Comparative Study of Useful Compounds Extracted from Lophanthus anisatus by Green Extraction
Abstract
1. Introduction
2. Results
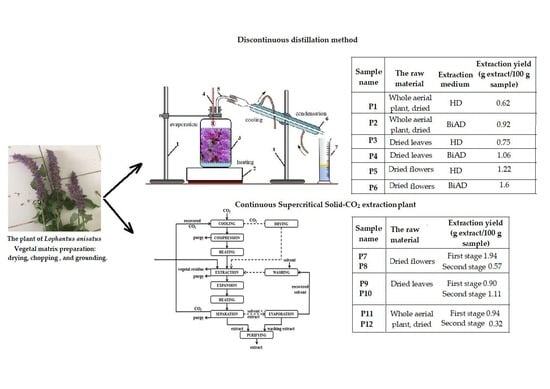
2.1. Green Oil Extraction Yields
2.1.1. HD and BiAD Oil Extraction Yields
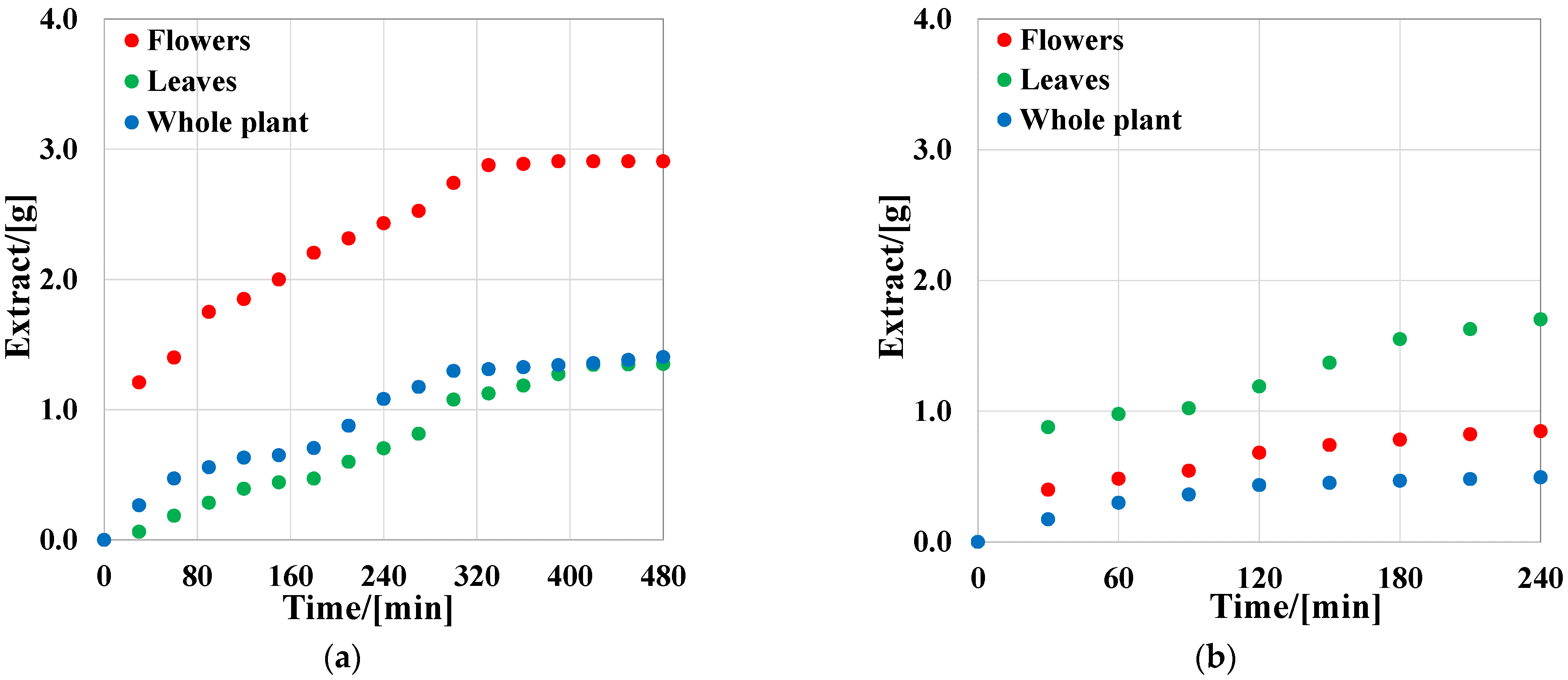
2.1.2. SFE Extraction
Supercritical Extraction Process Yield
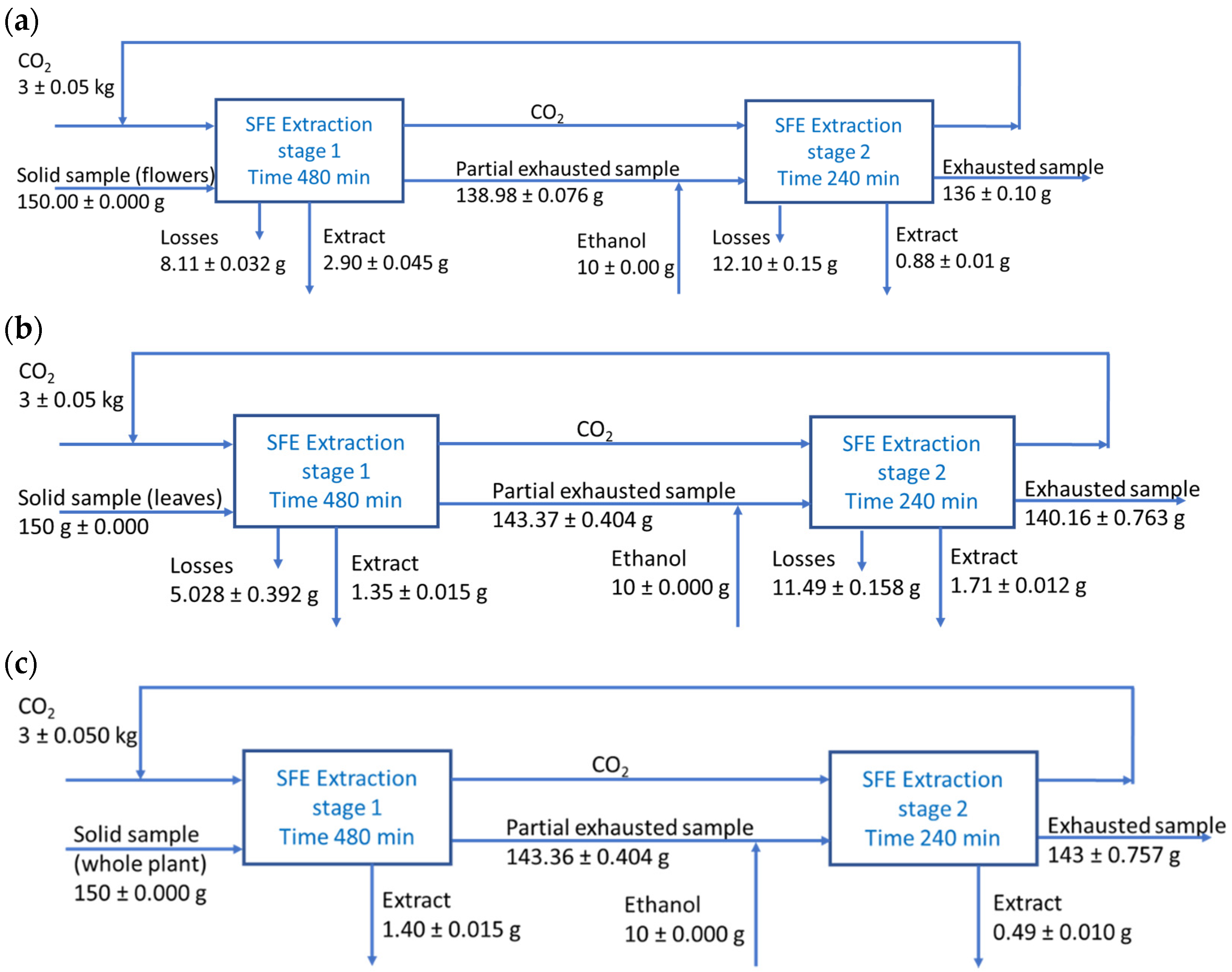
SFE Extraction Mass Balances
SFE Extract Qualitative Analysis
2.2. Total Polyphenolic Active Compounds Obtained by Green Extraction
Polyphenolic Compound Concentrations in the Extracts Obtained by HD, BiAD and SFE Determined Using the HPLC Method
2.3. The Composition of Other Active Compounds Obtained by Green Extraction
2.4. Antimicrobial Activity of Essential Oils from Lophanthus anisatus
3. Discussion
4. Materials and Methods
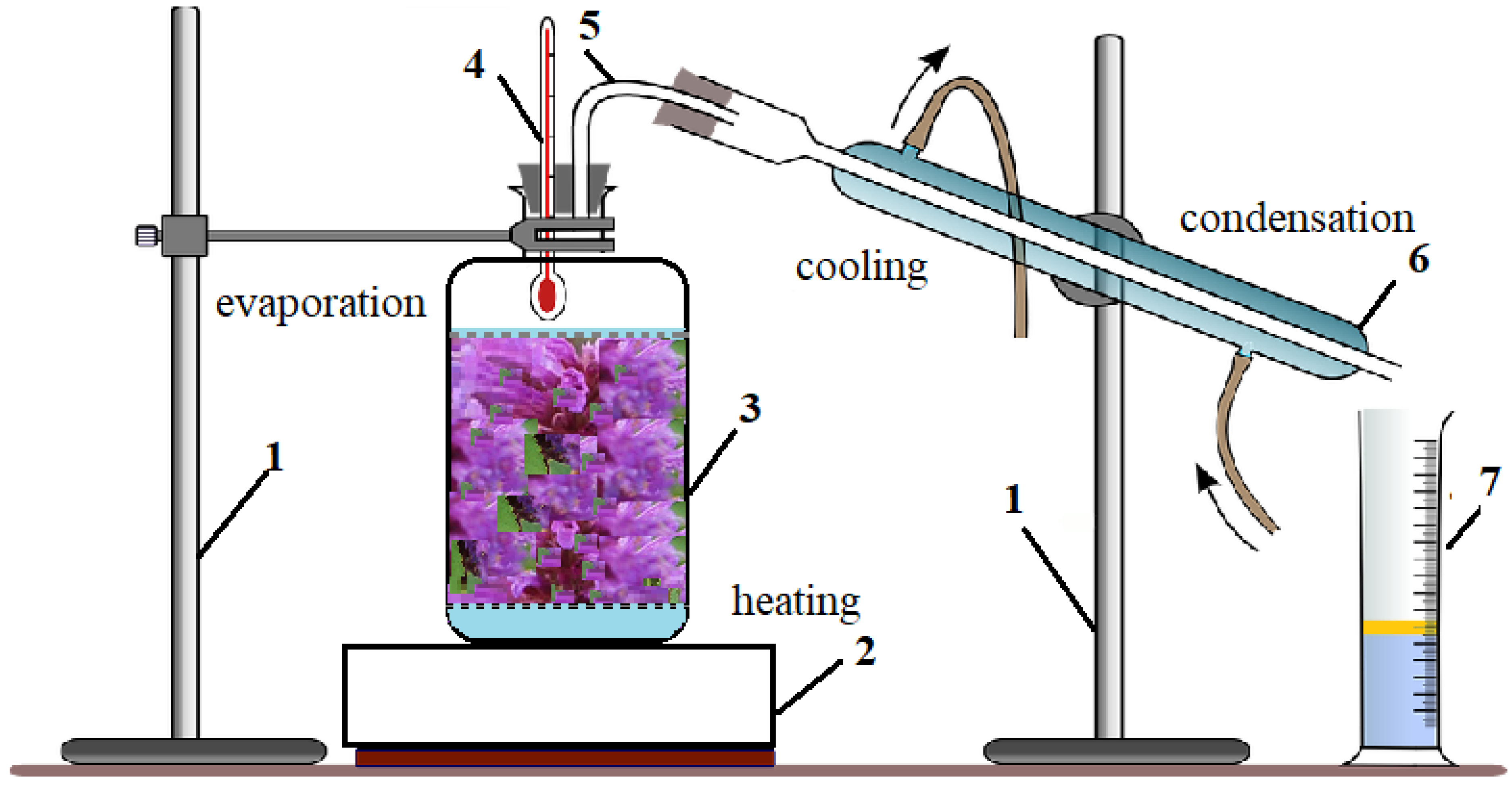
4.1. Sample Preparation
4.2. Extraction Methods
4.2.1. Obtaining the Essential Oil and Extracts by the Green Methods: Hydro-distillation and Bio-Alcoholic Solvent Distillation Methods
4.2.2. Supercritical CO2 Extraction, SFE
4.3. Physicochemical Determinations
4.3.1. Determination of Total Polyphenolic Compounds by UV-VIS Spectrometry
4.3.2. Determination of Polyphenolic Compounds by High Performance Liquid Chromatography (HPLC)
4.3.3. Mass Spectroscopy
4.4. Antimicrobial Activity of Essential Oils from Lophanthus anisatus
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Integrated Taxonomic Information System. Available online: https://www.itis.gov (accessed on 15 April 2021).
- Kumar, U.; Kumari, P.; Sinha, D.; Yadav, M.; Singh, D.; Singh, B.K. Antimicrobial Activity of Essential Oils Against Plant Pathogenic Fungi: A Review. Int. J. Incl. Dev. 2020, 6, 37–44. [Google Scholar] [CrossRef]
- Jomova, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Barbierikova, Z.; Malcek, M.; Alwasel, S.H.; Alhazza, I.M.; Rhodes, C.J.; Valko, M. The effect of Luteolin on DNA damage mediated by a copper catalyzed Fenton reaction. J. Inorg. Biochem. 2022, 226, 111635. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin—A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Kutchin, A.V.; Shishkina, L.N.; Weisfeld, L.I. Chemistry and Technology of Plant Substances: Chemical and Biochemical Aspects, 1st ed.; Apple Academic Press: Toronto, ON, Canada, 2017; pp. 329–332. [Google Scholar]
- Hossan, S.; Rahman, S.; Bashar, A.B.M.; Jahan, R.; Nahain, A.; Rahmatullah, M. Rosmarinic acid: A review of its anticancer action. World J. Pharm. Pharm. Sci. 2014, 3, 57–70. [Google Scholar]
- Strilbytska, O.M.; Zayachkivska, A.; Koliada, A.; Galeotti, F.; Volpi, N.; Storey, K.B.; Vaiserman, A.; Lushchak, O. Anise Hyssop Agastache foeniculum Increases Lifespan, Stress Resistance, and Metabolism by Affecting Free Radical Processes in Drosophila. Front. Physiol. 2020, 11, 596729. [Google Scholar] [CrossRef]
- Ownagh, A.; Hasani, A.; Mardani, K.; Ebrahimzadeh, S. Antifungal effects of thyme, agastache and satureja essential oils on Aspergillus fumigatus, Aspergillus flavus and Fusarium solani. Vet. Res. Forum. 2010, 1, 99–105. [Google Scholar]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison with Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00263/full (accessed on 15 May 2021). [CrossRef] [PubMed]
- Svoboda, K.P.; Gough, J.; Hampson, J.; Galambosi, B. Analysis of the essential oils of some Agastache species grown in Scotland from Various Seed Sources. Flavour Fragr. J. 1995, 10, 139–145. [Google Scholar] [CrossRef]
- Kotyuk, L.A. Biochemical Characteristics of Lophanthus Anisatus Adans. With Introduction in Polissya Conditions of Ukraine. Modern Scientific Research and Their Practical Application. 2013, p. J21301. Available online: http://www.sworld.com.ua/e-journal/J21301.pdf (accessed on 15 May 2021).
- Kovalenko, N.A.; Supichenko, G.N.; Leontiev, V.N.; Shutova, A.G. Composition of essential oil of plants of the genus Agastache L. introduced in Belarus. Proceedings of the National Academy of Sciences of Belarus. Biol. Ser. 2019, 64, 147–155. [Google Scholar] [CrossRef]
- Dapkevicius, A.; Venskutonis, R.; van Beek, T.A.; Linssen, J.P.H. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Pranskuniene, Z.; Ratkeviciute, K.; Simaitiene, Z.; Translunar, A.; Bernatoniene, J. Ethnobotanical Study of Cultivated Plants in Kaišiadorys District, Lithuania: Possible Trends for New Herbal Based Medicines. Evid. Based Complement. Altern. Med. 2019, 2019, 3940397. [Google Scholar] [CrossRef] [PubMed]
- Shtereva, L.; Vassilevska-Ivanova, R.; Stancheva, I.; Geneva, M.; Stoyanova, E. Evaluation of antioxidant activity of Agastache foeniculum and Agastache rugosa extracts. Comptes Rendus L ‘academie Bulg. Des Sci. 2016, 69, 295–302. [Google Scholar]
- Ivanov, I.G.; Vrancheva, R.Z.; Petkova, N.T.; Tumbarski, I.; Dincheva, I.N.; Ilian, K.; Badjakov, I.K. Phytochemical compounds of anise hyssop (Agastache foeniculum) and antibacterial, antioxidant, and acetylcholinesterase inhibitory properties of its essential oil. J. Appl. Pharm. Sci. 2019, 9, 72–78. [Google Scholar] [CrossRef]
- Nykanen, I.; Holm, Y.; Hiltunen, R. Composition of the EO of Agastache foeniculum. Planta Med. 1989, 55, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Ehsani, A.; Hassani, A.; Afshari, A.; Aminzare, M.; Sahranavard, T.; Azimzadeh, Z. Phytochemical, antibacterial, antifungal and antioxidant properties of Agastache foeniculum essential oil. J. Chem. Health Risks. 2017, 7, 95–104. Available online: http://www.jchr.org/article_544170.html (accessed on 15 June 2021).
- Mallavarapu, G.; Kulkarni, R.N.; Baskaran, K.; Ramesh, S. The EO composition of anise hyssop grown in India. Flavour Fragr. J. 2004, 19, 351–353. [Google Scholar] [CrossRef]
- Duda, M.M.; Matei, C.F.; Vârban, D.I.; Muntean, S.; Moldovan, C. The Results of Cultivating the Species Agastache foeniculum (Pursh) Kuntze at Jucu. CJ. Bull. USAMV Ser. Agric. 2013, 70, 214–217. [Google Scholar] [CrossRef]
- Chumakova, V.V.; Popova, O.I. Agastache foeniculum–A Perspective Source of Medical Products. Pharm. Pharmacol. 2013, 1, 39–43. [Google Scholar] [CrossRef][Green Version]
- Yurtaeva, E.A.; Remezova, I.P.; Tyrkov, A.G.; Luzhnova, S.A.; Popova, O.I. Dry leaf extract of Lophantus anisatum Benth: Obtaining and analysis of phenolic compounds. Pharmacy 2019, 68, 28–32. [Google Scholar] [CrossRef]
- Zielińska, S.; Matkowski, A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem Rev. 2014, 13, 391–416. [Google Scholar] [CrossRef]
- Duda, S.C.; Marghitas¸, L.A.; Dezmirean, D.; Duda, M.; Margaoan, R.; Bobis¸, O. Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of harvest time and plant species. Ind. Crops Prod. 2015, 77, 499–507. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Mahmoodi, M. Effect of Irrigation Regimes on the EO Content and Composition of Agastache foeniculum. J. Essent. Oil-Bear. Plants 2010, 13, 59–65. [Google Scholar] [CrossRef]
- Saeedfar, S.; Negahban, M.; Soorestani, M.M. The Effect of Drought Stress on the EO Content and Some of the Biochemical Characteristics of Anise Hyssop (Agastache foeniculum [Pursh] Kuntze). Eur. J. Mol. Biotechnol. 2015, 8, 103–114. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Kabudani, M.; Khoorang, M. Nitrogen fertilizer affecting herb yield, EO content and compositions of Agastache foeniculum purch. J. Essent. Oil-Bear. Plants. 2008, 11, 261–266. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Sefidkon, F. Essential Oil Composition of Agastache foeniculum Cultivated in Iran. J. Ess. Oil Res. 2003, 15, 52–53. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Sefidkon, F. Effect of sowing time on the essential oil content and composition of Agastache foeniculum. J. Essent. Oil-Bear. Plants 2004, 7, 190–194. [Google Scholar] [CrossRef]
- Moghaddam, M.; Estaji, A.; Farhadi, N. Effect of Organic and Inorganic Fertilizers on Morphological and Physiological Characteristics, Essential Oil Content and Constituents of Agastache (Agastache foeniculum). J. Essent. Oil-Bear. Plants 2015, 18, 1372–1381. [Google Scholar] [CrossRef]
- Sourestani, M.; Malekzadeh, M.; Tava, A. Influence of drying, storage and distillation times on EO yield and composition of anise hyssop [Agastache foeniculum (Pursh.) Kuntze]. J. Ess. Oil Res. 2014, 26, 177–184. [Google Scholar] [CrossRef]
- Shutava, H.G.; Shutava, T.G.; Kavalenka, N.A.; Supichenka, H.N. Antiradical and Antibacterial Activity of Essential Oils from the Lamiaceae Family Plants in Connection with their Composition and Optical Activity of Components. Int. J. Sec. Metab. 2018, 5, 109–122. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef]
- Polak, E.H.; Hixon, R.M. The volatile oil from Lophantus anisatus Benth. Am. Pharm. Assoc. 1945, 35, 240–243. [Google Scholar] [CrossRef]
- Lashkari, A.; Najafi, F.; Kavoosi, G.; Saeed, N.A. Evaluating the In vitro anti-cancer potential of Estragole from the EO of Agastache foeniculum [Pursh.] Kuntze. Biocatal. Agric. Biotechnol. 2020, 27, 101727. [Google Scholar] [CrossRef]
- Mazza, G.; Kiehn, F.A. Essential Oil of Agastache foeniculum, A Potential Source of Methyl Chavicol. J. Ess. Oil Res. 1992, 4, 295–299. [Google Scholar] [CrossRef]
- Charles, D.J.; Simon, J.E.; Widrlechner, M.P. Characterization of Essential Oil of Agastache Species. J. Agric. Food Chem. 1991, 39, 1946–1949. [Google Scholar] [CrossRef]
- Chae, S.C.; Lee, S.W.; Kim, J.K.; Park, W.T.; Uddin, M.R.; Kim, H.H.; Park, S.U. Variation of Carotenoid Content in Agastache rugosa and Agastache foeniculum. Asian J. Chem. 2013, 25, 4364–4366. [Google Scholar] [CrossRef]
- Woo Tae Park, W.T.; Kim, H.H.; Chae, S.C.; Cho, J.W.; Sang, U.; Park, S.U. Phenylpropanoids in Agastache foeniculum and Its Cultivar A. foeniculum ‘Golden Jubilee’. Asian J. Chem. 2014, 26, 4599–4601. [Google Scholar] [CrossRef]
- Ebadollahi, A. Chemical constituents and toxicity of Agastache foeniculum (Pursh) Kuntze EO against two stored-product insect pests. Chil. J. Agric. Res. 2011, 71, 212–217. [Google Scholar] [CrossRef]
- Talebi, S.F.; Saharkhiz, M.J.; Maryam, M.J.; Kermani, J.; Sharafi, Y.; Fard, F.R. Effect of Ploidy Level on the Nuclear Genome Content and Essential Oil Composition of Anise Hyssop (Agastache foeniculum [Pursh.] Kuntze). Anal. Chem. Lett. 2016, 6, 678–687. [Google Scholar] [CrossRef]
- Waller, S.B.; Cleff, M.B.; Serra, E.F.; Silva, A.L.; Gomes, A.R.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Plants from Lamiaceae family as source of antifungal molecules in humane and veterinary medicine. Microb. Pathog. 2017, 104, 232–237. [Google Scholar] [CrossRef]
- Derakhshani, Z.; Hassani, A.; Pirzad, A.; Abdollahi, R.; Dalkani, M. Evaluation of phenolic content and antioxidant capacity in some medicinal herbs cultivated in Iran. Bot. Serbica 2012, 36, 117–122. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. 2015 Essential Oils as Natural Food Antimicrobial Agents: A Review. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- de Souza, E.L. The effects of sublethal doses of essential oils and their constituents on antimicrobial susceptibility and antibiotic resistance among food-related bacteria: A review. Trends Food Sci. Technol. 2016, 56, 1–12. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Juárez-Becerra, G.P.; Sosa-Morales, M.E.; López-Malo, A. Microwave-assisted extraction of essential oils from herbs. J. Microw. Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidovic, S.; Redovnikovic, I.R.; Jokic, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Yadav, R.K.; Yadav, B.; Srivastav, G.; Jha, O.; Yadav, R.A. Conformational, structural and vibrational studies of estragole. Vib. Spectrosc. 2021, 117, 103317. [Google Scholar] [CrossRef]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of Eugenol against Escherichai coli. J. Herb. Med. 2020, 26, 100406. [Google Scholar] [CrossRef]
- Cui, X.; Gu, G.; Li, C.; Liu, N.; Gong, Y.; Liu, B. Synthesis and properties of biomass eugenol-functionalized isotactic poly (1-butene)s. Polymer 2020, 202, 122739. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, A.E.; Gonzalez-Trujano, M.E.; Hernandez-Leon, A.; Valle-Dorado, M.G.; Carballo-Villalobos, A.; Orozco-Suarez, S.; Alvarado-Vasquez, N.; Lopez-Munoz, F.J. Limonene from Agastache Mexicana essential oil antinociceptive effects, gastrointestinal protection and improves experimental ulcerative colitis. J. Ethnopharmacol. 2021, 280, 114462. [Google Scholar] [CrossRef]
- Cady, N.C.; McKean, K.A.; Behnke, J.; Kubec, R.; Mosier, A.P.; Kasper, S.H.; Burz, D.S.; Musah, R.A. Inhibition of Biofilm Formation, Quorum Sensing and Infection in Pseudomonas aeruginosa by Natural Products-Inspired Organosulfur Compounds. PLoS ONE 2012, 7, e38492. [Google Scholar] [CrossRef]



| Sample Type | Extraction Medium | Extraction Yield (g Extract/100 g Dried Plant ± SD) |
|---|---|---|
| Whole aerial plant, dried | HD | 0.62 ± 0.020 |
| Whole aerial plant, dried | BiAD | 0.92 ± 0.015 |
| Dried leaves | HD | 0.75 ± 0.008 |
| Dried leaves | BiAD | 1.06 ± 0.005 |
| Dried flowers | HD | 1.22 ± 0.011 |
| Dried flowers | BiAD | 1.60 ± 0.0049 |
| Sample Type | Dried Sample | Ground Sample | Extract | Extraction Yield (g Extract/100 g Sample ± SD) |
|---|---|---|---|---|
| Flowers |  |  |  | First stage 1.94 ± 0.030 Second stage 0.57 ± 0.003 |
| Leaves |  |  |  | First stage 0.90 ± 0.010 Second stage 1.14 ± 0.008 |
| Whole plant |  |  |  | First stage 0.94 ± 0.010 Second stage 0.32 ± 0.007 |
| Sample Name | Raw Material/Extraction Medium | Total Concentration Expressed as Gallic Acid Equivalent | |
|---|---|---|---|
| [mg/L Extract] | [mg/g Dried Plant] | ||
| P1 | Dried whole aerial plant/HD | 1.7 | 4.25 |
| P2 | Dried whole aerial plant/BiAD | 3.4 | 8.50 |
| P3 | Dried leaves/HD | 3.8 | 9.50 |
| P4 | Dried leaves/BiAD | 4.9 | 12.25 |
| P5 | Dried flowers/HD | 3.2 | 8.00 |
| P6 | Dried flowers/BiAD | 4.1 | 10.25 |
| P7 | Dried flowers/SFE stage 1 | 5.1 | 12.75 |
| P8 | Dried flowers/SFE stage 2 | 2.1 | 5.25 |
| P9 | Dried leaves/SFE stage 1 | 5.6 | 14.00 |
| P10 | Dried leaves/SFE stage 2 | 2.4 | 6.00 |
| P11 | Dried whole aerial plant/SFE stage 1 | 3.9 | 9.75 |
| P12 | Dried whole aerial plant/SFE stage 2 | 1.9 | 4.75 |
| Sample Name */Substance | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorogenic acid | 5.25 | 16.35 | 28.80 | 33.63 | 22.05 | 31.50 | 33.90 | 16.70 | 41.70 | 21.30 | 36.30 | 19.65 |
| Caffeic acid | 2.85 | 11.01 | 8.10 | 11.85 | 1.95 | 3.45 | 7.20 | 4.40 | 14.20 | 12.75 | 11.00 | 8.00 |
| Rosmarinic acid | 120.60 | 220.50 | 204.00 | 309.00 | 191.10 | 312.00 | 277.50 | 138.00 | 279.00 | 128.40 | 227.00 | 130.80 |
| Apigenin 7-glucoside | 3.90 | 21.90 | 18.15 | 25.50 | 4.05 | 8.43 | 26.10 | 10.80 | 29.10 | 23.70 | 9.60 | 6.30 |
| Ferulic acid | 1.30 | 2.80 | 1.67 | 2.25 | 2.34 | 3.15 | 4.35 | 2.40 | 3.45 | 0.90 | 3.75 | 13.5 |
| Crt. No. | Component Denomination | P1 | P2 | P3 | P4 | P5 | P6 | |
|---|---|---|---|---|---|---|---|---|
| 1 | Chavicol, (p-Allylphenol) | 1.04 | 1.27 | 14.22 | - | |||
| 2 | Estragole (methyl chavicol) | 66.48 | 89.32 | 30.16 | 18.17 | 88.09 | 91.31 | |
| 3 | Methoxy-eugenol | - | 0.22 | - | 17.30 | 0.68 | 0.05 | |
| 4 | 2-Allyl-4-methoxyphenol) | 1.22 | - | - | ||||
| 5 | Limonene | 5.24 | 5.40 | - | 8.01 | 7.07 | ||
| 6 | Methyl eugenol Eugenol methyl ether | 9.77 | 0.22 | - | 0.68 | 0.10 | ||
| 7 | Caryophyllene | 1.37 | 0.72 | - | ||||
| 9 | (o-Allylphenol) | 1.22 | - | - | ||||
| 10 | (n-Octyl Acetate) | 0.21 | 0.23 | - | 0.16 | |||
| 11 | Octanol | - | - | - | 0.64 | 0.21 | ||
| 12 | Eugenol | 0.04 | 13.96 | 17.96 | 0.37 | 0.17 | ||
| 13 | Benzaldehyde | 5.2 | 0.55 | 2.44 | 10.16 | 0.12 | ||
| 14 | Pentanol | 4.45 | 1.66 | 3.13 | 8.97 | 0.06 | ||
| 15 | Benzyl alcohol | - | 2.44 | 3.10 | 0.09 | |||
| 16 | Phenyl ethyl alcohol | - | 20.19 | 2.80 | 0.05 | |||
| 17 | Methyl jasmonate | - | 0.51 | |||||
| 18 | Ethyl lactate | 4.53 | 2.63 | |||||
| 19 | Cadinol α | - | 0.10 | 0.08 | ||||
| Crt. No. | Component Denomination | P7 | P8 | P9 | P10 | P11 | P12 |
|---|---|---|---|---|---|---|---|
| 1 | Estragole | 88.89 | 91.41 | 79.5 | 80.17 | 92.37 | 93.04 |
| 2 | Limonene | 8.01 | 3.91 | 0.35 | 2.71 | 3.24 | |
| 3 | Caryophyllene | 0.94 | 1.16 | 0.79 | 1.28 | ||
| 4 | Germacrene | 0.59 | 0.79 | 1.13 | 0.87 | ||
| 5 | Octanol acetate | 0.16 | 0.06 | 0.39 | 0.10 | ||
| 6 | Elemene τ | 0.20 | 0.14 | 0.48 | 0.37 | 0.18 | 0.26 |
| 7 | Phellandrene | 0.09 | 0.04 | 1.05 | 0.06 | ||
| 8 | Eugenol | 0.37 | 0.28 | 4.57 | 4.57 | 0.57 | 0.06 |
| 9 | Cadinol α | 0.10 | 0.04 | 0.30 | 0.11 | 0.14 | 0.06 |
| 10 | Phytol | 0.15 | 0.73 | 0.10 | |||
| 11 | Myrcene | 0.07 | 0.22 | 0.10 | |||
| 12 | 3 Octenone (Ethyl amyl ketone) | 0.07 | 0.05 | 0.09 | |||
| 13 | Octenol 3 Ol (Vinyl amyl carbinol) | 0.40 | 0.21 | 1.31 | 0.07 | ||
| 14 | Terpineol | 0.04 | 0.05 | ||||
| 15 | |||||||
| 16 | Methyl eugenol ether | 0.68 | 1.74 | 1.23 | 0.65 | ||
| 17 | Cadinene | 0.04 | 0.22 | 0.06 | |||
| 18 | Cubenol | 0.03 | 0.04 | ||||
| 19 | 2 Limonene | 8.25 | 10.31 | 0.25 | 3.40 | ||
| 20 | Phellandrene α | 0.06 | 0.13 | ||||
| 21 | Ethyl amyl ketone | ||||||
| 22 | Octenol-1-ol, acetate (oct-1-enyl acetate) | 0.21 | |||||
| 23 | Caryophyllene β | 1.06 | 1.42 | ||||
| 24 | Germacrene D | 0.95 | 1.02 | ||||
| 25 | Cadinadiene | 0.08 | |||||
| 26 | Germacrene D-4-ol | 0.07 | 0.10 | ||||
| 27 | 2 Eugenol | 0.17 | 0.06 | 0.09 |
| Tested Substances | Diameter of the Inhibition Zone (mm) | ||
|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | |
| EO Lophanthus anisatus flowers | 18.0 | 0 | 0 |
| EO Lophanthus anisatus leaves | 9.0 | 0 | 0 |
| EO Lophanthus anisatus whole plant | 10.5 | 0 | 0 |
| Lophanthus anisatus flowers (infusion) | 0 | 12 | 7 |
| Lophanthus anisatus flowers (soak) | 0 | 0 | 9 |
| Mint tea (infusion) (Maxi Pharma) (leaves and aerial part) | 12 | 0 | 0 |
| Mint tea (decoct) (Maxi Pharma) (leaves and aerial part) | 10 | 0 | 7 |
| Global interpretation | Intermediate | Resistant | Intermediate |
| Tested Substances | Antibiofilm Effect | ||
|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | |
| EO Lophanthus anisatus flowers | + | - | - |
| EO Lophanthus anisatus leaves | + | - | - |
| EO Lophanthus anisatus whole plant | + | - | - |
| Global interpretation | Active | Inactive | Inactive |
| Sample Name | Raw Material | Extraction Method | Plant/Extraction Medium Ratio |
|---|---|---|---|
| P1 | Whole aerial plant, dried | HD | 2000 g/10 L distilled water |
| P2 | Whole aerial plant, dried | BiAD | 2000 g/10 L bio-alcohol solvent, 35 % alcoholic degrees |
| P3 | Dried leaves | HD | 2000 g/10 L distilled water |
| P4 | Dried leaves | BiAD | 2000 g/10 L bio-alcohol solvent, 35 % alcoholic degrees |
| P5 | Dried flowers | HD | 2000 g/10 L distilled water |
| P6 | Dried flowers | BiAD | 2000 g/10 L bio-alcohol solvent, 35 % alcoholic degrees |
| Sample Name | Raw Material | Extraction Medium | Extraction Conditions |
|---|---|---|---|
| P7 | Dried flowers | Extraction stage 1 | 150 g dried flowers 8 h extraction time 13 kg/h CO2 flow rate 40 MPa pressure 40 °C temperature |
| P9 | Dried leaves | ||
| P11 | Whole aerial plant, dried | ||
| P8 | Dried flowers | Extraction stage 2 | The P7, P9, P11 sprayed with 10 g alcohol 4 h extraction time 13 kg/h CO2 flow rate 40 MPa pressure 40°C temperature |
| P10 | Dried leaves | ||
| P12 | Whole aerial plant, dried |
| Element | Time (min) | Temperature (°C) |
|---|---|---|
| Column | 0–10 | 40 |
| 10–45 | 40–220 | |
| 45–55 | 220 | |
| Injector | 200 | |
| Detector | 235 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan, D.-S.; Popescu, M.; Luntraru, C.-M.; Suciu, A.; Belcu, M.; Ionescu, L.-E.; Popescu, M.; Iancu, P.; Stefan, M. Comparative Study of Useful Compounds Extracted from Lophanthus anisatus by Green Extraction. Molecules 2022, 27, 7737. https://doi.org/10.3390/molecules27227737
Stefan D-S, Popescu M, Luntraru C-M, Suciu A, Belcu M, Ionescu L-E, Popescu M, Iancu P, Stefan M. Comparative Study of Useful Compounds Extracted from Lophanthus anisatus by Green Extraction. Molecules. 2022; 27(22):7737. https://doi.org/10.3390/molecules27227737
Chicago/Turabian StyleStefan, Daniela-Simina, Mariana Popescu, Cristina-Mihaela Luntraru, Alexandru Suciu, Mihai Belcu, Lucia-Elena Ionescu, Mihaela Popescu, Petrica Iancu, and Mircea Stefan. 2022. "Comparative Study of Useful Compounds Extracted from Lophanthus anisatus by Green Extraction" Molecules 27, no. 22: 7737. https://doi.org/10.3390/molecules27227737
APA StyleStefan, D.-S., Popescu, M., Luntraru, C.-M., Suciu, A., Belcu, M., Ionescu, L.-E., Popescu, M., Iancu, P., & Stefan, M. (2022). Comparative Study of Useful Compounds Extracted from Lophanthus anisatus by Green Extraction. Molecules, 27(22), 7737. https://doi.org/10.3390/molecules27227737