Cation Incorporation and Synergistic Effects on the Characteristics of Sulfur-Doped Manganese Ferrites S@Mn(Fe2O4) Nanoparticles for Boosted Sunlight-Driven Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
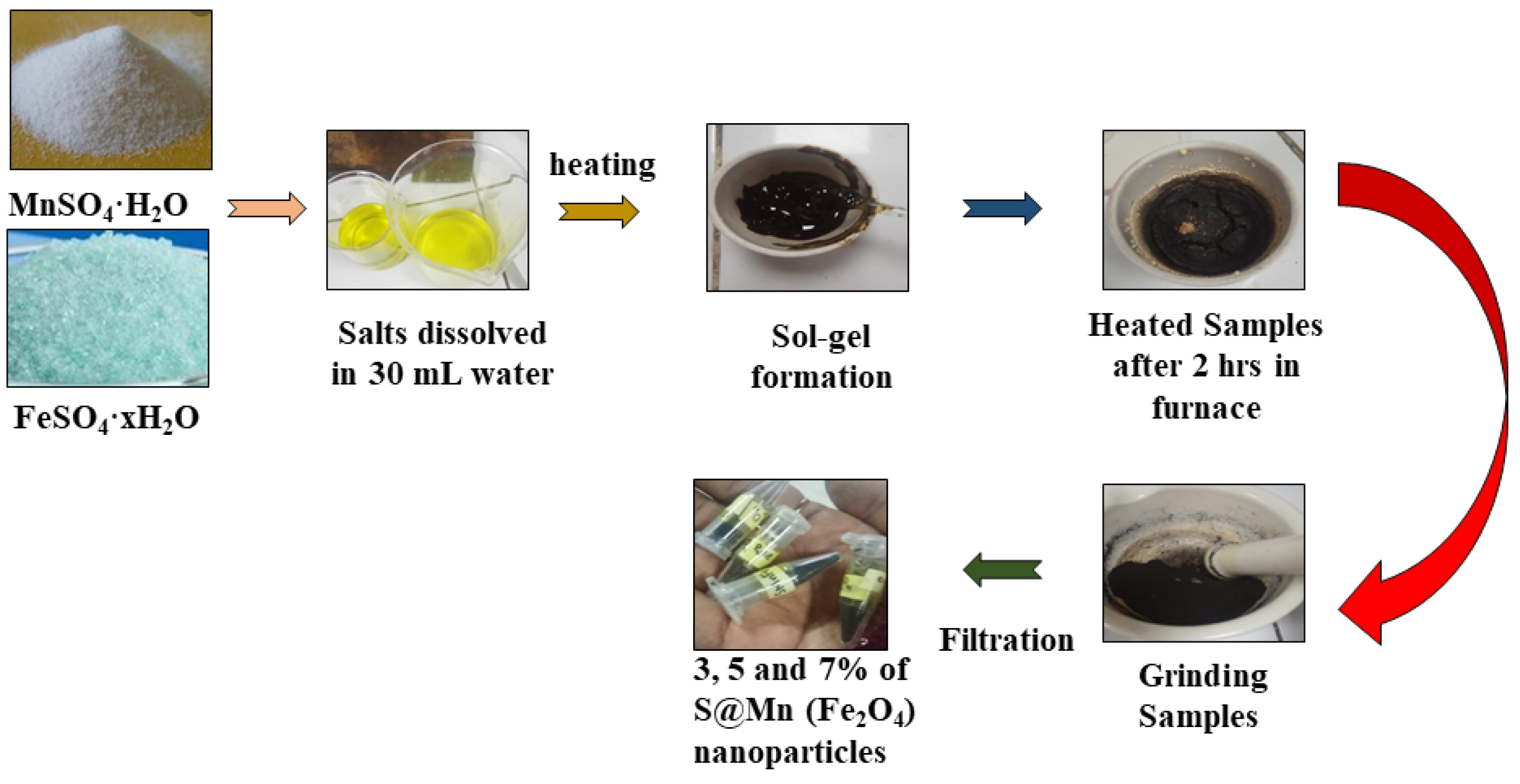
2.1. Synthesis of Sulfur-Doped Manganese Metal Ferrites Nanoparticles
2.2. Preparation of Methylene Blue and Chromium (IV) Solution
2.3. Characterization Methods
3. Results and Discussion
3.1. Scanning Electron Microscopy
3.2. Energy-Dispersive X-Ray Analysis (EDX)
3.3. Fourier-Transform Infrared Spectroscopy
3.4. XRD
3.5. UV–Vis Characterization of S@Mn(Fe2O4) Composite
3.6. Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hareendran, A.; Dais, E.; Shinoy, D.; Srikripa, S.; Shibu, G.M.; Kurian, M. Nitrogen- and sulfur-doped zinc ferrite nanoparticles as efficient heterogeneous catalysts in advanced oxidation processes. J. Phys. Chem. Solids 2021, 161, 110398. [Google Scholar] [CrossRef]
- Elsherif, K.; El-Dali, A.; Alkarewi, A.; Ewlad-Ahmed, A.; Treban, A. Adsorption of Crystal Violet Dye Onto Olive Leaves Powder: Equilibrium and Kinetic Studies; Elsherif, K.M., El-Dali, A., Alkarewi, A.A., Ewlad-Ahmed, A.M., Treban, A., Eds.; Chemistry International: New York, NY, USA, 2021; Volume 7, pp. 79–89. [Google Scholar]
- Shakir, I.; Agboola, P.O.; Haider, S. Manganese spinel ferrite-reduced graphene oxides nanocomposites for enhanced solar irradiated catalytic studies. Ceram. Int. 2021, 47, 28367–28376. [Google Scholar] [CrossRef]
- Abdi, J. Synthesis of Ag-doped ZIF-8 photocatalyst with excellent performance for dye degradation and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125330. [Google Scholar] [CrossRef]
- Morais, V.; Barrada, R.; Moura, M.; Almeida, J.; Moreira, T.; Gonçalves, G.; Ferreira, S.; Lelis, M.; Freitas, M. Synthesis of manganese ferrite from spent Zn–MnO2 batteries and its application as a catalyst in heterogeneous photo-Fenton processes. J. Environ. Chem. Eng. 2020, 8, 103716. [Google Scholar] [CrossRef]
- Yan, G.; Wang, P.; Li, Y.; Qin, Z.; Lan, S.; Yan, Y.; Zhang, Q.; Cheng, X. Adsorption-Oxidation Mechanism of δ-MnO2 to Remove Methylene Blue. Adsorpt. Sci. Technol. 2021, 2021, 3069392. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Lee, B.; Liu, C.; Kang, S.; Lee, T.; Park, Y.C.; Yoo, R.; Lee, W. Magnetically separable sulfur-doped SnFe2O4/graphene nanohybrids for effective photocatalytic purification of wastewater under visible light. J. Hazard. Mater. 2017, 338, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Samoila, P.; Cojocaru, C.; Mahu, E.; Ignat, M.; Harabagiu, V. Boosting catalytic wet-peroxide-oxidation performances of cobalt ferrite by doping with lanthanides for organic pollutants degradation. J. Environ. Chem. Eng. 2020, 9, 104961. [Google Scholar] [CrossRef]
- Irfan, R.M.; Tahir, M.H.; Maqsood, M.; Lin, Y.; Bashir, T.; Iqbal, S.; Zhao, J.; Gao, L.; Haroon, M. CoSe as non-noble-metal cocatalyst integrated with heterojunction photosensitizer for inexpensive H2 production under visible light. J. Catal. 2020, 390, 196–205. [Google Scholar] [CrossRef]
- Hussain, W.; Malik, H.; Hussain, R.A.; Hussain, H.; Green, I.R.; Marwat, S.; Bahadur, A.; Iqbal, S.; Farooq, M.U.; Li, H.; et al. Synthesis of MnS from Single- and Multi-Source Precursors for Photocatalytic and Battery Applications. J. Electron. Mater. 2019, 48, 2278–2288. [Google Scholar] [CrossRef]
- Adeola, A.; Forbes, P.B.C. Advances in water treatment technologies for removal of polycyclic aromatic hydrocarbons: Existing concepts, emerging trends, and future prospects. Water Environ. Res. 2020, 93, 343–359. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Mathimani, T.; Santhanam, N.; Phuong, T.N.; Brindhadevi, K.; Pugazhendhi, A. Green synthesis of cobalt-oxide nanoparticle using jumbo Muscadine (Vitis rotundifolia): Characterization and photo-catalytic activity of acid Blue-74. J. Photochem. Photobiol. B Biol. 2020, 211, 112011. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Unnarkat, A.; Patel, F.; Shah, M.; Shah, P.J.P.S.; Protection, E. A comprehensive review on spinel based novel catalysts for visible light assisted dye degradation Process Saf. Environ. Prot. 2022, 161, 703–722. [Google Scholar]
- Sher, M.; Javed, M.; Shahid, S.; Hakami, O.; Qamar, M.A.; Iqbal, S.; AL-Anazy, M.M.; Baghdadi, H.B. Designing of highly active g-C3N4/Sn doped ZnO heterostructure as a photocatalyst for the disinfection and degradation of the organic pollutants under visible light irradiation. J. Photochem. Photobiol. A Chem. 2021, 418, 113393. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Chen, A.; Huang, Z.; Shi, J.; Huang, T.; Peng, M.; Hu, L. Three-dimensional graphene supported catalysts for organic dyes degradation. Appl. Catal. B Environ. 2018, 228, 19–28. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Rahman, M.S.U.; Iqbal, S.; Arshad, M.I.; Tahir, M.A.; Mahmood, T. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers. J. Drug Deliv. Sci. Technol. 2017, 39, 277–282. [Google Scholar] [CrossRef]
- Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Anwer, S. Modulating the burst drug release effect of waterborne polyurethane matrix by modifying with polymethylmethacrylate. J. Appl. Polym. Sci. 2019, 136, 47253. [Google Scholar] [CrossRef]
- Islam, M.A.; Ali, I.; Karim, S.A.; Firoz, M.S.H.; Chowdhury, A.-N.; Morton, D.W.; Angove, M. Removal of dye from polluted water using novel nano manganese oxide-based materials. J. Water Process Eng. 2019, 32, 100911. [Google Scholar] [CrossRef]
- Slama, H.B.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Sonu; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar] [CrossRef]
- Chishti, A.N.; Guo, F.; Aftab, A.; Ma, Z.; Liu, Y.; Chen, M.; Gautam, J.; Chen, C.; Ni, L.; Diao, G. Synthesis of silver doped Fe3O4/C nanoparticles and its catalytic activities for the degradation and reduction of methylene blue and 4-nitrophenol. Appl. Surf. Sci. 2021, 546, 149070. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, A.; Xian, L.; Wang, Y.; Long, Y.; Fan, G. Facile fabrication of surface vulcanized Co-Fe spinel oxide nanoparticles toward efficient 4-nitrophenol destruction. J. Hazard. Mater. 2022, 430, 128433. [Google Scholar] [CrossRef]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Venditti, R.A. Impact of dyes and finishes on the microfibers released on the laundering of cotton knitted fabrics. Environ. Pollut. 2020, 272, 115998. [Google Scholar] [CrossRef] [PubMed]
- Warsi, M.F.; Bashir, B.; Zulfiqar, S.; Aadil, M.; Khalid, M.U.; Agboola, P.O.; Shakir, I.; Yousuf, M.A.; Shahid, M. Mn1-xCuxO2/reduced graphene oxide nanocomposites: Synthesis, characterization, and evaluation of visible light mediated catalytic studies. Ceram. Int. 2021, 47, 5044–5053. [Google Scholar] [CrossRef]
- Yao, B.; Chen, X.; Zhou, K.; Luo, Z.; Li, P.; Yang, Z.; Zhou, Y. p-Arsanilic acid decontamination over a wide pH range using biochar-supported manganese ferrite material as an effective persulfate catalyst: Performances and mechanisms. Biochar 2022, 4, 31. [Google Scholar] [CrossRef]
- Yue, Q.; Zhang, F.; Zhang, C.; Zhu, H.; Tang, Y.; Guo, P. A full fuzzy-interval credibility-constrained nonlinear programming approach for irrigation water allocation under uncertainty. Agric. Water Manag. 2019, 230, 105961. [Google Scholar] [CrossRef]
- Muhammad, G.; Mehmood, A.; Shahid, M.; Ashraf, R.S.; Altaf, M.; Hussain, M.A.; Raza, M.A. Biochemical Methods for Water Purification, Methods for Bioremediation of Water and Wastewater Pollution; Springer: Berlin/Heidelberg, Germany, 2020; pp. 181–212. [Google Scholar]
- Rafique, A.; Ikram, M.; Haider, A.; Ul-Hamid, A.; Naz, S.; Nabgan, W.; Haider, J.; Shahzadi, I. Dye degradation, antibacterial activity and molecular docking analysis of cellulose/polyvinylpyrrolidone-doped cadmium sulphide quantum dots. Int. J. Biol. Macromol. 2022, 214, 264–277. [Google Scholar] [CrossRef]
- Akbari, A.; Sabouri, Z.; Hosseini, H.A.; Hashemzadeh, A.; Khatami, M.; Darroudi, M. Effect of nickel oxide nanoparticles as a photocatalyst in dyes degradation and evaluation of effective parameters in their removal from aqueous environments. Inorg. Chem. Commun. 2020, 115, 107867. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Manhas, U.; Qadir, I.; Atri, A.K.; Singh, D. Different Fuel-Adopted Combustion Syntheses of Nano-Structured NiCrFeO4: A Highly Recyclable and Versatile Catalyst for Reduction of Nitroarenes at Room Temperature and Photocatalytic Degradation of Various Organic Dyes in Unitary and Ternary Solutions. ACS Omega 2022, 7, 19853–19871. [Google Scholar] [CrossRef]
- Baynosa, M.L.; Mady, A.H.; Kumar, D.R.; Sayed, M.S.; Tuma, D.; Shim, J.-J. Eco-friendly synthesis of recyclable mesoporous zinc ferrite@ reduced graphene oxide nanocomposite for efficient photocatalytic dye degradation under solar radiation. J. Colloid Interface Sci. 2020, 561, 459–469. [Google Scholar] [CrossRef]
- Majid, F.; Rauf, J.; Ata, S.; Bibi, I.; Malik, A.; Ibrahim, S.M.; Ali, A.; Iqbal, M. Synthesis and characterization of NiFe2O4 ferrite: Sol–gel and hydrothermal synthesis routes effect on magnetic, structural and dielectric characteristics. Mater. Chem. Phys. 2021, 258, 123888. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Priyadharsini, G.M.A.; Kaviyarasu, K.; Jothi, A.I.; Simiyon, G.G. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: Investigation of photocatalytic performance of congo red degradation dye. Surfaces Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Dou, R.; Cheng, H.; Ma, J.; Komarneni, S. Manganese doped magnetic cobalt ferrite nanoparticles for dye degradation via a novel heterogeneous chemical catalysis. Mater. Chem. Phys. 2019, 240, 122181. [Google Scholar] [CrossRef]
- Fatima, S.; Ali, S.I.; Iqbal, M.Z.; Rizwan, S. Congo Red Dye Degradation by Graphene Nanoplatelets/Doped Bismuth Ferrite Nanoparticle Hybrid Catalysts under Dark and Light Conditions. Catalysts 2020, 10, 367. [Google Scholar] [CrossRef]
- Kamal, S.; Pan, G.-T.; Chong, S.; Yang, T.C.-K. Ultrasonically Induced Sulfur-Doped Carbon Nitride/Cobalt Ferrite Nanocomposite for Efficient Sonocatalytic Removal of Organic Dyes. Processes 2020, 8, 104. [Google Scholar] [CrossRef]
- Jasrotia, R.; Kumar, G.; Batoo, K.M.; Adil, S.F.; Khan, M.; Sharma, R.; Kumar, A.; Singh, V.P. Synthesis and characterization of Mg-Ag-Mn nano-ferrites for electromagnet applications. Phys. B Condens. Matter 2019, 569, 1–7. [Google Scholar] [CrossRef]
- Gao, X.; Bi, J.; Gao, J.; Meng, L.; Xie, L.; Liu, C. Partial sulfur doping induced lattice expansion of NiFe2O4 with enhanced electrochemical capacity for supercapacitor application. Electrochimica Acta 2022, 426, 140739. [Google Scholar] [CrossRef]
- He, Y.; Yao, S.; Bi, M.; Yu, H.; Majeed, A.; Shen, X. Fabrication of ultrafine ZnFe2O4 nanoparticles decorated on nitrogen doped carbon nanofibers composite for efficient adsorption/electrocatalysis effect of lithium-sulfur batteries. Electrochimica Acta 2021, 394, 139126. [Google Scholar] [CrossRef]
- Yao, S.; Bi, M.; Yu, H.; Zhang, C.; Zhang, X.; Liu, H.; Zhang, T.; Xiang, J.; Shen, X. Spinel manganese-cobalt oxide nanospheres anchored on nitrogen-containing carbon nanofibers as a highly efficient redox electrocatalyst in lithium/polysulfides batteries. Appl. Surf. Sci. 2022, 598, 153787. [Google Scholar] [CrossRef]
- Vandhana, T.; Lourduraj, A.C. Biogenic synthesis of Mn-Ag co-doped FeO (Fe1-2xMnxAgx) nanoparticles: As an effective disinfectant and anticancer agent. Inorg. Chem. Commun. 2019, 112, 107712. [Google Scholar] [CrossRef]
- Kiani, M.; Zhang, J.; Chen, J.; Luo, Y.; Chen, Y.; Fan, J.; Wang, G.; Wang, R. Facile synthesis of magnesium ferrite nanoparticles supported on nitrogen and sulfur co-doped carbon black as an efficient electrocatalyst for oxygen reduction reaction. J. Nanoparticle Res. 2019, 21, 99. [Google Scholar] [CrossRef]
- Kuang, C.; Tan, P.; Javed, M.; Khushi, H.H.; Nadeem, S.; Iqbal, S.; Alshammari, F.H.; Alqahtani, M.D.; Alsaab, H.O.; Awwad, N.S.; et al. Boosting Photocatalytic interaction of Sulphur doped reduced graphene oxide-based S@ rGO/NiS2 nanocomposite for destruction of pathogens and organic pollutant degradation caused by visible light. Inorg. Chem. Commun. 2022, 141, 109575. [Google Scholar] [CrossRef]
- Maksoud, M.A.; El-Sayyad, G.S.; Ashour, A.; El-Batal, A.I.; Abd-Elmonem, M.S.; Hendawy, H.A.; Abdel-Khalek, E.; Labib, S.; Abdeltwab, E.; El-Okr, M. Synthesis and characterization of metals-substituted cobalt ferrite [Mx Co(1-x) Fe2O4; (M = Zn, Cu and Mn; x = 0 and 0.5)] nanoparticles as antimicrobial agents and sensors for Anagrelide determination in biological samples. Mater. Sci. Eng. C 2018, 92, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Tuan, D.D.; Da Oh, W.; Ghanbari, F.; Lisak, G.; Tong, S.; Lin, K.-Y.A. Coordination polymer-derived cobalt-embedded and N/S-doped carbon nanosheet with a hexagonal core-shell nanostructure as an efficient catalyst for activation of oxone in water. J. Colloid Interface Sci. 2020, 579, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, R.; Gholami, M.; Farzadkia, M.; Jonidi Jafari, A. Catalytic potential of CuFe2O4/GO for activation of peroxymonosulfate in metronidazole degradation: Study of mechanisms. J. Environ. Health Sci. Eng. 2020, 18, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; El-Nashar, D.; Agami, W.; Aly, M.A. Mechanical and dielectric properties of cobalt–zinc nanoferrite/nitrile butadiene rubber composites. J. Thermoplast. Compos. Mater. 2016, 31, 3–22. [Google Scholar] [CrossRef]
- Henry, J.; Mohanraj, K.; Kannan, S.; Barathan, S.; Sivakumar, G. Structural and optical properties of SnS nanoparticles and electron-beam-evaporated SnS thin films. J. Exp. Nanosci. 2013, 10, 78–85. [Google Scholar] [CrossRef]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion- and cation- doping of titanium dioxide in second-generation photocatalyst. J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef]
- Elahi, I.; Zahira, R.; Mehmood, K.; Jamil, A.; Amin, N. Co-precipitation synthesis, physical and magnetic properties of manganese ferrite powder. Afr. J. Pure Appl. Chem. 2012, 6, 1–5. [Google Scholar]









| Element | Apparent Concentration | Wt.% |
|---|---|---|
| C | 5.05 | 24.84 |
| O | 21.69 | 22.7 |
| S | 5.57 | 6.95 |
| Mn | 1.94 | 2.88 |
| Fe | 29.25 | 42.62 |
| Total: | 100 |
| Element | Apparent Concentration | Wt.% |
|---|---|---|
| C | 4.42 | 23.94 |
| O | 22.23 | 24.01 |
| S | 7.17 | 9.08 |
| Mn | 4.51 | 6.82 |
| Fe | 24.25 | 36.14 |
| Total: | 100 |
| Element | Apparent Concentration | Wt.% |
|---|---|---|
| C | 15.97 | 46.51 |
| O | 23.86 | 28.97 |
| S | 4.21 | 4.5 |
| Mn | 0.56 | 0.76 |
| Fe | 14.41 | 19.26 |
| Total: | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, S.; Bukhari, M.; Javed, M.; Iqbal, S.; Ahmad, M.N.; Alrbyawi, H.; Al-Anazy, M.M.; Elkaeed, E.B.; Hegazy, H.H.; Qayyum, M.A.; et al. Cation Incorporation and Synergistic Effects on the Characteristics of Sulfur-Doped Manganese Ferrites S@Mn(Fe2O4) Nanoparticles for Boosted Sunlight-Driven Photocatalysis. Molecules 2022, 27, 7677. https://doi.org/10.3390/molecules27227677
Nadeem S, Bukhari M, Javed M, Iqbal S, Ahmad MN, Alrbyawi H, Al-Anazy MM, Elkaeed EB, Hegazy HH, Qayyum MA, et al. Cation Incorporation and Synergistic Effects on the Characteristics of Sulfur-Doped Manganese Ferrites S@Mn(Fe2O4) Nanoparticles for Boosted Sunlight-Driven Photocatalysis. Molecules. 2022; 27(22):7677. https://doi.org/10.3390/molecules27227677
Chicago/Turabian StyleNadeem, Sohail, Mehak Bukhari, Mohsin Javed, Shahid Iqbal, Mirza Nadeem Ahmad, Hamad Alrbyawi, Murefah Mana Al-Anazy, Eslam B. Elkaeed, H. H. Hegazy, Muhammad Abdul Qayyum, and et al. 2022. "Cation Incorporation and Synergistic Effects on the Characteristics of Sulfur-Doped Manganese Ferrites S@Mn(Fe2O4) Nanoparticles for Boosted Sunlight-Driven Photocatalysis" Molecules 27, no. 22: 7677. https://doi.org/10.3390/molecules27227677
APA StyleNadeem, S., Bukhari, M., Javed, M., Iqbal, S., Ahmad, M. N., Alrbyawi, H., Al-Anazy, M. M., Elkaeed, E. B., Hegazy, H. H., Qayyum, M. A., Pashameah, R. A., Alzahrani, E., & Farouk, A.-E. (2022). Cation Incorporation and Synergistic Effects on the Characteristics of Sulfur-Doped Manganese Ferrites S@Mn(Fe2O4) Nanoparticles for Boosted Sunlight-Driven Photocatalysis. Molecules, 27(22), 7677. https://doi.org/10.3390/molecules27227677









