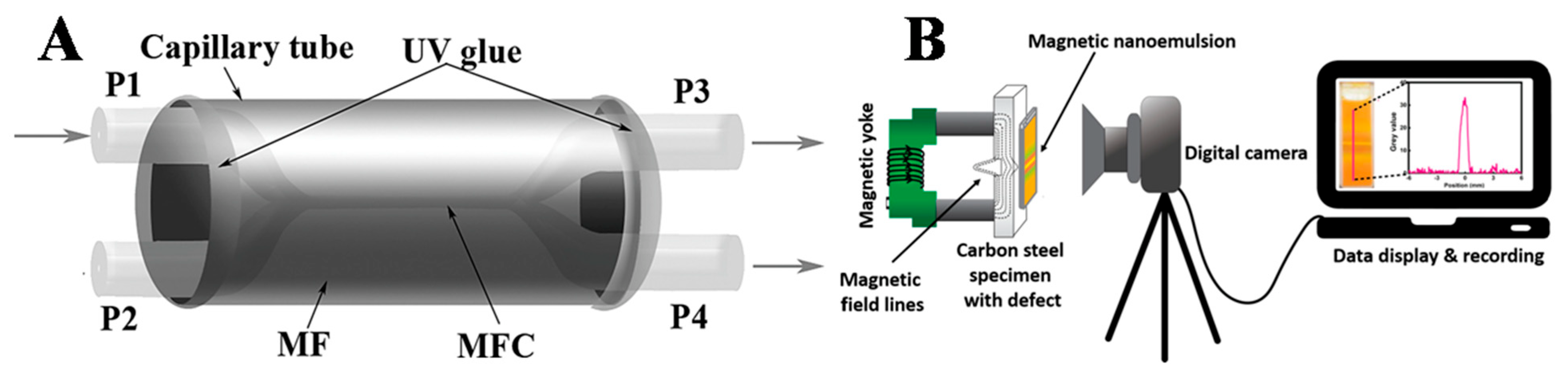
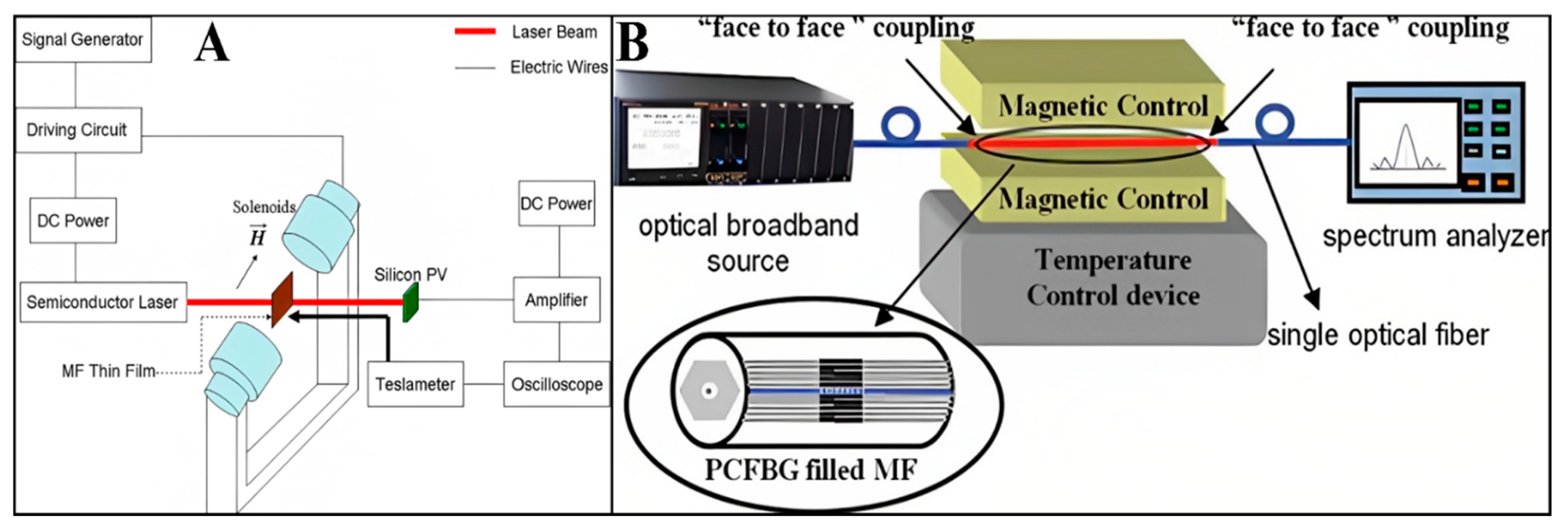
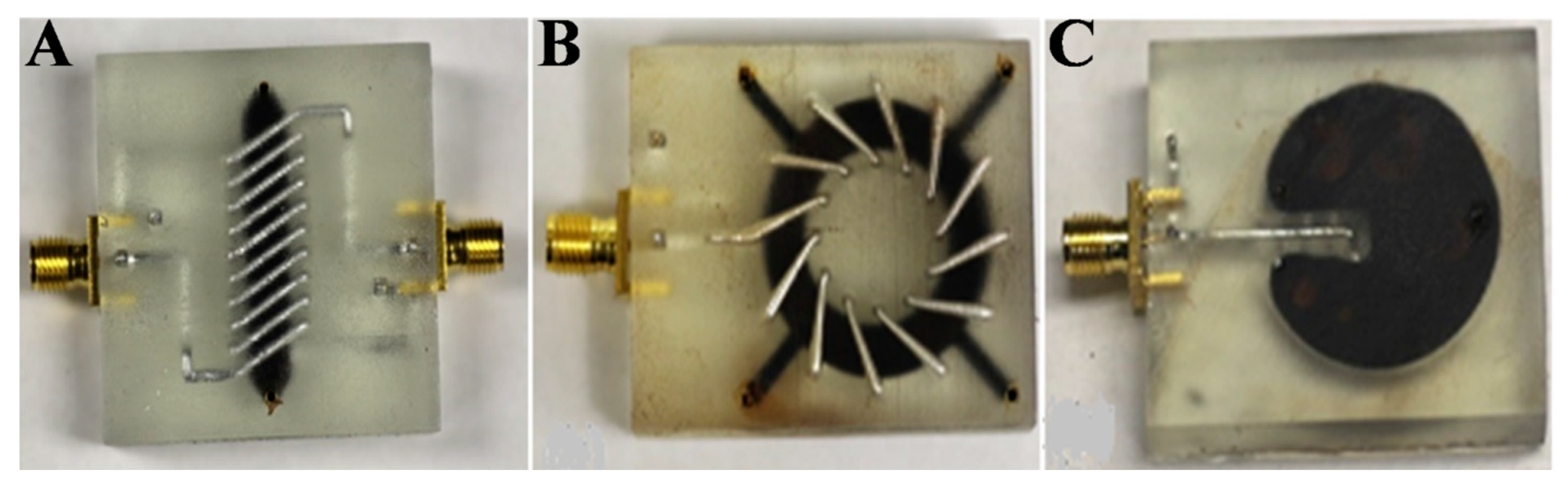
3.1. FN-Based Magnetic Field Sensors
3.2. FN-Based Temperature Sensors
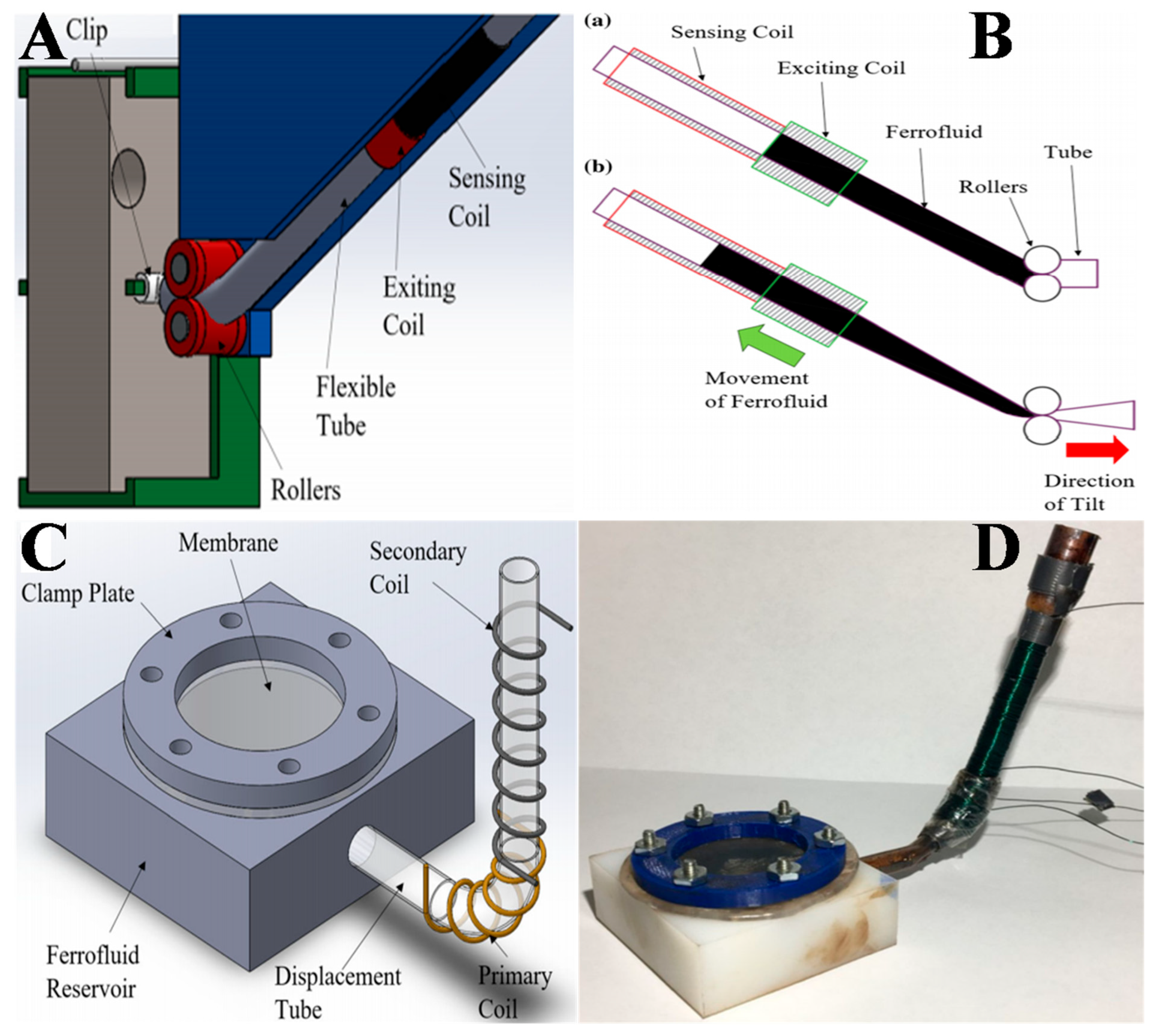
3.3. FN-Based Tilt Sensors/Inclinometers
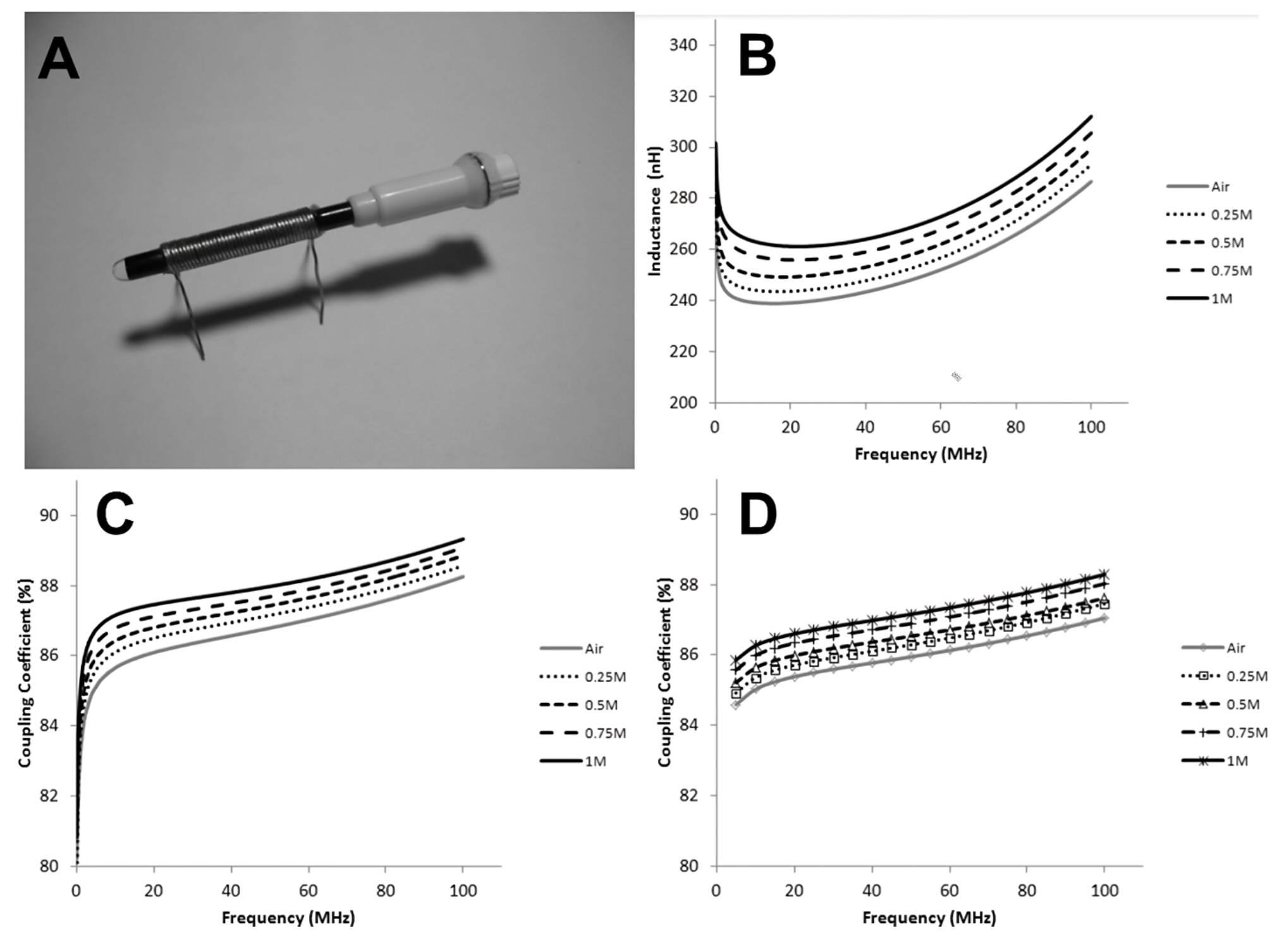
3.4. MEMS and On-Chip Components Using FN (FN Based On-Chip Transformers)
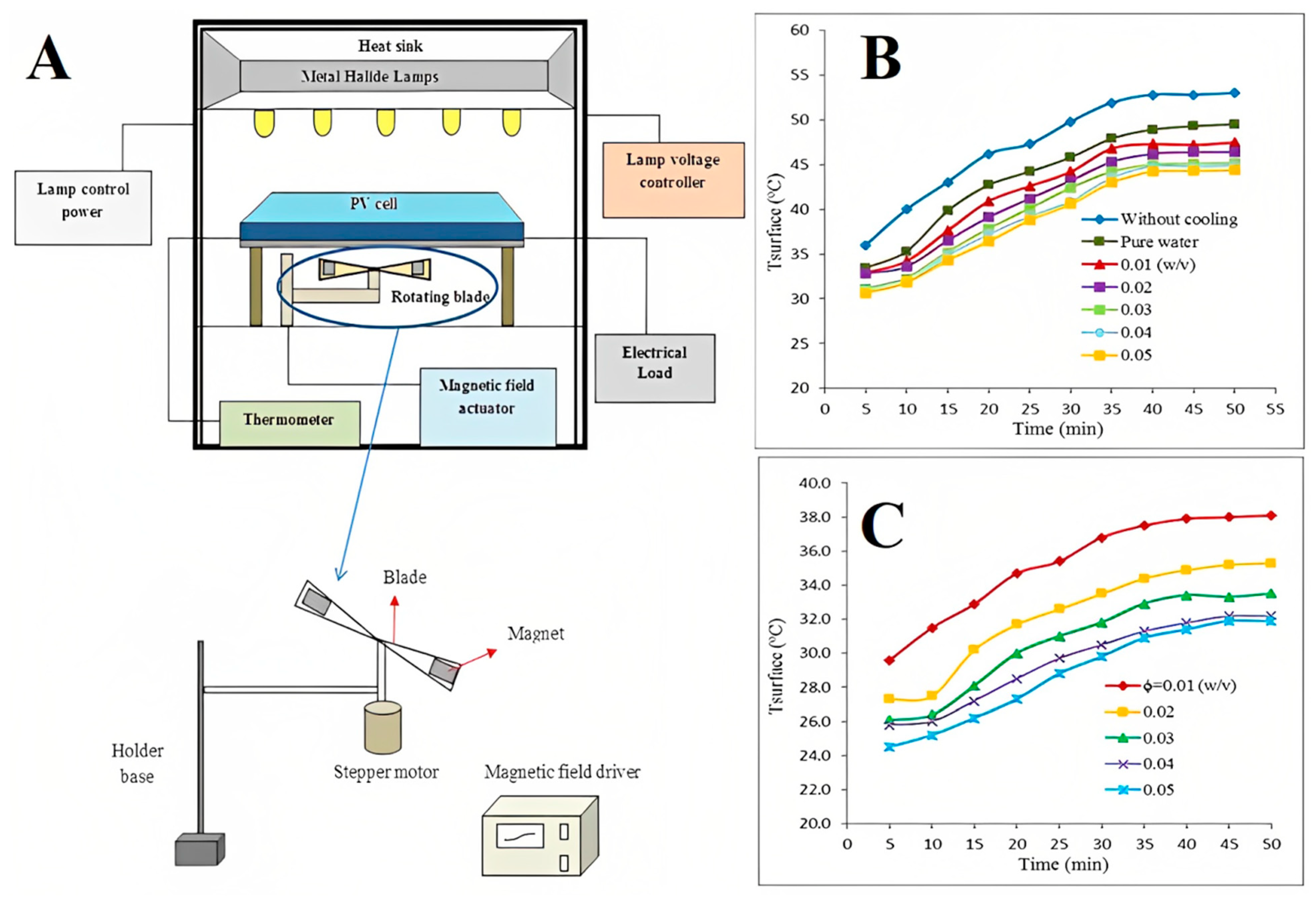
3.5. Cooling for Electronic Devices and Photovoltaic Thermal (PVT) Systems
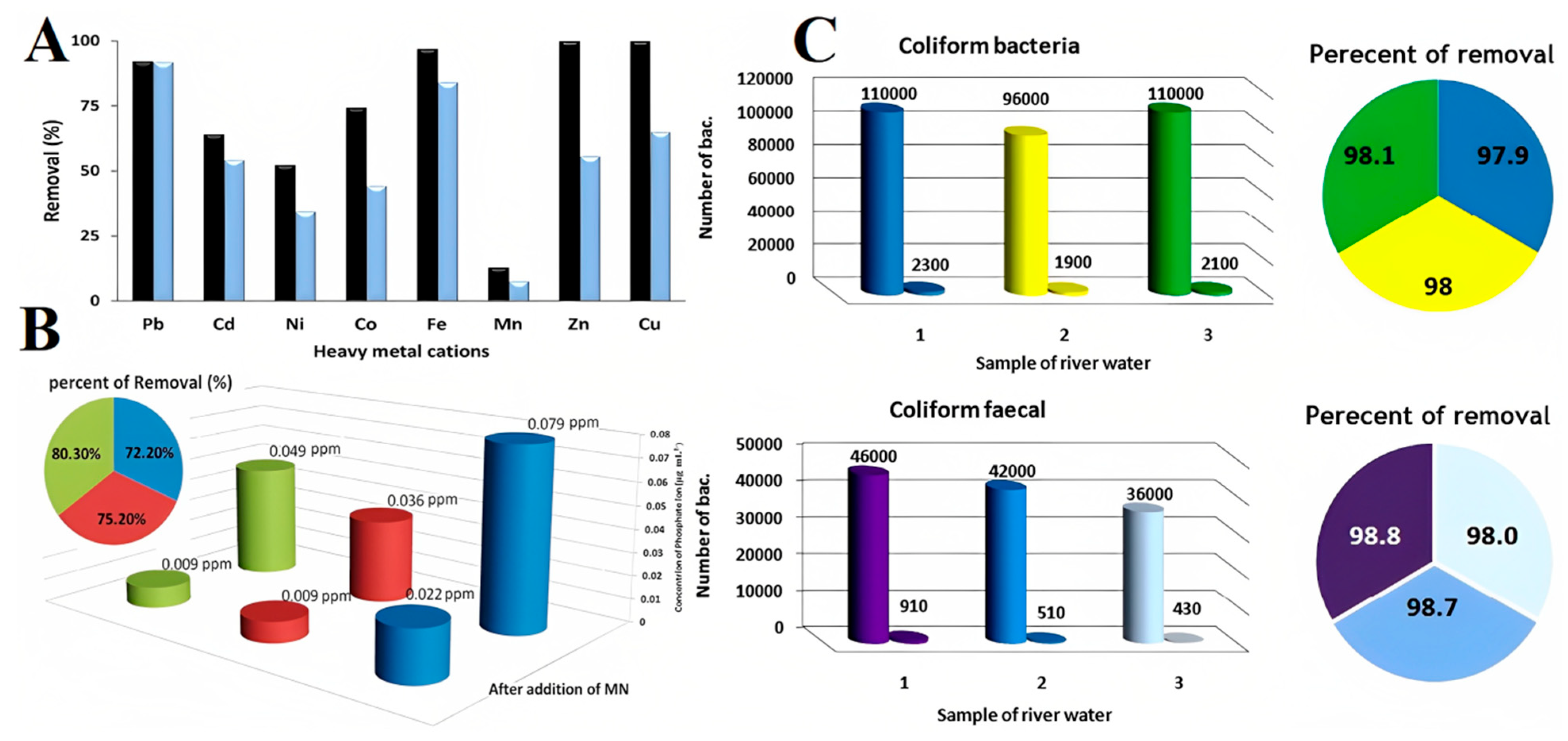
4. Environmental Engineering Applications
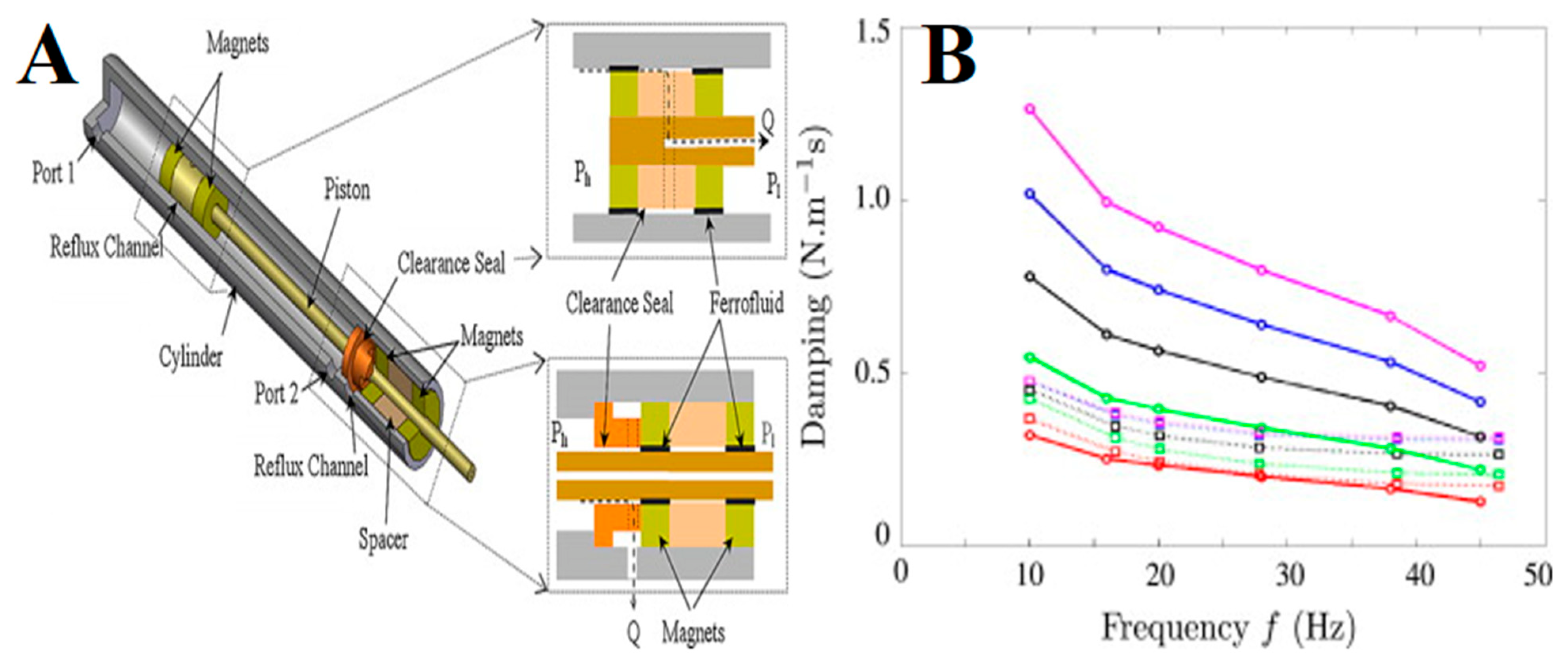
5. Mechanical Engineering or Magneto-Rheological Applications of FNs
6. Conclusions, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Alam, M.M.; Hussain, S.; Abutaleb, A.; Aziz, A.; Chandan, M.R.; Irshad, K.; Al-Hagri, A.M.A.; Bakather, O.Y.; Khan, A. Colloidal Fe3O4 nanoparticles-based oil blend ferro-nanofluid for heat transfer application. Eur. Phys. J. Plus 2021, 36, 752. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Afkhami, A.; Madrakian, T. Magnetic Nanomaterials in Analytical Chemistry; Elsevier B. V.: Amsterdam, The Netherlands, 2021; pp. 1–354. [Google Scholar]
- Dash, S.; Das, T.; Patel, P.; Panda, P.K.; Suar, M.; Verma, S.K. Emerging trends in the nanomedicine applications of functionalized magnetic nanoparticles as novel therapies for acute and chronic diseases. J. Nanobiotechnol. 2022, 20, 393. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Hussain, S.; Alam, M.M.; Imran, M.; Zouli, N.; Aziz, A.; Irshad, K.; Haider, M.; Khan, A. Fe3O4 nanoparticles decorated multi-walled carbon nanotubes based magnetic nanofluid for heat transfer application. Mater. Lett. 2020, 274, 128043. [Google Scholar] [CrossRef]
- Imran, M.; Zouli, N.; Ahamad, T.; Alshehri, S.M.; Chandan, M.R.; Hussain, S.; Aziz, A.; Dar, M.A.; Khan, A. Carbon-coated Fe3O4 core–shell super-paramagnetic nanoparticle-based ferrofluid for heat transfer applications. Nanoscale Adv. 2021, 3, 1962–1975. [Google Scholar] [CrossRef]
- Zamani, K.M.R.; Stadler, B.J. A guideline for effectively synthesizing and characterizing magnetic nanoparticles for advancing nanobiotechnology: A review. Sensors 2020, 20, 2554. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, Y.; Zhu, X.; Han, R.; Wang, X.; Luo, C. A nanocomposite prepared from bifunctionalized ionic liquid, chitosan, graphene oxide and magnetic nanoparticles for aptamer-based assay of tetracycline by chemiluminescence. Microchim. Acta 2020, 187, 63. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interf. Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Imran, M.; Anasri, A.R.; Shaik, A.H.; Aziz, A.; Hussain, S.; Khan, A.; Chandan, M.R. Ferrofluid synthesis using oleic acid coated Fe3O4 nanoparticles dispersed in mineral oil for heat transfer applications. Mater. Res. Express 2018, 5, 036108. [Google Scholar] [CrossRef]
- Imran, M.; Affandi, A.M.; Alam, M.M.; Khan, A.; Khan, A.I. Advanced biomedical applications of iron oxide nanostructures based ferrofluids. Nanotechnology 2021, 32, 422001. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Shaik, A.H.; Ansari, A.R.; Aziz, A.; Hussain, S.; Abouatiaa, A.F.F.; Khan, A.; Chandan, M.R. Synthesis of highly stable γ-Fe2O3 ferrofluid dispersed in liquid paraffin, motor oil and sunflower oil for heat transfer applications. RSC Adv. 2018, 8, 13970–13975. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rodriguez, A.; Rinaldi-Ramos, C.M. Emerging biomedical applications based on the response of magnetic nanoparticles to time-varying magnetic fields. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Monteserín, M.; Larumbe, S.; Martínez, A.V.; Burgui, S.; Martín, L.F. Recent advances in the development of magnetic nanoparticles for biomedical applications. J. Nanosci. Nanotechnol. 2021, 21, 2705–2741. [Google Scholar] [CrossRef]
- Javed, M.; Shaik, A.H.; Khan, T.A.; Imran, M.; Aziz, A.; Ansari, A.R.; Chandan, M.R. Synthesis of stable waste palm oil based CuO nanofluid for heat transfer applications. Heat Mass Transf. 2018, 54, 3739–3745. [Google Scholar] [CrossRef]
- Abutaleb, A.; Imran, M. Thermal conductivity enhancement for CuO nanoflakes in oil-based and oil blend-based nanofluids. J. Chin. Chem. Soc. 2021, 68, 1400–1404. [Google Scholar] [CrossRef]
- Cui, H.; Li, D. Fabrication and properties research on a novel perfluoropolyether based ferrofluid. J. Magn. Magn. Mater. 2019, 473, 341–347. [Google Scholar] [CrossRef]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Samykano, M.; Harun, W.S.; Najafi, G.; Mofijur, M.; Mazlan, M. A systematic review on graphene-based nanofluids application in renewable energy systems: Preparation, characterization, and thermophysical properties. Sustain. Energy Technol. Assess. 2021, 44, 101058. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Gao, H.; Yan, Y. Experimental study of viscosity and thermal conductivity of water based Fe3O4 nanofluid with highly disaggregated particles. Case Stud. Therm. Eng. 2022, 35, 102160. [Google Scholar] [CrossRef]
- Ratha, P.K.; Mishra, S.R.; Tripathy, R.S. Exploration of dissipative heat energy in conjunction with various thermophysical properties of nanofluids: Water and ethylene glycol base fluids. Int. Commun. Heat Mass Transf. 2022, 138, 106423. [Google Scholar] [CrossRef]
- Sarkar, S.; Ghosh, N.K. Theoretical study on enhancement of thermal conductivity and viscosity with volume fraction for ethylene glycol-based iron nanofluid. Mater. Today Proc. 2022, 66, 3377. [Google Scholar] [CrossRef]
- Khan, A.A.; Danish, M.; Rubaiee, S.; Yahya, S.M. Insight into the investigation of Fe3O4/SiO2 nanoparticles suspended aqueous nanofluids in hybrid photovoltaic/thermal system. Clean. Eng. Technol. 2022, 100572. [Google Scholar] [CrossRef]
- Elsaidy, A.; Vallejo, J.P.; Salgueiriño, V.; Lugo, L. Tuning the thermal properties of aqueous nanofluids by taking advantage of size-customized clusters of iron oxide nanoparticles. J. Mol. Liq. 2021, 344, 117727. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaushik, A.; Furukawa, H.; Khosla, A. Review-Towards 5th Generation AI and IoT Driven Sustainable Intelligent Sensors Based on 2D MXenes and Borophene. ECS Sens. Plus 2022, 1, 013601. [Google Scholar] [CrossRef]
- Ranjan, P.; Lee, J.M.; Kumar, P.; Vinu, A. Borophene: New Sensation in Flatland. Adv. Mater. 2020, 32, 2000531. [Google Scholar] [CrossRef]
- Ranjan, P.; Thomas, V.; Kumar, P. 2D Materials as a Diagnostic Platform for the Detection and Sensing of the SARS-CoV-2 Virus: A Bird’s-Eye View. J. Mater. Chem. B 2021, 9, 4608–4619. [Google Scholar] [CrossRef]
- Ranjan, P.; Sahu, T.K.; Bhushan, R.; Yamijala, S.S.; Late, D.J.; Kumar, P.; Vinu, A. Freestanding Borophene and Its Hybrids. Adv. Mater. 2019, 31, 1900353. [Google Scholar] [CrossRef]
- Chahal, S.; Ranjan, P.; Motlag, M.; Yamijala, S.S.R.K.C.; Late, D.J.; Sadki, E.H.S.; Cheng, G.J.; Kumar, P. Borophene via Micromechanical Exfoliation. Adv. Mater. 2021, 33, 2102039. [Google Scholar] [CrossRef]
- Sahu, T.K.; Ranjan, P.; Kumar, P. Chemical Exfoliation Synthesis of Boron Nitride and Molybdenum Disulfide 2D Sheets via Modified Hummers’ Method. Emergent Mater. 2021, 4, 645–654. [Google Scholar] [CrossRef]
- Ahmed, S.; Ansari, A.; Haidyrah, A.S.; Chaudhary, A.A.; Imran, M.; Khan, A. Hierarchical Molecularly Imprinted Inverse Opal-Based Platforms for Highly Selective and Sensitive Determination of Histamine. ACS Appl. Polym. Mater. 2022, 4, 2783–2793. [Google Scholar] [CrossRef]
- Ahmed, S.; Khatun, S.; Sallam, S.; Ansari, A.; Ansari, Z.A.; Kumar, R.R.; Hakami, J.; Khan, A. Photoresponse of Porous Silicon for Potential Optical Sensing. Europhys. Lett. 2022, 139, 36001. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, S.; Sun, B.-Y.; Chen, Y.-C.; Chuang, W.-T.; Chan, Y.-H.; Gupta, D.; Wu, P.-W.; Lin, H.-C. Self-Healable and Anti-Freezing Ion Conducting Hydrogel-Based Artificial Bioelectronic Tongue Sensing toward Astringent and Bitter Tastes. Biosens. Bioelectron. 2022, 198, 113811. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, G.; Tung, T.-W.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J.; Ansari, A.; Chuang, W.-T.; Lin, H.-C. UV-Enhanced Room-Temperature Ultrasensitive NO Gas Sensor with Vertical Channel Nano-Porous Organic Diodes. Sens. Actuators B Chem. 2020, 320, 128392. [Google Scholar] [CrossRef]
- Leonard, C.; Ferrasse, J.H.; Boutin, O.; Lefevre, S.; Viand, A. Bubble column reactors for high pressures and high temperatures operation. Chem. Eng. Res. Des. 2015, 100, 391–421. [Google Scholar] [CrossRef]
- Lin, T.J.; Tsuchiya, K.; Fan, L.S. Bubble flow characteristics in bubble columns at elevated pressure and temperature. AIChE J. 1998, 44, 545–560. [Google Scholar] [CrossRef]
- Suresh, A.K.; Bhalerao, S. Rate intensification of mass transfer process using ferrofluids. Indian J. Pure Appl. Phys. 2001, 40, 172–184. [Google Scholar]
- Komati, S.; Suresh, A.K. CO2 absorption into amine solutions: A novel strategy for intensification based on the addition of ferrofluids. J. Chem. Technol. Biotechnol. 2008, 83, 1094–1100. [Google Scholar] [CrossRef]
- Komati, S.; Suresh, A.K. Anomalous enhancement of interphase transport rates by nanoparticles: Effect of magnetic iron oxide on gas- liquid mass transfer. Ind. Eng. Chem. Res. 2010, 49, 390–405. [Google Scholar] [CrossRef]
- Saien, J.; Bamdadi, H. Mass transfer from nanofluid single drops in liquid–liquid extraction process. Ind. Eng. Chem. Res. 2012, 51, 5157–5166. [Google Scholar] [CrossRef]
- Wu, W.D.; Liu, G.; Chen, S.X.; Zhang, H. Nano-ferrofluid addition enhances ammonia/water bubble absorption in an external magnetic field. Energy Build. 2013, 57, 268–277. [Google Scholar] [CrossRef]
- Saien, J.; Bamdadi, H.; Daliri, S. Liquid–liquid extraction intensification with magnetite nanofluid single drops under oscillating magnetic field. J. Ind. Eng. Chem. 2015, 21, 1152–1159. [Google Scholar] [CrossRef]
- Saien, J.; Jafari, F. Mass transfer intensification strategies for liquid–liquid extraction with single drop investigations. Int. J. Heat Mass Transf. 2019, 144, 118603. [Google Scholar] [CrossRef]
- Hoseini, N.M.; Esfahany, N.; Etesami, N.; Afarani, H.T.; Fadaie, E. Investigation of the effect of electric field on CO2 absorption in water/Fe3O4 nanofluid. Braz. J. Chem. Eng. 2019, 36, 1333–1342. [Google Scholar] [CrossRef]
- Maiorov, M.M.; Zablotsky, D.; Blums, E.; Lickrastina, A. Magnetic field control of gas-liquid mass transfer in ferrofluids. J. Magn. Magn. Mater. 2020, 497, 165958. [Google Scholar] [CrossRef]
- Darvanjooghi, M.H.K.; Pahlevaninezhad, M.; Abdollahi, A.; Davoodi, S.M. Investigation of the effect of magnetic field on mass transfer parameters of CO2 absorption using Fe3O4-water nanofluid. AIChE J. 2017, 63, 2176–2186. [Google Scholar] [CrossRef]
- Pekel, J.-F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-resolution mapping of global surface water and its long-term changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Choi, S.; Chang, B.; Kim, S.; Lee, J.; Yoon, J.; Choi, J.W. Battery Electrode Materials with Omnivalent Cation Storage for Fast and Charge-Efficient Ion Removal of Asymmetric Capacitive Deionization. Adv. Funct. Mater. 2018, 28, 1802665. [Google Scholar] [CrossRef]
- He, F.; Biesheuvel, P.; Bazant, M.; Hatton, T.A. Theory of water treatment by capacitive deionization with redox active porous electrodes. Water Res. 2018, 132, 282–291. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Wan, K.; Huang, L.; Yan, J.; Ma, B.; Huang, X.; Luo, Z.; Zhang, H.; Xiao, T. Removal of fluoride from industrial wastewater by using different adsorbents: A review. Sci. Total Environ. 2021, 773, 145535. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Morris, G.; Qi, D. Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination 2008, 225, 329–340. [Google Scholar] [CrossRef]
- Chen, S.; Fan, F.; Chang, S.; Miao, Y.; Chen, M.; Li, J.; Wang, X.; Lin, L. Tunable optical and magneto-optical properties of ferrofluid in the terahertz regime. Opt. Express 2014, 22, 6313–6321. [Google Scholar] [CrossRef]
- Xu, M.; Ridler, P.J. Linear dichroism and birefringence effects in magnetic fluids. J. Appl. Phys. 1997, 82, 326–332. [Google Scholar] [CrossRef]
- Lin, J.; Sheu, J.; Lee, M.; Lin, Y. Linear birefringence and dichroism measurements for silica coated iron oxide ferrofluids. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Yang, S.Y.; Chieh, J.J.; Horng, H.E.; Hong, C.Y.; Yang, H.C. Origin and applications of magnetically tunable refractive index of magnetic fluid films. Appl. Phys. Lett. 2004, 84, 5204–5206. [Google Scholar] [CrossRef]
- Wang, H.; Pu, S.; Gharibi, A.; Wang, N. Generation and versatile transmission properties of ring-shaped beams based on thermal lens effect of magnetic fluids and ring-limited windows. Opt. Commun. 2013, 286, 211–216. [Google Scholar] [CrossRef]
- Cecelja, F.; Rakowski, R.; Martinez, L. A ferrofluid-based magnetic field sensor. In Proceedings of the Instrumentation and Measurement Technology Conference, IMTC, Proceedings IEEE, Ottawa, ON, Canada, 16–19 May 2005; Volume 1, pp. 612–616. [Google Scholar]
- Nair, S.S.; Rajesh, S.; Abraham, V.S.; Anantharaman, M.R. Ferrofluid thin films as optical gauss meters proposed for field and magnetic moment sensing. Bull. Mater. Sci. 2011, 34, 245–249. [Google Scholar] [CrossRef][Green Version]
- Budker, D. A new spin on magnetometry. Nature 2003, 422, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Schwindt, P.D.; Knappe, S.; Shah, V.; Hollberg, L.; Kitching, J. Chip-scale atomic magnetometer. Appl. Phys. Lett. 2004, 85, 6409–6411. [Google Scholar] [CrossRef]
- Auster, H.U.; Glassmeier, K.H.; Magnes, W.; Aydogar, O.; Baumjohann, W.; Constantinescu, D.; Fischer, D.; Fornacon, K.H.; Georgescu, E.; Harvey, P.; et al. The THEMIS fluxgate magnetometer. Space Sci. Rev. 2008, 141, 235–264. [Google Scholar] [CrossRef]
- Singh, K.; Sharma, A.; Rajput, G.; Kasturia, D. Ferro Fluids: - Properties and Applications. Int. J. Res. IJR 2014, 1, 343. [Google Scholar]
- Martinez, L.; Cecelja, F.; Rakowski, R. A novel magneto-optic ferrofluid material for sensor applications. Sensor. Actuat. A Phys. 2005, 123–124, 438–443. [Google Scholar] [CrossRef]
- Zu, P.; Chan, C.C.; Lew, W.S.; Jin, Y.; Zhang, Y.; Liew, H.F.; Chen, L.H.; Wong, W.C.; Dong, X. Magneto-optical fiber sensor based on magnetic fluid. Opt. Lett. 2012, 37, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Pu, S.; Wang, X.; Yu, G. Magnetic field sensing based on V-shaped groove filled with magnetic fluids. Appl. Opt. 2012, 51, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Pu, S.; Wang, X.; Yu, G.; Wang, N.; Wang, H. Magnetic field sensing based on capillary filled with magnetic fluids. Appl. Opt. 2012, 51, 6528–6538. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Q.; Liu, T.; Lan, X.; Xiao, H. Optical fiber magnetic field sensor based on single-mode–multimode–single-mode structure and magnetic fluid. Opt. Lett. 2013, 38, 3999–4001. [Google Scholar] [CrossRef]
- Candiani, A.; Bravo, M.; Pissadakis, S.; Cucinotta, A.; Lopez-Amo, M.; Selleri, S. Magnetic field sensor based on backscattered intensity using ferrofluid. IEEE Photon. Technol. Lett. 2013, 25, 1481–1484. [Google Scholar] [CrossRef]
- Thakur, H.V.; Nalawade, S.M.; Gupta, S.; Kitture, R.; Kale, S.N. Photonic crystal fiber injected with Fe3O4 nanofluid for magnetic field detection. Appl. Phys. Lett. 2011, 99, 161101. [Google Scholar] [CrossRef]
- Homa, D.; Pickrell, G. Magnetic sensing with ferrofluid and fiber optic connectors. Sensors 2014, 14, 3891–3896. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhou, B.; Chen, Z.; Jiang, H.; Xing, X. Magnetic-field sensor utilizing the ferrofluid and thin-core fiber modal interferometer. IEEE Sens. J. 2014, 15, 333–336. [Google Scholar] [CrossRef]
- Dudus, A.; Blue, R.; Uttamchandani, D. Transmissive optical fiber magnetic field sensor based on ferrofluids. IEEE Sens. 2017, 1–3. [Google Scholar]
- Mitu, S.A.; Dey, D.K.; Ahmed, K.; Paul, B.K.; Luo, Y.; Zakaria, R.; Dhasarathan, V. Fe3O4 nanofluid injected photonic crystal fiber for magnetic field sensing applications. J. Magn. Magn. Mater. 2020, 494, 165831. [Google Scholar] [CrossRef]
- Luo, L.; Pu, S.; Tang, J.; Zeng, X.; Lahoubi, M. Highly sensitive magnetic field sensor based on microfiber coupler with magnetic fluid. Appl. Phys. Lett. 2015, 106, 193507. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, Z.; Yuan, B.; Yuan, Z.; Xue, H. The influence of incident power on the magnetic fluid sensor sensitivity based on optical transmission properties. Math. Probl. Eng. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Samian; Zaidan, A.H.; Arifianto, A.S.; Taufiq, A. Magnetic field detection using fiber bundle and magnetic fluid as sensors. Microw. Opt. Technol. 2021, 63, 975. [Google Scholar] [CrossRef]
- Nandy, M.; Lahiri, B.B.; Philip, J. Visual detection of defects in carbon steel using magnetic nanoemulsions: Effect of stabilizing moieties on the defect detection sensitivity. Sens. Actuators A Phys. 2020, 314, 112220. [Google Scholar] [CrossRef]
- Zhang, D.; Di, Z.; Zou, Y.; Chen, X. Temperature sensor using ferrofluid thin film. Microfluid. Nanofluid. 2009, 7, 141. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Wu, D.; Wang, Q. Magnetic field and temperature measurements with a magnetic fluid-filled photonic crystal fiber Bragg grating. Instrum. Sci. Technol. 2013, 41, 463. [Google Scholar] [CrossRef]
- Pathak, S.; Jain, K.; Kumar, V.; Pant, R.P. Magnetic fluid based high precision temperature sensor. IEEE Sens. J. 2017, 17, 2670. [Google Scholar] [CrossRef]
- Perreard, I.M.; Reeves, D.B.; Zhang, X.; Kuehlert, E.; Forauer, E.R.; Weaver, J.B. Temperature of the magnetic nanoparticle microenvironment: Estimation from relaxation times. Phys. Med. Bio. 2014, 59, 1109. [Google Scholar] [CrossRef] [PubMed]
- Draack, S.; Viereck, T.; Nording, F.; Janssen, K.J.; Schilling, M.; Ludwig, F. Determination of dominating relaxation mechanisms from temperature-dependent Magnetic Particle Spectroscopy measurements. J. Magnet. Mag. Mater. 2019, 474, 570. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Saha, R.; Wong, D.; Wang, J.P. Magnetic particle spectroscopy-based bioassays: Methods, applications, advances, and future opportunities. J. Phys. D Appl. Phys. 2019, 52, 173001. [Google Scholar] [CrossRef]
- Ando, B.; Baglio, S.; Beninato, A. Can ferrohydrodynamic instabilities be useful in transducers? [Instrumentation Notes]. IEEE instrum. Meas. Magaz. 2011, 14, 38. [Google Scholar] [CrossRef]
- Ando, B.; Ascia, A.; Baglio, S.; Beninato, A. A ferrofluidic inertial sensor exploiting the Rosensweig effect. IEEE Trans. Instrum. Meas. 2010, 59, 1471. [Google Scholar] [CrossRef]
- Ando, B.; Baglio, S.; Beninato, A. A flow sensor exploiting magnetic fluids. Sens. Actuators A Phys. 2013, 189, 17. [Google Scholar] [CrossRef]
- Andò, B.; Baglio, S.; Beninato, A.; Graziani, S.; Pagano, F.; Umana, E. A seismic sensor based on IPMC combined with ferrofluids. IEEE Trans. Instrum. Meas. 2013, 62, 1292. [Google Scholar] [CrossRef]
- Li, L.; Han, Q.; Chen, Y.; Liu, T.; Zhang, R. An all-fiber optic current sensor based on ferrofluids and multimode interference. IEEE Sens. J. 2014, 14, 1749. [Google Scholar]
- DeGraff, A.; Rashidi, R. Ferrofluid transformer-based tilt sensor. Microsyst. Technol. 2020, 26, 2499. [Google Scholar] [CrossRef]
- Martin, J.; Rashidi, R. A differential transformer-based force sensor utilizing a magnetic fluid core. Microsyst. Technol. 2020, 27, 115. [Google Scholar] [CrossRef]
- Zhu, H.; Fohlerová, Z.; Pekárek, J.; Basova, E.; Neužil, P. Recent advances in lab-on-a-chip technologies for viral diagnosis. Biosens. Bioelectron. 2020, 153, 112041. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liao, N.; Fang, X.; Sin, J.K.O. A Silicon-Embedded Transformer for High-Efficiency, High-Isolation, and Low-Frequency On-Chip Power Transfer. IEEE Trans. Electron. Devices 2015, 62, 220. [Google Scholar] [CrossRef]
- Khorsandi, D.; Nodehi, M.; Waqar, T.; Shabani, M.; Kamare, B.; Zare, E.N.; Ersoy, S.; Annabestani, M.; Çelebi, M.F.; Kafadenk, A. Manufacturing of microfluidic sensors utilizing 3d printing technologies: A production system. J. Nanomater. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, W.S.; Wu, W.J.; Wang, D.W.; Gao, H.; Hu, Y.; Liu, J. Miniaturized Microwave Microfluidic Sensor Based on Quarter-Mode 2.5-D Spoof Plasmons. Sens. Actuators A Phys. 2022, 342, 113621. [Google Scholar] [CrossRef]
- Jian, F.; Niansong, M.; Yumei, H.; Zhiliang, H. CMOS high linearity PA driver with an on-chip transformer for W-CDMA application. J. Semicond. 2011, 32, 1. [Google Scholar]
- Ercoli, M.; Dragomirescu, D.; Plana, R. Reduced size high performance transformer balun at 60 GHz in CMOS 65nm Technology. Microelectron. J. 2012, 43, 737. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Yin, W.Y.; Li, X. Three-solenoid windings transformer baluns on CMOS-grade silicon substrate. Microelectron. Eng. 2010, 87, 150. [Google Scholar] [CrossRef]
- Bajwa, R.; Yapici, M.K. Integrated On-Chip Transformers: Recent Progress in the Design, Layout, Modeling and Fabrication. Sensors 2019, 19, 3535. [Google Scholar] [CrossRef]
- Kordzadeh-Kermani, V.; Madadelahi, M.; Ashrafizadeh, S.N.; Kulinsky, L.; Martinez-Chapa, S.O.; Madou, M.J. Electrified lab on disc systems: A comprehensive review on electrokinetic applications. Biosens. Bioelectron. 2022, 214, 114381. [Google Scholar] [CrossRef] [PubMed]
- Boulouard, A.; Rouzic, M.L. Analysis of Rectangular Spiral Transformers for MMIC applications. IEEE Trans. Microw. Theory Tech. 1989, 37, 1257. [Google Scholar] [CrossRef]
- Long, J.R. Monolithic Transformers for Silicon RF IC Design. IEEE J. Solid State Circuit. 2000, 35, 1368. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chen, P.H.; Lee, D.S.; Yang, C.T. Investigation of electrical and magnetic properties of ferro-nanofluid on transformers. Nanoscale Res. Lett. 2011, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, N.; Bedair, S.S.; Smith, G.L. Creating 3D printed magnetic devices with ferrofluids and liquid metals. Addit. Manuf. 2019, 26, 15. [Google Scholar] [CrossRef]
- Keneth, E.S.; Epstein, A.R.; Harari, M.S.; Pierre, R.S.; Magdassi, S.; Bergbreiter, S. 3D printed ferrofluid based soft actuators. In Proceedings of the 2019 International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; p. 7569. [Google Scholar]
- Doganay, S.; Cetin, L.; Ezan, M.A.; Turgut, A. A rotating permanent magnetic actuator for micropumping devices with magnetic nanofluids. J. Micromech. Microeng. 2020, 30, 075012. [Google Scholar] [CrossRef]
- Sardarabadi, M.; Hosseinzadeh, M.; Kazemian, A.; Passandideh-Fard, M. Experimental investigation of the effects of using metal-oxides/water nanofluids on a photovoltaic thermal system (PVT) from energy and energy view points. Energy 2017, 138, 682. [Google Scholar] [CrossRef]
- Rostami, Z.; Rahimi, M.; Azimi, N. Using high-frequency ultrasound waves and nanofluid for increasing the efficiency and cooling performance of a PV module. Energy Convers. Manag. 2018, 160, 141. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Chaichan, M.T.; Kazem, H.A.; Sopian, K.; Ibrahim, A.; Mat, S.; Ruslan, M.H. Comparison study of indoor/outdoor experiments of a photovoltaic thermal PV/T system containing SiC nanofluid as a coolant. Energy 2018, 151, 33. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chung, H.H.; Lee, Y.H.; Yang, L.Q. Heat dissipation and electrical conduction of an LED by using a microfluidic channel with a graphene solution. Appl. Therm. Eng. 2020, 175, 115383. [Google Scholar] [CrossRef]
- Nazari, M.A.; Ghasempour, R.; Ahmadi, M.H.; Heydarian, G.; Shafii, M.B. Experimental investigation of graphene oxide nanofluid on heat transfer enhancement of pulsating heat pipe. Int. Commun. Heat Mass Transf. 2018, 91, 90. [Google Scholar] [CrossRef]
- Ghadiri, M.; Sardarabadi, M.; Pasandideh-fard, M.; Moghadam, A.J. Experimental investigation of a PVT system performance using nano ferrofluids. Energy Convers. Manag. 2015, 103, 468. [Google Scholar] [CrossRef]
- Heidari, N.; Rahimi, M.; Azimi, N. Experimental investigation on using ferrofluid and rotating magnetic field (RMF) for cooling enhancement in a photovoltaic cell. Int. Commun. Heat Mass Transf. 2018, 94, 32. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, M.Y. Illuminance and Heat Transfer Characteristics of High Power LED Cooling System with Heat Sink filled with Ferrofluid. Appl. Therm. Eng. 2018, 143, 438–449. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—an overview. RSC Adv. 2012, 2, 6380. [Google Scholar] [CrossRef]
- Guibert, C.; Fresnais, J.; Peyre, V.; Dupuis, V. Magnetic fluid hyperthermia probed by both calorimetric and dynamic hysteresis measurements. J. Magnet. Magn. Mater. 2017, 421, 384. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, Y.; Wang, X.; Liu, M.; Xia, S.Q.; Yin, D.Q.; Zhang, Y.L.; Zhao, J.F. Microwave-assisted preparation of bamboo charcoal-based iron-containing adsorbents for Cr (VI) removal. Chem. Eng. J. 2011, 174, 326. [Google Scholar] [CrossRef]
- Ali, I. New generation adsorbents for water treatment. Chem. Rev. 2012, 112, 5073. [Google Scholar] [CrossRef]
- Saranya, P.S.; Ramesh, T.; Gandhimathi, R. Effectiveness of natural coagulants from non-plant-based sources for water and wastewater treatment—A review. Desal. Water Treat. 2014, 52, 6030. [Google Scholar] [CrossRef]
- Oladoja, N.A. Headway on natural polymeric coagulants in water and wastewater treatment operations. J. Water Proc. Eng. 2015, 6, 174. [Google Scholar] [CrossRef]
- Kimura, M.; Matsui, Y.; Kondo, K.; Ishikawa, T.B.; Matsushita, T.; Shirasaki, N. Minimizing residual aluminum concentration in treated water by tailoring properties of polyaluminum coagulants. Water Res. 2013, 47, 2075. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, S.; Gao, B.; Yue, Q.; Wang, Y.; Li, Q. Coagulation behavior and floc properties of compound bioflocculant–polyaluminum chloride dual-coagulants and polymeric aluminum in low temperature surface water treatment. J. Environ. Sci. 2015, 30, 215. [Google Scholar] [CrossRef] [PubMed]
- Fatombi, J.K.; Lartiges, B.; Aminou, T.; Barres, O.; Caillet, C. A natural coagulant protein from copra (Cocos nucifera): Isolation, characterization, and potential for water purification. Sep. Purif. Technol. 2013, 116, 35. [Google Scholar] [CrossRef]
- Keeley, J.; Smith, A.D.; Judd, S.J.; Jarvis, P. Reuse of recovered coagulants in water treatment: An investigation on the effect coagulant purity has on treatment performance. Sep. Purif. Technol. 2014, 131, 69. [Google Scholar] [CrossRef]
- Li, R.; He, C.; He, Y. Preparation and characterization of poly-silicic-cation coagulant from industrial wastes. Desalination 2013, 319, 85. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Gao, B.Y.; Qi, Q.B.; Wang, Y.; Phuntsho, S.; Kim, J.H.; Yue, Q.Y.; Li, Q.; Shon, H.K. Cationic polyacrylamide as coagulant aid with titanium tetrachloride for low molecule organic matter removal. J. Hazard. Mater. 2013, 258, 84. [Google Scholar] [CrossRef]
- Jeon, J.R.; Kim, E.J.; Kim, Y.M.; Murugesan, K.; Kim, J.H.; Chang, Y.S. Use of grape seed and its natural polyphenol extracts as a natural organic coagulant for removal of cationic dyes. Chemosphere 2009, 77, 1090. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Zeng, Z.; Pearce, P. Preparation and use of modified clay coagulants for wastewater treatment. Water Air Soil Poll. 2004, 158, 53. [Google Scholar] [CrossRef]
- Al-Maghrabi, M.N.; Imran, M.; Sharma, C. Top Down Activation Enhancement of Bentonite for the Removal of Emulsified Oil from Wastewater. J. Nanoeng. Nanomanuf. 2016, 6, 288. [Google Scholar] [CrossRef]
- Cheng, W.P.; Chi, F.H.; Yu, R.F.; Lee, Y.C. Using chitosan as a coagulant in recovery of organic matters from the mash and lauter wastewater of brewery. J. Polym. Environ. 2005, 13, 383. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Dávila-Acedo, M.A. Optimization of the synthesis of a new coagulant from a tannin extract. J. Hazard. Mater. 2011, 186, 1704. [Google Scholar] [CrossRef]
- Hatamie, A.; Parham, H.; Zargar, B.; Heidari, Z. Evaluating magnetic nano-ferrofluid as a novel coagulant for surface water treatment. J. Mol. Liq. 2016, 219, 694–702. [Google Scholar] [CrossRef]
- Jarvis, P.; Sharp, E.; Pidou, M.; Molinder, R.; Parsons, S.A.; Jefferson, B. Comparison of coagulation performance and floc properties using a novel zirconium coagulant against traditional ferric and alum coagulants. Water Res. 2012, 46, 4179. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317. [Google Scholar] [CrossRef] [PubMed]
- Uheida, A.; Salazar-Alvarez, G.; Björkman, E.; Yu, Z.; Muhammed, M. Fe3O4 and γ-Fe2O3 nanoparticles for the adsorption of Co2+ from aqueous solution. J. Colloid Interface Sci. 2006, 298, 501. [Google Scholar] [CrossRef]
- Kuwahara, T. Fundamental characteristics of low-resistive particulate matter removal using a magnetic fluid and nonthermal plasma. J. Magn. Magn. Mater. 2020, 498, 166161. [Google Scholar] [CrossRef]
- Urreta, H.; Aguirre, G.; Kuzhir, P.; Lopez de Lacalle, L.N. Seals based on magnetic fluids for high precision spindles of machine tools. Int. J. Precis. Eng. Manufact. 2018, 19, 495. [Google Scholar] [CrossRef]
- Boots, A.S.T.; Krijgsman, L.E.; de Ruiter, B.J.; Lampaert, S.G.; Spronck, J.W. Increasing the load capacity of planar ferrofluid bearings by the addition of ferromagnetic material. Tribol. Int. 2019, 129, 46. [Google Scholar] [CrossRef]
- Van der Wal, K.; van Ostayen, R.A.; Lampaert, S.G. Ferrofluid rotary seal with replenishment system for sealing liquids. Tribol. Int. 2020, 150, 106372. [Google Scholar] [CrossRef]
- Sammaiah, A.; Dai, Q.; Huang, W.; Wang, X. Synthesis of GO-Fe3O4-based ferrofluid and its lubrication performances. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2020, 234, 1160. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Uzun, B.B.; Önal, E.; Pütün, A.E. Synthetic fuel production from cottonseed: Fast pyrolysis and a TGA/FT-IR/MS study. J. Anal. Appl. Pyrolysis 2014, 105, 83. [Google Scholar] [CrossRef]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl. Surf. Sci. 2010, 256, 3093. [Google Scholar] [CrossRef]
- Korolev, V.V.; Ramazanova, A.G.; Blinov, A.V. Adsorption of surfactants on superfine magnetite. Russ. Chem. Bull. 2002, 51, 2044. [Google Scholar] [CrossRef]
- Scherer, C.; FigueiredoNeto, A.M. Ferrofluids: Properties and Applications. Braz. J. Phys. 2005, 35, 718. [Google Scholar] [CrossRef]
- Raj, K.; Moskowitz, R. Commercial applications of ferrofluids. J. Magn. Magn. Mater. 1990, 85, 233. [Google Scholar] [CrossRef]
- De Volder, M.; Reynaerts, D. Development of a hybrid ferrofluid seal technology for miniature pneumatic and hydraulic actuators. Sens. Actuators A Phys. 2009, 152, 234. [Google Scholar] [CrossRef]
- Kumbhar, B.K.; Patil, S.R.; Sawant, S.M. Synthesis and characterization of magneto-rheological (MR) fluids for MR brake application. Int. J. Eng. Sci. 2015, 18, 432. [Google Scholar] [CrossRef]
- Wang, N.; Liu, X.; Zhang, X. Squeeze-strengthening effect of silicone oil-based magnetorheological fluid with nanometer Fe3O4 addition in high-torque magnetorheological brakes. J. Nanosci. Nanotechnol. 2019, 19, 2633. [Google Scholar] [CrossRef]
- Hu, G.; Ru, Y.; Li, W. Design and development of a novel displacement differential self-induced magnetorheological damper. J. Intell. Mater. Syst. Struct. 2015, 26, 527. [Google Scholar] [CrossRef]
- Pinho, M.; Génevaux, J.M.; Dauchez, N.; Brouard, B.; Collas, P.; Mézière, H. Damping induced by ferrofluid seals in ironless loudspeaker. J. Magn. Magn. Mater. 2014, 356, 125. [Google Scholar] [CrossRef]
- Lampaert, S.G.; Fellinger, B.J.; Spronck, J.W.; Van Ostayen, R.A.J. In-plane friction behaviour of a ferrofluid bearing. Precis. Eng. 2018, 54, 163. [Google Scholar] [CrossRef]



| ION Size (nm) | Concentration | Amplitude of Mag. Field | Applications | Results | Ref. |
|---|---|---|---|---|---|
| 15 | 0.39% | 100–1000 G | Mass transfer | 92.8% enhancement | [39] |
| 13.2 | 0.3% | - | Mass transfer | 164% enhancement | [40] |
| 17 | 0.002 wt.% | - | Mass transfer | 72% enhancement | [41] |
| 17 | 0.001–0.005 wt.% | 0.36–1.45 T | Mass transfer | 121% enhancement | [43] |
| 14 | - | 200×10−6 T | Magneto-optic sensor | Sensitivity of 276 mT·A−1 | [67] |
| 10 | 0.39 vol.% | 2013 Oe (perpendicular) and 2167 Oe (parallel) | Magneto-optic sensor | Sensitivity 0.3998 dB/Oe (perpendicular) and 0.0017 dB/Oe (parallel) | [68] |
| 10 | - | 0–220 Oe | Optical fiber magnetic field sensor | Sensitivities up to 905 pm/mT and 0.748 dB/mT | [71] |
| 20 | 0.3–0.6 wt.% | 0–80 mT | Magnetic field sensor | Higher sensitivity of 155.7 mT and 242 pm/mT for 0.3 wt.% and 0.6 wt.%, respectively | [73] |
| 10 | 7.9 and 17.7 vol.% | −3000 to 3000 mG | Fiber-optic magnetic sensor | Higher sensitivity of 0.3 to 2.3 nm/mT | [74] |
| 10 | - | 0–240 Oe | Magnetic field optical fiber sensor | Sensitivity of 11.8 pm/Oe | [75] |
| 9 | 2.5% | 25.28–489.56 mT | Magnetic field sensor | Sensitivity of 25.3 to 83.5 mT | [80] |
| 10 | 6.47 vol.% | 40.9 mT | Temperature sensor | Detected above 60 °C | [82] |
| - | 3 vol.% | 20–300 Oe | Temperature sensor | Detection limit ±0.82 °C | [83] |
| 10–15 | 3 vol.% | - | Temperature sensor | Sensitivity 3.7 mK | [84] |
| - | - | - | Force sensor | 68.3 mV/N | [94] |
| 10 | 0.5–1 M | - | Transformer | Improved inductance and coupling efficiencies | [96] |
| <15 | - | - | Transformer | Coupling improvements and nearly threefold increase in inductance density | [107] |
| 15–20 | 1–3 vol.% | 135–160 mT | Micropump device | Cell sorting devices (6.1 µL/min), low-flow drug delivery systems (1–10 µL/min), and pathogen detection systems (3–5.83 µL/min). | [109] |
| 45 | 3 wt.% | 300 G | Coolant | Overall cooling efficiency increased by 79% | [115] |
| 25 | 0.05% (w/v) | 880 mT | Coolant | Power (P) increased by 47.5% and thermal efficiency increased by 30% | [116] |
| 30–50 | - | - | Water purification | 98% removal of fecal coliform bacteria | [136] |
| 9.5–11.3 | - | - | Water purification | 86% efficiency of Co2+ adsorption | [139] |
| 9.5–11.3 | - | Water purification | 30% efficiency of Co2+ adsorption | [139] | |
| 10 | 0.3 vol.% | 490 mT | Air purification | PM removal efficiencies (η) of44% for PMs with diameters dp > 0.3 μm, 85% for dp > 0.5 μm, 99% for dp > 1.0 μm, and 100% for dp > 2.0 μm | [140] |
| - | - | - | Seal | Sealing capacity improved | [143] |
| 0.5 wt.% | - | Lubricant | 19.4% reduction in friction coefficient | [144] | |
| 20 | 30 vol.% | 10–650 mT | Brake | Braking torque increased by1300 N·m and 410 N·m for axial and radial squeezing stress, respectively. | [152] |
| - | - | 1000 kA·m−1 | Damper | Higher magnetic saturation and higher damping | [154] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Chaudhary, A.A.; Ahmed, S.; Alam, M.M.; Khan, A.; Zouli, N.; Hakami, J.; Rudayni, H.A.; Khan, S.-U.-D. Iron Oxide Nanoparticle-Based Ferro-Nanofluids for Advanced Technological Applications. Molecules 2022, 27, 7931. https://doi.org/10.3390/molecules27227931
Imran M, Chaudhary AA, Ahmed S, Alam MM, Khan A, Zouli N, Hakami J, Rudayni HA, Khan S-U-D. Iron Oxide Nanoparticle-Based Ferro-Nanofluids for Advanced Technological Applications. Molecules. 2022; 27(22):7931. https://doi.org/10.3390/molecules27227931
Chicago/Turabian StyleImran, Mohd, Anis Ahmad Chaudhary, Shahzad Ahmed, Md. Mottahir Alam, Afzal Khan, Nasser Zouli, Jabir Hakami, Hassan Ahmad Rudayni, and Salah-Ud-Din Khan. 2022. "Iron Oxide Nanoparticle-Based Ferro-Nanofluids for Advanced Technological Applications" Molecules 27, no. 22: 7931. https://doi.org/10.3390/molecules27227931
APA StyleImran, M., Chaudhary, A. A., Ahmed, S., Alam, M. M., Khan, A., Zouli, N., Hakami, J., Rudayni, H. A., & Khan, S.-U.-D. (2022). Iron Oxide Nanoparticle-Based Ferro-Nanofluids for Advanced Technological Applications. Molecules, 27(22), 7931. https://doi.org/10.3390/molecules27227931








