Enhanced Performance of Camphorsulfonic Acid-Doped Perovskite Solar Cells
Abstract
1. Introduction
2. Results and Discussion
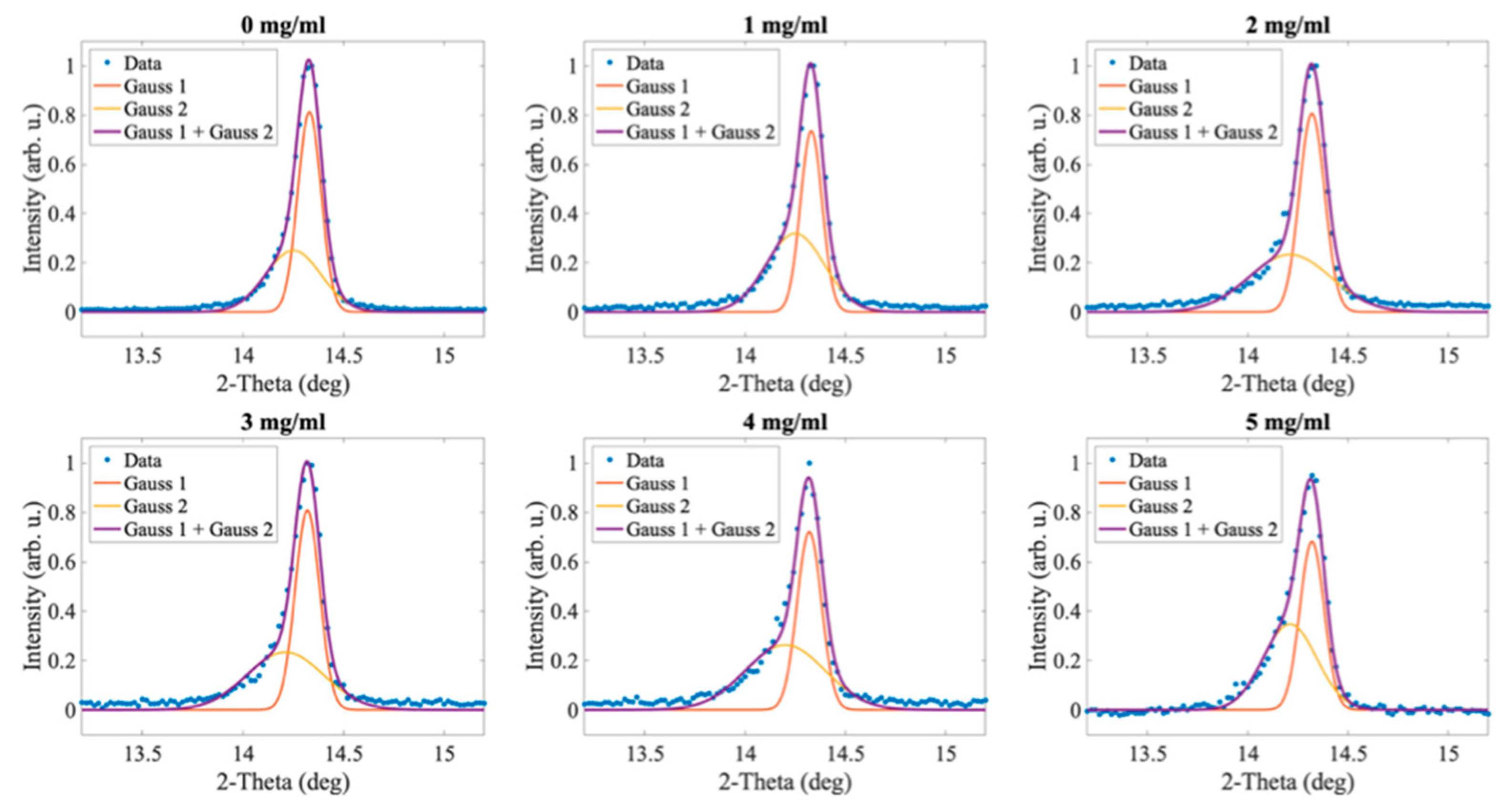
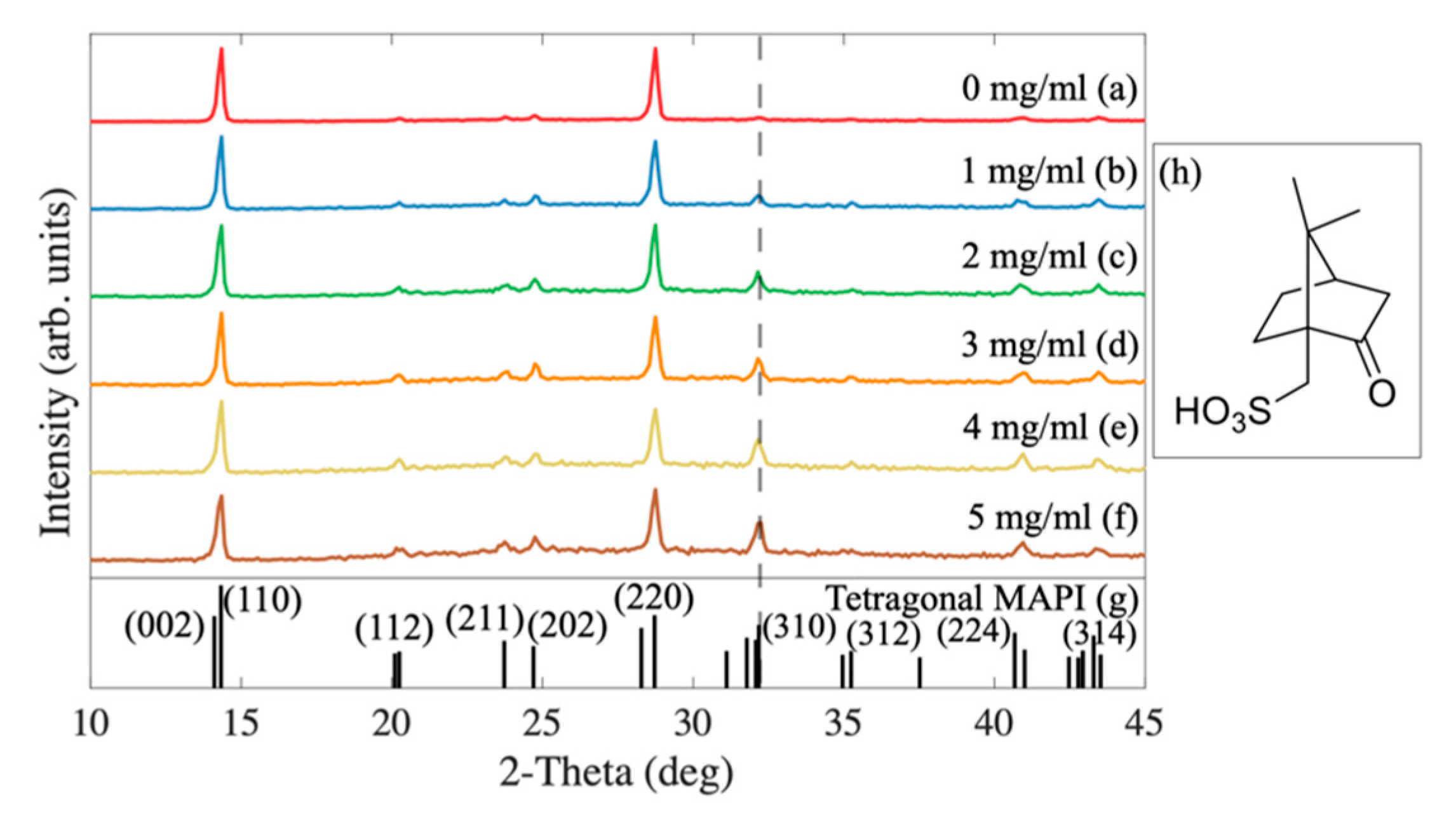
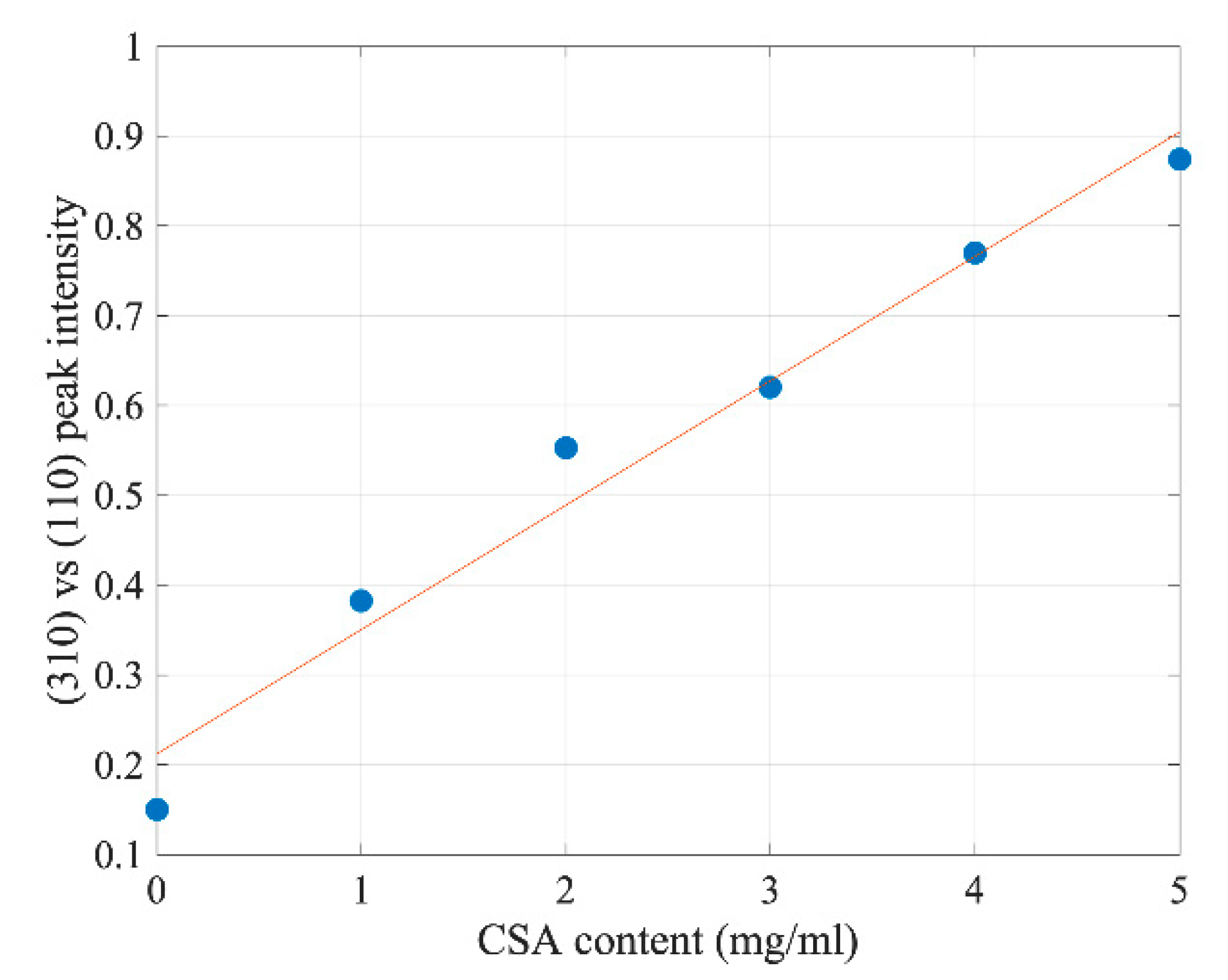
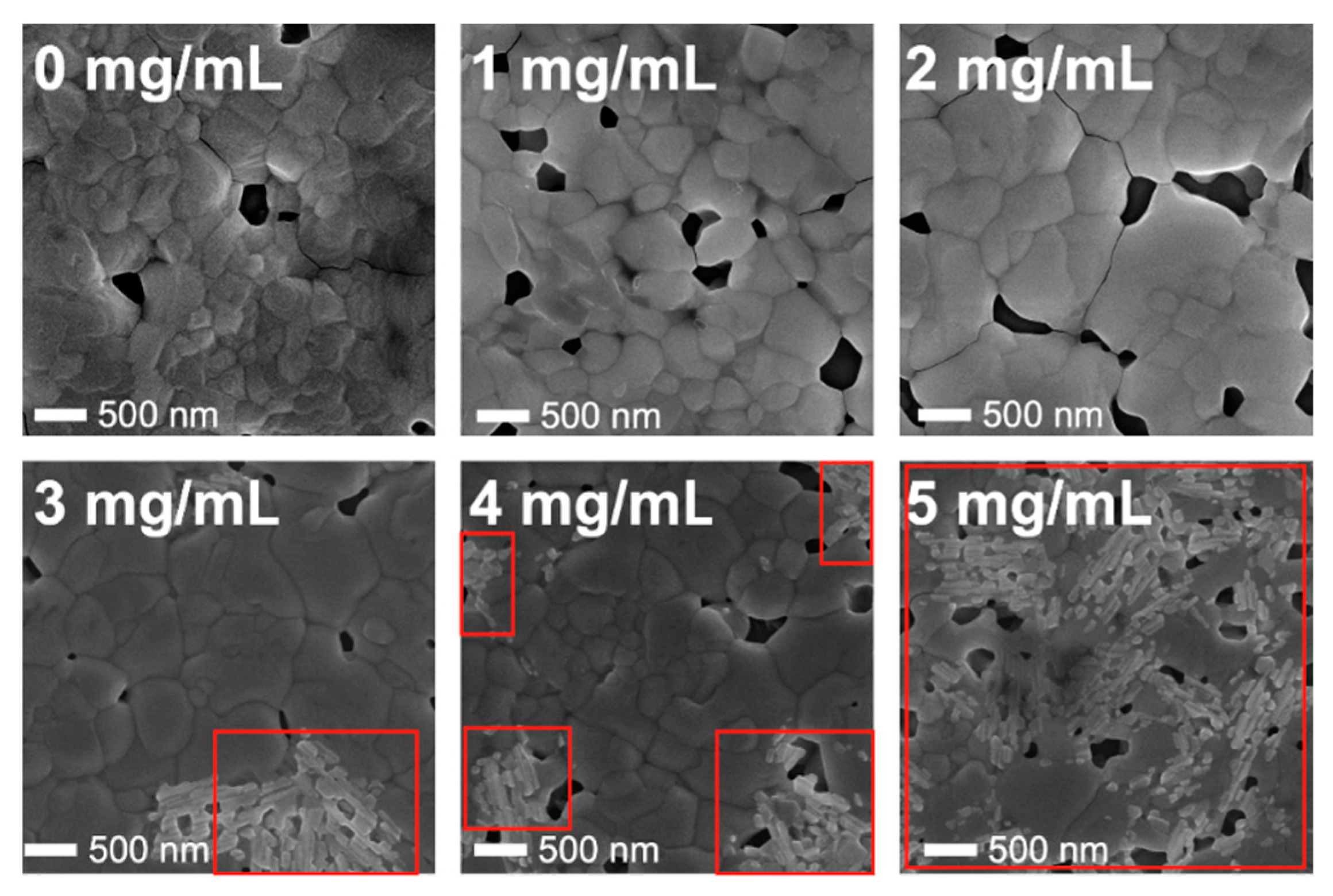
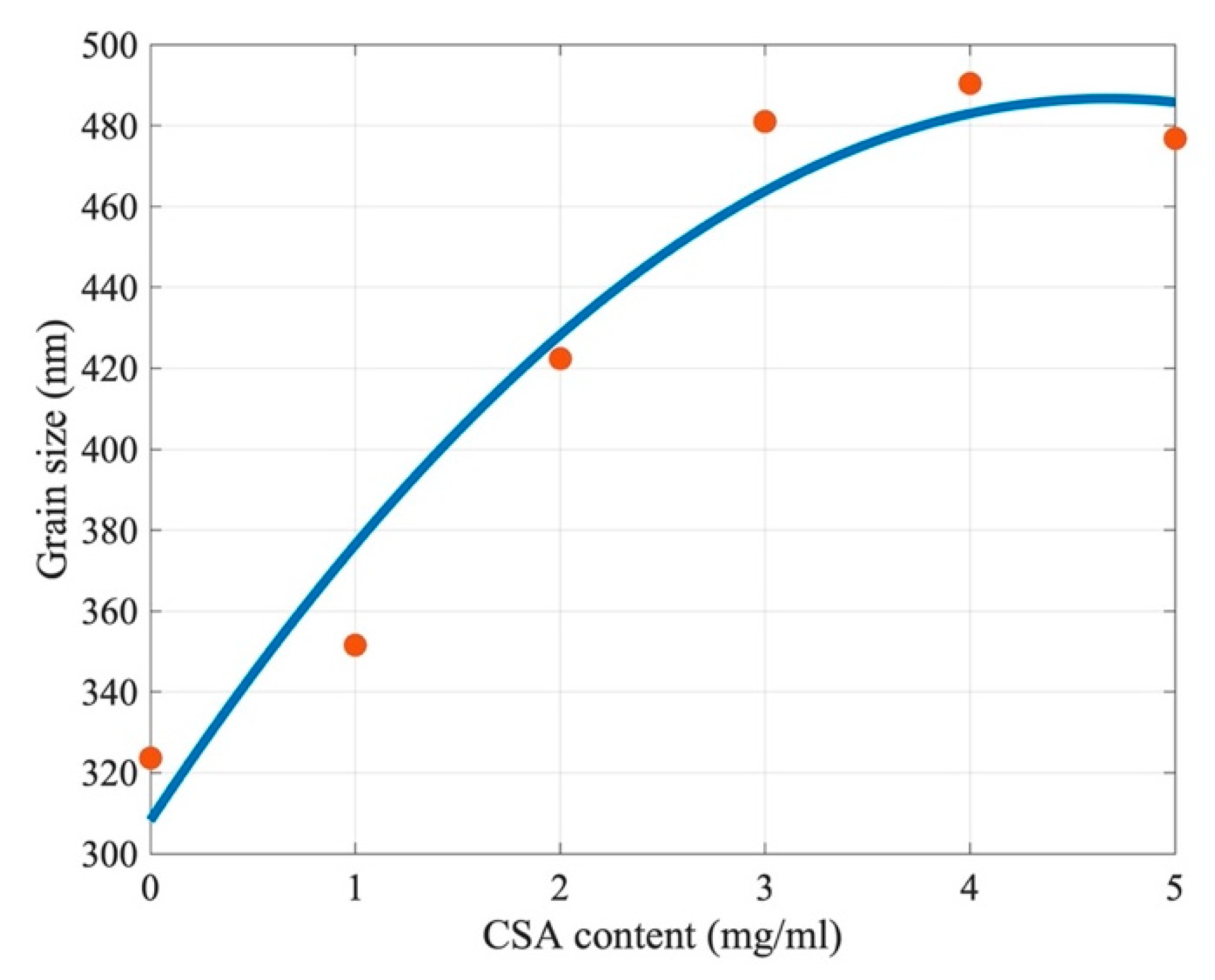
2.1. The Influence of Camphorsulfonic Acid (CSA) on the Perovskite Structure
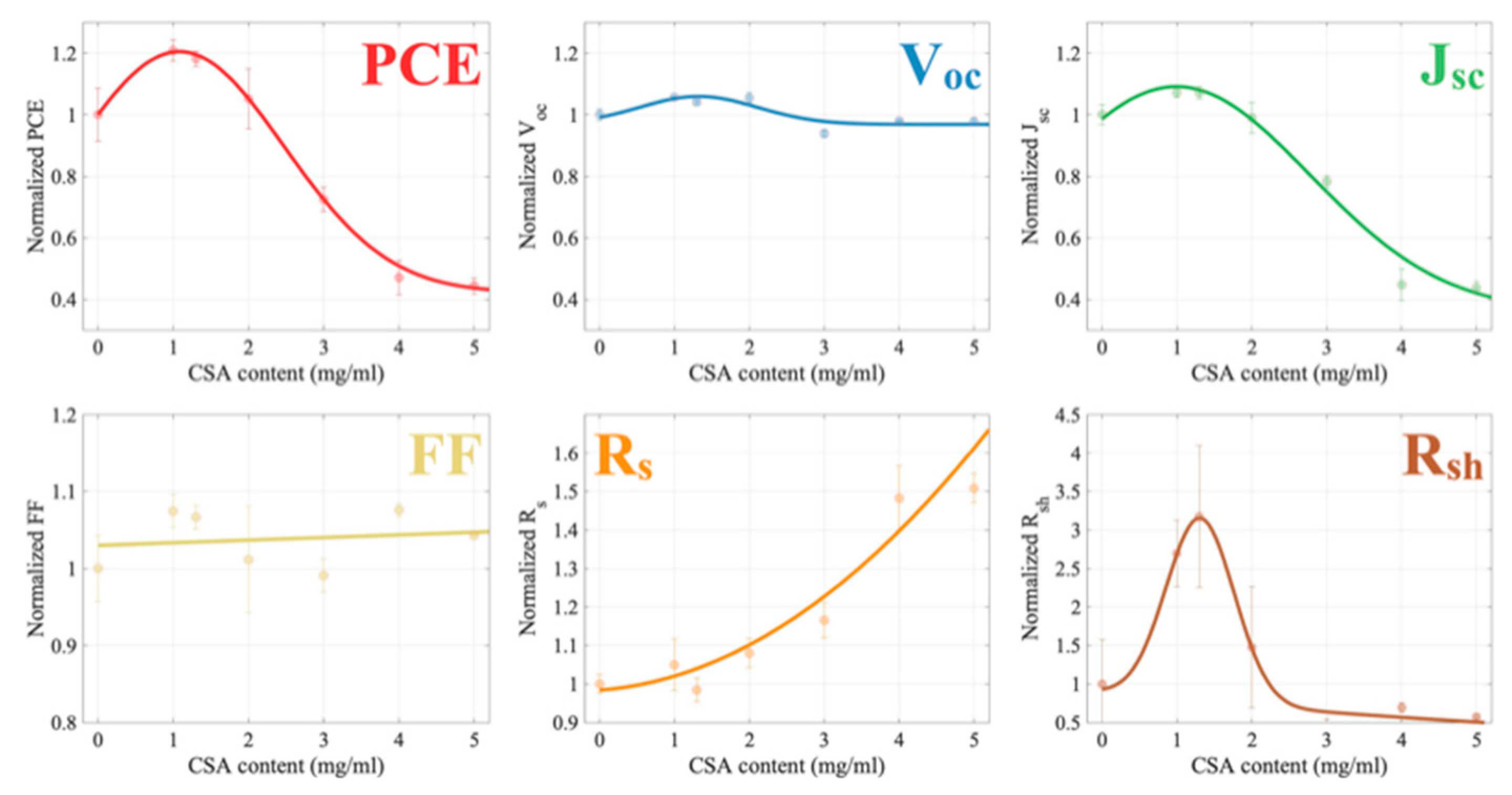
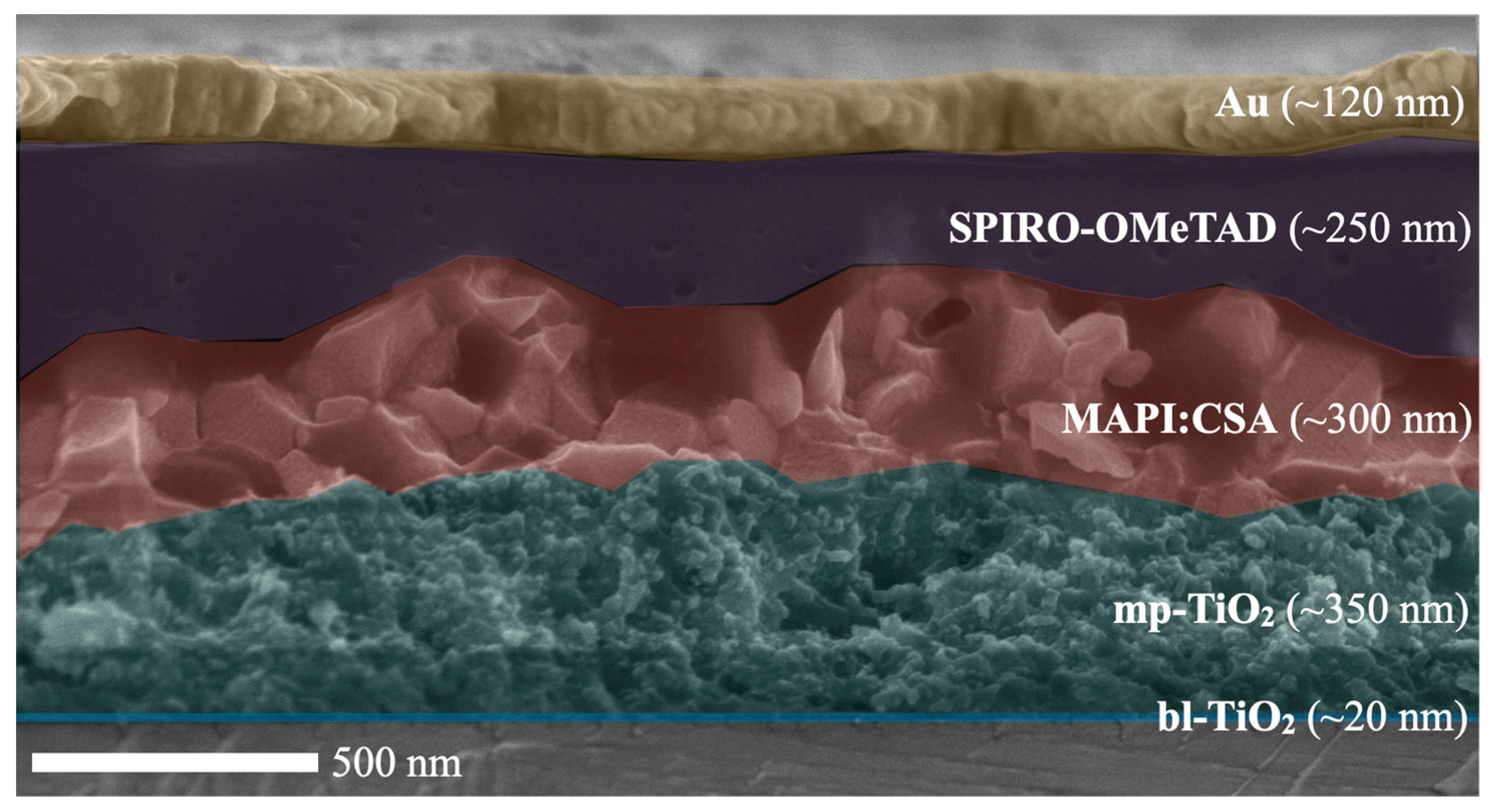
2.2. Photovoltaic Properties
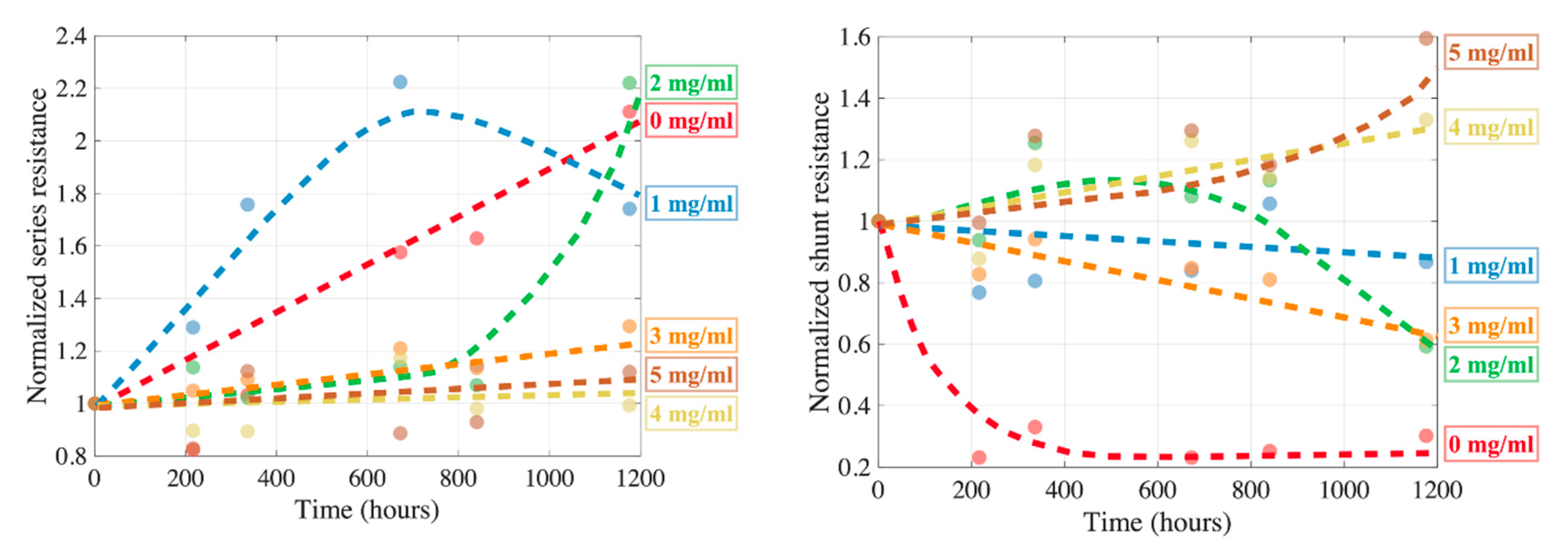
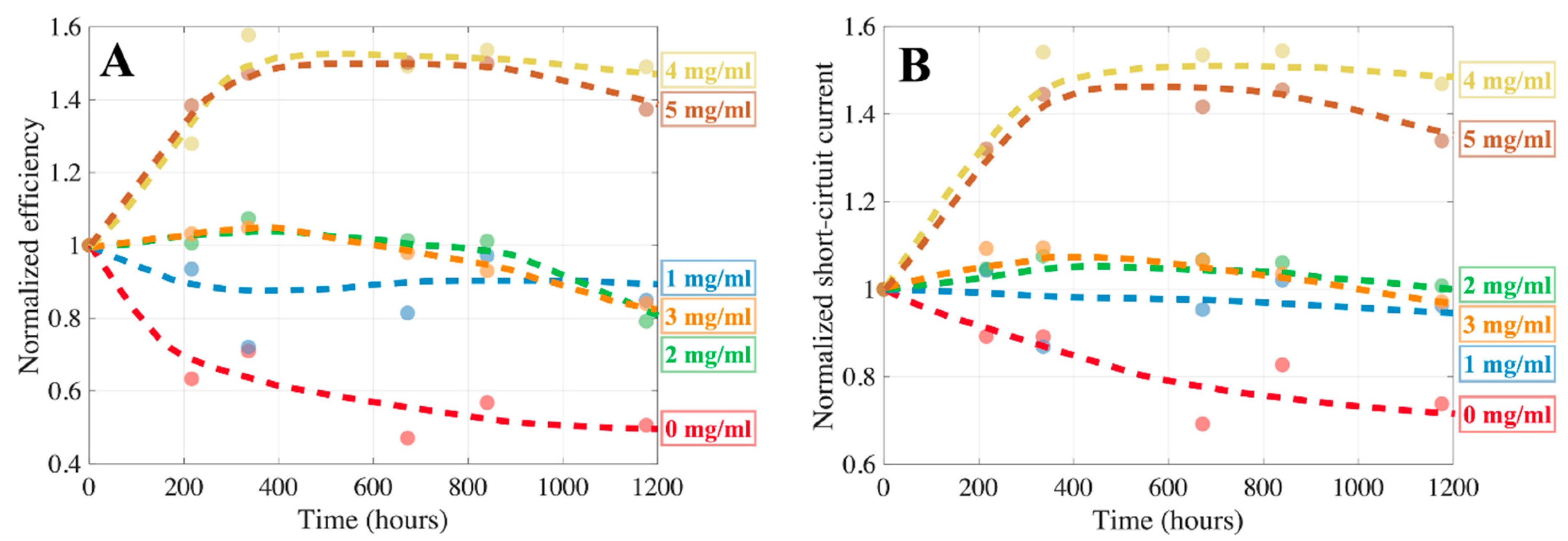
2.3. Degradation
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Perovskite Thin Films
3.3. Preparation of Solar Cells
3.3.1. Cleaning of ITO Substrates
3.3.2. Preparation of ETL
3.3.3. Preparation of Perovskite Layer
3.3.4. Preparation of HTL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| CSA Content (mg/mL) | PCE | Voc | Jsc | FF | ρs | ρsh |
|---|---|---|---|---|---|---|
| 0 | 8.74 | 0.93 | 16.41 | 57.14 | 11.71 | 359.12 |
| 1 | 10.56 | 0.98 | 17.58 | 61.41 | 12.29 | 1222.72 |
| 1.3 | 10.32 | 0.96 | 17.58 | 60.95 | 11.53 | 1042.64 |
| 2 | 9.19 | 0.98 | 16.25 | 57.80 | 12.66 | 670.35 |
| 3 | 6.34 | 0.87 | 12.84 | 56.63 | 13.65 | 577.20 |
| 4 | 4.11 | 0.91 | 7.36 | 61.48 | 17.36 | 451.93 |
| 5 | 3.89 | 0.91 | 7.19 | 59.60 | 17.67 | 414.53 |
References
- Bera, S.; Saha, A.; Mondal, S.; Biswas, A.; Mallick, S.; Chatterjee, R.; Roy, S. Review of Defect Engineering in Perovskites for Photovoltaic Application. Mater. Adv. 2022, 3, 5234–5247. [Google Scholar] [CrossRef]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and Oxygen Induced Degradation Limits the Operational Stability of Methylammonium Lead Triiodide Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef]
- Yuan, H.; Debroye, E.; Janssen, K.; Naiki, H.; Steuwe, C.; Lu, G.; Moris, M.; Orgiu, E.; Uji-I, H.; De Schryver, F.; et al. Degradation of Methylammonium Lead Iodide Perovskite Structures through Light and Electron Beam Driven Ion Migration. J. Phys. Chem. Lett. 2016, 7, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Shang, Y.; Fang, Z.; Hu, W.; Zuo, C.; Li, B.; Li, X.; Wang, M.; Ding, L.; Yang, S.; Shang, Y.; et al. Efficient and Photostable CsPbI2Br Solar Cells Realized by Adding PMMA. J. Semicond. 2021, 42, 050501–050504. [Google Scholar] [CrossRef]
- Liu, S.; Guan, Y.; Sheng, Y.; Hu, Y.; Rong, Y.; Mei, A.; Han, H. A Review on Additives for Halide Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902492. [Google Scholar] [CrossRef]
- Kim, K.; Han, J.; Maruyama, S.; Balaban, M.; Jeon, I. Role and Contribution of Polymeric Additives in Perovskite Solar Cells: Crystal Growth Templates and Grain Boundary Passivators. Sol. RRL 2021, 5, 2000783. [Google Scholar] [CrossRef]
- Pan, J.; Mu, C.; Li, Q.; Li, W.; Ma, D.; Xu, D. Room-Temperature, Hydrochloride-Assisted, One-Step Deposition for Highly Efficient and Air-Stable Perovskite Solar Cells. Adv. Mater. 2016, 28, 8309–8314. [Google Scholar] [CrossRef]
- Heo, J.H.; Song, D.H.; Im, S.H. Planar CH3NH3PbBr3 Hybrid Solar Cells with 10.4% Power Conversion Efficiency, Fabricated by Controlled Crystallization in the Spin-Coating Process. Adv. Mater. 2014, 26, 8179–8183. [Google Scholar] [CrossRef]
- Xu, X.; Chueh, C.C.; Yang, Z.; Rajagopal, A.; Xu, J.; Jo, S.B.; Jen, A.K.Y. Ascorbic Acid as an Effective Antioxidant Additive to Enhance the Efficiency and Stability of Pb/Sn-Based Binary Perovskite Solar Cells. Nano Energy 2017, 34, 392–398. [Google Scholar] [CrossRef]
- Su, L.; Xiao, Y.; Han, G.; Lu, L.; Li, H.; Zhu, M. Performance Enhancement of Perovskite Solar Cells Using Trimesic Acid Additive in the Two-Step Solution Method. J. Power Sources 2019, 426, 11–15. [Google Scholar] [CrossRef]
- Han, F.; Luo, J.; Ashraf Malik, H.; Zhao, B.; Wan, Z.; Jia, C. A Functional Sulfonic Additive for High Efficiency and Low Hysteresis Perovskite Solar Cells. J. Power Sources 2017, 359, 577–584. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Mech, W.; Wincukiewicz, A.; Korona, K.P.; Zarębska, K.; Kamińska, M.; Skompska, M. Investigation of Polyaniline Doped with Camphorsulfonic Acid in Chloroform Solution as a Hole Transporting Layer in PTB7: PCBM and Perovskite-Based Solar Cells. Electrochim. Acta 2021, 380, 138264. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Fang, Y.; Zhou, Y.; Ni, Z.; Xiao, X.; Chen, S.; Huang, J. Ligand Assisted Growth of Perovskite Single Crystals with Low Defect Density. Nat. Commun. 2021, 12, 4–11. [Google Scholar] [CrossRef]
- Wang, S.; Pang, S.; Chen, D.; Zhu, W.; Xi, H.; Zhang, C. Improving Perovskite Solar Cell Performance by Compositional Engineering via Triple-Mixed Cations. Sol. Energy 2021, 220, 412–417. [Google Scholar] [CrossRef]
- (1S)-(+)-Camphor-10-Sulphonic Acid|3144-16-9. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0395928.htm (accessed on 8 October 2022).
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Liu, Z.; Chen, Q.; Wang, X.; Zhou, H. The Additive Coordination Effect on Hybrids Perovskite Crystallization and High-Performance Solar Cell. Adv. Mater. 2016, 28, 9862–9868. [Google Scholar] [CrossRef]
- Nukunudompanich, M.; Budiutama, G.; Suzuki, K.; Hasegawa, K.; Ihara, M. Dominant Effect of the Grain Size of the MAPbI3 Perovskite Controlled by the Surface Roughness of TiO2 on the Performance of Perovskite Solar Cells. CrystEngComm 2020, 22, 2718–2727. [Google Scholar] [CrossRef]
- Kim, H.D.; Ohkita, H.; Benten, H.; Ito, S. Photovoltaic Performance of Perovskite Solar Cells with Different Grain Sizes. Adv. Mater. 2016, 28, 917–922. [Google Scholar] [CrossRef]
- Sangeetha, P.; Mullainathan, S.; Muthu, S.; Rajaraman, B.R.; Saral, A.; Selvakumari, S. Investigation of Spectroscopic (FT-IR, FT-Raman), Reactive Charge Transfer and Docking Properties of (1S) -(+)-10-Camphorsulfonic Acid by Density Functional Method. Mater. Today Proc. 2020, 50, 2768–2776. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast Oxygen Diffusion and Iodide Defects Mediate Oxygen-Induced Degradation of Perovskite Solar Cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tinkham, J.; Schulz, P.; Yang, M.; Kim, D.H.; Berry, J.; Sellinger, A.; Zhu, K. Acid Additives Enhancing the Conductivity of Spiro-OMeTAD Toward High-Efficiency and Hysteresis-Less Planar Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1601451. [Google Scholar] [CrossRef]
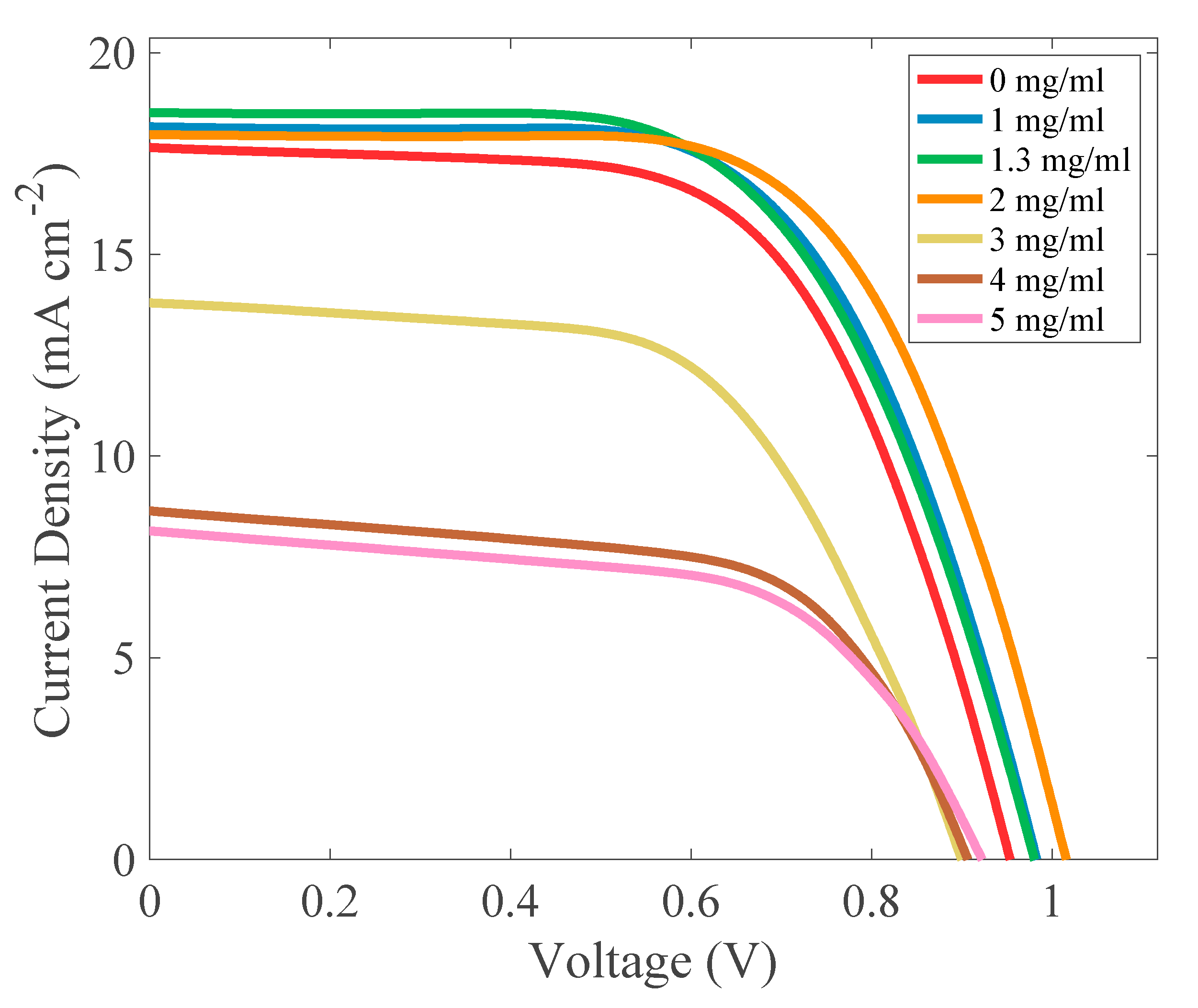
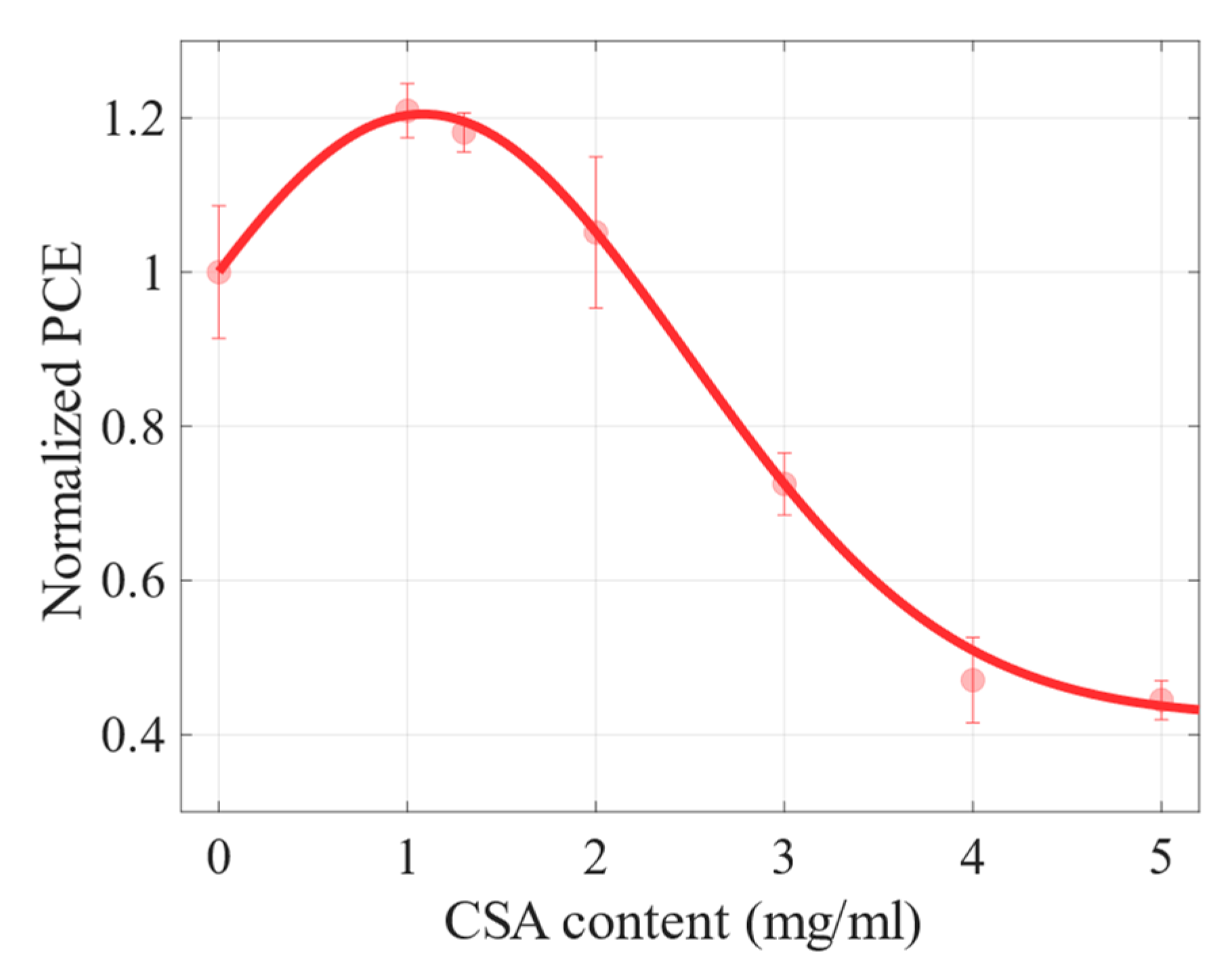
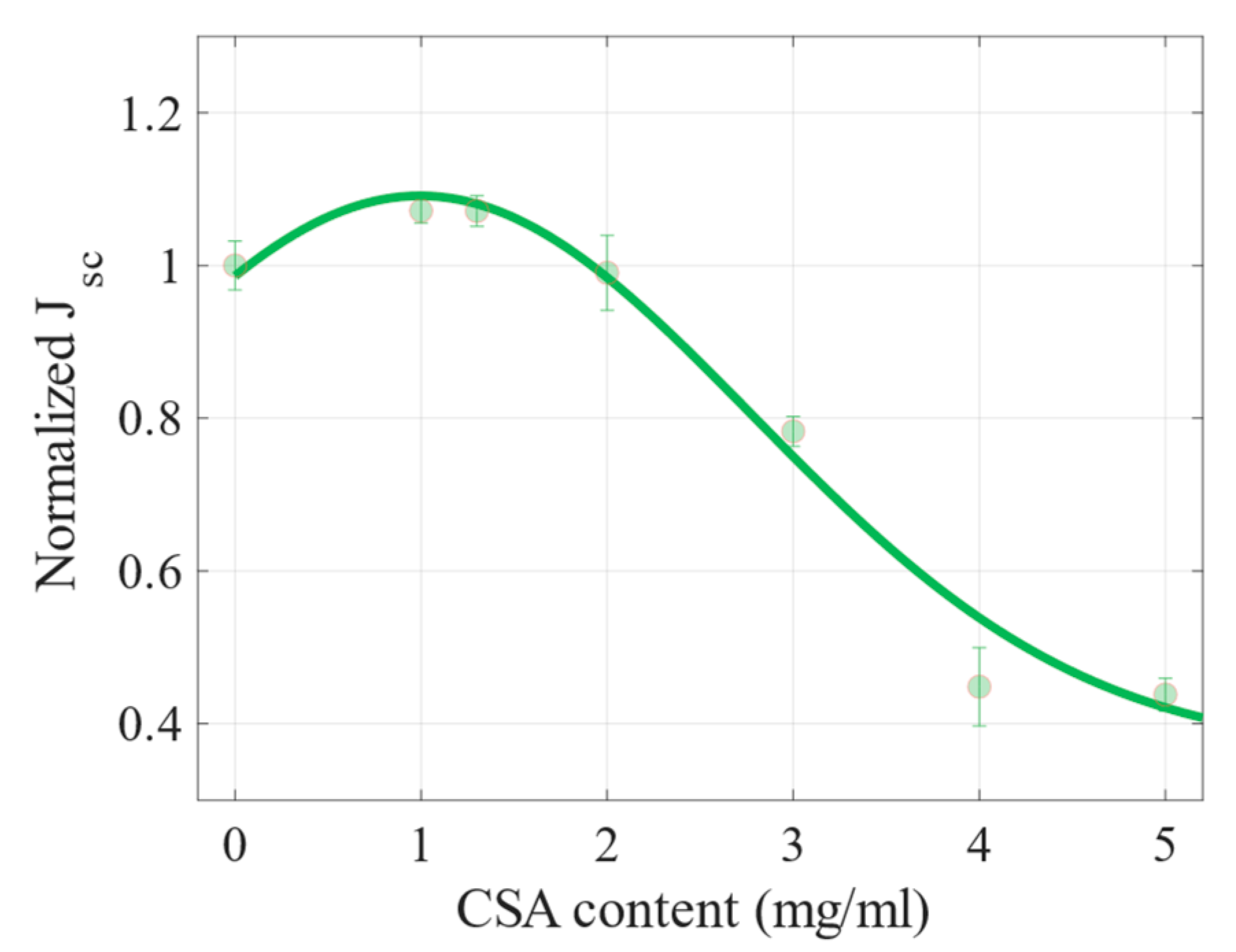
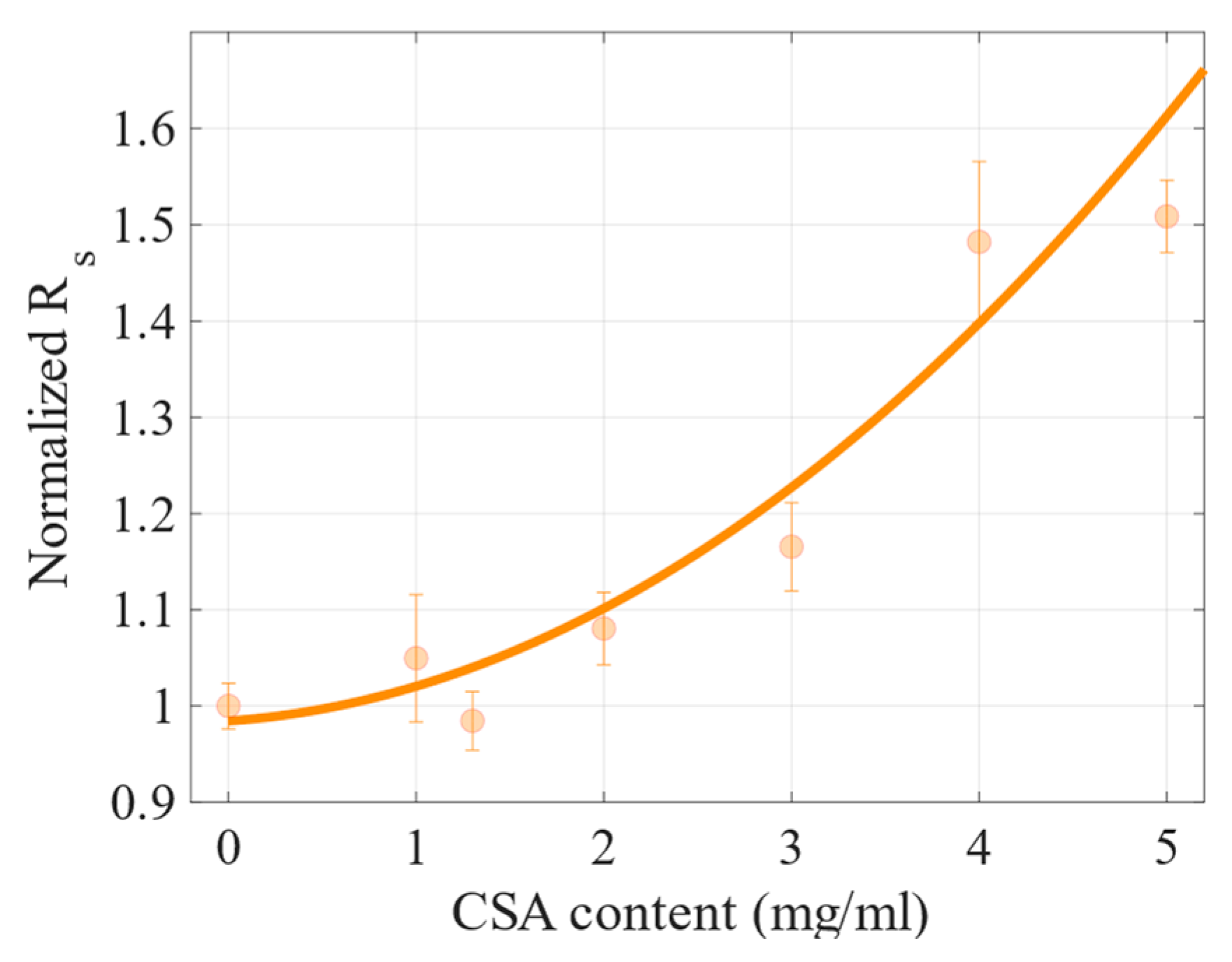
| CSA Content (mg/mL) | Mean PCE (%) | Max PCE (%) | Voc (V) | Jsc (mA cm−2) | FF (%) | ρs (Ω cm2) | ρsh (Ω cm2) |
|---|---|---|---|---|---|---|---|
| 0 | 8.74 | 10.40 | 0.95 | 17.65 | 61.83 | 6.40 | 1297.56 |
| 1 | 10.56 | 11.17 | 0.98 | 18.16 | 62.65 | 6.57 | 3880.28 |
| 1.3 | 10.32 | 11.03 | 0.98 | 18.51 | 60.81 | 6.84 | 7026.6 |
| 2 | 9.19 | 11.73 | 1.02 | 17.96 | 64.34 | 7.03 | 5370.4 |
| 3 | 6.34 | 7.35 | 0.90 | 13.80 | 59.25 | 8.72 | 816.52 |
| 4 | 4.11 | 4.78 | 0.91 | 8.64 | 61.08 | 8.79 | 585.32 |
| 5 | 3.89 | 4.47 | 0.92 | 8.15 | 59.52 | 10.90 | 562.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wincukiewicz, A.; Wierzyńska, E.; Bohdan, A.; Tokarczyk, M.; Korona, K.P.; Skompska, M.; Kamińska, M. Enhanced Performance of Camphorsulfonic Acid-Doped Perovskite Solar Cells. Molecules 2022, 27, 7850. https://doi.org/10.3390/molecules27227850
Wincukiewicz A, Wierzyńska E, Bohdan A, Tokarczyk M, Korona KP, Skompska M, Kamińska M. Enhanced Performance of Camphorsulfonic Acid-Doped Perovskite Solar Cells. Molecules. 2022; 27(22):7850. https://doi.org/10.3390/molecules27227850
Chicago/Turabian StyleWincukiewicz, Adam, Ewelina Wierzyńska, Aliaksei Bohdan, Mateusz Tokarczyk, Krzysztof P. Korona, Magdalena Skompska, and Maria Kamińska. 2022. "Enhanced Performance of Camphorsulfonic Acid-Doped Perovskite Solar Cells" Molecules 27, no. 22: 7850. https://doi.org/10.3390/molecules27227850
APA StyleWincukiewicz, A., Wierzyńska, E., Bohdan, A., Tokarczyk, M., Korona, K. P., Skompska, M., & Kamińska, M. (2022). Enhanced Performance of Camphorsulfonic Acid-Doped Perovskite Solar Cells. Molecules, 27(22), 7850. https://doi.org/10.3390/molecules27227850







