Physico-Chemical and Antimicrobial Efficacy of Encapsulated Dhavana Oil: Evaluation of Release and Stability Profile from Base Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methodology
2.3. Experimental Details
2.3.1. Physio-Chemical Analysis of Dhavana Oil
2.3.2. Formation of an Emulsion of Dhavana Oil
2.3.3. Encapsulation of Dhavana Oil
2.3.4. Evaluation of Total Oil Content
- Psd = Percentage (v/w) of Steam distilled oil.
- P = Real oil content in an Encapsulated product (%v/w)
- Vsample = Final volume in ml of oil collected in a sidearm of Clevenger’s apparatus.
- X = Weight of encapsulate sample taken before steam distillation. i.e., sample weight.
2.3.5. Evaluation of Surface Oil
2.3.6. Thermal Analysis
- Batch no. 001: Neat Dhavana oil (1% loading) in TALC.
- Batch no. 002: Encapsulated Dhavana oil in TALC (3.35% loading as encapsulated Dhavana oil contains a 30% neat oil)
- Batch no. 003: Neat Dhavana oil (1%) in CaCO3.
- Batch no. 004: Encapsulated Dhavana oil in CaCO3 (3.35% loading as encapsulated Dhavana oil contains a 30% neat oil)
- Neat Dhavana oil @ 1% in TALC and CaCO3.
- 3.35% of encapsulated Dhavana oil in TALC and CaCO3.
- Encapsulated oil contains 30% oil loading, i.e., (3.35 × 30% = 1.005), to keep the oil ratio approximately the same in both formats.
- For stability studies in TALC
- Batch no. 001/1–Neat Dhavana oil in TALC for 45 °C/1 month
- Batch no. 001/2–Neat Dhavana oil in TALC for 45 °C/2 month
- Batch no. 002/1–Encapsulated Dhavana oil in TALC for 45 °C/1 month
- Batch no. 002/2–Encapsulated Dhavana oil in TALC for 45 °C/2 month
- For stability studies for the CaCO3
- Batch no. 003/1–Neat Dhavana oil in CaCO3 for 45 °C/1 month
- Batch no. 003/2–Neat Dhavana oil in CaCO3 for 45 °C/2 month
- Batch no. 004/1–Encapsulated Dhavana oil in CaCO3 for 45 °C/1 month
- Batch no. 004/2–Encapsulated Dhavana oil in CaCO3 for 45 °C/2 month
- Batch no. 001A–Neat Dhavana oil in TALC for 22 ), one-week, open petri dish;
- Batch no. 001B–Neat Dhavana oil in TALC for 45 , one-week, open petri dish;
- Batch no. 002 A–Encapsulated Dhavana oil in TALC for 22 ), one-week, open petri dish;
- Batch no. 002B–Encapsulated Dhavana oil in TALC for 45 , one-week, open petri dish;
- Batch no. 003A–Neat Dhavana oil in CaCO3 for 22 , (RT) one-week, open petri dish;
- Batch no. 003B–Neat Dhavana oil in CaCO3 for 45 , one-week, open petri dish;
- Batch no. 004A–Encapsulated Dhavana oil in CaCO3 for 22 ), one-week, open petri dish;
- Batch no. 004B–Encapsulated Dhavana oil in CaCO3 for 45 , one-week, open petri dish.
2.3.7. Sensory/Olfactive Analysis
- The intensity scale of neat v/s encapsulated Dhavana oil in both the bases;
- Intensity scale of neat Dhavana oil in TALC v/s neat Dhavana oil in CaCO3;
- Intensity scale of neat Dhavana oil in TALC and CaCO3 v/s encapsulated Dhavana oil in TALC and CaCO3 when dissolved in water solution.
- Batch no.-001-Dhavana oil in TALC-oil loading @ 1.00%;
- Batch no.-002-Encapsulated Dhavana oil in TALC-loaded at 3.35% (encapsulation at 30% of oil);
- Batch no.-003-Dhavana oil in CaCO3 -oil loading @1.00%;
- Batch no.-004-Encapsulated Dhavana oil in CaCO3 -loaded at 3.35% (encapsulation at 30% of oil).
2.3.8. Fourier Transform InfraRed (FTIR) Analysis
2.3.9. Antimicrobial Testing
Preparation of Dhavana Oil Stock Solution for Antibacterial and Antifungal Study
Preparation of Encapsulated Product Stock Solution
Growth and Maintenance of Bacterial Strains
Antibacterial Agar Well Assay
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Assay
Antifungal Testing
3. Results and Discussion
3.1. Physico-Chemical Properties of Neat Dhavana Oil
3.2. Physico-Chemical Properties of Encapsulated Dhavana Oil
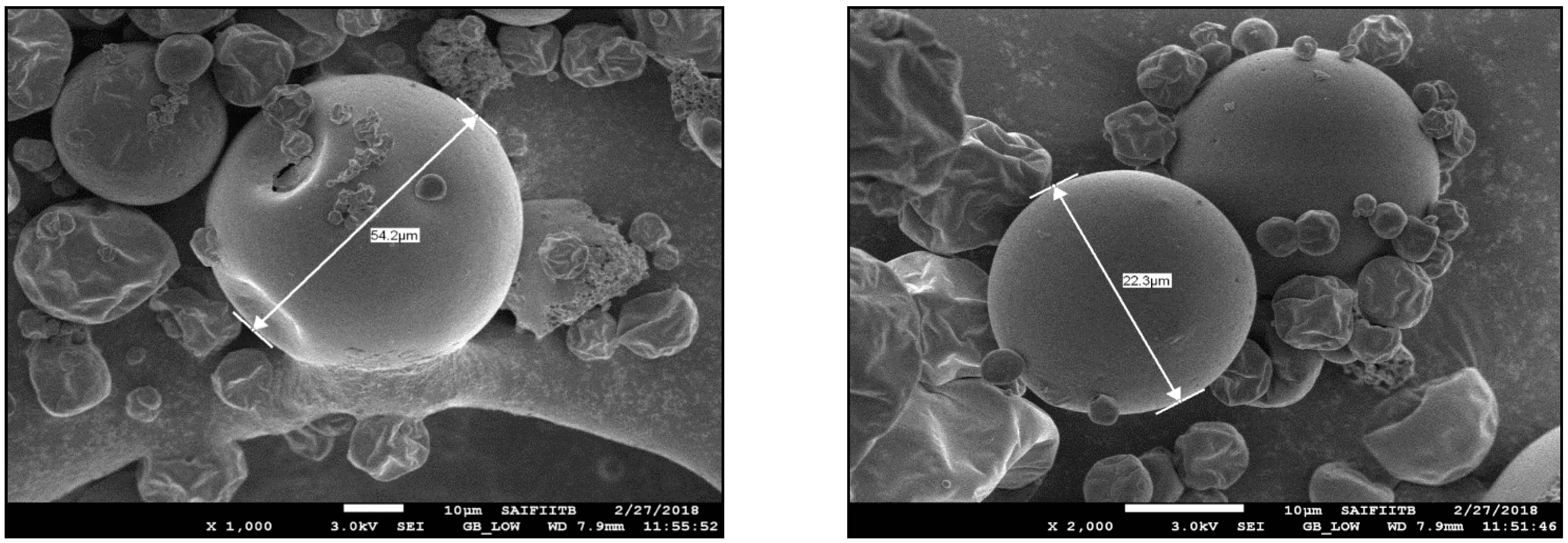
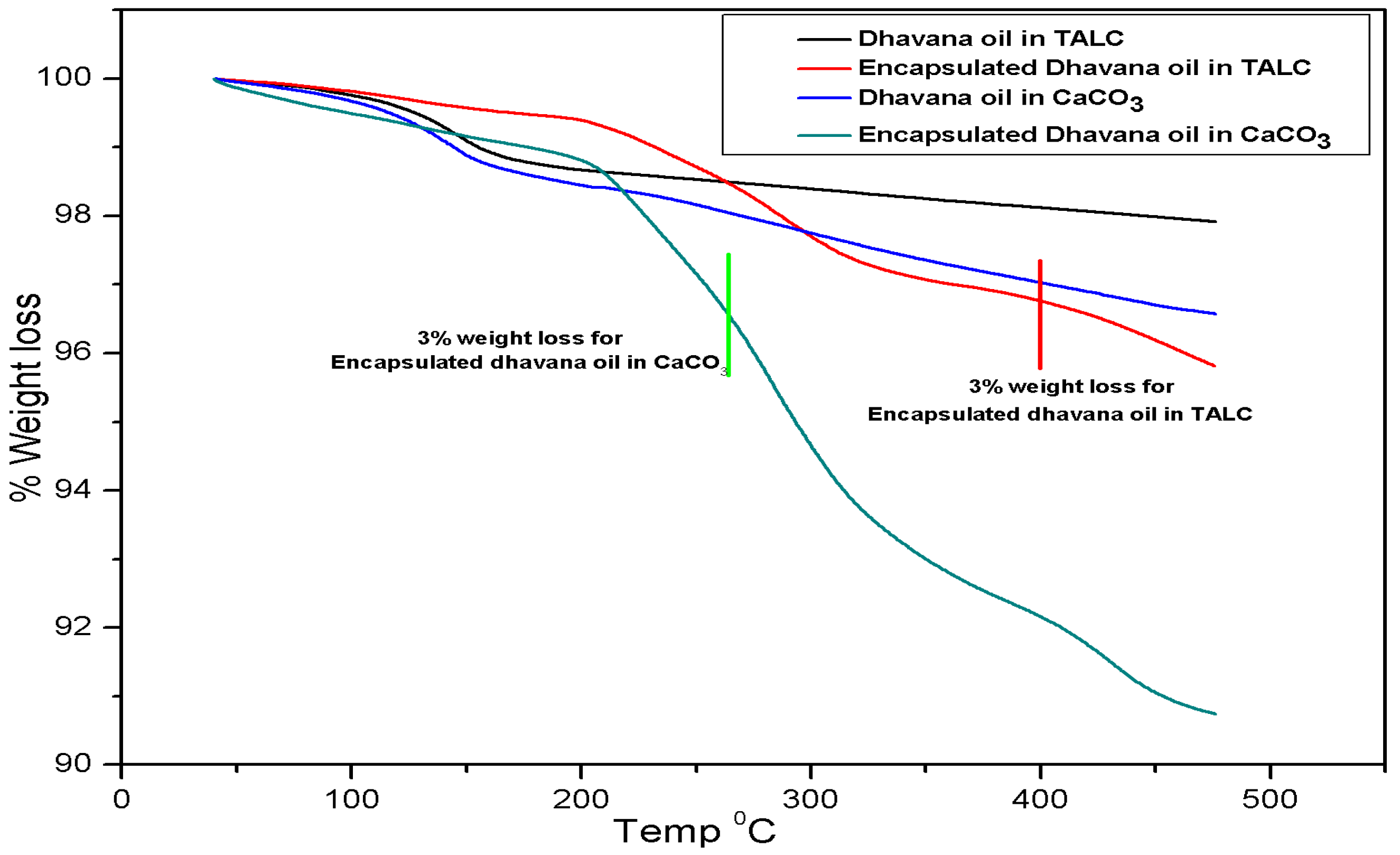
3.2.1. Thermal Analysis
3.2.2. GC-MS Analysis
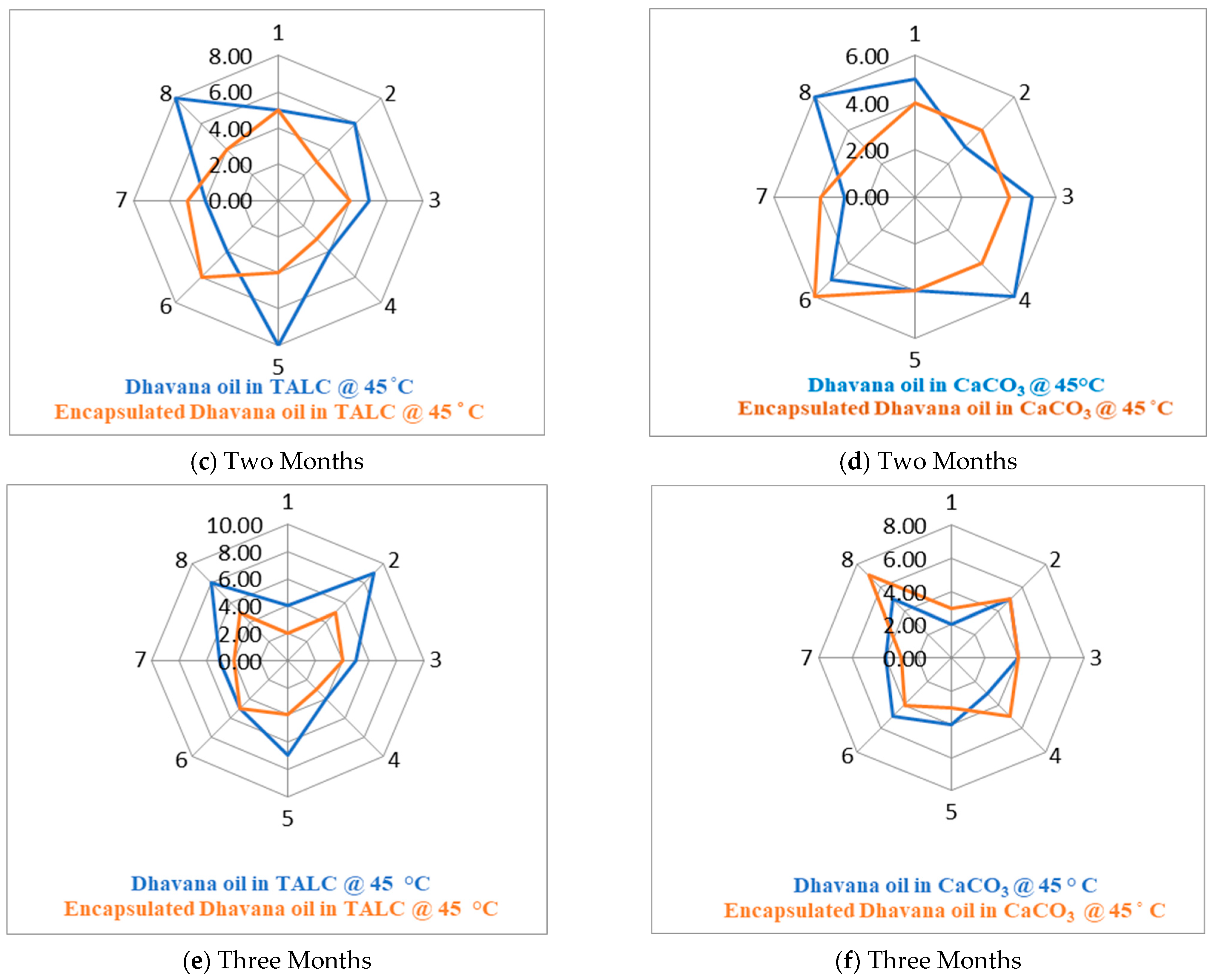
3.2.3. Stability Study and Olfactory Analysis
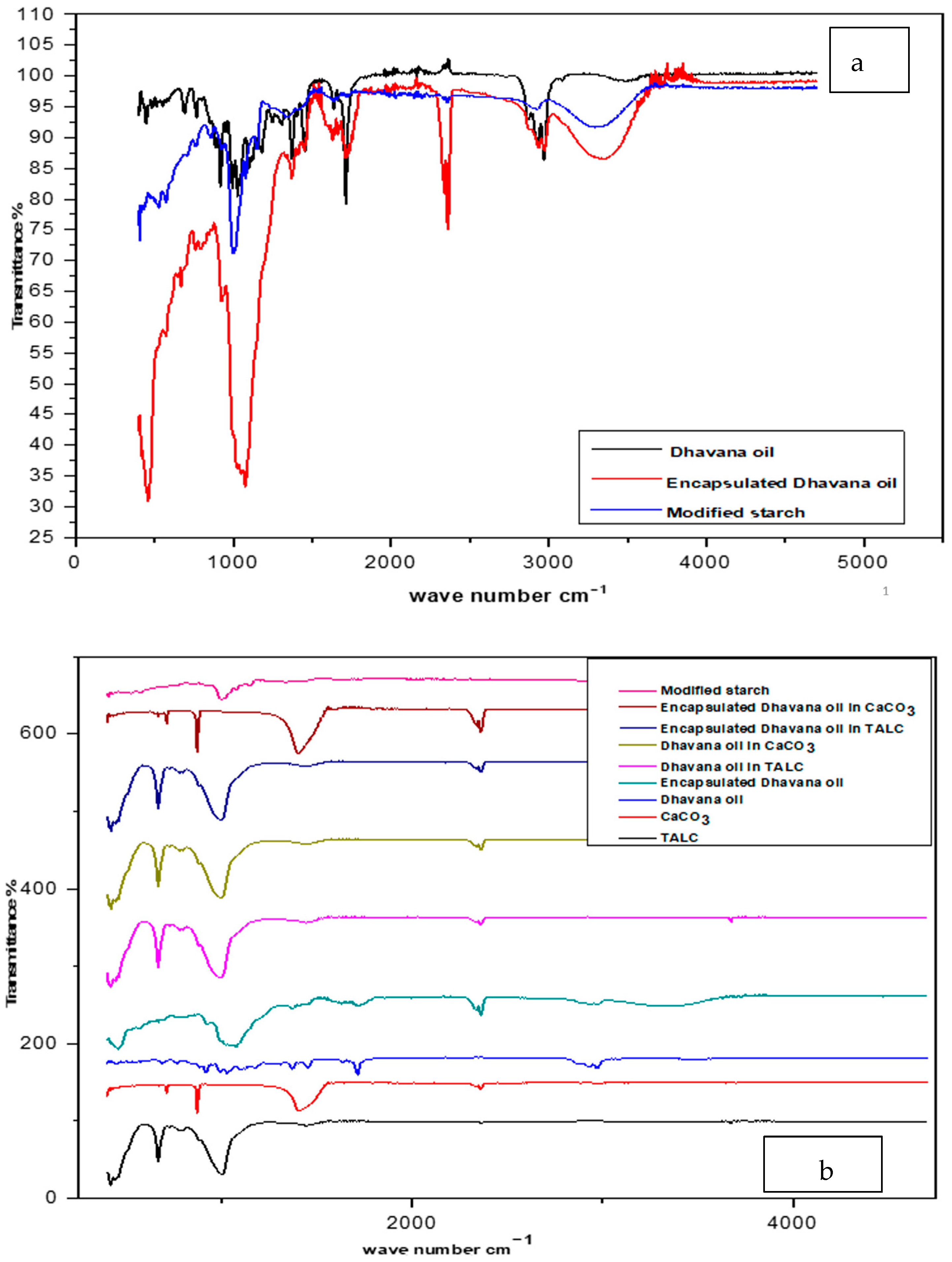
3.2.4. FTIR Analysis
3.2.5. Antimicrobial Activity of Dhavana Oil and Its Encapsulated Product
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mehdizadeh, L.; Pirbalouti, A.G.; Moghaddam, M. Storage stability of essential oil of cumin (Cuminum cyminum L.) a function of temperature. Int. J. Food Prop. 2017, 20 (Suppl. S2), S1742–S1750. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of flavours and fragrances into polymeric capsules and cyclodextrins inclusion complexes. Molecules 2020, 25, 5878. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. Micro/nanoencapsulation of essential oils and fragrances: Focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J. Microencapsul. 2016, 3, 497–510. [Google Scholar] [CrossRef]
- Misra, L.N.; Chandra, A.; Thakur, R.S. Fragrant components of oil from Artemisia pallens. Phytochemistry 1991, 30, 549–552. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Bautista-Baños, S.; García, O.D.; Cabrera-Barjas, G. Corn-starch-based materials incorporated with cinnamon oil emulsion: Physico-chemical characterization and biological activity. Foods 2020, 9, 475. [Google Scholar] [CrossRef]
- Rakmaia, J.; Cheirsilp, B.; Mejuto, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocoll. 2017, 65, 157–164. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.-L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed]
- Kausadikara, S.; Gadhave, A.D.; Waghmare, J. Microencapsulation of lemon oil by spray drying and its application in Tea flavour. Adv. Appl. Sci. Res. 2015, 6, 69–78. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Potential use of polymers and their complexes as media for storage and delivery of fragrances. J. Control. Release 2018, 285, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Baranauskieneu, R.; Bylaiteú, E.; Ukauskateú, J.Z.; Venskutonis, R.P. Flavor retention of peppermint (Mentha piperita L.) essential oil spray-dried in modified starches during encapsulation and storage. J. Agric. Food Chem. 2007, 55, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Hu, Q.; Li, X.; Chen, F.; Wan, R.; Yu, C.-W.; Li, J.; McClements, D.J.; Deng, Z. Microencapsulation of an essential oil (cinnamon oil) by spray drying: Effects of wall materials and storage conditions on microcapsule properties. J. Food Process. Preserv. 2020, 44, e14805. [Google Scholar] [CrossRef]
- Costa, P.; Velasco, C.V.; Loureiro, J.M.; Rodrigues, A.E. Effect of cosmetic matrices on the release and odour profiles of the supercritical CO2 extract of Origanum majorana. Int. J. Cosmet. Sci. 2016, 38, 325–431. [Google Scholar] [CrossRef]
- Hermanto, R.F.; Khasanah, L.U.; Kawiji; Atmaka, W.; Manuhara, G.J.; Utami, R. Physical characteristics of cinnamon oil microcapsule. Mater. Sci. Eng. 2016, 107, 012064. [Google Scholar] [CrossRef]
- Baila, S.; Buchbauer, G.; Schmidt, E.; Wanner, J.; Slavchev, A.; Stoyanova, A.; Denkova, Z.; Geissler, M.; Jirovetz, L. GC-MS-analysis antimicrobial activities and olfactory evaluation of essential Dhavana (Artemisia pallens Wall. ex DC) oil from India. Nat. Prod. Commun. 2008, 3, 1057–1063. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils: Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Ruikar, D.; Kamble, G.S.; Puranik, V.G.; Deshpande, N.R.; Ingle, T.R. Antimicrobial Screening of Medicinal Plant—Artemisia pallens. Int. J. PharmTech Res. 2009, 1, 1164–1166. [Google Scholar]
- Suresh, J.; Singh, A.; Vasavi, A.; Ihsanullah, M.; Mary, S. Phytochemical and pharmacological properties of artemisia. Int. J. Pharm. Sci. Res. 2011, 2, 3081–3090. [Google Scholar] [CrossRef]
- Gajjar, P.; Deshpande, R.; Kukreja, T. Study of evaluation of antimicrobial property of different concentrations of Artemisia pallens (Dhavana) extract against Streptococcus mutans serotype c (ATCC 25175). Indian J. Basic Appl. Med. Res. 2019, 8, 154–157. [Google Scholar]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro- and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Łopusiewicz, Ł. Formulation and evaluation of spray-dried reconstituted flaxseed oil-in-water emulsions based on flaxseed oil cake extract as emulsifying and stabilizing agent. Foods 2021, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Gomez, M.; Galicia-García, T.; Marquez-Melendez, R.; Ruiz-Gutierrez, M.A. Quintero-Ramos, Spray-dried microencapsulation of orange essential oil using modified rice starch as wall material. J. Food Process. Preserv. 2017, 42, e13428. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 12, 40–53. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the application of microcapsules as carriers of functional compounds for food products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef]
- Cortez-Pereira, C.S.; Baby, A.R.; Kaneko, T.M.; Velasco, M.V.R. Sensory approach to measure fragrance intensity on the skin. J. Sens. Stud. 2009, 24, 871–901. [Google Scholar] [CrossRef]
- Carranza, K.; Rodriguez, C.; Esenarro, D.; Veliz, M.; Arteaga, J. Sensory evaluation of a perfume made of orange essential oil. Int. J. Chem. Eng. Appl. 2020, 11, 89–92. [Google Scholar] [CrossRef]
- Mascheroni, E.; Fuenmayor, C.A.; Cosio, M.S.; Di Silvestro, G.; Piergiovanni, L.; Mannino, S.; Schiraldi, A. Encapsulation of volatiles in nanofibrous polysaccharide membranes for humidity-triggered release. Carbohydr. Polym. 2013, 98, 17–25. [Google Scholar] [CrossRef]
- Demirpolat, A.; Akman, F.; Kazachenko, A.S. An Experimental and Theoretical Study on Essential Oil of Aethionema sancakense—Characterization, Molecular Properties and RDG Analysis. Molecules 2022, 27, 6129. [Google Scholar] [CrossRef]
- Yekeler, M.; Ulusoy, U.; Hiçyılmaz, C. Effect of particle shape and roughness of TALC mineral ground by different mills on the wettability and floatability. Powder Technol. 2004, 140, 68–78. [Google Scholar] [CrossRef]
- Arsoy, Z.; Ersoy, B.; Evcin, A.; Icduygu, M.G. Influence of dry grinding on physicochemical and surface properties of TALC. Physicochem. Probl. Miner. Process. 2017, 53, 288–306. [Google Scholar] [CrossRef]
- Xiao, Z.; Kang, Y.; Hou, W.; Niu, Y.; Kou, X. Microcapsules based on octenyl succinic anhydride (OSA)-modified starch and maltodextrins changing the composition and release property of rose essential oil. Int. J. Biol. Macromol. 2019, 137, 132–138. [Google Scholar] [CrossRef]


| Sr.no. | Name of Ingredient | 100% w/w |
|---|---|---|
| 1 | Citric Acid Anhydrous | 2.00~5.00 |
| 2 | Modified starch | 50.00~55.00 |
| 3 | Tri potassium citrate | 3.00~8.00 |
| 4 | Sucrose | 5.00~12.00 |
| 5 | Oil (Dhavana oil) | 30.000 |
| 6 | DM Water | 150.000 |
| Sr. No. | Parameter | Reading |
|---|---|---|
| 1 | Inlet temperature | 160–170 °C |
| 2 | Outlet temperature | 92 °C |
| 3 | Aspirator | 45% (1350 rpm) |
| 4 | Vacuum | 50 |
| 5 | Air pressure | 2 bar |
| 6 | Total time to complete a spray-drying | 40 to 45 min. |
| Sr. No. | Test | Observation | Specification |
|---|---|---|---|
| 1 | Color | Brownish -Yellow viscous liquid | Brownish Yellow viscous liquid |
| 2 | Odor | Aromatic, Balsamic, Fruity, Woody, Sweet | Aromatic, Balsamic, Fruity, Woody, Sweet |
| 3 | Solubility | Clearly soluble in less than 1.5 volumes of 99% ethyl alcohol (Hyman grade) | Alcohol & Hexane soluble |
| 4 | Refractive index at 25 °C (Abbe refractometer Atago) | 1.486 | 1.4794 to 1.4917 |
| 5 | Specific gravity at 25 °C | 0.9591 | 0.9394 to 0.9560 |
| 6 | Acid value | 2.95 | ≤3.5 |
| 7 | Flashpoint Seta Multiflash (closed cup) | 110 | ≥97 |
| 8 | Boiling Point (capillary method) | 190 | |
| 9 | GC-MS analysis (Firmenich) | Complete (Report as attached) | |
| 10 | Total ketones content as Davanone (GCMS-Firmenich) | 56.00% | 36 to 56.0% |
| 11 | Free Davanone as per GC | Passes | 25 to 52.5% |
| 12 | Viscosity BF at 25 °C (100 rpm, spindle 3, BF RVT) | 28 cps | N.A. |
| 13 | pH @1% solution of Dhavana oil in water. | 4.88 | Less than 7.00 |
| 14 | Saponification Value | 57.45 mg KOH/g |
| Sr. no | Test | Observation |
|---|---|---|
| 1 | 10% particles below | 6.71 µm |
| 2 | 50% particles below | 19.35 µm |
| 3 | 90% of particles below | 59.36 µm |
| 4 | 100% particles below | 236.78 µm |
| Sr. No. | Surface Oil (%) | Average Surface Oil (%) | Total Oil (%) | Average Total Oil (%) | Encapsulation Efficiency (%) | Average Encapsulation Efficiency (%) | Entrapment Efficiency (%) | Average Entrapment Efficiency |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.44 | 3.526% | 28.92 | 29.02% | 96.40% | 88.10% | ||
| 2 | 3.57 | 29.17 | 97.23% | 96.69% | 87.76% | 87.84% | ||
| 3 | 3.57 | 28.94 | 96.46% | 87.66% |
| Sr.no | Component | Matrix | Duration | Retention Time | Area % |
|---|---|---|---|---|---|
| 1 | Neat Dhavana oil | TALC | One Month | 44.0583 | 30.1827 |
| 2 | Encapsulated Dhavana oil | TALC | One Month | 44.0395 | 30.8049 |
| 3 | Neat Dhavana oil | TALC | Two Months | 44.0453 | 23.5616 |
| 4 | Encapsulated Dhavana oil | TALC | Two Months | 44.0364 | 26.7507 |
| 5 | Neat Dhavana oil | CaCO3 | One Month | 43.1950 | 10.6000 |
| 6 | Encapsulated Dhavana oil | CaCO3 | One Month | 43.1960 | 25.0000 |
| 7 | Neat Dhavana oil | CaCO3 | Two Months | 43.1870 | 10.7100 |
| 8 | Encapsulated Dhavana oil | CaCO3 | Two Months | 43.1870 | 24.5200 |
| Sr. No. | Component | Matrix | Duration | % Of Hydroxydhavanone |
|---|---|---|---|---|
| 1 | Encapsulated Dhavana Oil | TALC | Two months | 26.75 |
| 2 | Neat Dhavana oil | TALC | Two months | 23.56 |
| 3 | Encapsulated Dhavana Oil | TALC | One month | 30.8 |
| 4 | Encapsulated Dhavana Oil | CaCO3 | Two months | 24.52 |
| 5 | Neat Dhavana oil | CaCO3 | Two months | 10.71 |
| 6 | Encapsulated Dhavana Oil | CaCO3 | One month | 25 |
| Sr.no. | Component | Matrix | Temperature | Occurrence of the Active Ingredient |
|---|---|---|---|---|
| 001A | Neat Dhavana oil | TALC | RT | Present |
| 001B | Neat Dhavana oil | TALC | 45 | Absent |
| 002A | Encapsulated Dhavana oil | TALC | RT | Present |
| 002B | Encapsulated Dhavana oil | TALC | 45 | Present |
| 003A | Neat Dhavana oil | CaCO3 | RT | Present |
| 003B | Neat Dhavana oil | CaCO3 | 45 | Absent |
| 004A | Encapsulated Dhavana oil | CaCO3 | RT | Present |
| 004B | Encapsulated Dhavana oil | CaCO3 | 45 | Present |
| Respondent Number | Intensity Score | Intensity Score | Intensity Score | Intensity Score | Intensity Score | Intensity Score |
|---|---|---|---|---|---|---|
| Dhavana Oil in TALC 1M | Dhavana Oil in CaCO3 1M | Dhavana Oil in TALC 2M | Dhavana Oil in CaCO3 2M | Dhavana Oil in TALC-3M | Dhavana Oil in CaCO3-3M | |
| 1 | 7 | 6 | 5 | 5 | 4 | 3 |
| 2 | 5 | 3 | 6 | 3 | 9 | 5 |
| 3 | 6 | 5 | 5 | 5 | 5 | 4 |
| 4 | 6 | 5 | 4 | 6 | 4 | 5 |
| 5 | 6 | 5 | 8 | 4 | 7 | 3 |
| 6 | 7 | 6 | 4 | 5 | 5 | 4 |
| 7 | 8 | 4 | 4 | 3 | 5 | 3 |
| 8 | 8 | 5 | 8 | 6 | 8 | 7 |
| Total | 53 | 39 | 44 | 37 | 47 | 34 |
| Name of Bacterial Strain | With Neat Dhavana Oil Zone Size (mm) | |||
| 300 mg/mL | 150 mg/mL | 75 mg/mL | 37.5 mg/mL | |
| S. aureus | 17 ± 0.2 | 14 ± 0.3 | 12 ± 0.1 | - |
| Micrococcus spp. | 18 ± 0.4 | 16 ± 0.2 | 13 ± 0.1 | - |
| E. coli | 15 ± 0.2 | 13 ± 0.1 | 11 ± 0.2 | - |
| Serratia spp. | 16 ± 0.2 | 13 ± 0.1 | - | - |
| K. pneumoniae | 16 ± 0.3 | 13 ± 0.4 | - | - |
| B. subtilis | 12 ± 0.1 | - | - | - |
| S. typhi | 15 ± 0.3 | - | - | - |
| S. mutans | 14 ± 0.2 | 13 ± 0.1 | 11 ± 0.2 | - |
| P. aeruginosa | - | - | - | - |
| Name of Bacterial Strain | With Encapsulated Dhavana oil Zone size (mm) | |||
| 300 mg/mL | 150 mg/mL | 75 mg/mL | 37.5 mg/mL | |
| E. coli | 16 ± 0.2 | 13 ± 0.2 | - | - |
| S. aureus | 16 ± 0.4 | 13 ± 0.3 | - | - |
| Micrococcus spp. | 15 ± 0.1 | 13 ± 0.2 | - | - |
| S. mutans | 18 ± 0.3 | 16 ± 0.1 | 15 ± 0.2 | 10 ± 0.1 |
| Name of Fungal Strain | With neat Dhavana oil Zone size (mm) | |||
| 200 μL | ||||
| Aureobasidium pullulans | 18.5 | |||
| NCIM 1049 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phanse, S.K.; Sawant, S.; Singh, H.; Chandra, S. Physico-Chemical and Antimicrobial Efficacy of Encapsulated Dhavana Oil: Evaluation of Release and Stability Profile from Base Matrices. Molecules 2022, 27, 7679. https://doi.org/10.3390/molecules27227679
Phanse SK, Sawant S, Singh H, Chandra S. Physico-Chemical and Antimicrobial Efficacy of Encapsulated Dhavana Oil: Evaluation of Release and Stability Profile from Base Matrices. Molecules. 2022; 27(22):7679. https://doi.org/10.3390/molecules27227679
Chicago/Turabian StylePhanse, Shirish K., Shriya Sawant, Harinder Singh, and Sudeshna Chandra. 2022. "Physico-Chemical and Antimicrobial Efficacy of Encapsulated Dhavana Oil: Evaluation of Release and Stability Profile from Base Matrices" Molecules 27, no. 22: 7679. https://doi.org/10.3390/molecules27227679
APA StylePhanse, S. K., Sawant, S., Singh, H., & Chandra, S. (2022). Physico-Chemical and Antimicrobial Efficacy of Encapsulated Dhavana Oil: Evaluation of Release and Stability Profile from Base Matrices. Molecules, 27(22), 7679. https://doi.org/10.3390/molecules27227679







