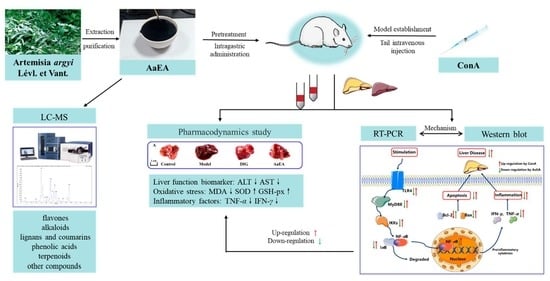
Ethyl Acetate Extract from Artemisia argyi Prevents Liver Damage in ConA-Induced Immunological Liver Injury Mice via Bax/Bcl-2 and TLR4/MyD88/NF-κB Signaling Pathways
Abstract
1. Introduction
2. Results
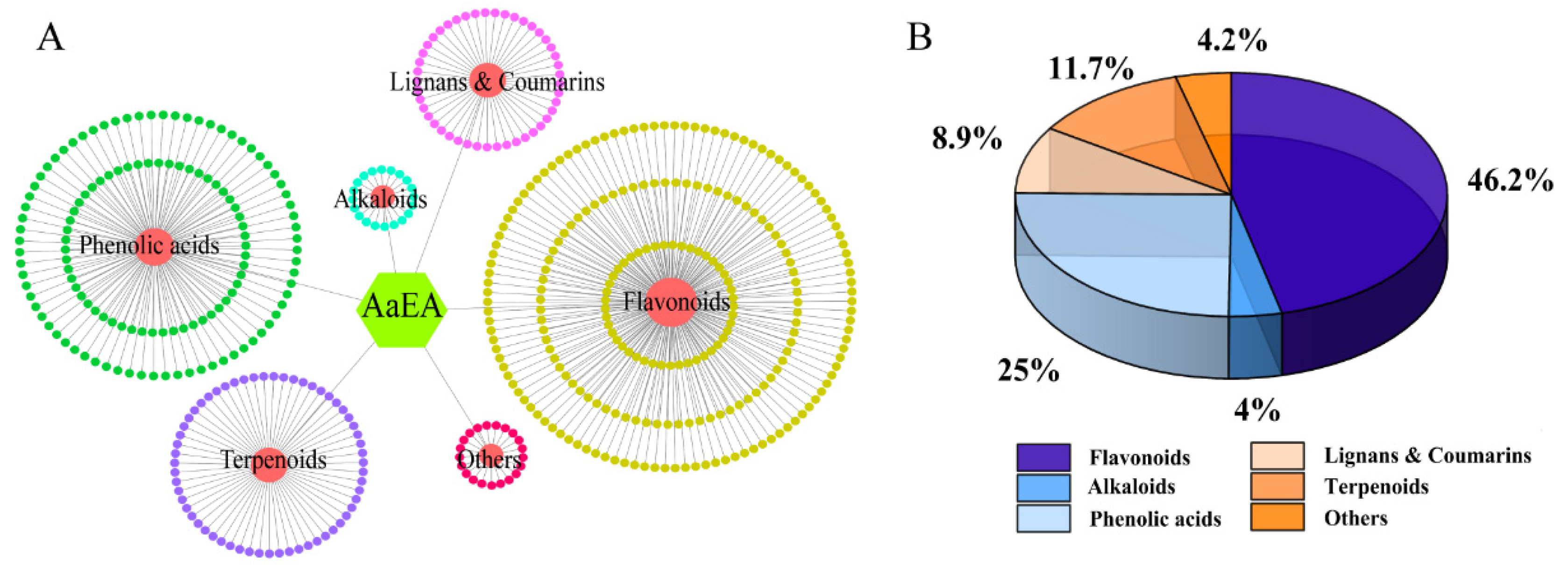
2.1. LC-MS Analysis of AaEA
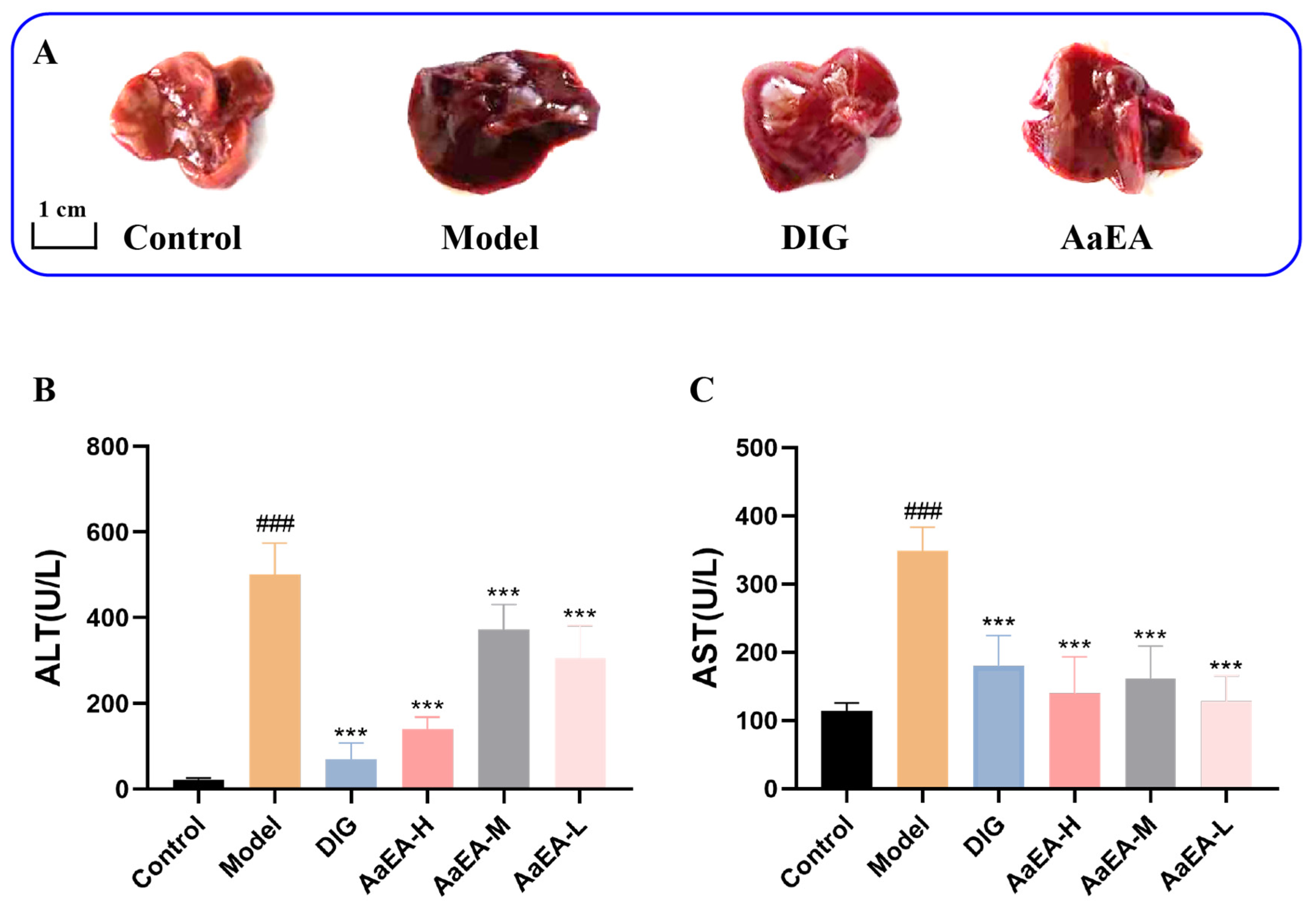
2.2. Effect of AaEA on ConA-Induced Liver Damage and Serum Transaminase Levels
2.3. Effects of AaEA on Liver Oxidative Stress
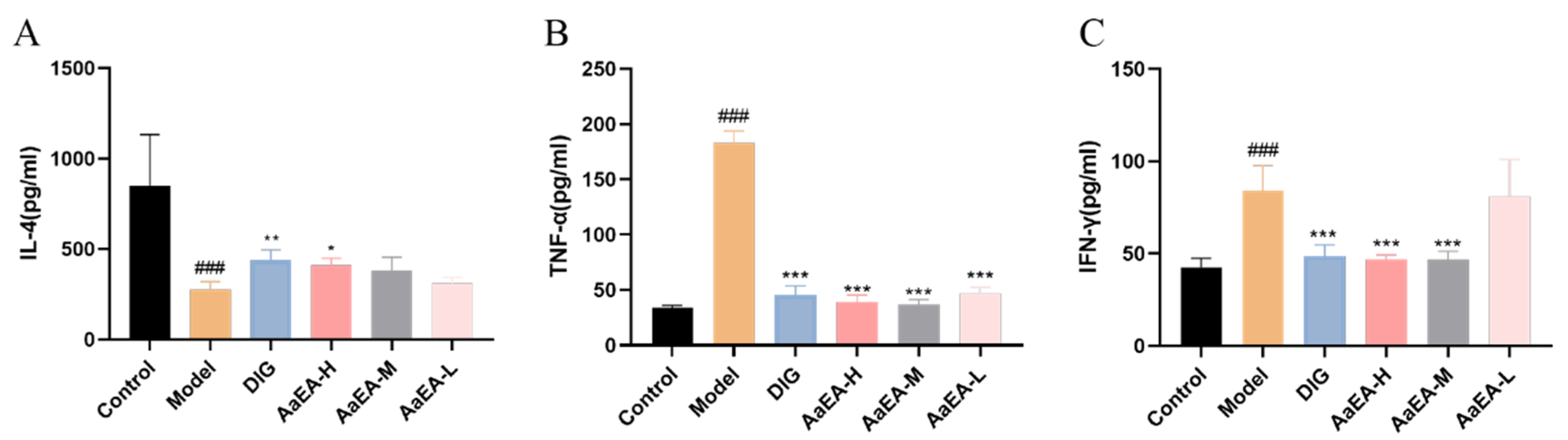
2.4. Effects of AaEA on IL-4, TNF-α, and IFN-γ
2.5. Histopathological Features of Mice Livers
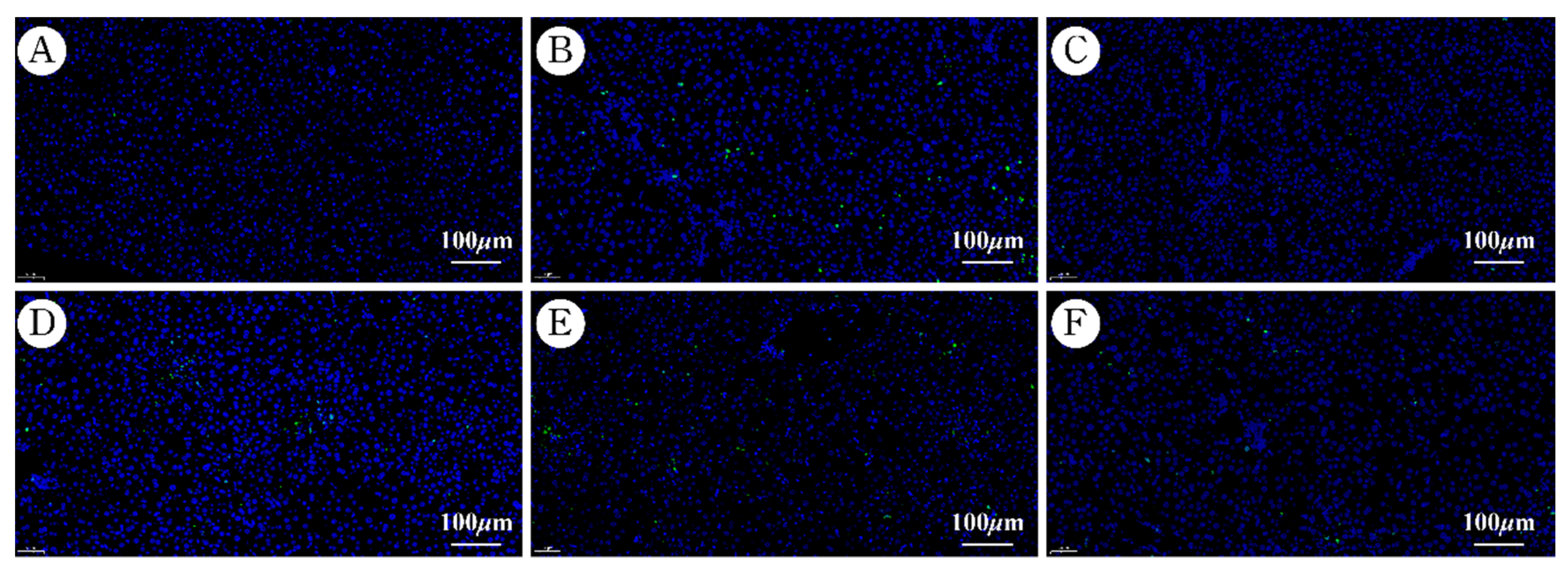
2.6. Effects of AaEA on Liver Cell Apoptosis
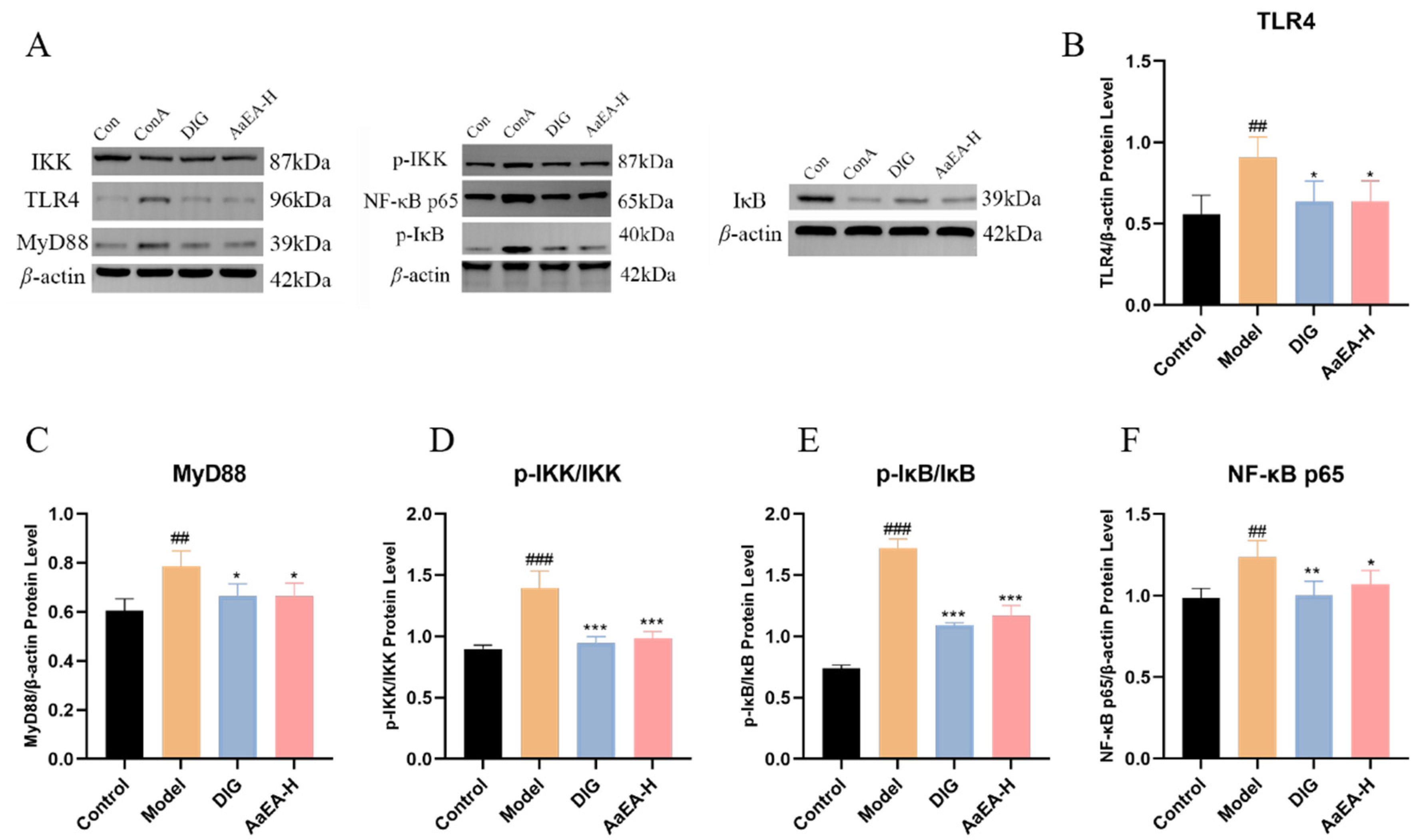
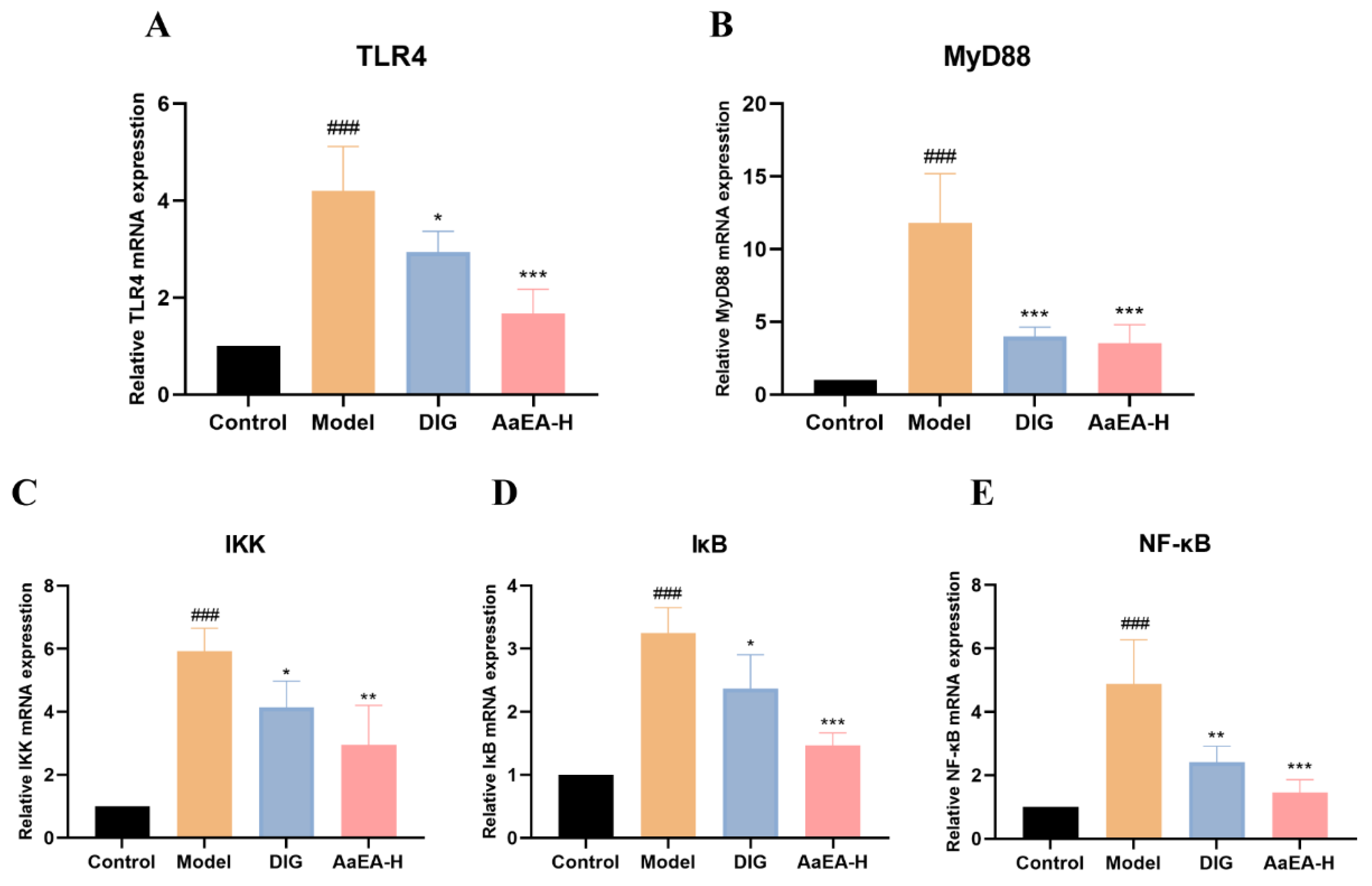
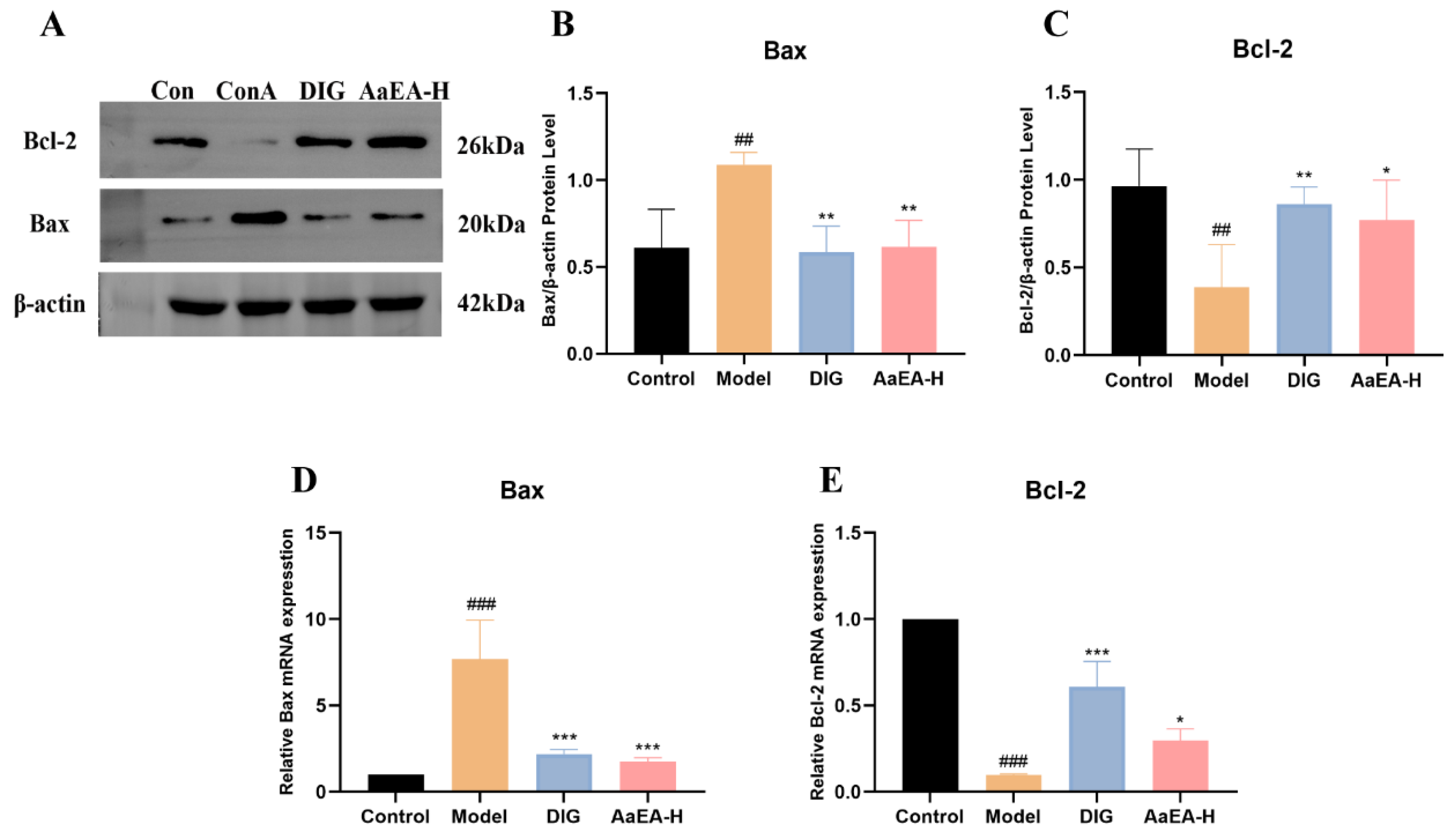
2.7. AaEA Inhibited the Bax/Bcl-2 and TLR4/MyD88/NF-κB Signaling Pathways
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation
3.3. LC-MS Analysis
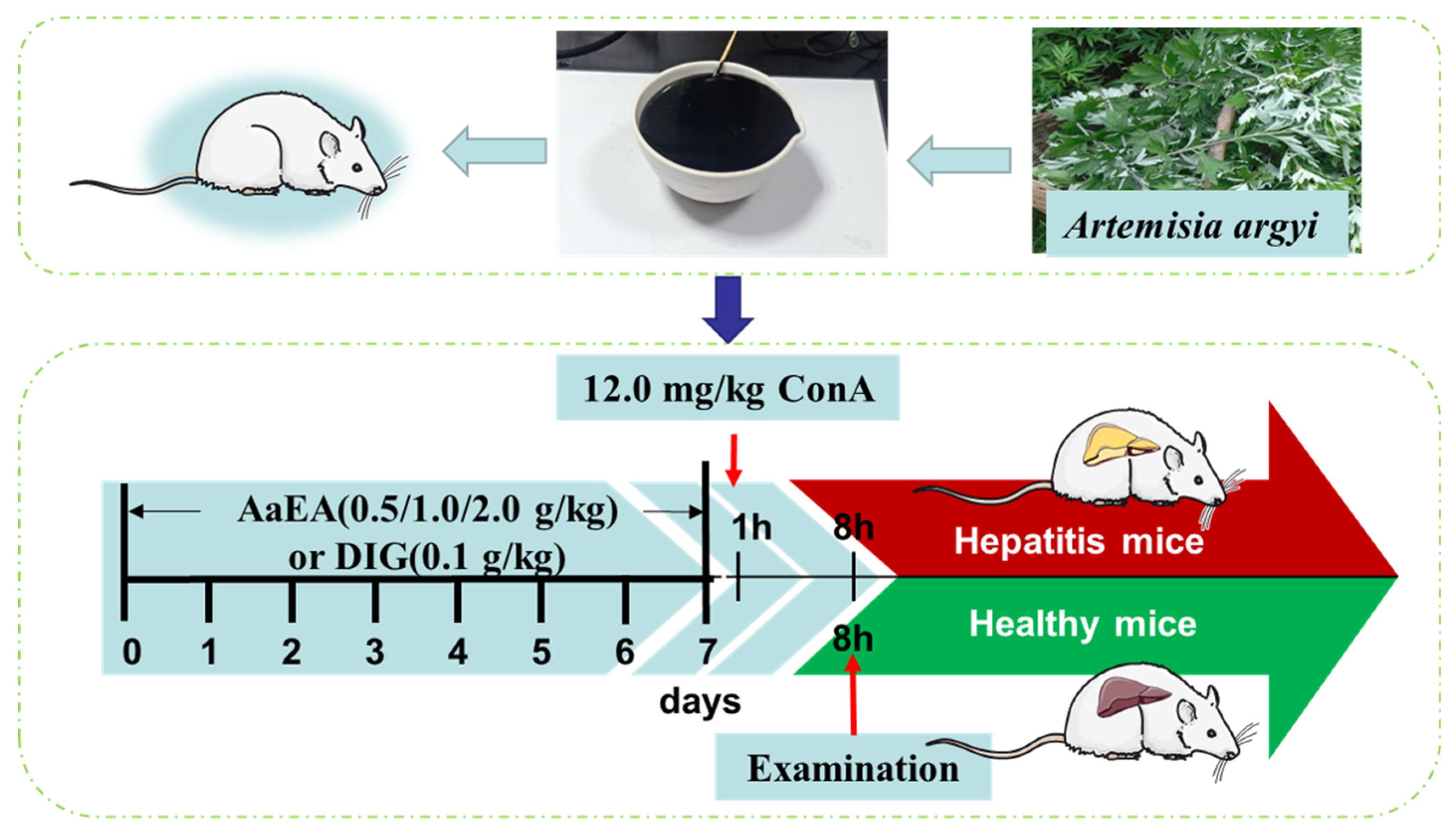
3.4. Animals and Experimental Design
3.5. Serum Biomarker Assays
3.6. Oxidative Stress-Related Factors
3.7. Inflammatory Factors
3.8. Histopathological Analysis
3.9. TdT-Mediated dUTP Nick-End Labeling (TUNEL) Staining
3.10. Real-Time PCR Assay
3.11. Western Blotting Analysis
3.12. Statistical Analysis
4. Conclusions and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhang, Y.; Xiao, F.; Zhou, Q.; Diao, T.; Zhang, M.; Liu, D.; Wang, Z.; Huang, T.; Wu, Y.; Bai, Y.; et al. The Potential Protective Effect of Iridoid Glycosides Isolated from Osmanthus fragrans Seeds Against the Development of Immune Liver Injury in Mice. Front. Pharmacol. 2021, 12, 760338. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, H.; Gores, G.J. Mechanisms of Liver Injury: An Overview. Curr. Mol. Med. 2003, 3, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lamba, M.; Ngu, J.H.; Stedman, C.A. Trends in Incidence of Autoimmune Liver Diseases and Increasing Incidence of Autoimmune Hepatitis. Clin. Gastroenterol. Hepatol. 2020, 19, 573–579.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, X. Food therapy and medical diet therapy of Traditional Chinese Medicine. Clin. Nutr. Exp. 2018, 18, 1–5. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Ma, L.; Cao, X.; Xiao, J.; Chen, J.; Jiao, S.; Gao, Y.; Liu, C.; Duan, Z.; et al. Activation of Vascular Endothelial Growth Factor Receptor-3 in Macrophages Restrains TLR4-NF-κB Signaling and Protects against Endotoxin Shock. Immunity 2014, 40, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mohan, C. Toll-Like Receptor Signaling Pathways—Therapeutic Opportunities. Mediat. Inflamm. 2010, 2010, 781235. [Google Scholar] [CrossRef]
- Lai, J.-L.; Liu, Y.-H.; Liu, C.; Qi, M.P.; Liu, R.-N.; Zhu, X.-F.; Zhou, Q.-G.; Chen, Y.-Y.; Guo, A.-Z.; Hu, C.-M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2016, 40, 1–12. [Google Scholar] [CrossRef]
- Rashidian, A.; Muhammadnejad, A.; Dehpour, A.-R.; Mehr, S.E.; Akhavan, M.M.; Shirkoohi, R.; Chamanara, M.; Mousavi, S.-E.; Rezayat, S.-M. Atorvastatin attenuates TNBS-induced rat colitis: The involvement of the TLR4/NF-kB signaling pathway. Inflammopharmacology 2016, 24, 109–118. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, P.; Chen, Z.-L.; Zhang, S.-J.; Wang, Y.-Q.; Cai, X.; Luo, L.; Zhou, X.; Zhao, L. Emodin Attenuates Lipopolysaccharide-Induced Acute Liver Injury via Inhibiting the TLR4 Signaling Pathway in vitro and in vivo. Front. Pharmacol. 2018, 9, 962. [Google Scholar] [CrossRef]
- Yang, K.; Zhan, L.; Lu, T.; Zhou, C.; Chen, X.; Dong, Y.; Lv, G.; Chen, S. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-κB signaling pathway. Cytokine 2020, 130, 155058. [Google Scholar] [CrossRef]
- Song, X.; Wen, X.; He, J.; Zhao, H.; Li, S.; Wang, M. Phytochemical components and biological activities of Artemisia argyi. J. Funct. Foods 2018, 52, 648–662. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Wang, F.; Xu, R.; Yang, M.; Ci, Z.; Wu, Z.; Zhang, D.; Lin, J. From longevity grass to contemporary soft gold: Explore the chemical constituents, pharmacology, and toxicology of Artemisia argyi H.Lév. & vaniot essential oil. J. Ethnopharmacol. 2021, 279, 114404. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, M.; Wang, T.; Liu, T.; Guo, Y.; Granato, D. Green tea polyphenols mitigate the plant lectins-induced liver inflammation and immunological reaction in C57BL/6 mice via NLRP3 and Nrf2 signaling pathways. Food Chem. Toxicol. 2020, 144, 111576. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.F.; Hu, H.; Xing, C.Z.; Li, H.C.; Zhang, C.Z. Effect of Artemisia argyi extract solution on expression of Cyclin D1 in rat with liver fibrosis. Chin. J. Integr. Tradit. West. Med. Dig. 2011, 19, 4–6. [Google Scholar]
- Yang, C.L.; Wang, H.L.; Yu, X.; Li, Z.J. Study on the mechanism of Artemisia argyi polysaccharide preventing acetaminophen liver poisoning. Lishizhen Med. Mater. Med. Res. 2012, 23, 10. [Google Scholar]
- Wang, J.; Chu, H.; Li, H.; Yang, W.; Zhao, Y.; Shen, T.; Cary, J.; Zhang, L. A Network Pharmacology Approach to Investigate the Mechanism of Erjing Prescription in Type 2 Diabetes. Evidence-Based Complement. Altern. Med. 2021, 2021, 9933236. [Google Scholar] [CrossRef]
- Chu, H.; Wang, J.; Wang, Q.; Chen, J.; Li, J.; Li, H.; Zhang, L. Protective Effect of n -Butanol Extract from Viola yedoensis on Immunological Liver Injury. Chem. Biodivers. 2021, 18, e2001043. [Google Scholar] [CrossRef]
- Ye, S.; Feng, L.; Zhang, S.; Lu, Y.; Xiang, G.; Nian, B.; Wang, Q.; Zhang, S.; Song, W.; Yang, L.; et al. Integrated Metabolomic and Transcriptomic Analysis and Identification of Dammarenediol-II Synthase Involved in Saponin Biosynthesis in Gynostemma longipes. Front. Plant Sci. 2022, 13, 852377. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Liu, X.; Gao, S.; Sun, X. Glycyrrhizic acid ammonium salt alleviates Concanavalin A-induced immunological liver injury in mice through the regulation of the balance of immune cells and the inhibition of hepatocyte apoptosis. Biomed. Pharmacother. 2019, 120, 109481. [Google Scholar] [CrossRef]
- Shan, Y.; Jiang, B.; Yu, J.; Wang, J.; Wang, X.; Li, H.; Wang, C.; Chen, J.; Sun, J. Protective Effect of Schisandra chinensis Polysaccharides Against the Immunological Liver Injury in Mice Based on Nrf2/ARE and TLR4/NF-κB Signaling Pathway. J. Med. Food 2019, 22, 885–895. [Google Scholar] [CrossRef]
- Pan, L.; Liu, C.; Liu, Q.; Li, Y.; Du, C.; Kang, X.; Dong, S.; Zhou, Z.; Chen, H.; Liang, X.; et al. Human Wharton’s jelly-derived mesenchymal stem cells alleviate concanavalin A-induced fulminant hepatitis by repressing NF-κB signaling and glycolysis. Stem Cell Res. Ther. 2021, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Desta, Y.T.; Wu, M.; Bai, L.; Wu, X.; Xiong, M.; Weng, X. Mitochondrial-targeted ubiquinone alleviates concanavalin A-induced hepatitis via immune modulation. Int. Immunopharmacol. 2020, 84, 106518. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Li, P.-Y.; Wang, J.-B.; Zhou, H.-Q.; Yang, Z.-R.; Yang, R.-C.; Bai, Z.-F.; Wang, L.-F.; Li, J.-Y.; Liu, H.-H.; et al. Inhibition of Sophocarpine on Poly I: C/D-GalN-Induced Immunological Liver Injury in Mice. Front. Pharmacol. 2016, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Baeck, C.; Tacke, F. Balance of inflammatory pathways and interplay of immune cells in the liver during homeostasis and injury. EXCLI J. 2014, 13, 67–81. [Google Scholar] [CrossRef]
- Hou, X.; Zhou, R.; Wei, H.; Sun, R.; Tian, Z. NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis. Hepatology 2008, 49, 940–949. [Google Scholar] [CrossRef]
- Xing, J.-J.; Hou, J.-G.; Liu, Y.; Zhang, R.-B.; Jiang, S.; Ren, S.; Wang, Y.-P.; Shen, Q.; Li, W.; Li, X.-D.; et al. Supplementation of Saponins from Leaves of Panax quinquefolius Mitigates Cisplatin-Evoked Cardiotoxicity via Inhibiting Oxidative Stress-Associated Inflammation and Apoptosis in Mice. Antioxidants 2019, 8, 347. [Google Scholar] [CrossRef]
- Han, B.; Xin, Z.; Ma, S.; Liu, W.; Zhang, B.; Ran, L.; Yi, L.; Ren, D. Comprehensive characterization and identification of antioxidants in Folium Artemisiae Argyi using high-resolution tandem mass spectrometry. J. Chromatogr. B 2017, 1063, 84–92. [Google Scholar] [CrossRef]
- Lan, X.Y.; Zhang, Y.; Zhu, L.B.; Liu, D.H.; Huang, X.Z.; Zhou, L.; Kang, L.P. Research progress on chemical constituents from Artemisiae Argyi Folium and their pharmacological activities and quality control. China J. Chin. Mater. Med. 2020, 45, 4017–4030. [Google Scholar] [CrossRef]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Morath, A.; Schamel, W.W.; Steinberger, P.; Leitner, J.; Huber, R.; Hamburger, M.; Gründemann, C. Immunosuppressive Activity of Artemisia argyi Extract and Isolated Compounds. Front. Pharmacol. 2020, 11, 402. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Z.; Guo, Y.; Lu, S.; Du, H.; Cao, Y. Antioxidant capacity of flavonoids from Folium Artemisiae Argyi and the molecular mechanism in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 279, 114398. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; Jiang, Z.; Zafar, S.; Xie, Q.; Yang, Y.; Liu, Y.; Yuan, H.; Jian, Y.; Wang, W. Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus. Molecules 2021, 26, 3038. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Bueno, B.G.; Caso, J.; Madrigal, J.; Leza, J. Innate immune receptor Toll-like receptor 4 signalling in neuropsychiatric diseases. Neurosci. Biobehav. Rev. 2016, 64, 134–147. [Google Scholar] [CrossRef]
- Sang, R.; Yu, Y.; Ge, B.; Xu, L.; Wang, Z.; Zhang, X. Taraxasterol from Taraxacum prevents concanavalin A-induced acute hepatic injury in mice via modulating TLRs/NF-κB and Bax/Bc1-2 signalling pathways. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3929–3937. [Google Scholar] [CrossRef]
- Wang, Y.L. Toxicological Safety of Artemisia Argyi Extract and Its Effect on Immune and Antioxidant Functions in Mice. Master’s Thesis, Inner Mongolia Agricultural University, Inner Mongolia Autonomous Region, China, 2018. (In Chinese). [Google Scholar]
- Wang, X.-Z.; Xue, R.; Zhang, S.-Y.; Zheng, Y.-T.; Zhang, L.-Y.; Jiang, Z.-Z. Activation of natural killer T cells contributes to triptolide-induced liver injury in mice. Acta Pharmacol. Sin. 2018, 39, 1847–1854. [Google Scholar] [CrossRef]
- Bonder, C.S.; Ajuebor, M.N.; Zbytnuik, L.D.; Kubes, P.; Swain, M.G. Essential Role for Neutrophil Recruitment to the Liver in Concanavalin A-Induced Hepatitis. J. Immunol. 2003, 172, 45–53. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Shao, F.; Wang, J.; Shen, T.; Zhao, Y.; Fu, X.; Zhang, L.; Li, H. Ethyl Acetate Extract from Artemisia argyi Prevents Liver Damage in ConA-Induced Immunological Liver Injury Mice via Bax/Bcl-2 and TLR4/MyD88/NF-κB Signaling Pathways. Molecules 2022, 27, 7883. https://doi.org/10.3390/molecules27227883
Yang W, Shao F, Wang J, Shen T, Zhao Y, Fu X, Zhang L, Li H. Ethyl Acetate Extract from Artemisia argyi Prevents Liver Damage in ConA-Induced Immunological Liver Injury Mice via Bax/Bcl-2 and TLR4/MyD88/NF-κB Signaling Pathways. Molecules. 2022; 27(22):7883. https://doi.org/10.3390/molecules27227883
Chicago/Turabian StyleYang, Wenqian, Fei Shao, Jiexin Wang, Tong Shen, Yu Zhao, Xueyan Fu, Liming Zhang, and Hangying Li. 2022. "Ethyl Acetate Extract from Artemisia argyi Prevents Liver Damage in ConA-Induced Immunological Liver Injury Mice via Bax/Bcl-2 and TLR4/MyD88/NF-κB Signaling Pathways" Molecules 27, no. 22: 7883. https://doi.org/10.3390/molecules27227883
APA StyleYang, W., Shao, F., Wang, J., Shen, T., Zhao, Y., Fu, X., Zhang, L., & Li, H. (2022). Ethyl Acetate Extract from Artemisia argyi Prevents Liver Damage in ConA-Induced Immunological Liver Injury Mice via Bax/Bcl-2 and TLR4/MyD88/NF-κB Signaling Pathways. Molecules, 27(22), 7883. https://doi.org/10.3390/molecules27227883