Octahedral Rhenium Cluster Complexes with 1,2-Bis(4-pyridyl)ethylene and 1,3-Bis(4-pyridyl)propane as Apical Ligands
Abstract
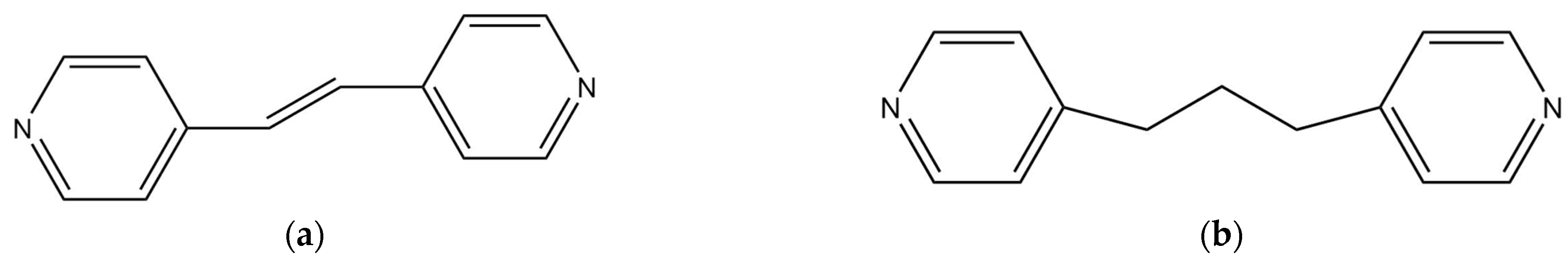
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Preparation of Trans-[Re6S8(bpe)4Cl2] (1)
2.3. Preparation of Trans-[Re6S8(bpe)4Br2] (2)
2.4. Preparation of Trans-[Re6Se8(bpe)4Cl2] (3)
2.5. Preparation of Trans-[Re6Se8(bpe)4Br2] (4)
2.6. Preparation of Trans-[Re6S8(bpp)4Cl2] (5)
2.7. Preparation of Trans-[Re6S8(bpp)4Br2] (6)
2.8. Preparation of Trans-[Re6Se8(bpp)4Cl2] (7)
2.9. Preparation of Trans-[Re6Se8(bpp)4Br2] (8)
2.10. The X-ray Diffraction (XRD) Analysis
2.11. Calculation Details
3. Results and Discussion
3.1. Synthesis
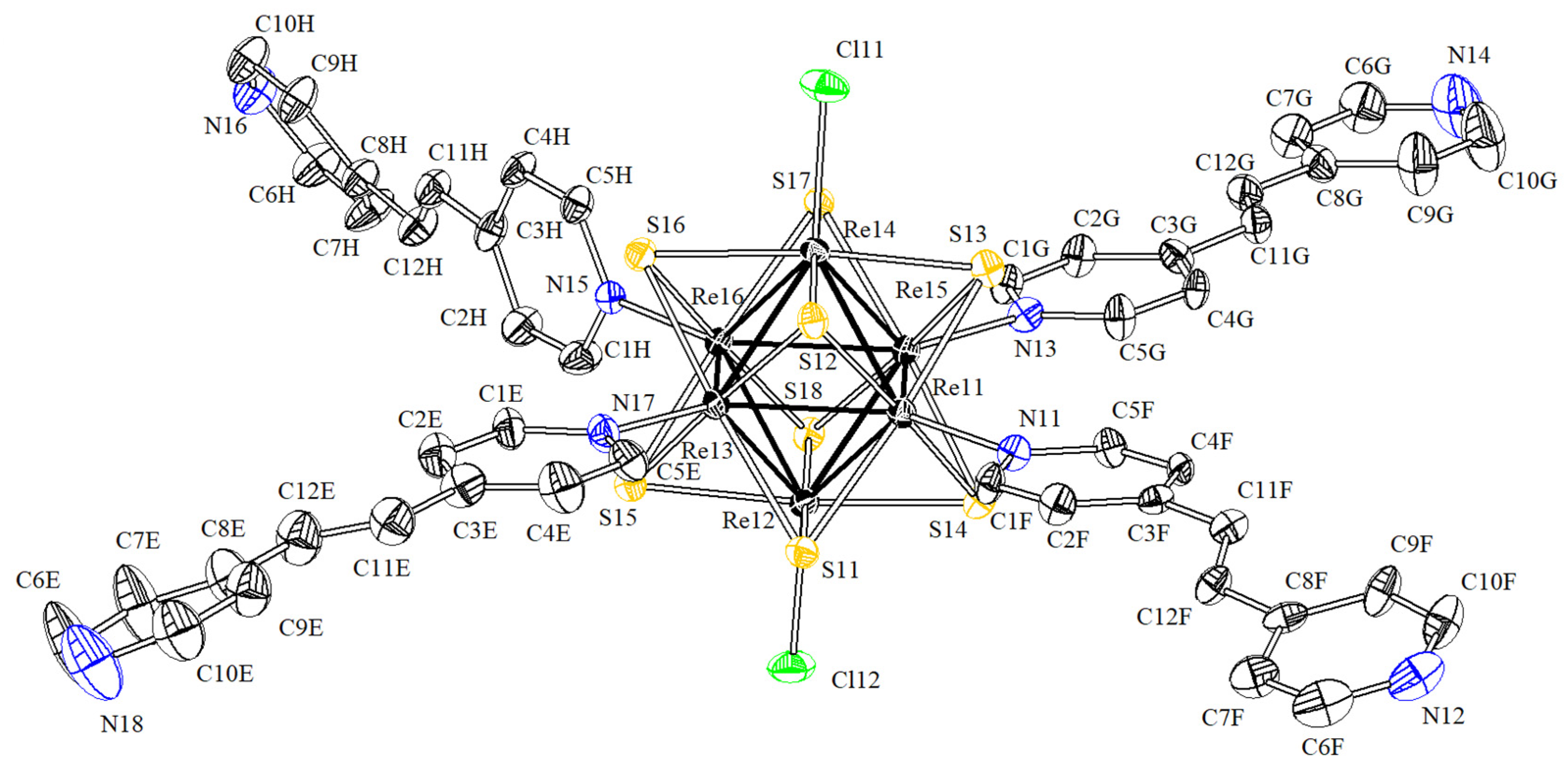
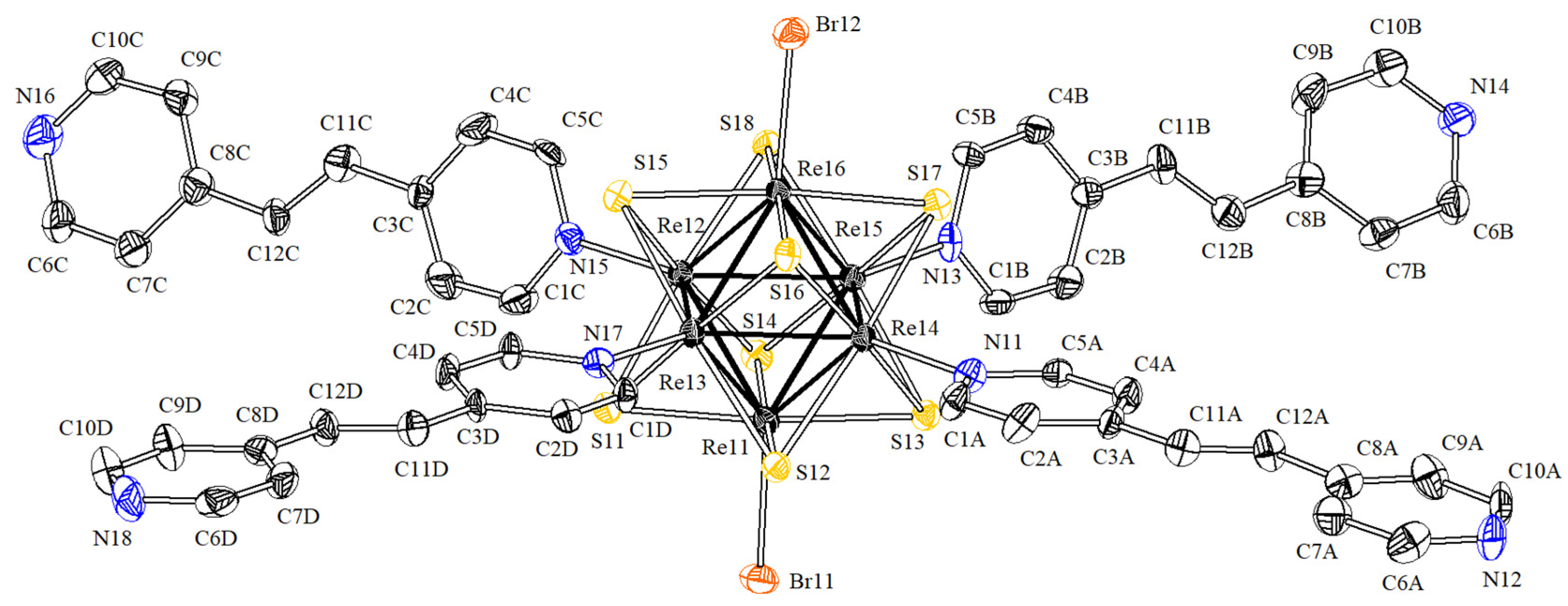
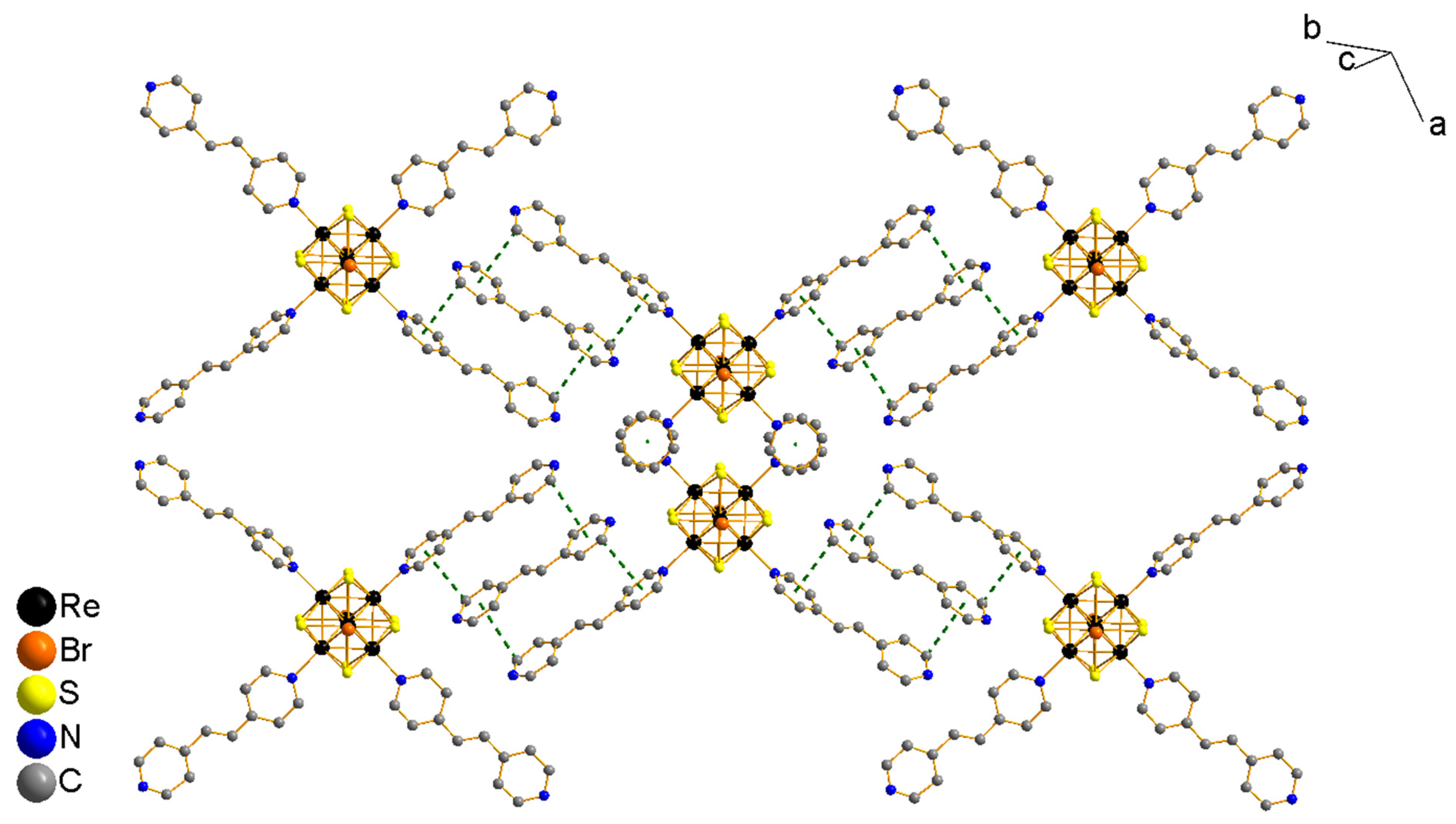
3.2. Crystal Structures
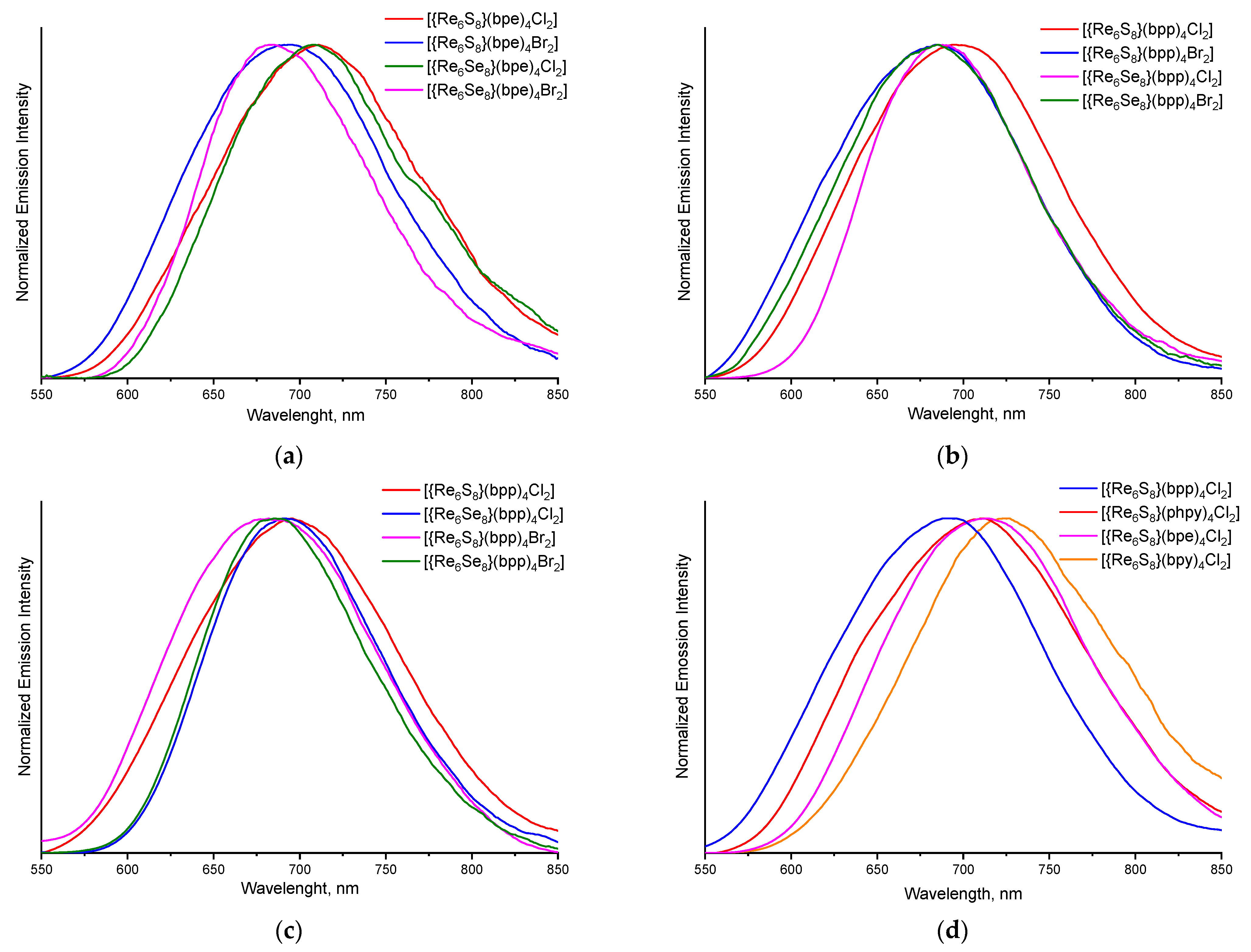
3.3. Luminescent Properties
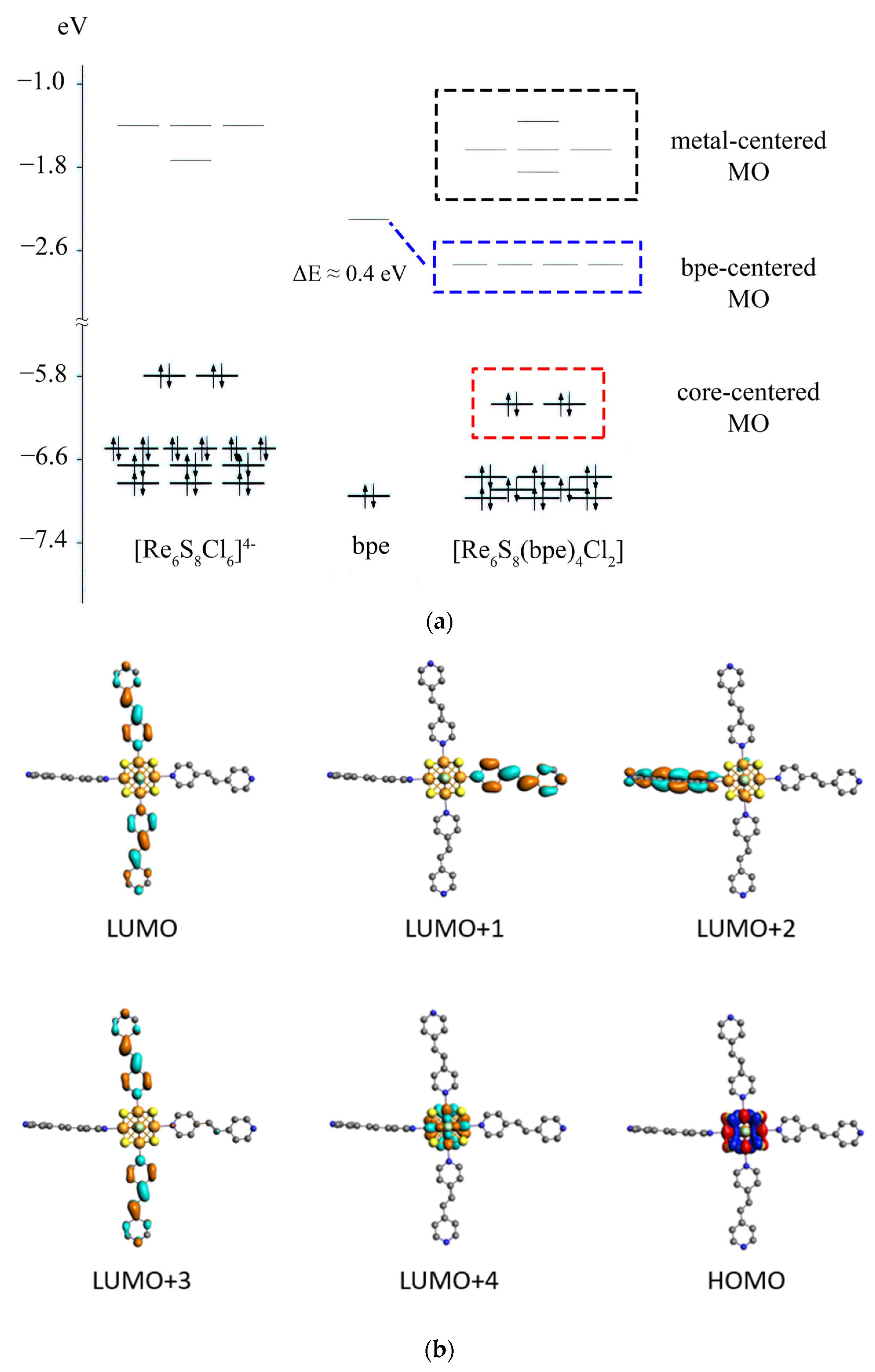
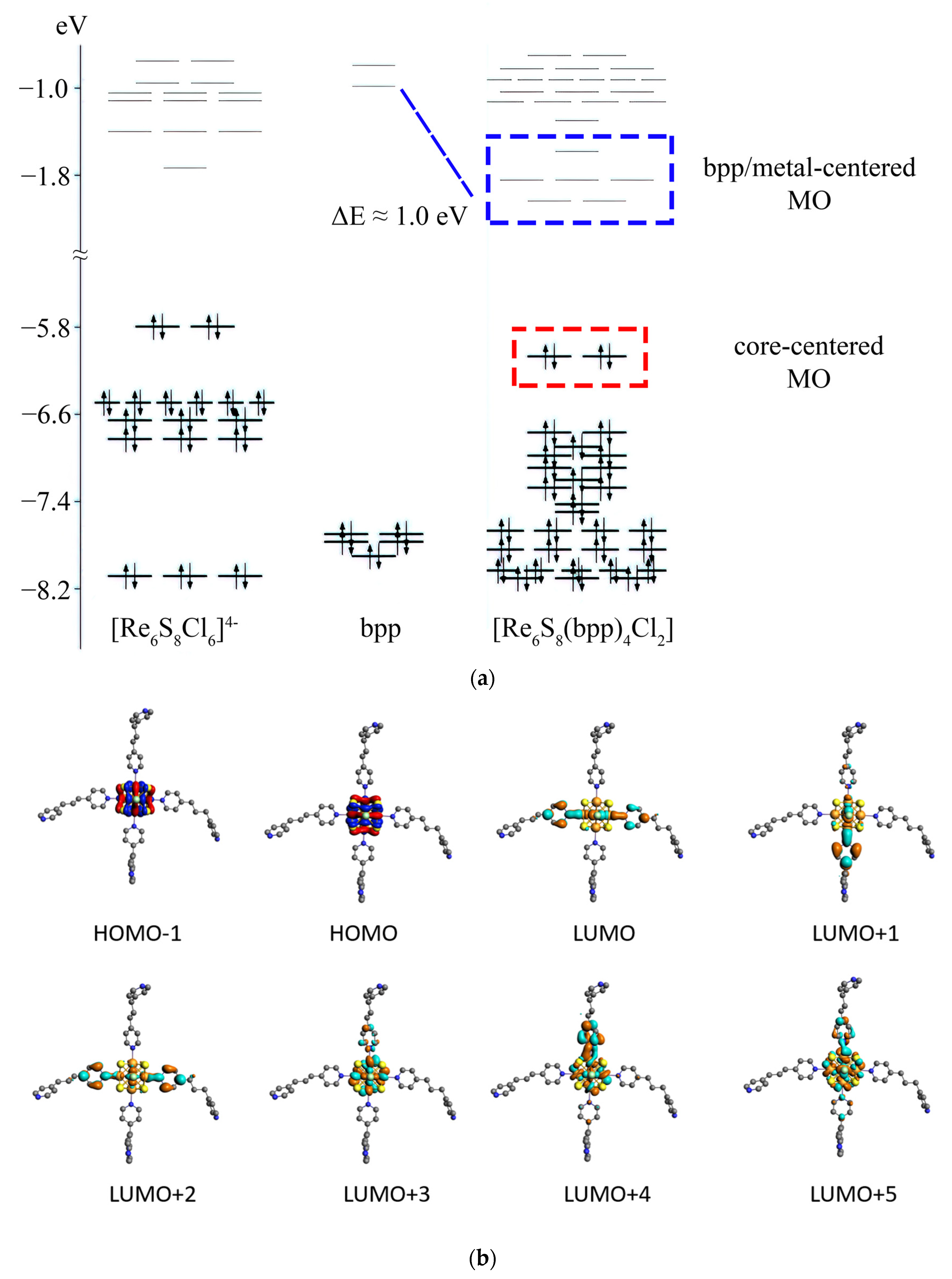
3.4. DFT Calculations
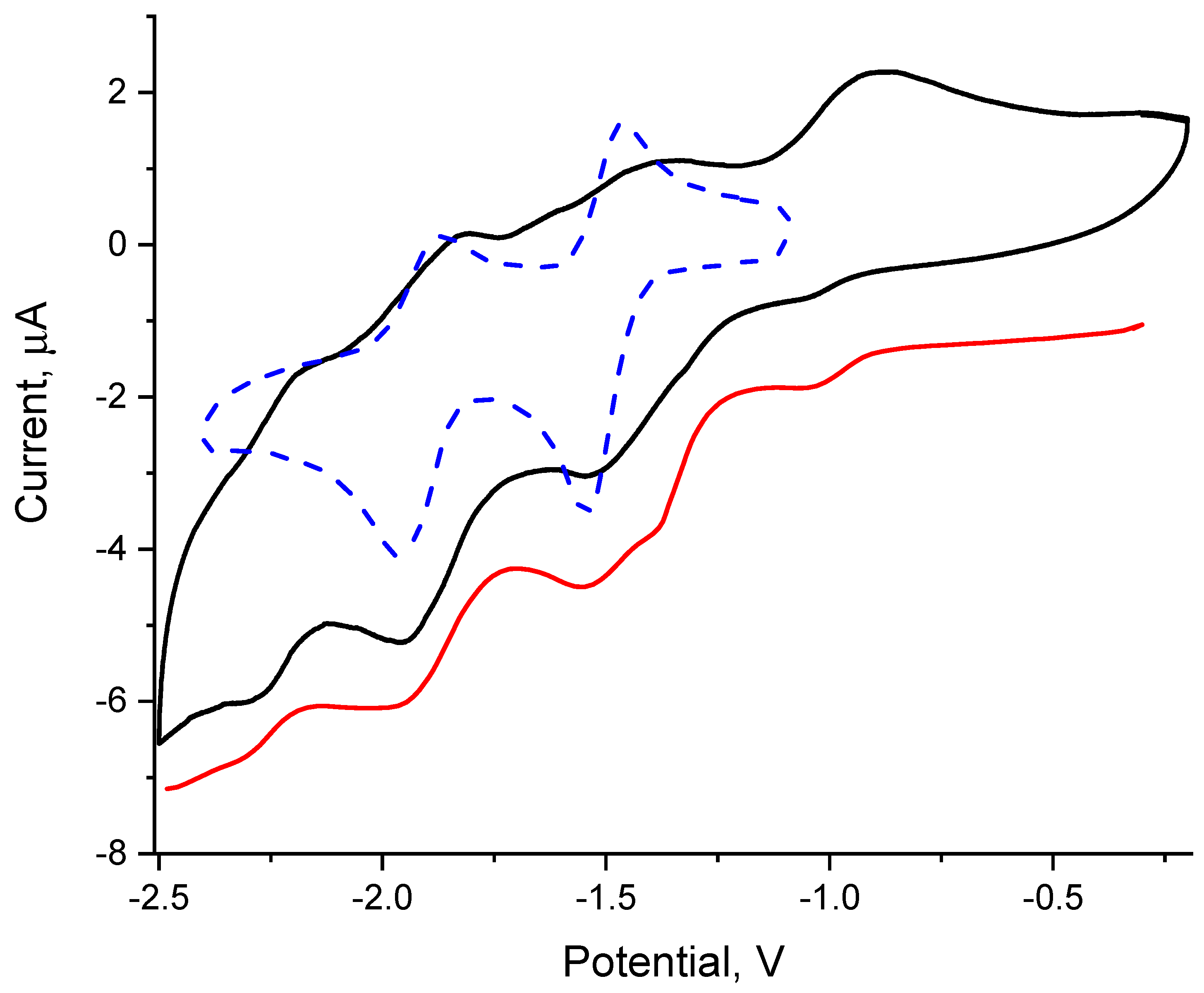
3.5. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gabriel, J.-C.P.; Boubekeur, K.; Uriel, S.; Batail, P. Chemistry o f Hexanuclear Rhenium Chalcohalide Clusters. Chem. Rev. 2001, 101, 2037–2066. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Naumov, N.G.; Samoylov, P.P.; Fedin, V.P. Clusters and Cluster Assemblies. In Comprehensive Inorganic Chemistry II; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Oxford, UK, 2013; Volume 2, pp. 271–310. [Google Scholar] [CrossRef]
- Naumov, N.G.; Brylev, K.A.; Mironov, Y.V.; Cordier, S.; Fedorov, V.E. Octahedral clusters with mixed inner ligand environment: Self-assembly, modification and isomerism. J. Struct. Chem. 2014, 55, 1371–1389. [Google Scholar] [CrossRef]
- Selby, H.D.; Zheng, Z. New Directions of Cluster Chemistry—The Story of the [Re6(µ3-Se)8]2+ Clusters. Comments Inorg. Chem. 2005, 26, 75–102. [Google Scholar] [CrossRef]
- Doud, E.A.; Voevodin, A.; Hochuli, T.J.; Champsaur, A.M.; Nuckolls, C.; Roy, X. Superatoms in Materials Science. Nat. Rev. Mater. 2020, 5, 371–387. [Google Scholar] [CrossRef]
- Reed, D.A.; Hochuli, T.J.; Gadjieva, N.A.; He, S.; Wiscons, R.A.; Bartholomew, A.K.; Champsaur, A.M.; Steigerwald, M.L.; Roy, X.; Nuckolls, C. Controlling Ligand Coordination Spheres and Cluster Fusion in Superatoms. J. Am. Chem. Soc. 2022, 144, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.E.; Naumov, N.G. Octahedral Chalcogenide Rhenium Clusters: From Solids to Isolated Cluster Complexes. In Ligated Transition Metal Clusters in Solid-State Chemistry. Structure and Bonding; Halet, J.F., Ed.; Springer: Cham, Switzerland, 2019; Volume 180, pp. 31–74. [Google Scholar] [CrossRef]
- Konovalov, D.I.; Ivanov, A.A.; Vorotnikov, Y.A.; Kuratieva, N.V.; Eltsov, I.V.; Kovalenko, K.A.; Shestopalov, M.A. Self-Assembled Microporous M-HOFs Based on an Octahedral Rhenium Cluster with Benzimidazole. Inorg. Chem. 2021, 60, 14687–14696. [Google Scholar] [CrossRef]
- Szczepura, L.F.; Soto, E. Exploring the Breadth of Terminal Ligands Coordinated in [Mo6X8]4+- and [Re6Q8]2+-Based Cluster Complexes. In Ligated Transition Metal Clusters in Solid-State Chemistry. Structure and Bonding; Halet, J.F., Ed.; Springer: Cham, Switzerland, 2019; Volume 180, pp. 75–108. [Google Scholar] [CrossRef]
- Cordier, S.; Molard, Y.; Brylev, K.A.; Mironov, Y.V.; Grasset, F.; Fabre, B.; Naumov, N.G. Advances in the Engineering of Near Infrared Emitting Liquid Crystals and Copolymers, Extended Porous Frameworks, Theranostic Tools and Molecular Junctions Using Tailored Re6 Cluster Building Blocks. J. Cluster Sci. 2015, 26, 53–81. [Google Scholar] [CrossRef]
- Yoshimura, T.; Umakoshi, K.; Sasaki, Y.; Ishizaka, S.; Kim, H.B.; Kitamura, N. Emission and Metal- and Ligand-Centered-Redox Characteristics of the Hexarhenium(III) Clusters Trans- and Cis-[Re6(µ3-S)8Cl4(L)2]2–, Where L Is a Pyridine Derivative or Pyrazine. Inorg. Chem. 2000, 39, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T. Synthesis, Structures, and Redox Properties of Octa(µ3-Sulfido)Hexarhenium(III) Complexes Having Terminal Pyridine Ligands. Inorg. Chem. 1999, 38, 5557–5564. [Google Scholar] [CrossRef]
- Yoshimura, T.; Suo, C.; Tsuge, K.; Ishizaka, S.; Nozaki, K.; Sasaki, Y.; Kitamura, N.; Shinohara, A. Excited-State Properties of Octahedral Hexarhenium(Lll) Complexes with Redox-Active N-Heteroaromatic Ligands. Inorg. Chem. 2010, 49, 531–540. [Google Scholar] [CrossRef]
- Ulantikov, A.A.; Gayfulin, Y.M.; Ivanov, A.A.; Sukhikh, T.S.; Ryzhikov, M.R.; Brylev, K.A.; Smolentsev, A.I.; Shestopalov, M.A.; Mironov, Y.V. Soluble Molecular Rhenium Cluster Complexes Exhibiting Multistage Terminal Ligands Reduction. Inorg. Chem. 2020, 59, 6460–6470. [Google Scholar] [CrossRef]
- Yarovoi, S.S.; Mironov, Y.V.; Naumov, D.Y.; Gatilov, Y.V.; Kozlova, S.G.; Kim, S.J.; Fedorov, V.E. Octahedral Hexahydroxo Rhenium Cluster Complexes [Re6Q8(OH)6]4–.(Q=S, Se): Synthesis, Structure, and Properties. Eur. J. Inorg. Chem. 2005, 8, 3945–3949. [Google Scholar] [CrossRef]
- Bruker, A.X.S. APEX2 (Version 2.0), SAINT (Version 8.18 c), SADABS (Version 2.11); Bruker Advanced X-ray Solutions; Bruker: Madison, WA, USA, 2000. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Fonseca Guerra, C.; Snijders, J.G.; Te Velde, G.; Baerends, E.J. Towards an Order-N DFT Method. Theor. Chem. Acc. 1998, 99, 391–403. [Google Scholar] [CrossRef]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Erratum: Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy (Physical Review B (1992) 45 (13244). https://doi.org/10.1103/PhysRevB.45.13244). Phys. Rev. B 2018, 98, 244–249. [Google Scholar] [CrossRef]
- Swart, M. A New Family of Hybrid Density Functionals. Chem. Phys. Lett. 2013, 580, 166–171. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-Type Basis Sets for the Elements 1-118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.C.; Ziegler, T. An Implementation of the Conductor-like Screening Model of Solvation within the Amsterdam Density Functional Package. Theor. Chem. Acc. 1999, 101, 396–408. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. WIREs Comput. Mol. Sci. 2011, 1, 699–709. [Google Scholar] [CrossRef]
- Van Lenthe, E. Geometry Optimizations in the Zero Order Regular Approximation for Relativistic Effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Ulantikov, A.A.; Gayfulin, Y.M.; Sukhikh, T.S.; Ryadun, A.A.; Ryzhikov, M.R.; Mironov, Y.V. Synthesis, structure, and physicochemical properties of molecular rhenium cluster complexes with 4-phenylpyridine molecules as terminal ligands. J. Struct. Chem. 2021, 62, 1009–1019. [Google Scholar] [CrossRef]
- Shestopalov, M.A.; Cordier, S.; Hernandez, O.; Molard, Y.; Perrin, C.; Perrin, A.; Fedorov, V.E.; Mironov, Y.V. Self-Assembly of Ambivalent Organic/Inorganic Building Blocks Containing Re6 Metal Atom Cluster: Formation of a Luminescent Honeycomb, Hollow, Tubular Metal-Organic Framework. Inorg. Chem. 2009, 48, 1482–1489. [Google Scholar] [CrossRef]
- El Osta, R.; Demont, A.; Audebrand, N.; Molard, Y.; Nguyen, T.T.; Gautier, R.; Brylev, K.A.; Mironov, Y.V.; Naumov, N.G.; Kitamura, N.; et al. Supramolecular Frameworks Built up from Red-Phosphorescent Trans-Re6 Cluster Building Blocks: One Pot Synthesis, Crystal Structures, and DFT Investigations. Zeitschrift Anorg. Allg. Chem. 2015, 641, 1156–1163. [Google Scholar] [CrossRef]
- Konovalov, D.I.; Ivanov, A.A.; Frolova, T.S.; Eltsov, I.V.; Gayfulin, Y.M.; Plunkett, L.; Bazzar, M.; Adawi, A.M.; Bouillard, J.S.G.; Baiborodin, S.I.; et al. Water-Soluble Rhenium Clusters with Triazoles: The Effect of Chemical Structure on Cellular Internalization and the DNA Binding of the Complexes. Chem.-A Eur. J. 2020, 26, 13904–13914. [Google Scholar] [CrossRef]
- Ulantikov, A.A.; Sukhikh, T.S.; Gribov, E.N.; Maltseva, N.V.; Brylev, K.A.; Mironov, Y.V.; Gayfulin, Y.M. Thermally Controlled Synthesis of Octahedral Rhenium Clusters with 4,4′-Bipyridine and CN−Apical Ligands. Symmetry 2021, 13, 2187. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuda, A.; Ito, Y.; Ishizaka, S.; Shinoda, S.; Tsukube, H.; Kitamura, N.; Shinohara, A. Photoluminescent Properties of Chalcobromide-Capped Octahedral Hexarhenium(III) Complexes [{Re6Q8-nBrn}Br6]n-4 (Q = Se, n=1-3; Q = S, n = 1, 2). Inorg. Chem. 2010, 49, 3473–3481. [Google Scholar] [CrossRef]
- Yoshimura, T.; Nishizawa, H.; Nagata, K.; Ito, A.; Sakuda, E.; Ishizaka, S.; Kitamura, N.; Shinohara, A. Tuning the Ground- and Excited-State Redox Potentials of Octahedral Hexanuclear Rhenium(III) Complexes by the Combination of Terminal Halide and N-Heteroaromatic Ligands. ACS Omega 2022, 7, 26965–26982. [Google Scholar] [CrossRef] [PubMed]


| λem, nm | Φem | τem, μs | |
|---|---|---|---|
| [Re6S8(bpe)4Cl2] (1) | 710 | <0.005 | 0.57 |
| [Re6S8(bpe)4Br2] (2) | 690 | <0.005 | 0.44 |
| [Re6Se8(bpe)4Cl2] (3) | 705 | <0.005 | n/a |
| [Re6Se8(bpe)4Br2] (4) | 685 | <0.005 | n/a |
| [Re6S8(bpp)4Cl2] (5) | 695 (solid) 695 (solution) | 0.04 (solid) 0.05 (solution) | 6.38 (solid) 6.80 (solution) |
| [Re6S8(bpp)4Br2] (6) | 685 (solid) 682 (solution) | 0.01 (solid) 0.02 (solution) | 2.30 (solid) 7.10 (solution) |
| [Re6Se8(bpp)4Cl2] (7) | 690 (solid) 692 (solution) | 0.02 (solid) 0.02 (solution) | 4.54 (solid) 4.71 (solution) |
| [Re6Se8(bpp)4Br2] (8) | 685 (solid) 685 (solution) | 0.005 (solid) 0.02 (solution) | 3.48 (solid) n/a (solution) |
| [Re6S8(phpy)4Cl2] | 708 | 0.028 | 4.3 |
| [Re6S8(phpy)4Br2] | 715 | 0.013 | 4.5 |
| [Re6Se8(phpy)4Cl2] | 708 | 0.015 | 3.8 |
| [Re6Se8(phpy)4Br2] | 718 | 0.013 | 4.0 |
| [Re6S8(bpy)4Cl2] | 725 | <0.005 | n/a |
| [Re6S8(bpy)4Br2] | 730 | <0.005 | n/a |
| [Re6Se8(bpy)4Cl2] | 725 | 0.02 | n/a |
| [Re6Se8(bpy)4Br2] | 730 | 0.01 | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulantikov, A.A.; Brylev, K.A.; Sukhikh, T.S.; Mironov, Y.V.; Muravieva, V.K.; Gayfulin, Y.M. Octahedral Rhenium Cluster Complexes with 1,2-Bis(4-pyridyl)ethylene and 1,3-Bis(4-pyridyl)propane as Apical Ligands. Molecules 2022, 27, 7874. https://doi.org/10.3390/molecules27227874
Ulantikov AA, Brylev KA, Sukhikh TS, Mironov YV, Muravieva VK, Gayfulin YM. Octahedral Rhenium Cluster Complexes with 1,2-Bis(4-pyridyl)ethylene and 1,3-Bis(4-pyridyl)propane as Apical Ligands. Molecules. 2022; 27(22):7874. https://doi.org/10.3390/molecules27227874
Chicago/Turabian StyleUlantikov, Anton A., Konstantin A. Brylev, Taisiya S. Sukhikh, Yuri V. Mironov, Viktoria K. Muravieva, and Yakov M. Gayfulin. 2022. "Octahedral Rhenium Cluster Complexes with 1,2-Bis(4-pyridyl)ethylene and 1,3-Bis(4-pyridyl)propane as Apical Ligands" Molecules 27, no. 22: 7874. https://doi.org/10.3390/molecules27227874
APA StyleUlantikov, A. A., Brylev, K. A., Sukhikh, T. S., Mironov, Y. V., Muravieva, V. K., & Gayfulin, Y. M. (2022). Octahedral Rhenium Cluster Complexes with 1,2-Bis(4-pyridyl)ethylene and 1,3-Bis(4-pyridyl)propane as Apical Ligands. Molecules, 27(22), 7874. https://doi.org/10.3390/molecules27227874







