Lycopene Improves Metabolic Disorders and Liver Injury Induced by a Hight-Fat Diet in Obese Rats
Abstract
1. Introduction
Obesity: Treatments, Conceptualizations, and Future Directions for a Growing Problem
2. Results
2.1. Effect of LYC on Body Weight and Abdominal Fat
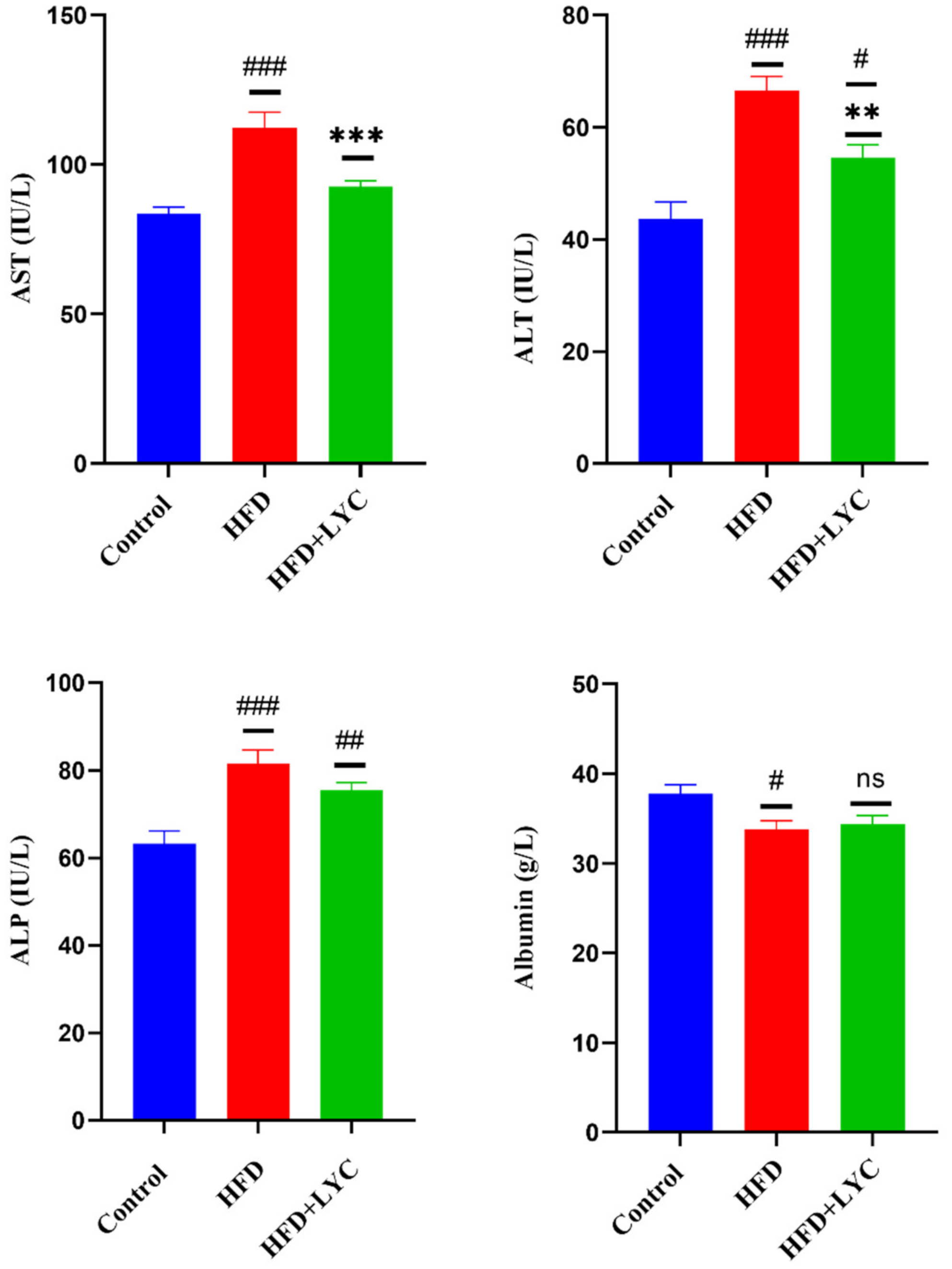
2.2. Effect of LYC on Liver Function Biomarkers
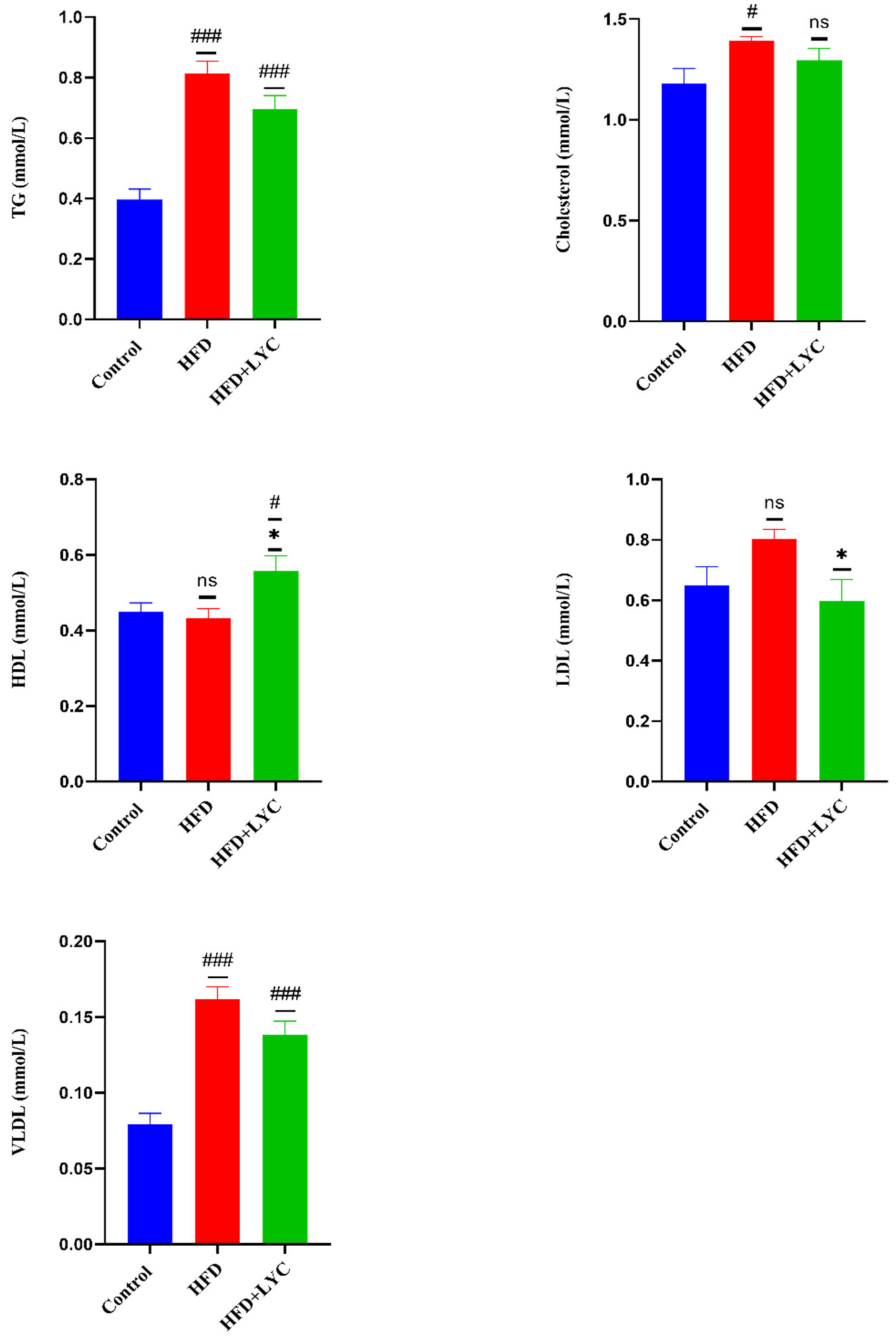
2.3. Effect of LYC on Lipid Profiles
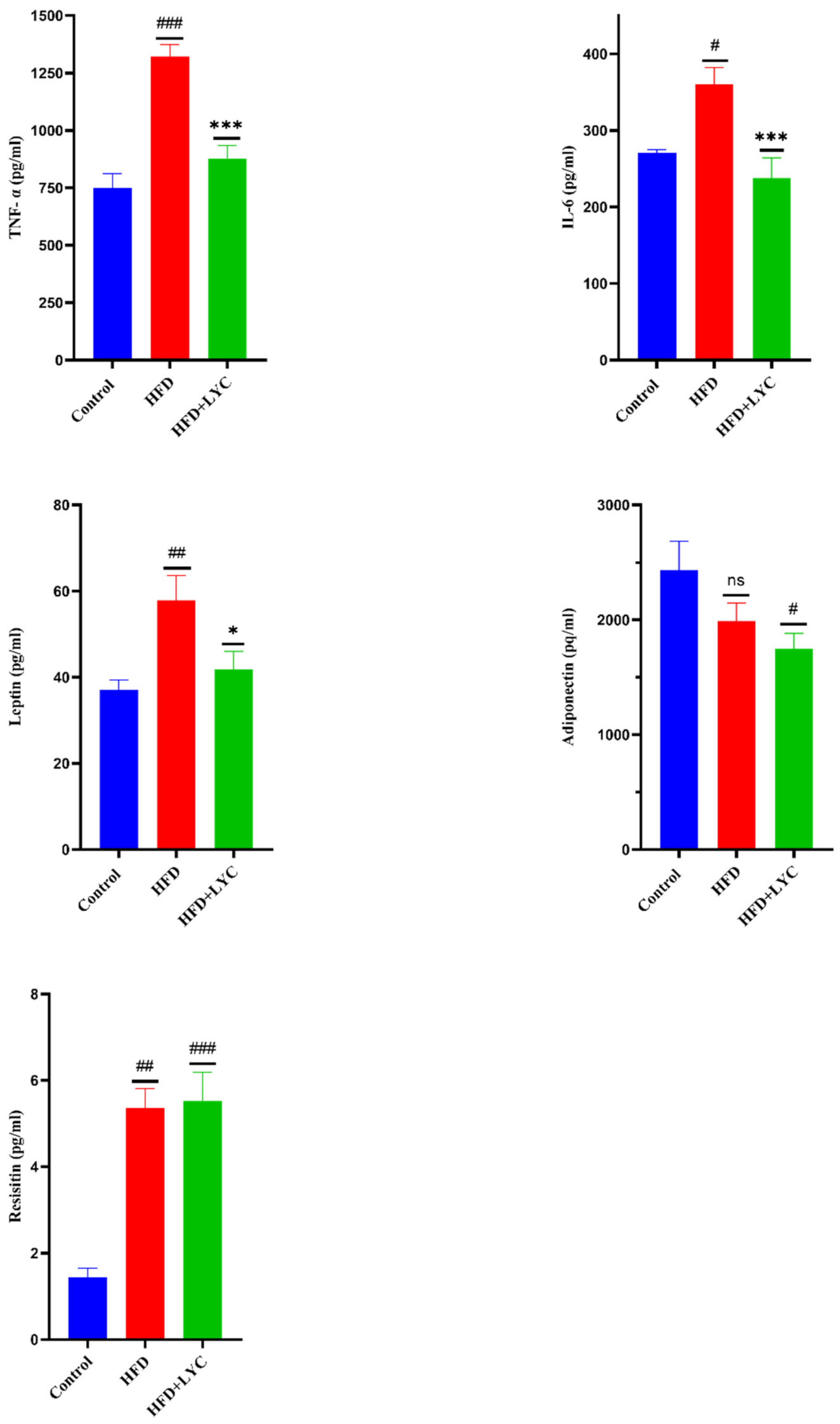
2.4. Effect of LYC on Inflammatory Biomarkers
2.5. Effect of LYC on Hepatic Oxidative and Antioxidant Status
2.6. Histopathological Findings
2.6.1. Liver
2.6.2. White Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Statement
4.2. Standard Animal Diet
4.3. Preparation of the High-Fat Diet (HFD)
4.4. Preparation of LYC
4.5. Experimental Design
4.6. Sampling and Tissue Preparation
4.7. Serum Biochemical Parameters
4.7.1. Liver Function Tests and Lipid Profile
4.7.2. Determination of Inflammatory Markers
4.8. Oxidative Stress Markers
4.9. Antioxidant Enzymatic Activities
4.10. Histological Examination
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Caputo, T.; Gilardi, F.; Desvergne, B. From chronic overnutrition to metaflammation and insulin resistance: Adipose tissue and liver contributions. FEBS Lett. 2017, 591, 3061–3088. [Google Scholar] [CrossRef] [PubMed]
- Alramah, T.Y. Novel Metabolic Risk Markers in Obesity and Type Two Diabetes Mellitus. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2020. [Google Scholar]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2021, 143, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Overby, H.B.; Ferguson, J.F. Gut microbiota-derived short-chain fatty acids facilitate microbiota: Host cross talk and modulate obesity and hypertension. Curr. Hypertens. Rep. 2021, 23, 8. [Google Scholar] [CrossRef]
- Lazarus, E.; Bays, H.E. Cancer and Obesity: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes. Pillars 2022, 3, 100026. [Google Scholar] [CrossRef]
- Alqarni, S.M. A review of prevalence of obesity in Saudi Arabia. J. Obes. Eat. Disord. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Baker, J.S.; Supriya, R.; Dutheil, F.; Gao, Y. Obesity: Treatments, conceptualizations, and future directions for a growing problem. Biology 2022, 11, 160. [Google Scholar] [CrossRef]
- Yokota-Nakagi, N.; Omoto, S.; Tazumi, S.; Kawakami, M.; Takamata, A.; Morimoto, K. Estradiol replacement improves high-fat diet-induced insulin resistance in ovariectomized rats. Physiol. Rep. 2022, 10, 15193. [Google Scholar] [CrossRef]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef]
- Algarni, S.M.; Yousef, J.M.; Gashlan, H.M. Ameliorative effect of blackberry cultivated from taif city on serum and liver tissue in hyperlipidemic rats. Life Sci. J. 2016, 13, 3. [Google Scholar]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.I.; Alhoshy, M.; Wang, T.; Mohsin, M.; Wang, J.; Wang, X.; Han, T.; Wang, Y.; Zhang, Z. Dietary supplementations modulate the physiological parameters, fatty acids profile and the growth of red claw crayfish (Cherax quadricarinatus). J. Anim. Physiol. Anim. Nutr. 2022, 106, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Peng, N.; Hu, C.; Zhong, L.; Zhong, M.; Zou, Y. The albumin-to-alkaline phosphatase ratio as an independent predictor of future non-alcoholic fatty liver disease in a 5-year longitudinal cohort study of a non-obese Chinese population. Lipids Health Dis. 2021, 20, 50. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Youssef, W.I.; McCullough, A.J. Steatohepatitis in obese individuals. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 733–747. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Mirza, M. Obesity, visceral fat, and NAFLD: Querying the role of adipokines in the progression of nonalcoholic fatty liver disease. Int. Sch. Res. Not. 2011, 2011, 592404. [Google Scholar] [CrossRef]
- Suriano, F.; Van Hul, M.; Cani, P.D. Gut microbiota and regulation of myokine-adipokine function. Curr. Opin. Pharmacol. 2020, 52, 9–17. [Google Scholar] [CrossRef]
- Hauner, H. Secretory factors from human adipose tissue and their functional role. Proc. Nutr. Soc. 2005, 64, 163–169. [Google Scholar] [CrossRef]
- Baltieri, L.; Chaim, E.A.; Chaim, F.D.M.; Utrini, M.P.; Gestic, M.A.; Cazzo, E. Correlation between nonalcoholic fatty liver disease features and levels of adipokines and inflammatory cytokines among morbidly obese individuals. Arq. Gastroenterol. 2018, 55, 247–251. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative stress in non-alcoholic fatty liver disease. An updated mini review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef] [PubMed]
- Yesilova, Z.; Yaman, H.; Oktenli, C.; Ozcan, A.; Uygun, A.; Cakir, E.; Sanisoglu, S.Y.; Erdil, A.; Ates, Y.; Aslan, M. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Off. J. Am. Coll. Gastroenterol. ACG 2005, 100, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Azarmehr, N.; Mansourian, M.; Doustimotlagh, A.H. Inactivation of the superoxide dismutase by malondialdehyde in the nonalcoholic fatty liver disease: A combined molecular docking approach to clinical studies. Arch. Physiol. Biochem. 2021, 127, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Von Lintig, J. Colors with functions: Elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 2010, 30, 35–56. [Google Scholar] [CrossRef]
- Jia, K.-P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef]
- Mounien, L.; Tourniaire, F.; Landrier, J.-F. Anti-obesity effect of carotenoids: Direct impact on adipose tissue and adipose tissue-driven indirect effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef]
- Bayramoglu, A.; Bayramoglu, G.; Senturk, H. Lycopene partially reverses symptoms of diabetes in rats with streptozotocin-induced diabetes. J. Med. Food 2013, 16, 128–132. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Zigangirova, N.A.; Pristensky, D.; Chernyshova, M.; Tsibezov, V.V.; Chalyk, N.E.; Morgunova, E.Y.; Kyle, N.H.; Bashmakov, Y.K. Non-invasive immunofluorescence assessment of lycopene supplementation status in skin smears. Monoclon. Antibodies Immunodiagn. Immunother. 2018, 37, 139–146. [Google Scholar] [CrossRef]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of dietary and supplemental lycopene on cardiovascular risk factors: A systematic review and meta-analysis. Adv. Nutr. 2020, 11, 1453–1488. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Wang, X.-D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214S–1222S. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; O’Driscoll, C.M.; Griffin, B.T. Bioavailability of lycopene in the rat: The role of intestinal lymphatic transport. J. Pharm. Pharmacol. 2010, 62, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Albrahim, T.; Alonazi, M.A. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed. Pharmacother. 2021, 141, 111831. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Salwe, K.J.; Kumarappan, M. Evaluation of antioxidant, hypolipidemic, and antiatherogenic property of lycopene and astaxanthin in atherosclerosis-induced rats. Pharmacogn. Res. 2017, 9, 161. [Google Scholar]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Wadie, W.; Mohamed, A.H.; Masoud, M.A.; Rizk, H.A.; Sayed, H.M. Protective impact of lycopene on ethinylestradiol-induced cholestasis in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 447–455. [Google Scholar] [CrossRef]
- Ip, B.C.; Liu, C.; Lichtenstein, A.H.; von Lintig, J.; Wang, X.-D. Lycopene and apo-10′-lycopenoic acid have differential mechanisms of protection against hepatic steatosis in β-carotene-9′, 10′-oxygenase knockout male mice. J. Nutr. 2015, 145, 268–276. [Google Scholar] [CrossRef]
- Róvero Costa, M.; Leite Garcia, J.; Cristina Vágula de Almeida Silva, C.; Junio Togneri Ferron, A.; Valentini Francisqueti-Ferron, F.; Kurokawa Hasimoto, F.; Schmitt Gregolin, C.; Henrique Salomé de Campos, D.; Roberto de Andrade, C.; dos Anjos Ferreira, A.L. Lycopene modulates pathophysiological processes of non-alcoholic fatty liver disease in obese rats. Antioxidants 2019, 8, 276. [Google Scholar] [CrossRef]
- Kilany, O.E.; Abdelrazek, H.M.; Aldayel, T.S.; Abdo, S.; Mahmoud, M.M. Anti-obesity potential of Moringa olifera seed extract and lycopene on high fat diet induced obesity in male Sprauge Dawely rats. Saudi J. Biol. Sci. 2020, 27, 2733–2746. [Google Scholar] [CrossRef]
- Licholai, J.A.; Nguyen, K.P.; Fobbs, W.C.; Schuster, C.J.; Ali, M.A.; Kravitz, A.V. Why Do Mice Overeat High-Fat Diets? How High-Fat Diet Alters the Regulation of Daily Caloric Intake in Mice. Obes. (Silver Spring Md.) 2018, 26, 1026–1033. [Google Scholar] [CrossRef]
- Santos, E.W.; de Oliveira, D.C.; Hastreiter, A.; Beltran, J.S.d.O.; Rogero, M.M.; Fock, R.A.; Borelli, P. High-fat diet or low-protein diet changes peritoneal macrophages function in mice. Nutrire 2016, 41, 6. [Google Scholar] [CrossRef]
- Santos, E.W.; Oliveira, D.C.; Hastreiter, A.; Silva, G.B.; Beltran, J.S.d.O.; Rogero, M.M.; Fock, R.A.; Borelli, P. Short-term high-fat diet affects macrophages inflammatory response, early signs of a long-term problem. Braz. J. Pharm. Sci. 2019, 55, e17561. [Google Scholar] [CrossRef]
- Kostrycki, I.M.; Wildner, G.; Donato, Y.H.; Dos Santos, A.B.; Beber, L.C.C.; Frizzo, M.N.; Ludwig, M.S.; Keane, K.N.; Cruzat, V.; Rhoden, C.R. Effects of high-fat diet on eHSP72 and extra-to-intracellular HSP70 levels in mice submitted to exercise under exposure to fine particulate matter. J. Diabetes Res. 2019, 2019, 4858740. [Google Scholar] [CrossRef]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.-S.; Mestry, S.N.; Gursahani, M.S.; Juvekar, A.R. Plumbagin reduces obesity and nonalcoholic fatty liver disease induced by fructose in rats through regulation of lipid metabolism, inflammation and oxidative stress. Biomed. Pharmacother. 2019, 111, 686–694. [Google Scholar] [CrossRef]
- Koubaa-Ghorbel, F.; Chaâbane, M.; Turki, M.; Makni-Ayadi, F.; El Feki, A. The protective effects of Salvia officinalis essential oil compared to simvastatin against hyperlipidemia, liver, and kidney injuries in mice submitted to a high-fat diet. J. Food Biochem. 2020, 44, 13160. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Alkhuriji, A.F.; Yousef, A.O.S.; Metwally, D.M.; Habotta, O.A.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Antagonistic efficacy of luteolin against lead acetate exposure-associated with hepatotoxicity is mediated via antioxidant, anti-inflammatory, and anti-apoptotic activities. Antioxidants 2019, 9, 10. [Google Scholar] [CrossRef]
- Al Aboud, D.; Baty, R.S.; Alsharif, K.F.; Hassan, K.E.; Zhery, A.S.; Habotta, O.A.; Elmahallawy, E.K.; Amin, H.K.; Abdel Moneim, A.E.; Kassab, R.B. Protective efficacy of thymoquinone or ebselen separately against arsenic-induced hepatotoxicity in rat. Environ. Sci. Pollut. Res. 2021, 28, 6195–6206. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, M.-H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180. [Google Scholar] [CrossRef]
- Zidani, S.; Benakmoum, A.; Ammouche, A.; Benali, Y.; Bouhadef, A.; Abbeddou, S. Effect of dry tomato peel supplementation on glucose tolerance, insulin resistance, and hepatic markers in mice fed high-saturated-fat/high-cholesterol diets. J. Nutr. Biochem. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- Schreiber, G.; Urban, J.; Edwards, K.; Dryburgh, H.; Inglis, A.S. Mechanism and regulation of albumin synthesis in liver and hepatomas. Adv. Enzym. Regul. 1976, 14, 163–184. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Albumin synthesis. In Albumin: Structure, Function and Uses; Rosenoer, V.M., Oratz, M., Rothschild, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 227–253. [Google Scholar]
- Dooley, J.S.; Lok, A.S.; Garcia-Tsao, G.; Pinzani, M. Sherlock’s Diseases of the Liver and Biliary System, 13th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 47–65. [Google Scholar]
- Sheinenzon, A.; Shehadeh, M.; Michelis, R.; Shaoul, E.; Ronen, O. Serum albumin levels and inflammation. Int. J. Biol. Macromol. 2021, 184, 857–862. [Google Scholar] [CrossRef]
- Thoenes, M.; Reil, J.-C.; Khan, B.V.; Bramlage, P.; Volpe, M.; Kirch, W.; Böhm, M. Abdominal obesity is associated with microalbuminuria and an elevated cardiovascular risk profile in patients with hypertension. Vasc. Health Risk Manag. 2009, 5, 577. [Google Scholar]
- Fathy, E.; Aisha, H.A.A.; Abosayed, A.K.; ElAnsary, A.M.S.E.O.; Al Aziz, A.A. Effect of Bariatric Surgery on Albuminuria in Non-Diabetic Non-Hypertensive Patients with Severe Obesity: A Short-Term Outcome. Obes. Surg. 2022, 32, 2397–2402. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.-L.; Mira, J.-P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef]
- Guo, X.-x.; Wang, Y.; Wang, K.; Ji, B.-p.; Zhou, F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. -Sci. B 2018, 19, 559–569. [Google Scholar] [CrossRef]
- Udomkasemsab, A.; Ngamlerst, C.; Kwanbunjun, K.; Krasae, T.; Amnuaysookkasem, K.; Chunthanom, P.; Prangthip, P. Maoberry (Antidesma bunius) improves glucose metabolism, triglyceride levels, and splenic lesions in high-fat diet-induced hypercholesterolemic rats. J. Med. Food 2019, 22, 29–37. [Google Scholar] [CrossRef]
- Xia, H.M.; Wang, J.; Xie, X.J.; Xu, L.J.; Tang, S.Q. Green tea polyphenols attenuate hepatic steatosis, and reduce insulin resistance and inflammation in high-fat diet-induced rats. Int. J. Mol. Med. 2019, 44, 1523–1530. [Google Scholar] [CrossRef]
- Zeng, Z.; He, W.; Jia, Z.; Hao, S. Lycopene improves insulin sensitivity through inhibition of STAT3/Srebp-1c-mediated lipid accumulation and inflammation in mice fed a high-fat diet. Exp. Clin. Endocrinol. Diabetes 2017, 125, 610–617. [Google Scholar] [CrossRef]
- Brito, A.K.d.S.; Lima, G.d.M.; Farias, L.M.d.; Rodrigues, L.A.R.L.; Carvalho, V.B.L.d.; Pereira, C.F.d.C.; Frota, K.d.M.G.; Conde-Júnior, A.M.; Silva, A.M.O.; Rizzo, M.d.S. Lycopene-rich extract from red guava (Psidium guajava L.) decreases plasma triglycerides and improves oxidative stress biomarkers on experimentally-induced dyslipidemia in hamsters. Nutrients 2019, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Inoue, T.; Suganuma, H. A Possible Relationship between Tomatoes, Lycopene, and Level of High-Density Lipoprotein-Cholesterol. In Lycopene Tomatoes Human Nutrition Health; CRC Press: Boca Raton, FL, USA, 2018; pp. 41–50. [Google Scholar]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Catalano, A.; Simone, R.E.; Mele, M.C.; Cittadini, A. Effect of lycopene and tomato products on cholesterol metabolism. Ann. Nutr. Metab. 2012, 61, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Campbell, C.L.; Yu, R.; Li, F.; Zhou, Q.; Chen, D.; Qi, C.; Yin, Y.; Sun, J. Modulation of fat metabolism and gut microbiota by resveratrol on high-fat diet-induced obese mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 97. [Google Scholar] [CrossRef]
- Luvizotto, R.d.A.M.; Nascimento, A.F.; Imaizumi, E.; Pierine, D.T.; Conde, S.J.; Correa, C.R.; Yeum, K.-J.; Ferreira, A.L.A. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br. J. Nutr. 2013, 110, 1803–1809. [Google Scholar] [CrossRef]
- Ip, B.C.; Hu, K.-Q.; Liu, C.; Smith, D.E.; Obin, M.S.; Ausman, L.M.; Wang, X.-D. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet–promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev. Res. 2013, 6, 1304–1316. [Google Scholar] [CrossRef]
- Ribot, J.; Rodríguez, A.M.; Rodríguez, E.; Palou, A. Adiponectin and resistin response in the onset of obesity in male and female rats. Obesity 2008, 16, 723–730. [Google Scholar] [CrossRef]
- Saucillo, D.C.; Gerriets, V.A.; Sheng, J.; Rathmell, J.C.; MacIver, N.J. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 2014, 192, 136–144. [Google Scholar] [CrossRef]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedűs, C.; Kovács, D.; Peitl, B.; Gál, F.; Stündl, L.; Szilvássy, Z.; Remenyik, J. Effect of anthocyanin-rich tart cherry extract on inflammatory mediators and adipokines involved in type 2 diabetes in a high fat diet induced obesity mouse model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef]
- Enriori, P.J.; Evans, A.E.; Sinnayah, P.; Jobst, E.E.; Tonelli-Lemos, L.; Billes, S.K.; Glavas, M.M.; Grayson, B.E.; Perello, M.; Nillni, E.A. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007, 5, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Tominaga, N.; Wakagi, M.; Ishikawa-Takano, Y. Consumption of lycopene-rich tomatoes improved glucose homeostasis in rats via an increase in leptin levels. J. Nat. Med. 2020, 74, 252–256. [Google Scholar] [CrossRef]
- Iikuni, N.; Lam, Q.L.; Lu, L.; Matarese, G.; La Cava, A. Leptin and Inflammation. Curr. Immunol. Rev. 2008, 4, 70–79. [Google Scholar] [CrossRef]
- Boyapati, R.; Chintalapani, S.; Ramisetti, A.; Salavadhi, S.S.; Ramachandran, R. Evaluation of serum leptin and adiponectin in obese individuals with chronic periodontitis. Contemp. Clin. Dent. 2018, 9, 210. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, X.; Chong, L.; Kong, L.; Wen, S.; Zhang, H.; Zhang, W.; Li, C. Adiponectin alleviates exacerbation of airway inflammation and oxidative stress in obesity-related asthma mice partly through AMPK signaling pathway. Int. Immunopharmacol. 2019, 67, 396–407. [Google Scholar] [CrossRef]
- Cavaliere, G.; Viggiano, E.; Trinchese, G.; De Filippo, C.; Messina, A.; Monda, V.; Valenzano, A.; Cincione, R.I.; Zammit, C.; Cimmino, F. Long feeding high-fat diet induces hypothalamic oxidative stress and inflammation, and prolonged hypothalamic AMPK activation in rat animal model. Front. Physiol. 2018, 9, 818. [Google Scholar] [CrossRef]
- Wei, Z.; Wong, W. Resistin as an Adipokine. In Adipokines, 1st ed.; Preedy, V.R., Hunter, R.J., Eds.; Science Publishers: Enfield, NH, USA; Boca Raton, FL, USA, 2016; pp. 102–109. [Google Scholar]
- Chen, W.C.; Lu, Y.C.; Kuo, S.J.; Lin, C.Y.; Tsai, C.H.; Liu, S.C.; Chen, Y.L.; Wang, S.W.; Tang, C.H. Resistin enhances IL-1β and TNF-α expression in human osteoarthritis synovial fibroblasts by inhibiting miR-149 expression via the MEK and ERK pathways. FASEB J. 2020, 34, 13671–13684. [Google Scholar] [CrossRef]
- Singh, D.; Khare, P.; Zhu, J.; Kondepudi, K.; Singh, J.; Baboota, R.; Boparai, R.; Khardori, R.; Chopra, K.; Bishnoi, M. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. Int. J. Obes. 2016, 40, 487–496. [Google Scholar] [CrossRef]
- Fenni, S.; Hammou, H.; Astier, J.; Bonnet, L.; Karkeni, E.; Couturier, C.; Tourniaire, F.; Landrier, J.F. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol. Nutr. Food Res. 2017, 61, 1601083. [Google Scholar] [CrossRef]
- Emami, S.R.; Jafari, M.; Haghshenas, R.; Ravasi, A. Impact of eight weeks endurance training on biochemical parameters and obesity-induced oxidative stress in high fat diet-fed rats. J. Exerc. Nutr. Biochem. 2016, 20, 29. [Google Scholar] [CrossRef]
- Piña-Zentella, R.M.; Rosado, J.L.; Gallegos-Corona, M.A.; Madrigal-Pérez, L.A.; García, O.P.; Ramos-Gomez, M. Lycopene improves diet-mediated recuperation in rat model of nonalcoholic fatty liver disease. J. Med. Food 2016, 19, 607–614. [Google Scholar] [CrossRef]
- Agrawal, N.; Singh, S.K. Obesity: An independent risk factor for oxidative stress. Int. J. Adv. Med. 2017, 4, 718–721. [Google Scholar] [CrossRef]
- Yefsah-Idres, A.; Benazzoug, Y.; Otman, A.; Latour, A.; Middendorp, S.; Janel, N. Hepatoprotective effects of lycopene on liver enzymes involved in methionine and xenobiotic metabolism in hyperhomocysteinemic rats. Food Funct. 2016, 7, 2862–2869. [Google Scholar] [CrossRef]
- Mozos, I.; Borzak, G.; Caraba, A.; Mihaescu, R. Arterial stiffness in hematologic malignancies. OncoTargets Ther. 2017, 10, 1381. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Bahcecioglu, I.H.; Kuzu, N.; Metin, K.; Ozercan, I.H.; Ustündag, B.; Sahin, K.; Kucuk, O. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Vet. Med. Int. 2010, 2010, 262179. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sanchez-Zapata, E.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.; Fernández-López, J. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef]
- Pereira, B.L.; Reis, P.P.; Severino, F.E.; Felix, T.F.; Braz, M.G.; Nogueira, F.R.; Silva, R.A.; Cardoso, A.C.; Lourenço, M.A.; Figueiredo, A.M. Tomato (Lycopersicon esculentum) or lycopene supplementation attenuates ventricular remodeling after myocardial infarction through different mechanistic pathways. J. Nutr. Biochem. 2017, 46, 117–124. [Google Scholar] [CrossRef]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef]
- Bandeira, A.C.B.; da Silva, R.C.; Júnior, J.V.R.; Figueiredo, V.P.; Talvani, A.; Cangussú, S.D.; Bezerra, F.S.; Costa, D.C. Lycopene pretreatment improves hepatotoxicity induced by acetaminophen in C57BL/6 mice. Bioorganic Med. Chem. 2017, 25, 1057–1065. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Bayramoglu, A.; Altuner, Y.; Uyanoglu, M.; Colak, S. The effects of lycopene on hepatic ischemia/reperfusion injury in rats. Cytotechnology 2015, 67, 487–491. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ali, S.; Bahcecioglu, I.H.; Guler, O.; Ozercan, I.; Ilhan, N.; Kucuk, O. Orally administered lycopene attenuates diethylnitrosamine-induced hepatocarcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/mTOR pathways. Nutr. Cancer 2014, 66, 590–598. [Google Scholar] [CrossRef]
- Sheik Abdulazeez, S.; Thiruvengadam, D. Effect of lycopene on oxidative stress induced during D-galactosamine/lipopolysaccharide-sensitized liver injury in rats. Pharm. Biol. 2013, 51, 1592–1599. [Google Scholar] [CrossRef]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef]
- Pierine, D.; Navarro, M.; Minatel, I.; Luvizotto, R.; Nascimento, A.; Ferreira, A.; Yeum, K.; Corrêa, C. Lycopene supplementation reduces TNF-α via RAGE in the kidney of obese rats. Nutr. Diabetes 2014, 4, 142. [Google Scholar] [CrossRef]
- Yang, W.; Shen, Z.; Wen, S.; Wang, W.; Hu, M. Mechanisms of multiple neurotransmitters in the effects of Lycopene on brain injury induced by Hyperlipidemia. Lipids Health Dis. 2018, 17, 13. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; González, E.; Kafarov, V. Kinetics of Lycopene Degradation in Sunflower and Grape Seed Oils. Orient. J. Chem. 2018, 34, 2229–2235. [Google Scholar] [CrossRef]
- Abdel-Rahman, H.G.; Abdelrazek, H.; Zeidan, D.W.; Mohamed, R.M.; Abdelazim, A.M. Lycopene: Hepatoprotective and antioxidant effects toward bisphenol A-induced toxicity in female Wistar rats. Oxidative Med. Cell. Longev. 2018, 2018, 5167524. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Control | HFD | HFD + LYC |
|---|---|---|---|
| Food intake (g/rat/day) | 29.34 ± 0.35 | 23.61 ± 0.39 ### | 23.26 ± 0.75 ### |
| Weight gain (%) | 58.47 ± 4.62 | 104.94 ± 8.22 ### | 109.29 ± 8.45 ### |
| Liver weight (g) | 11.48 ± 0.30 | 11.58 ± 0.29 ns | 12.32 ± 0.40 NS |
| Liver weight index (%) | 3.12 ± 0.10 | 2.47 ± 0.06 ### | 2.61 ± 0.13 ## |
| Abdominal fat (g) | 4.70 ± 0.52 | 24.89 ± 2.51 ### | 20.33 ± 1.35 ### |
| Abdominal fat index (%) | 1.47 ± 0.20 | 5.23 ± 0.48 ### | 4.02 ± 0.27 ###, * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baz, L.; Algarni, S.; Al-thepyani, M.; Aldairi, A.; Gashlan, H. Lycopene Improves Metabolic Disorders and Liver Injury Induced by a Hight-Fat Diet in Obese Rats. Molecules 2022, 27, 7736. https://doi.org/10.3390/molecules27227736
Baz L, Algarni S, Al-thepyani M, Aldairi A, Gashlan H. Lycopene Improves Metabolic Disorders and Liver Injury Induced by a Hight-Fat Diet in Obese Rats. Molecules. 2022; 27(22):7736. https://doi.org/10.3390/molecules27227736
Chicago/Turabian StyleBaz, Lina, Salha Algarni, Mona Al-thepyani, Abdullah Aldairi, and Hana Gashlan. 2022. "Lycopene Improves Metabolic Disorders and Liver Injury Induced by a Hight-Fat Diet in Obese Rats" Molecules 27, no. 22: 7736. https://doi.org/10.3390/molecules27227736
APA StyleBaz, L., Algarni, S., Al-thepyani, M., Aldairi, A., & Gashlan, H. (2022). Lycopene Improves Metabolic Disorders and Liver Injury Induced by a Hight-Fat Diet in Obese Rats. Molecules, 27(22), 7736. https://doi.org/10.3390/molecules27227736








