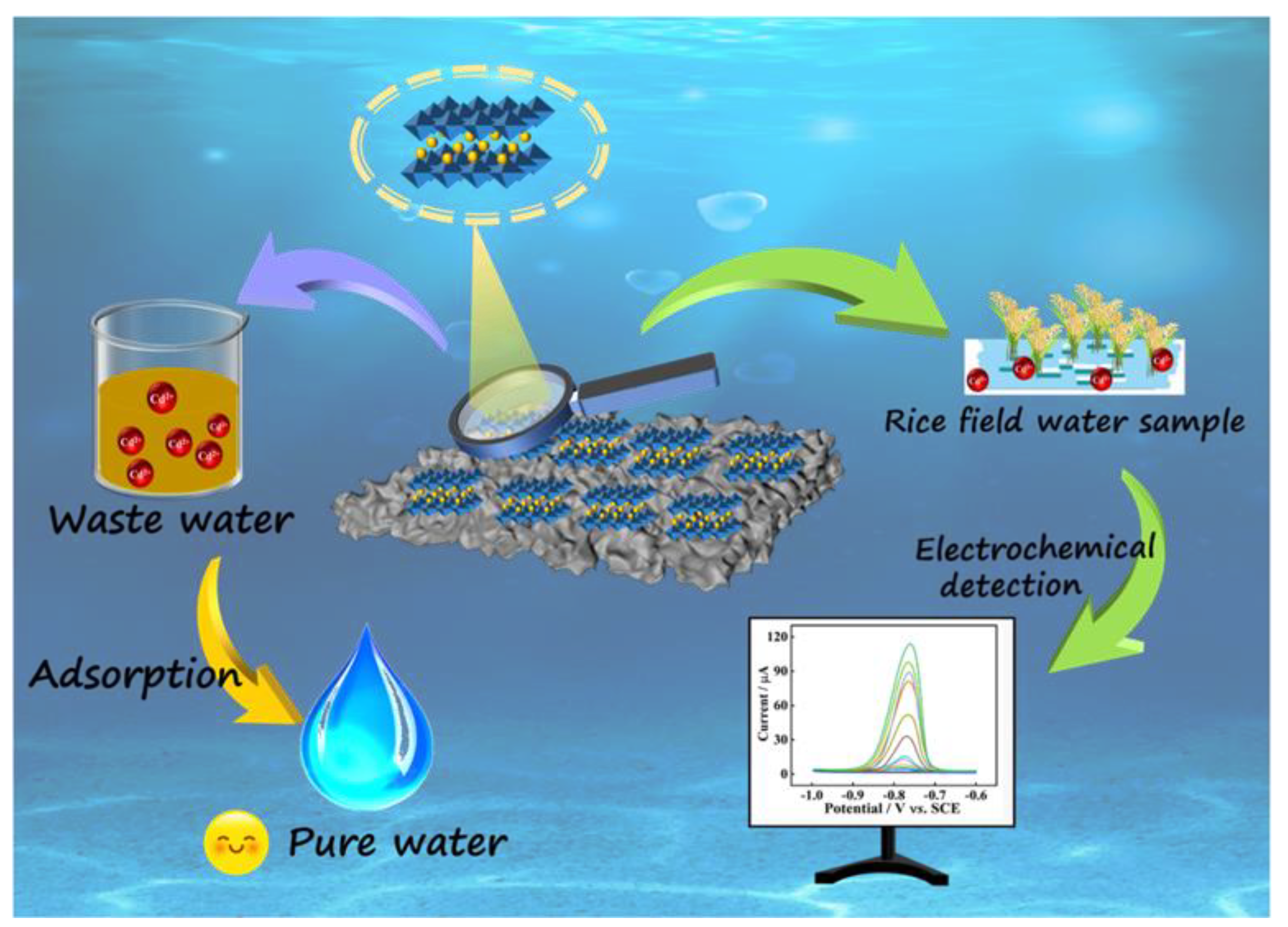
In Situ Synthesis of MnMgFe-LDH on Biochar for Electrochemical Detection and Removal of Cd2+ in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
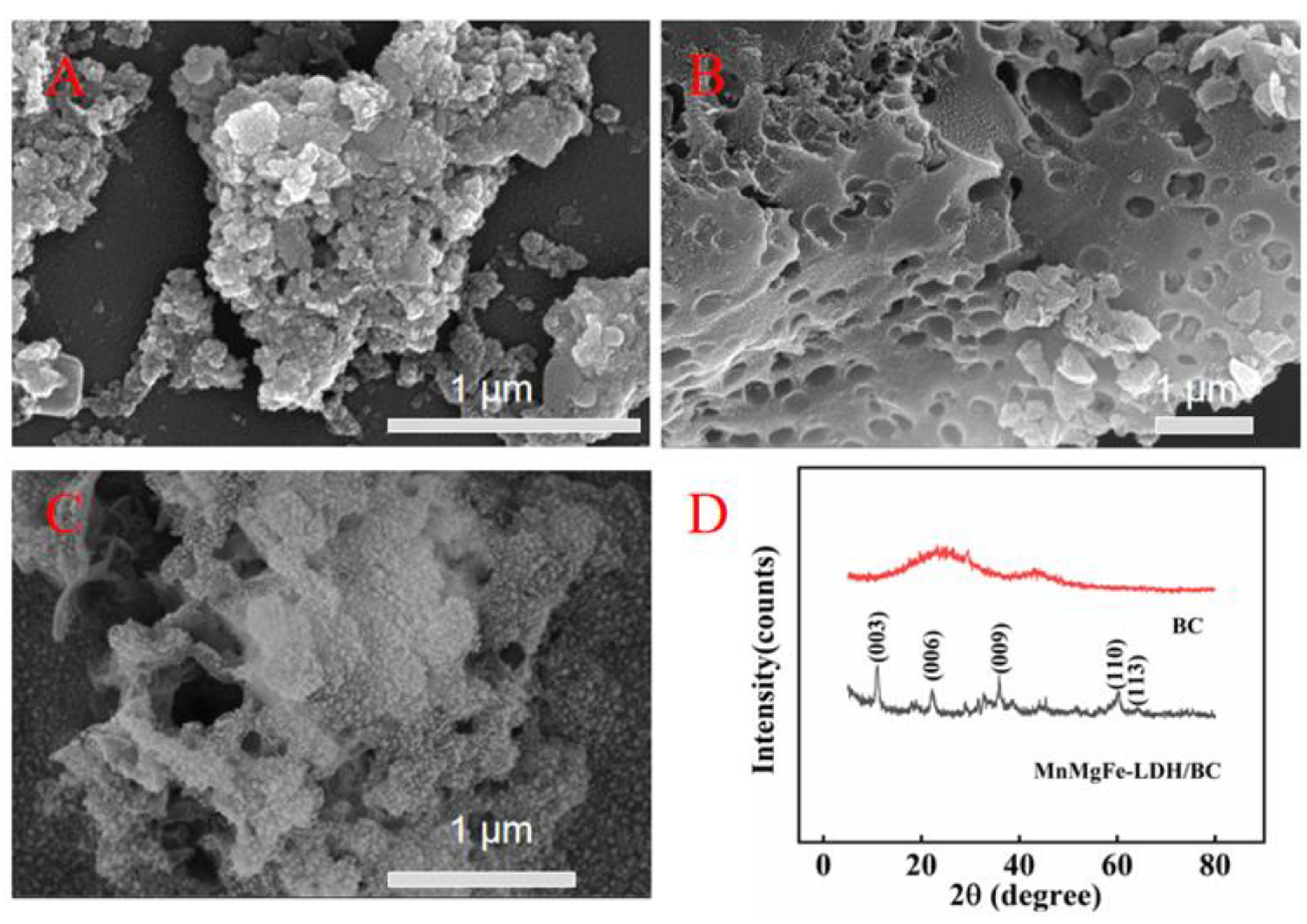
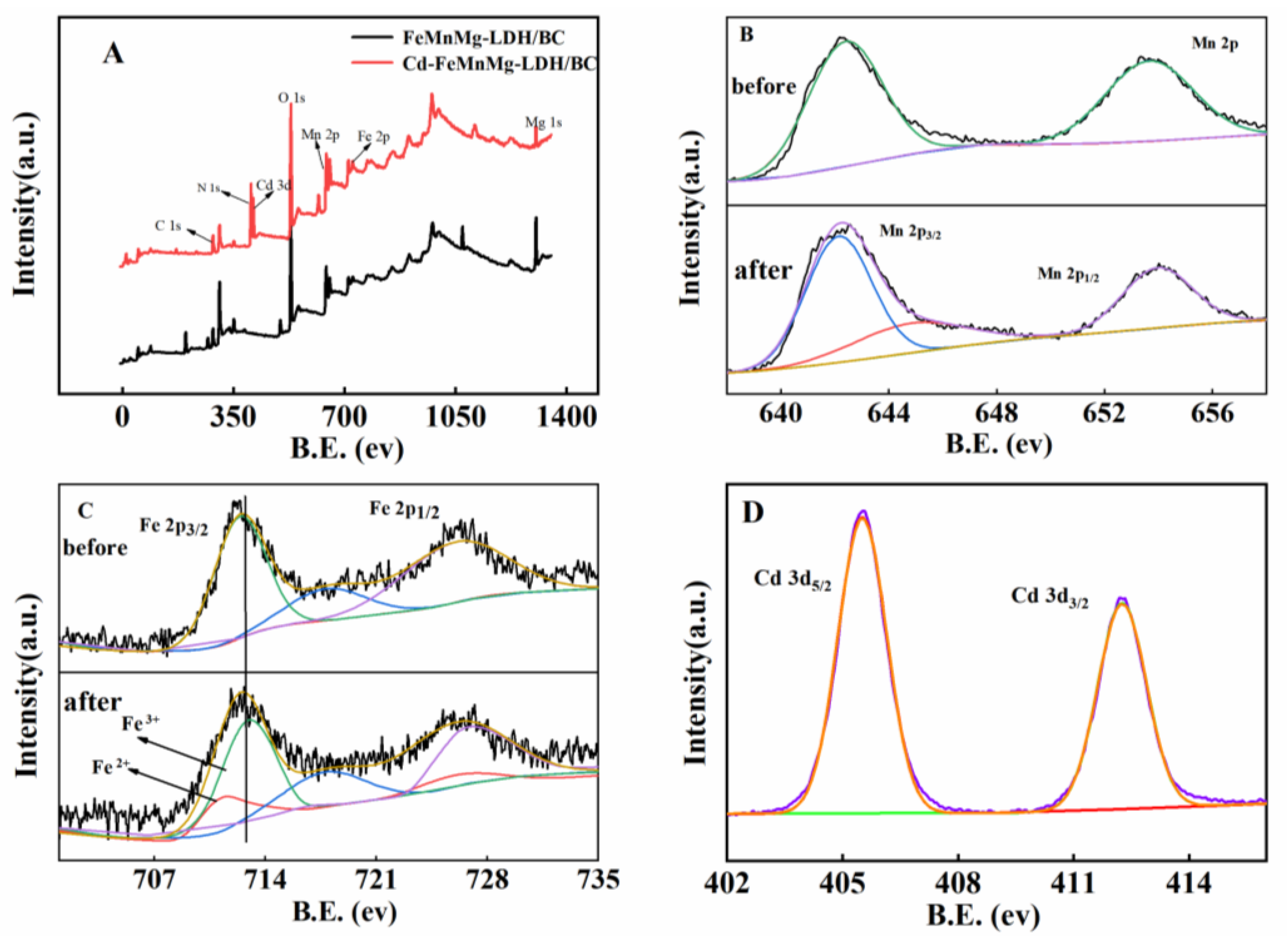
2.1. Morphology and Structure Characterization
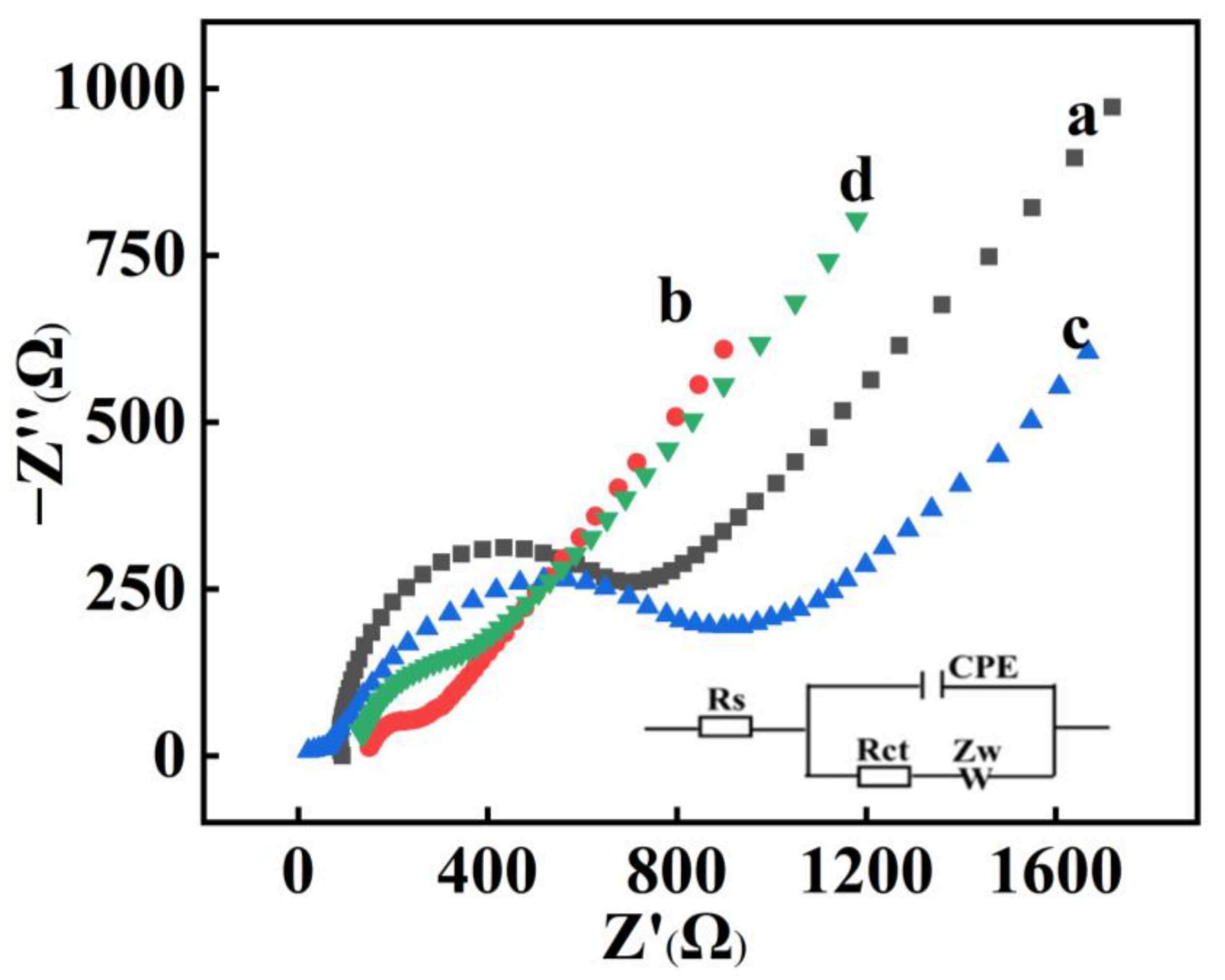
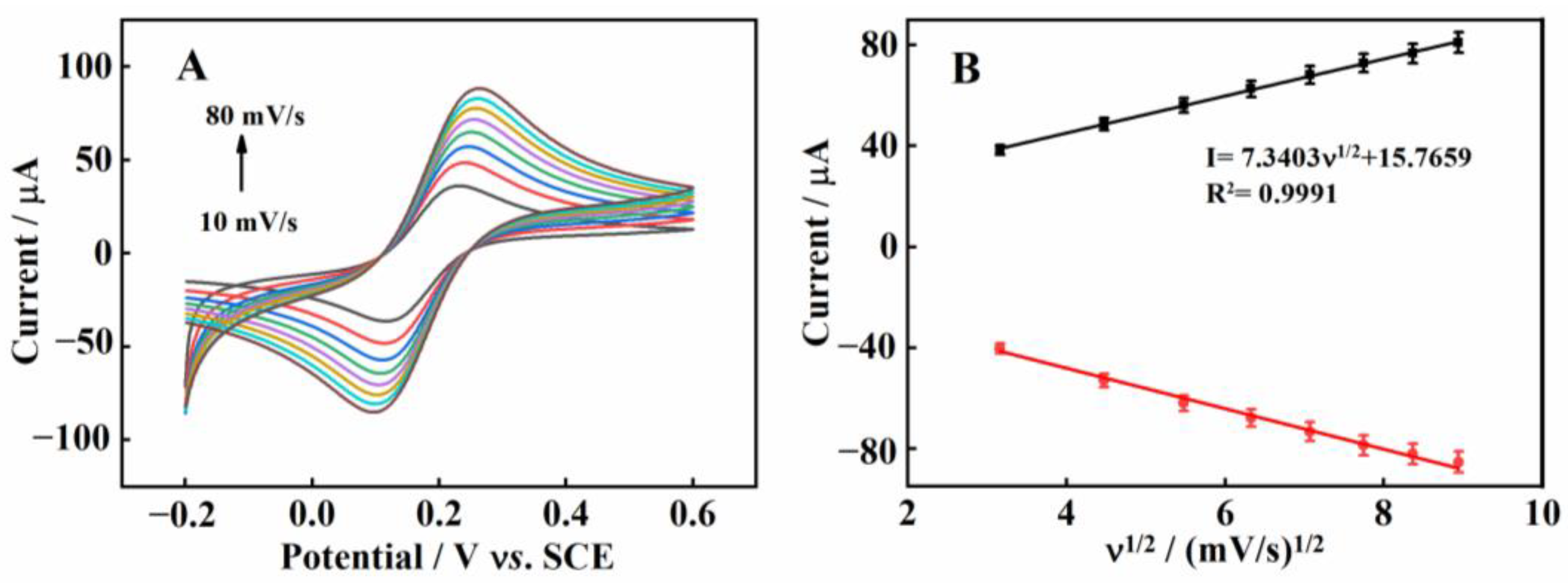
2.2. Electrochemical Characterizations
2.3. Electrochemical Behaviors of Different Electrodes
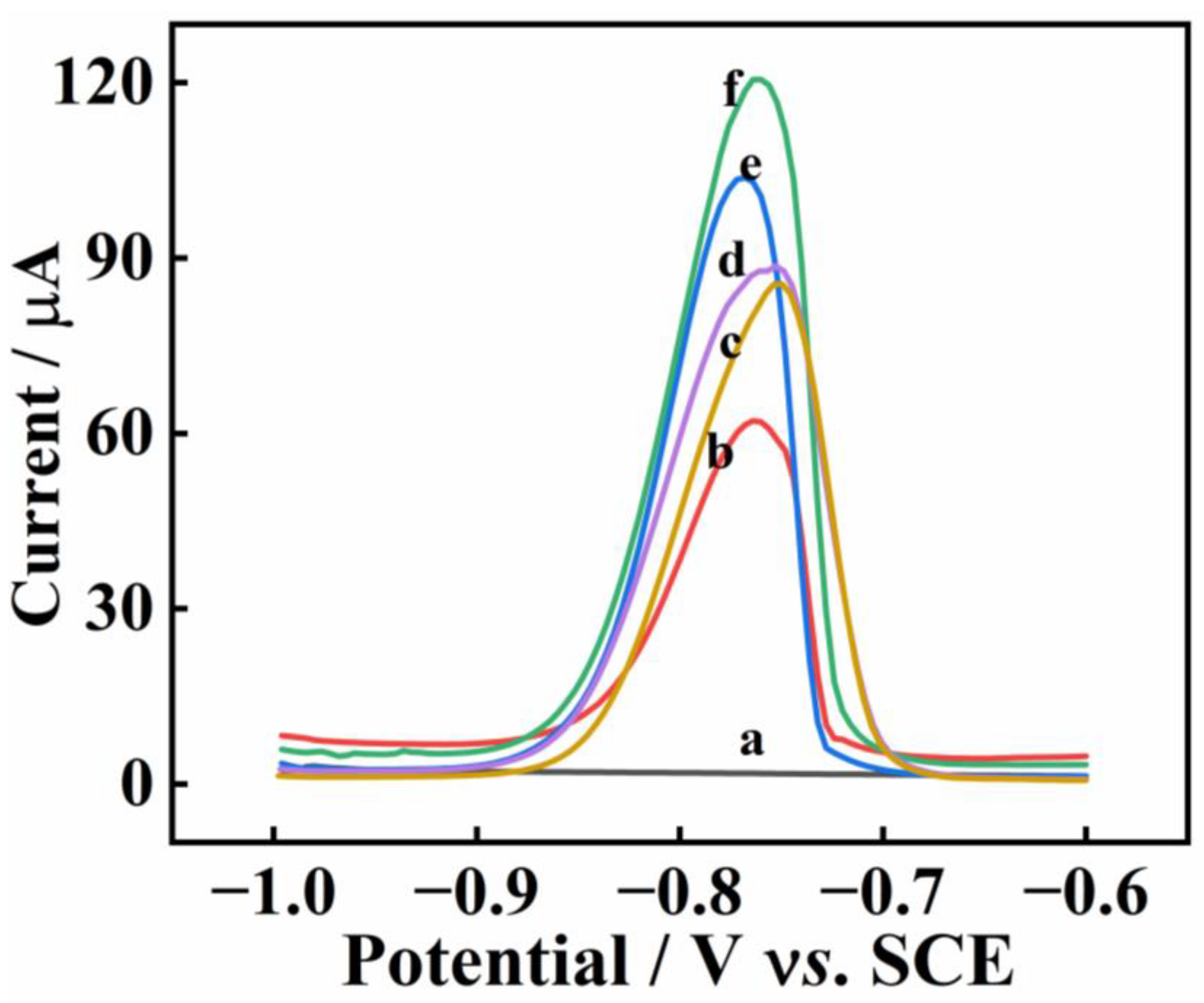
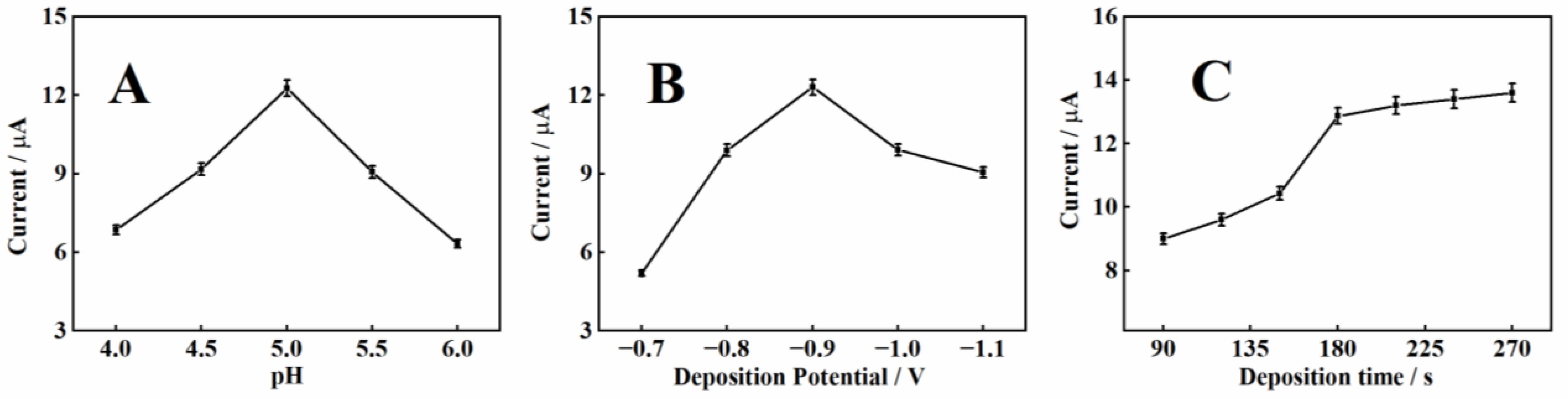
2.4. Optimization of Experimental Parameters
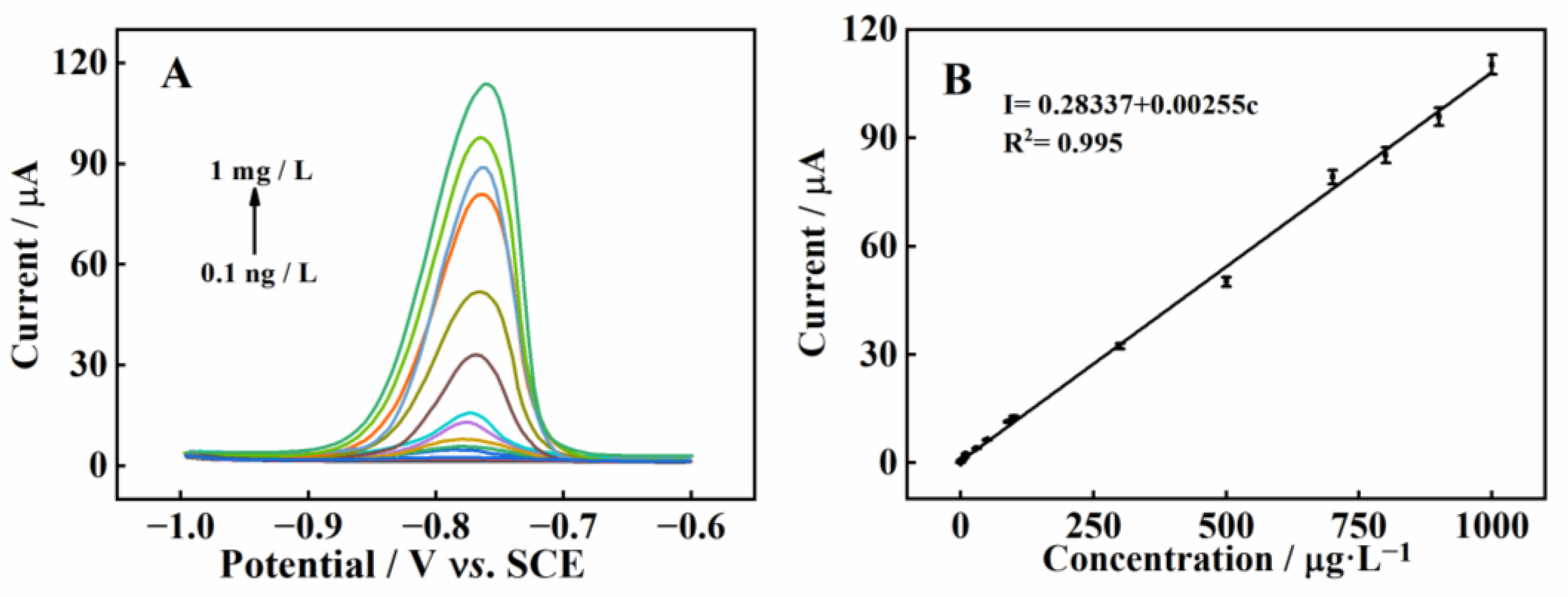
2.5. DPASV Detection of Cd2+ at MnMgFe-LDHs/BC/GCE
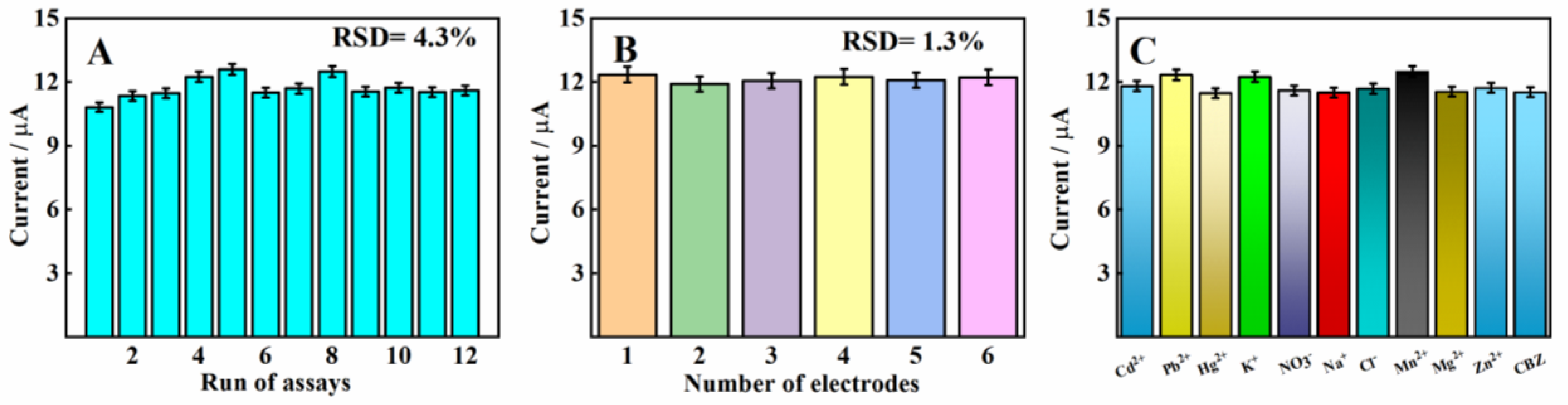
2.6. Repeatability, Reproducibility, and Selectivity of MnMgFe-LDHs/BC/GCE
2.7. Application of MnMgFe-LDHs/BC/GCE for Cd2+ Detection in Real Sample Analysis
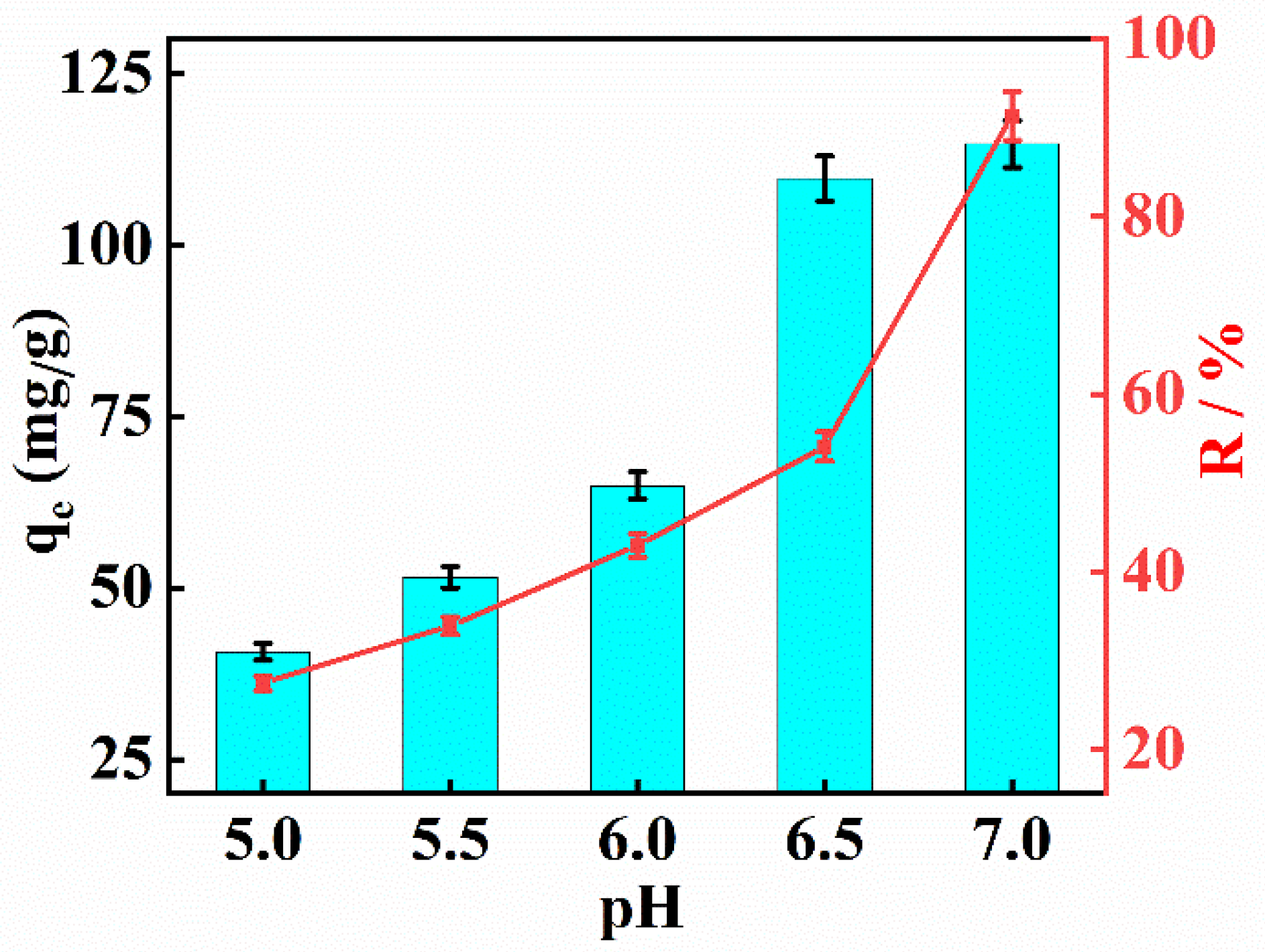
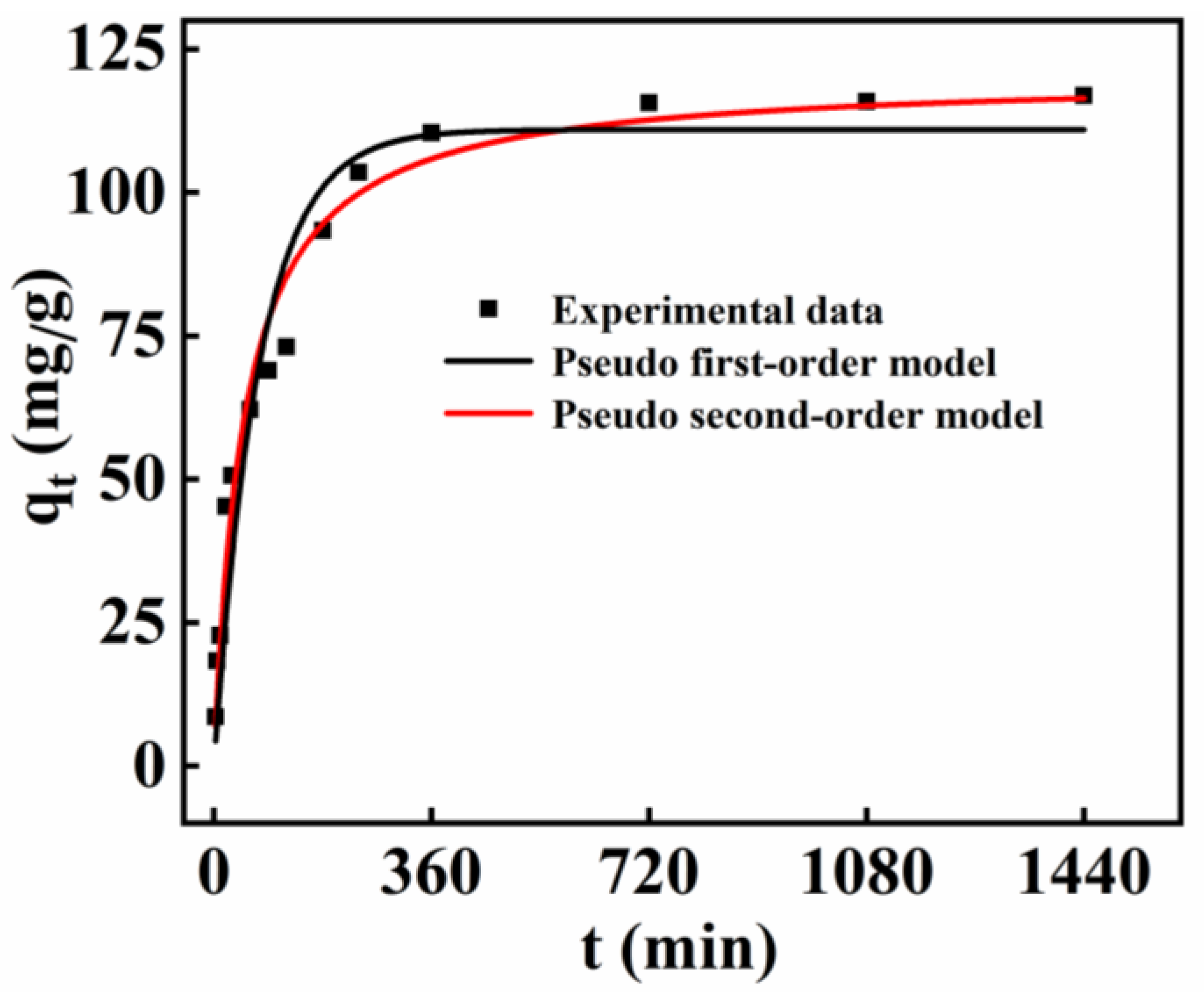
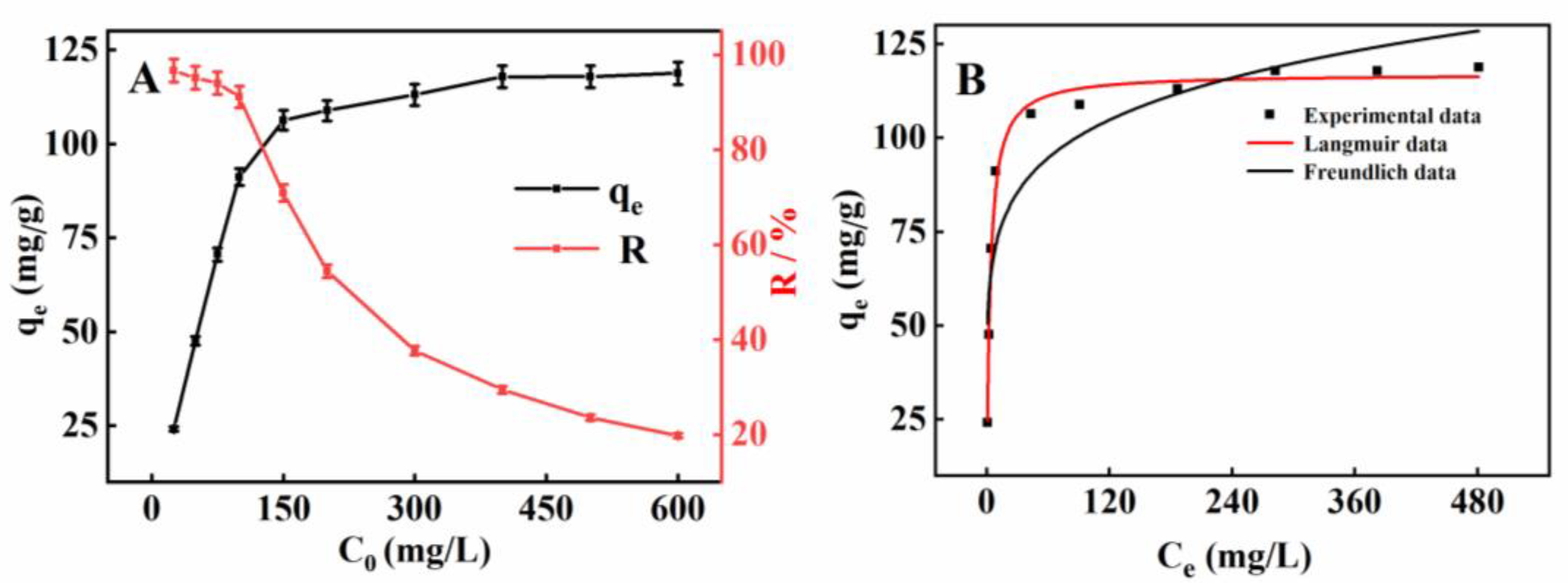
2.8. Adsorption Properties of MnMgFe-LDHs/BC toward Cd2+
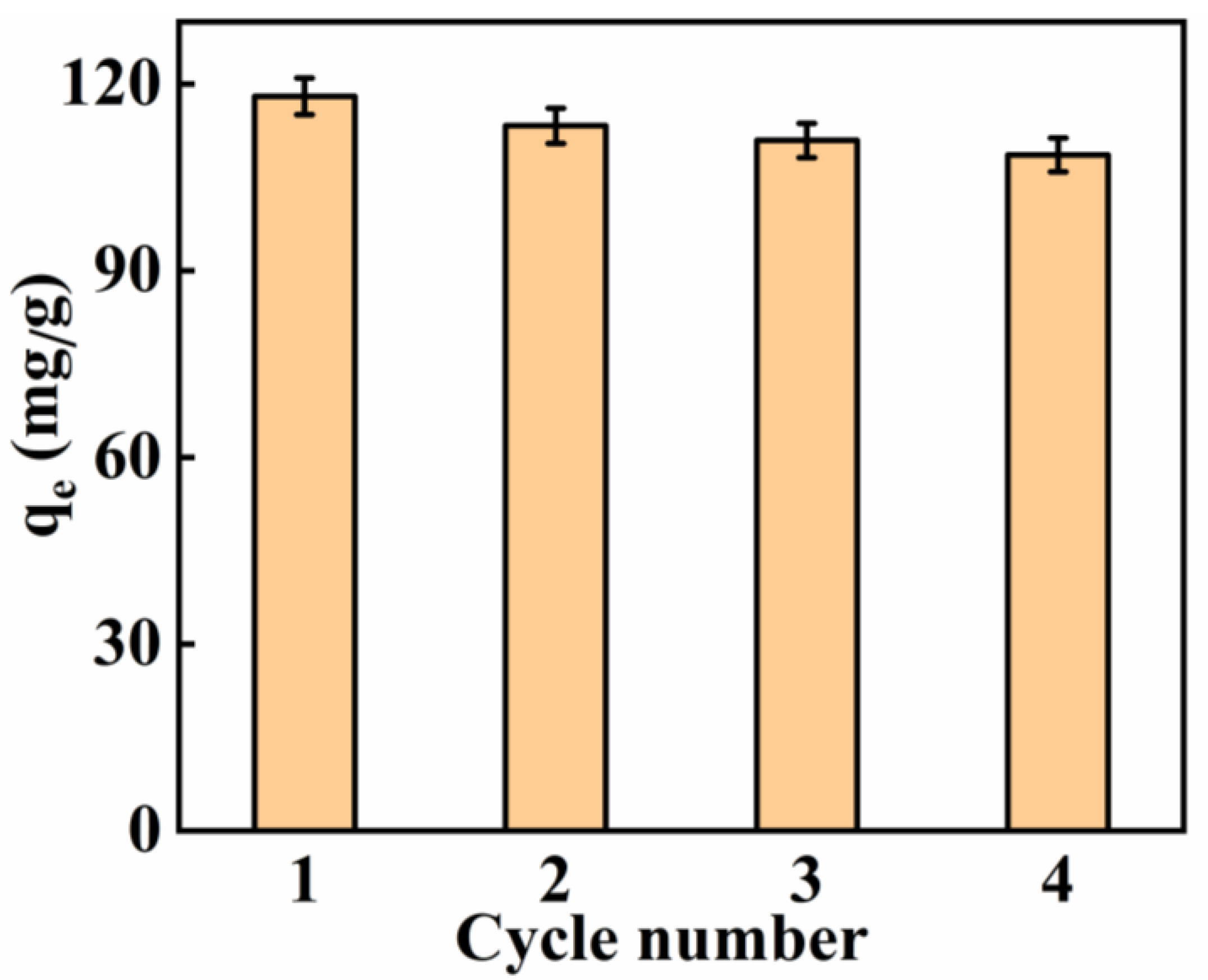
2.9. Regeneration and Stability
3. Experimental Section
3.1. Materials
3.2. Apparatus
3.3. Preparation of BC and LDHs/BC Composite
3.4. Preparation of the Modified Electrodes
3.5. Analytical Procedure
3.6. Adsorption Studies of Cd2+
3.7. TCLP Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, Q.; Sheng, Y.; Liu, X. Efficacy of in situ active capping Cd highly contaminated sediments with nano-Fe2O3 modified biochar. Environ. Pollut. 2021, 290, 118134–118145. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, D.; Mishra, U.; Biswas, S. A comprehensive review on Cd(II) removal from aqueous solution. J. Water Process Eng. 2014, 2, 105–128. [Google Scholar] [CrossRef]
- Memon, A.F.; Ameen, S.; Qambrani, N.; Buledi, J.A.; Khand, N.H.; Solangi, A.R.; Taqvi, S.I.H.; Karaman, C.; Karimi, F.; Afsharmanesh, E. An improved electrochemical sensor based on triton X-100 functionalized SnO2 nanoparticles for ultrasensitive determination of cadmium. Chemosphere 2022, 300, 134634–134641. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, Z.; Yu, X.; Shi, X.; Jiang, H.; Li, X.; Kong, Y.; Xu, Q.; Chen, J. Raspberry-Like Bismuth Oxychloride on Mesoporous Siliceous Support for Sensitive Electrochemical Stripping Analysis of Cadmium. Molecules 2017, 22, 797. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, D.; Wang, D.; Lu, L.; Wang, X.; Guo, G. A carbon-supported BiSn nanoparticles based novel sensor for sensitive electrochemical determination of Cd (II) ions. Talanta 2019, 202, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, B.; Ji, L.; Wang, F.; Yuan, Q.; Hu, G.; Dong, A.; Gan, W. An efficient electrochemical sensor based on three-dimensionally interconnected mesoporous graphene framework for simultaneous determination of Cd(II) and Pb(II). Electrochim. Acta 2016, 222, 1371–1377. [Google Scholar] [CrossRef]
- Yu, Q.; Zou, J.; Peng, G.; Gao, F.; Gao, Y.; Fan, G.; Chen, S.; Lu, L. A facile fabrication of ratiometric electrochemical sensor for sensitive detection of riboflavin based on hierarchical porous biochar derived from KOH-activated Soulangeana sepals. Nanotechnology 2022, 33, 445501–445514. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in Preparation and Application of Modified Biochar for Improving Heavy Metal Ion Removal From Wastewater. Bioresour. Technol. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Zou, J.; Qian, W.; Li, Y.; Yu, Q.; Yu, Y.; Chen, S.; Qu, F.; Gao, Y.; Lu, L. Multilayer activated biochar/UiO-66-NH2 film as intelligent sensing platform for ultra-sensitive electrochemical detection of Pb2+ and Hg2+. Appl. Surf. Sci. 2021, 569, 151006–151019. [Google Scholar] [CrossRef]
- Li, A.; Xie, H.; Qiu, Y.; Liu, L.; Lu, T.; Wang, W.; Qiu, G. Resource utilization of rice husk biomass: Preparation of MgO flake-modified biochar for simultaneous removal of heavy metals from aqueous solution and polluted soil. Environ. Pollut. 2022, 310, 119869–119879. [Google Scholar] [CrossRef]
- Rohit, R.C.; Jagadale, A.D.; Shinde, S.K.; Kim, D.Y.; Kumbhar, V.S.; Nakayama, M. Hierarchical nanosheets of ternary CoNiFe layered double hydroxide for supercapacitors and oxygen evolution reaction. J. Alloys Compd. 2021, 863, 158081–158089. [Google Scholar] [CrossRef]
- Aliahmadi, Z.; Mohadesi, A.; Ranjbar, M.; Javanshah, A. Preparation and evaluation of Ca/Mg-layered double hydroxide as a novel modifier for electrochemical determination of gibberellic acid. J. Mol. Struct. 2021, 1246, 131200–131207. [Google Scholar] [CrossRef]
- Feng, X.; Long, R.; Wang, L.; Liu, C.; Bai, Z.; Liu, X. A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Sep. Purif. Technol. 2022, 284, 120099–120145. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Y.; Ge, W.; Zhang, Y.; Liu, L.; Qiu, G. Removal of heavy metals from wastewaters with biochar pyrolyzed from MgAl-layered double hydroxide-coated rice husk: Mechanism and application. Bioresour. Technol. 2022, 347, 126425–126435. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Pelalak, R.; Pishnamazi, M.; Marjani, A.; Shirazian, S. A water-stable functionalized NiCo-LDH/MOF nanocomposite: Green synthesis, characterization, and its environmental application for heavy metals adsorption. Arab. J. Chem. 2021, 14, 103052–103063. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, Z.; Wei, S. A new hydrotalcite-like absorbent FeMnMg-LDH and its adsorption capacity for Pb2+ ions in water. Appl. Clay Sci. 2018, 153, 29–37. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, Z.; Wei, S.; Liang, J. Adsorption of Cd(II) from Aqueous Solutions by a Novel Layered Double Hydroxide FeMnMg-LDH. Water Air Soil Pollut. 2018, 229, 78–94. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Xie, D.; Gu, Y.; Zhu, X.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Hierarchical MgFe-layered double hydroxide microsphere/graphene composite for simultaneous electrochemical determination of trace Pb(II) and Cd(II). Chem. Eng. J. 2018, 347, 953–962. [Google Scholar] [CrossRef]
- Xiang, X.; Pan, F.; Du, Z.; Feng, X.; Gao, C.; Li, Y. MgAl-layered double hydroxide flower arrays grown on carbon paper for efficient electrochemical sensing of nitrite. J. Electroanal. Chem. 2019, 855, 113632–113640. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Srivastava, P.; Naidu, R.; Vinu, A. Single step synthesis of activated bio-carbons with a high surface area and their excellent CO2 adsorption capacity. Carbon 2017, 116, 448–455. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, J.; Xu, Q.; Duan, X.; Jiang, F.; Lu, L.; Jia, H.; Jia, Y.; Li, Y.; Yu, Y. A poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)-based electrochemical sensor for tert.-butylhydroquinone. Microchim. Acta 2019, 186, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yu, Q.; Gao, Y.; Chen, S.; Huang, X.; Hu, D.; Liu, S.; Lu, L. Bismuth Nanoclusters/Porous Carbon Composite: A Facile Ratiometric Electrochemical Sensing Platform for Pb2+ Detection with High Sensitivity and Selectivity. ACS Omega 2022, 7, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, P.; Hu, Z.; Liang, Y.; Han, H.; Yang, M.; Luo, X.; Hou, C.; Huo, D. Amino-Functionalized Multilayer Ti3C2Tx Enabled Electrochemical Sensor for Simultaneous Determination of Cd2+ and Pb2+ in food samples. Food Chem. 2022, 402, 134269. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, Y.; Yu, Z.; Lu, L.; Wang, X. Simultaneous determination of Cd2+ and Pb2+ by an electrochemical sensor based on Fe3O4/Bi2O3/C3N4 nanocomposites. Talanta Open 2021, 3, 100024–100031. [Google Scholar] [CrossRef]
- Li, Y.; Huang, H.; Cui, R.; Wang, D.; Yin, Z.; Wang, D.; Zheng, L.; Zhang, J.; Zhao, Y.; Yuan, H.; et al. Electrochemical sensor based on graphdiyne is effectively used to determine Cd2+ and Pb2+ in water. Sens. Actuators B Chem. 2021, 332, 129519–129527. [Google Scholar] [CrossRef]
- Ding, L.; Luo, X.; Shao, P.; Yang, J.; Sun, D. Thiol-Functionalized Zr-Based Metal–Organic Framework for Capture of Hg(II) through a Proton Exchange Reaction. ACS Sustain. Chem. Eng. 2018, 6, 8494–8502. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, X.; Wang, C.; Sun, H.; Ma, A.; Zhang, G.; Wang, N. Sorption of cadmium onto Mg-Fe layered double hydroxide (LDH)-Kiwi branch biochar. Environ. Pollut. Bioavailab. 2019, 31, 189–197. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, Q.; Khan, S.; Liu, Y.; Liao, Z.; Li, G.; Ok, Y.S. Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol. Eng. 2016, 87, 240–245. [Google Scholar] [CrossRef]
- Shan, R.; Yan, L.; Yang, K.; Hao, Y.; Du, B. Adsorption of Cd(II) by Mg-Al-CO3- and magnetic Fe3O4/Mg-Al-CO3-layered double hydroxides: Kinetic, isothermal, thermodynamic and mechanistic studies. J. Hazard. Mater. 2015, 299, 42–49. [Google Scholar] [CrossRef]
- Liao, W.; Bao, D.; Li, H.; Yang, P. Cu(II) and Cd(II) removal from aqueous solution with LDH@GO-NH2 and LDH@GO-SH: Kinetics and probable mechanism. Environ. Sci. Pollut. Res. 2021, 28, 65848–65861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Han, C.; Ren, Y.; Ji, Y.; Ge, Y.; Li, Z.; He, J. Preparation, characteristics and mechanisms of Cd (II) adsorption from aqueous solution by mango kernel-derived biochar. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Cui, S.; Ke, Y.; Fu, Q.; Hough, R.; Zhang, Z.; Shen, Z.; An, L.; Li, Y. Optimization preparation of biochar from garden waste and quantitative analysis for Cd2+ adsorption mechanism in aqueous solution. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Cao, B.; Qu, J.; Yuan, Y.; Zhang, W.; Miao, X.; Zhang, X.; Xu, Y.; Han, T.; Song, H.; Ma, S.; et al. Efficient scavenging of aqueous Pb(II)/Cd(II) by sulfide-iron decorated biochar: Performance, mechanisms and reusability exploration. J. Environ. Chem. Eng. 2022, 10, 107531–107539. [Google Scholar] [CrossRef]
- Mcintyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
| Sample | Added (μg/L) | Found (μg/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0 | - | - | - |
| 2 | 0.05 | 0.05 ± 0.002 | 97.3 | 4.3 |
| 3 | 0.50 | 0.50 ± 0.021 | 100.6 | 4.1 |
| 4 | 5.00 | 5.00 ± 0.140 | 99.6 | 2.9 |
| 5 | 50.00 | 50.00 ± 1.320 | 102.3 | 1.8 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||
|---|---|---|---|---|---|---|
| qe,exp (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g/mg·min) | R2 | qe (mg/g) |
| 118.00 | 0.0133 | 0.9354 | 110.97 | 0.000168 | 0.9750 | 117.94 |
| Adsorbents | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| Kiwi branch BC/MgFe-LDH | 25.60 | [28] |
| Peanut husk BC | 28.99 | [29] |
| Mg-Al-CO3-LDH | 70.20 | [30] |
| MgAL-LDH@graphene oxide-SH | 102.77 | [31] |
| Mango kernel BC | 27.81 | [32] |
| Garden waste BC | 40.00 | [33] |
| BC-Fe-S | 57.71 | [34] |
| MnMgFe-LDH/BC | 118.00 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Yang, W.; Wang, H.; Huang, G. In Situ Synthesis of MnMgFe-LDH on Biochar for Electrochemical Detection and Removal of Cd2+ in Aqueous Solution. Molecules 2022, 27, 7875. https://doi.org/10.3390/molecules27227875
Yu Y, Yang W, Wang H, Huang G. In Situ Synthesis of MnMgFe-LDH on Biochar for Electrochemical Detection and Removal of Cd2+ in Aqueous Solution. Molecules. 2022; 27(22):7875. https://doi.org/10.3390/molecules27227875
Chicago/Turabian StyleYu, Yongfang, Wenting Yang, Haocheng Wang, and Guoqin Huang. 2022. "In Situ Synthesis of MnMgFe-LDH on Biochar for Electrochemical Detection and Removal of Cd2+ in Aqueous Solution" Molecules 27, no. 22: 7875. https://doi.org/10.3390/molecules27227875
APA StyleYu, Y., Yang, W., Wang, H., & Huang, G. (2022). In Situ Synthesis of MnMgFe-LDH on Biochar for Electrochemical Detection and Removal of Cd2+ in Aqueous Solution. Molecules, 27(22), 7875. https://doi.org/10.3390/molecules27227875







