Transformation of Residual Açai Fruit (Euterpe oleracea) Seeds into Porous Adsorbent for Efficient Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide from Waters
Abstract
1. Introduction
2. Results and Discussion
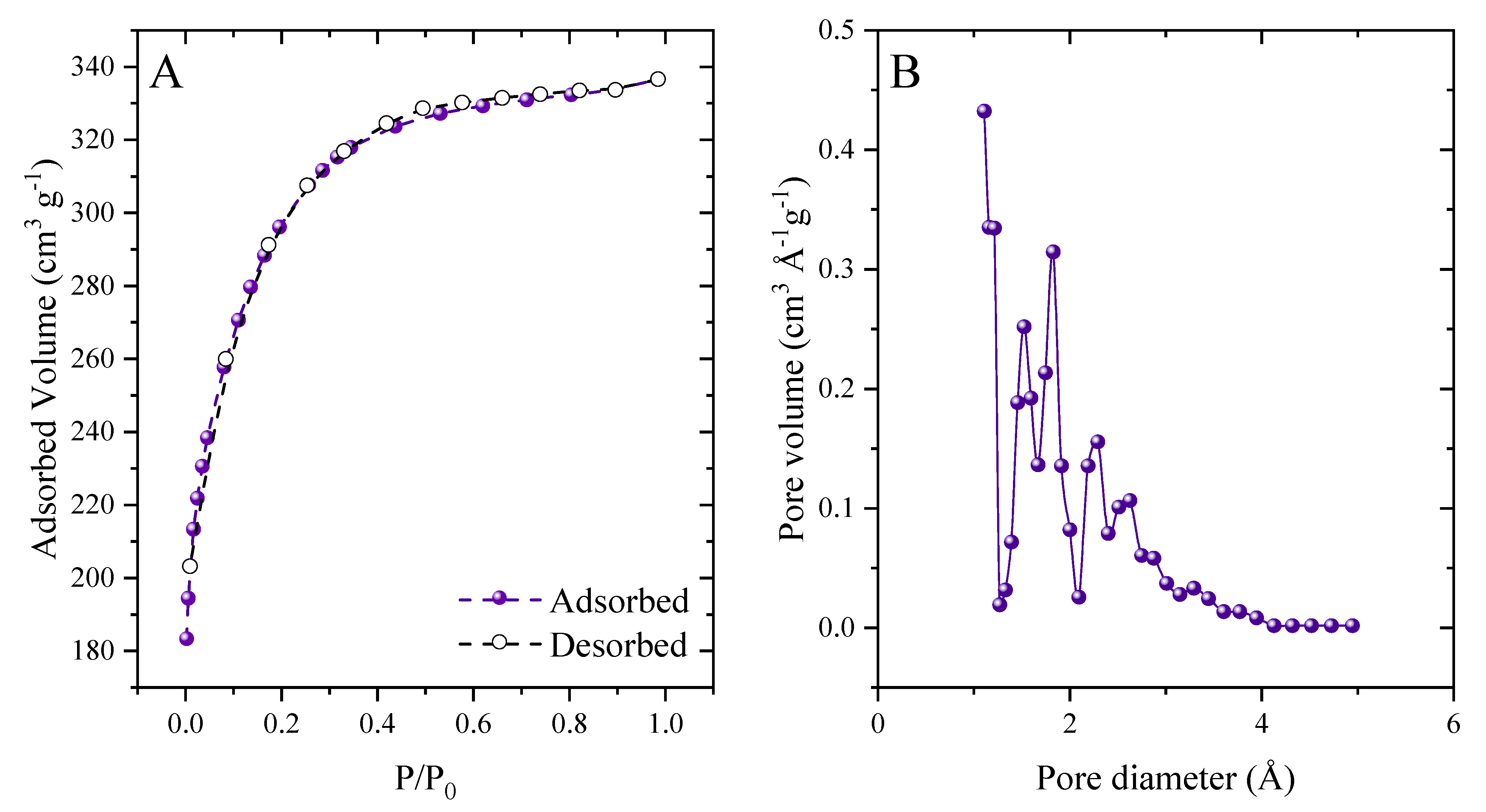
2.1. Features of Açai-Derived AC
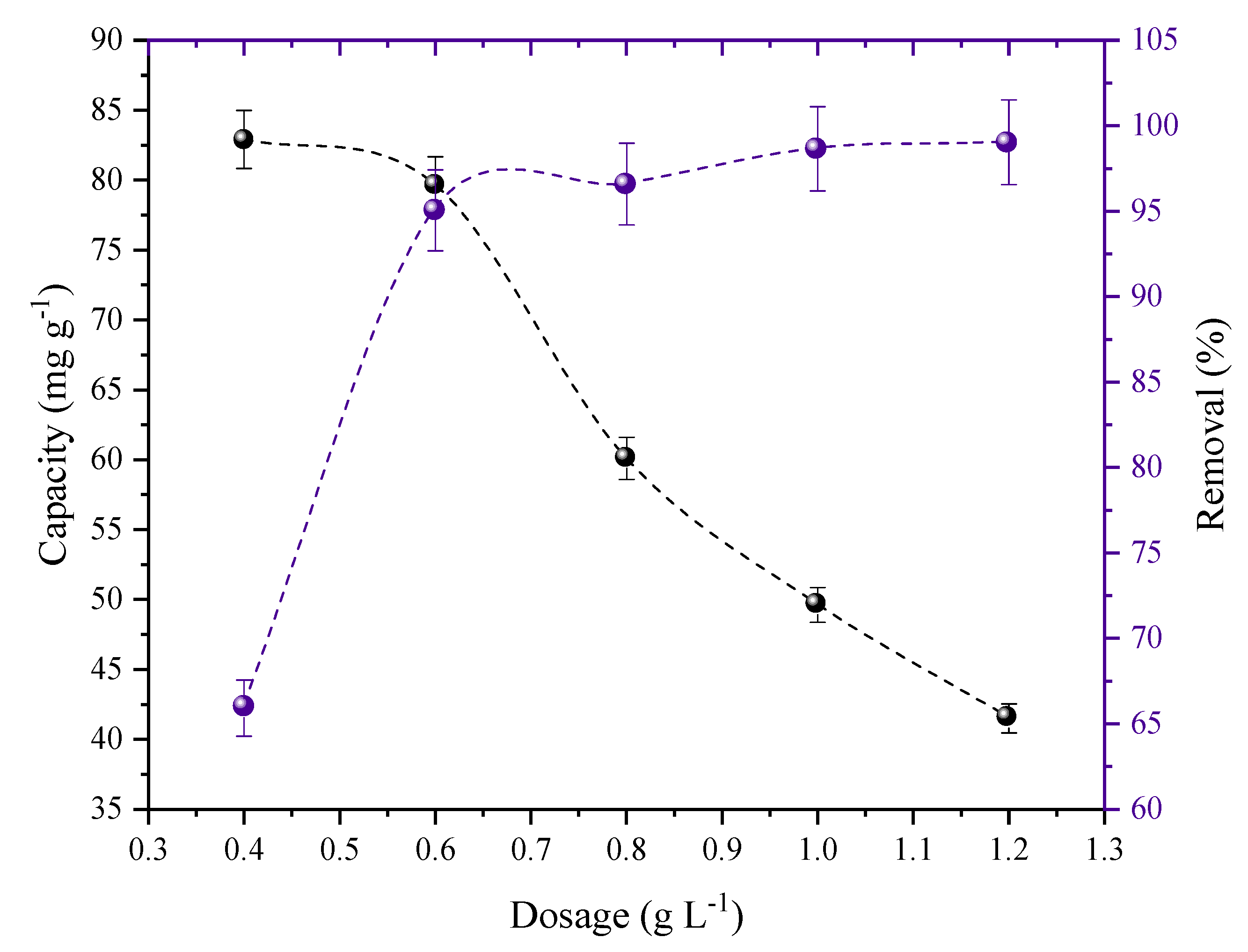
2.2. Effect of Adsorbent Dosage
2.3. Isothermal and Thermodynamic Studies
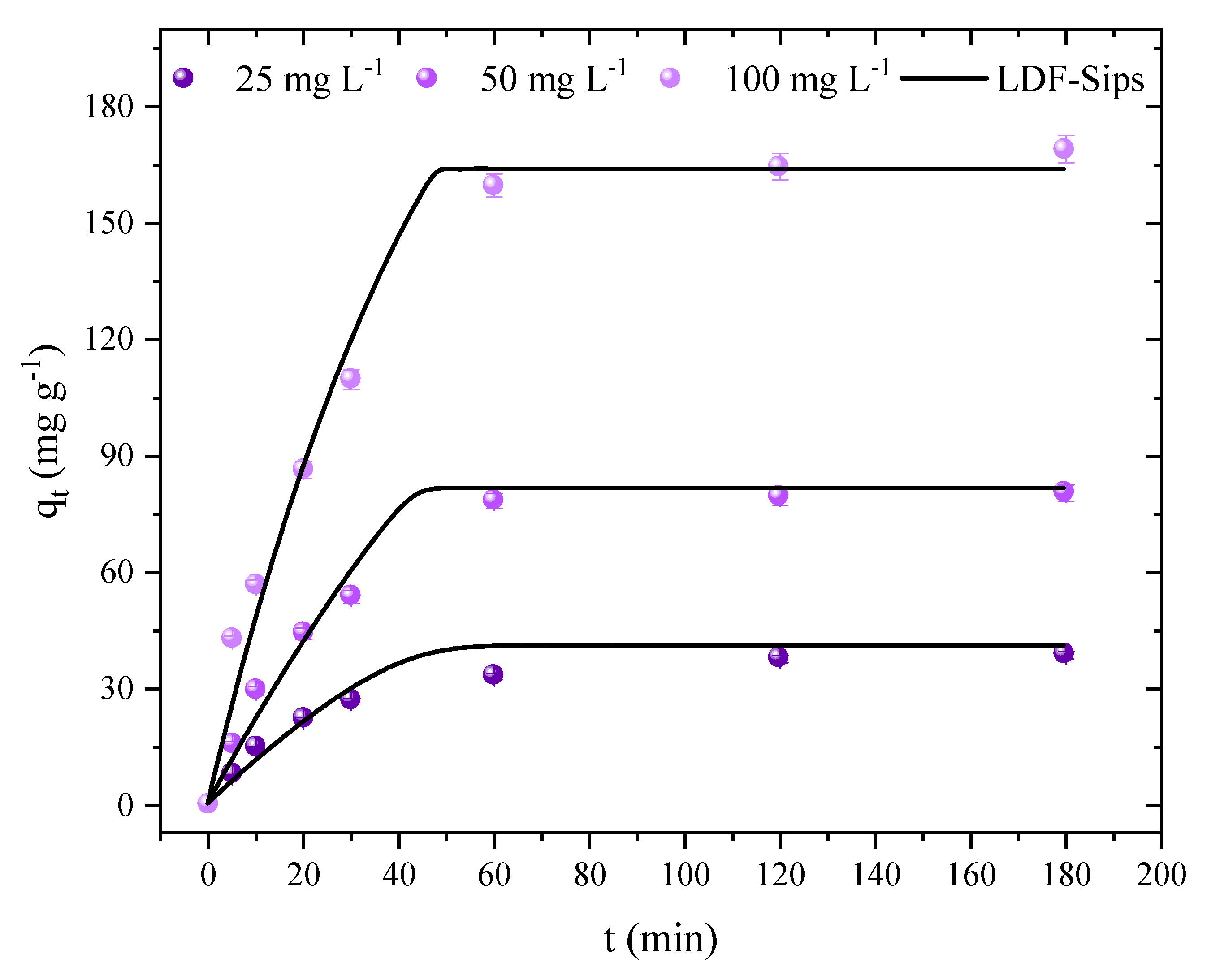
2.4. Kinetic Studies and Application of the Linear Driving Force Model (LDF)
3. Materials and Methods
3.1. Chemicals and Reagents Used
3.2. Preparation of AC and Characterization
3.3. Adsorption Experiments
3.4. Equilibrium Models and Thermodynamic Parameters
3.5. Adsorption Kinetics
3.6. Parameter Estimation, Differential Equation Solution, and Model Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Binh, Q.A.; Nguyen, H.H. Investigation the Isotherm and Kinetics of Adsorption Mechanism of Herbicide 2,4-Dichlorophenoxyacetic Acid (2,4-D) on Corn Cob Biochar. Bioresour. Technol. Rep. 2020, 11, 100520. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Q.; Yang, Z.; Wang, W. Adsorption of 2,4-D on Magnetic Graphene and Mechanism Study. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 367–375. [Google Scholar] [CrossRef]
- Kearns, J.P.; Wellborn, L.S.; Summers, R.S.; Knappe, D.R.U. 2,4-D Adsorption to Biochars: Effect of Preparation Conditions on Equilibrium Adsorption Capacity and Comparison with Commercial Activated Carbon Literature Data. Water Res. 2014, 62, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-F.; Chen, P.-C.; Wang, S.-L. Adsorption of 2,4-D on Mg/Al–NO3 Layered Double Hydroxides with Varying Layer Charge Density. Appl. Clay Sci. 2008, 40, 193–200. [Google Scholar] [CrossRef]
- Yamil, L.D.O.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. High-Performance Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide in Water Using Activated Carbon Derived from Queen Palm Fruit Endocarp (Syagrus romanzoffiana). J. Environ. Chem. Eng. 2021, 9, 104911. [Google Scholar] [CrossRef]
- Bakhtiary, S.; Shirvani, M.; Shariatmadari, H. Adsorption-Desorption Behavior of 2,4-D on NCP-Modified Bentonite and Zeolite: Implications for Slow-Release Herbicide Formulations. Chemosphere 2013, 90, 699–705. [Google Scholar] [CrossRef]
- El Harmoudi, H.; El Gaini, L.; Daoudi, E.; Rhazi, M.; Boughaleb, Y.; El Mhammedi, M.A.; Migalska-Zalas, A.; Bakasse, M. Removal of 2,4-D from Aqueous Solutions by Adsorption Processes Using Two Biopolymers: Chitin and Chitosan and Their Optical Properties. Opt. Mater. 2014, 36, 1471–1477. [Google Scholar] [CrossRef]
- Sayğili, H.; Güzel, F. High Surface Area Mesoporous Activated Carbon from Tomato Processing Solid Waste by Zinc Chloride Activation: Process Optimization, Characterization and Dyes Adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Carter, A.D. Herbicide Movement in Soils: Principles, Pathways and Processes. Weed Res. 2000, 40, 113–122. [Google Scholar] [CrossRef]
- Calisto, J.S.; Pacheco, I.S.; Freitas, L.L.; Santana, L.K.; Fagundes, W.S.; Amaral, F.A.; Canobre, S.C. Adsorption Kinetic and Thermodynamic Studies of the 2, 4—Dichlorophenoxyacetate (2,4-D) by the [Co–Al–Cl] Layered Double Hydroxide. Heliyon 2019, 5, e02553. [Google Scholar] [CrossRef]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Dotto, G.L.; Duran-Valle, C.J. Adsorption in Water Treatment. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Vieira, Y.; Schnorr, C.; Piazzi, A.C.; Netto, M.S.; Piccini, W.M.; Franco, D.S.P.; Mallmann, E.S.; Georgin, J.; Silva, L.F.O.; Dotto, G.L. An Advanced Combination of Density Functional Theory Simulations and Statistical Physics Modeling in the Unveiling and Prediction of Adsorption Mechanisms of 2,4-D Pesticide to Activated Carbon. J. Mol. Liq. 2022, 361, 119639. [Google Scholar] [CrossRef]
- Ngoc, P.K.; Mac, T.K.; Nguyen, H.T.; Viet, D.T.; Thanh, T.D.; Van Vinh, P.; Phan, B.T.; Duong, A.T.; Das, R. Superior Organic Dye Removal by CoCr2O4 Nanoparticles: Adsorption Kinetics and Isotherm. J. Sci. Adv. Mater. Devices 2022, 7, 100438. [Google Scholar] [CrossRef]
- Sbizzaro, M.; Sampaio, S.C.; dos Reis, R.R.; de Assis Beraldi, F.; Rosa, D.M.; de Freitas Maia, C.M.B.; do Nascimento, C.T.; da Silva, E.A.; Borba, C.E. Effect of Production Temperature in Biochar Properties from Bamboo Culm and Its Influences on Atrazine Adsorption from Aqueous Systems. J. Mol. Liq. 2021, 343, 117667. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Lima, E.C.; Dotto, G.L. Application of Araçá Fruit Husks (Psidium Cattleianum) in the Preparation of Activated Carbon with FeCl3 for Atrazine Herbicide Adsorption. Chem. Eng. Res. Des. 2022, 180, 67–78. [Google Scholar] [CrossRef]
- Hernandes, P.T.; Franco, D.S.P.; Georgin, J.; Salau, N.P.G.; Dotto, G.L. Investigation of Biochar from Cedrella Fissilis Applied to the Adsorption of Atrazine Herbicide from an Aqueous Medium. J. Environ. Chem. Eng. 2022, 10, 107408. [Google Scholar] [CrossRef]
- Ndjientcheu Yossa, L.M.; Ouiminga, S.K.; Sidibe, S.S.; Ouedraogo, I.W.K. Synthesis of a Cleaner Potassium Hydroxide-Activated Carbon from Baobab Seeds Hulls and Investigation of Adsorption Mechanisms for Diuron: Chemical Activation as Alternative Route for Preparation of Activated Carbon from Baobab Seeds Hulls and Adsorption. Sci. Afr. 2020, 9, e00476. [Google Scholar] [CrossRef]
- Tongur, T.; Ayranci, E. Adsorption and Electrosorption of Paraquat, Diquat and Difenzoquat from Aqueous Solutions onto Activated Carbon Cloth as Monitored by in-Situ Uv-Visible Spectroscopy. J. Environ. Chem. Eng. 2021, 9, 105566. [Google Scholar] [CrossRef]
- Li, Y.; Tsend, N.; Li, T.K.; Liu, H.; Yang, R.; Gai, X.; Wang, H.; Shan, S. Microwave Assisted Hydrothermal Preparation of Rice Straw Hydrochars for Adsorption of Organics and Heavy Metals. Bioresour. Technol. 2019, 273, 136–143. [Google Scholar] [CrossRef]
- Zavalloni, C.; Alberti, G.; Biasiol, S.; Vedove, G.D.; Fornasier, F.; Liu, J.; Peressotti, A. Microbial Mineralization of Biochar and Wheat Straw Mixture in Soil: A Short-Term Study. Appl. Soil Ecol. 2011, 50, 45–51. [Google Scholar] [CrossRef]
- Martins, M.A.; Mattoso, L.H.C.; Pessoa, J.D.C. Thermogravimetric Evaluation of Açaí Fruit (Euterpe Oleracea Mart.) Agro Industry Waste. Rev. Bras. Frutic. 2009, 31, 1150–1157. [Google Scholar] [CrossRef]
- Freitas, M.A.B.; Magalhães, J.L.L.; Carmona, C.P.; Arroyo-Rodríguez, V.; Vieira, I.C.G.; Tabarelli, M. Intensification of Açaí Palm Management Largely Impoverishes Tree Assemblages in the Amazon Estuarine Forest. Biol. Conserv. 2021, 261, 109251. [Google Scholar] [CrossRef]
- Melo, P.S.; Selani, M.M.; Gonçalves, R.H.; de Oliveira Paulino, J.; Massarioli, A.P.; de Alencar, S.M. Açaí Seeds: An Unexplored Agro-Industrial Residue as a Potential Source of Lipids, Fibers, and Antioxidant Phenolic Compounds. Ind. Crops Prod. 2021, 161, 113204. [Google Scholar] [CrossRef]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Pedroso, A.J.S.; de Freitas Maia, C.M.B. Biochar from Acai Agroindustry Waste: Study of Pyrolysis Conditions. Waste Manag. 2019, 96, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Vasconcelos De Almeida, A.; Vieira, W.T.; Bispo, M.D.; De Melo, S.F.; Da Silva, T.L.; Balliano, T.L.; Vieira, M.G.A.; Soletti, J.I. Caffeine Removal Using Activated Biochar from Acai Seed (Euterpe Oleracea Mart): Experimental Study and Description of Adsorbate Properties Using Density Functional Theory (DFT). J. Environ. Chem. Eng. 2021, 9, 104891. [Google Scholar] [CrossRef]
- Gonçalves Junior, A.C.; Coelho, G.F.; Schwantes, D.; Rech, A.L.; Campagnolo, M.Â.; Miola, A.J. Biossorção de Cu (II) e Zn (II) Utilizando o Endocarpo de Açaí Euterpe Oleracea M. Em Solução Aquosa Contaminada. Acta Sci. Technol. 2016, 38, 361–370. [Google Scholar] [CrossRef]
- de Lima, L.D.R.; da Costa, O.F.; da Fonseca Alves, B.S.; Dantas, K.D.G.F.; Lemos, V.P.; Pinheiro, M.H.T. Removal of Cu (II), Zn (II) and Ni (II) Using Açaí Residue (Euterpe Oleracea Mart.) as a Biosorbent in Aqueous Solution. Rev. Virtual Quim. 2020, 12, 1066–1078. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Schwantes, D.; Campagnolo, M.A.; Dragunski, D.C.; Tarley, C.R.T.; Dos Santos Silva, A.K. Removal of Toxic Metals Using Endocarp of Açaí Berry as Biosorbent. Water Sci. Technol. 2018, 77, 1547–1557. [Google Scholar] [CrossRef]
- Dias, Y.N.; Souza, E.S.; da Costa, H.S.C.; Melo, L.C.A.; Penido, E.S.; do Amarante, C.B.; Teixeira, O.M.M.; Fernandes, A.R. Biochar Produced from Amazonian Agro-Industrial Wastes: Properties and Adsorbent Potential of Cd2+ and Cu2+. Biochar 2019, 1, 389–400. [Google Scholar] [CrossRef]
- de Sousa, A.A.O.; Oliveira, T.S.; de Azevedo, L.E.C.; Nobre, J.R.C.; Stefanelli, W.F.R.; de Sousa Costa, T.A.P.; da Silva, J.P.S.; Barral, A.V.S. Adsorption of the Basic Dye Malachite Green via Activated Carbon from Açaí Seed. Res. Soc. Dev. 2021, 10, e49110212871. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Calvete, T.; Pinto, I.S.; Amavisca, C.V.; Fernandes, T.H.M.; Pinto, R.B.; Alencar, W.S. Application of Aqai Stalks as Biosorbents for the Removal of the Dyes Reactive Black 5 and Reactive Orange 16 from Aqueous Solution. J. Chem. Eng. Data 2011, 56, 1857–1868. [Google Scholar] [CrossRef]
- de Sousa Ribeiro, L.A.; Thim, G.P.; Alvarez-Mendez, M.O.; dos Reis Coutinho, A.; de Moraes, N.P.; Rodrigues, L.A. Preparation, Characterization, and Application of Low-Cost Açaí Seed-Based Activated Carbon for Phenol Adsorption. Int. J. Environ. Res. 2018, 12, 755–764. [Google Scholar] [CrossRef]
- Georgin, J.; da Boit Martinello, K.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Yilmaz, M.; Silva, L.F.O.; Dotto, G.L. Residual Peel of Pitaya Fruit (Hylocereus Undatus) as a Precursor to Obtaining an Efficient Carbon-Based Adsorbent for the Removal of Metanil Yellow Dye from Water. J. Environ. Chem. Eng. 2022, 10, 107006. [Google Scholar] [CrossRef]
- Georgin, J.; Yamil, L.D.O.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Perondi, D.; Silva, L.F.O.; Foletto, E.L.; Dotto, G.L. Development of Highly Porous Activated Carbon from Jacaranda Mimosifolia Seed Pods for Remarkable Removal of Aqueous-Phase Ketoprofen. J. Environ. Chem. Eng. 2021, 9, 105676. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Netto, M.S.; Allasia, D.; Oliveira, M.L.S.; Foletto, E.L.; Dotto, G.L. Highly Effective Adsorption of Synthetic Phenol Effluent by a Novel Activated Carbon Prepared from Fruit Wastes of the Ceiba Speciosa Forest Species. J. Environ. Chem. Eng. 2021, 9, 105927. [Google Scholar] [CrossRef]
- Üner, O.; Bayrak, Y. The Effect of Carbonization Temperature, Carbonization Time and Impregnation Ratio on the Properties of Activated Carbon Produced from Arundo Donax. Microporous Mesoporous Mater. 2018, 268, 225–234. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T.; Hashim, R.; Said, N.; Akhtar, M.N.; Mohamad-Saleh, J.; Sulaiman, O. Comparison of Surface Properties of Wood Biomass Activated Carbons and Their Application against Rhodamine B and Methylene Blue Dye; Elsevier: Amsterdam, The Netherlands, 2018; Volume 11, ISBN 6065512001. [Google Scholar]
- Cruz, G.; Santiago, P.A.; Braz, C.E.M.; Seleghim, P.; Crnkovic, P.M. Investigation into the Physical–Chemical Properties of Chemically Pretreated Sugarcane Bagasse. J. Therm. Anal. Calorim. 2018, 132, 1039–1053. [Google Scholar] [CrossRef]
- Mohd Din, A.T.; Hameed, B.H.; Ahmad, A.L. Batch Adsorption of Phenol onto Physiochemical-Activated Coconut Shell. J. Hazard. Mater. 2009, 161, 1522–1529. [Google Scholar] [CrossRef]
- Anchieta, C.; Cancelier, A.; Mazutti, M.; Jahn, S.; Kuhn, R.; Gündel, A.; Chiavone-Filho, O.; Foletto, E. Effects of Solvent Diols on the Synthesis of ZnFe2O4 Particles and Their Use as Heterogeneous Photo-Fenton Catalysts. Materials 2014, 7, 6281–6290. [Google Scholar] [CrossRef]
- Yamil, L.D.O.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Grassi, P.; Piccilli, D.G.A.; Oliveira, M.L.S.; Dotto, G.L. Powdered Biosorbent from Pecan Pericarp (Carya Illinoensis) as an Efficient Material to Uptake Methyl Violet 2B from Effluents in Batch and Column Operations. Adv. Powder Technol. 2020, 31, 2843–2852. [Google Scholar] [CrossRef]
- de Salomón, Y.L.O.O.; Georgin, J.; Franco, D.S.P.P.; Netto, M.S.; Foletto, E.L.; Allasia, D.; Dotto, G.L. Application of Seed Residues from Anadenanthera Macrocarpa and Cedrela Fissilis as Alternative Adsorbents for Remarkable Removal of Methylene Blue Dye in Aqueous Solutions. Environ. Sci. Pollut. Res. 2020, 28, 2342–2354. [Google Scholar] [CrossRef]
- Georgin, J.; Dotto, G.L.; Mazutti, M.A.; Foletto, E.L. Preparation of Activated Carbon from Peanut Shell by Conventional Pyrolysis and Microwave Irradiation-Pyrolysis to Remove Organic Dyes from Aqueous Solutions. J. Environ. Chem. Eng. 2016, 4, 266–275. [Google Scholar] [CrossRef]
- Li, W.; Mo, W.; Kang, C.; Zhang, M.; Meng, M.; Chen, M. Adsorption of Nitrate from Aqueous Solution onto Modified Cassava (Manihot Esculenta) Straw. Ecol. Chem. Eng. S 2012, 19, 629–638. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of Naphthalene and 1-Naphthol by Biochars of Orange Peels with Different Pyrolytic Temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Elena, A.; Gozescu, I.; Dabici, A.; Sfirloaga, P.; Szabadai, Z. Organic Compounds FT-IR Spectroscopy. In Macro To Nano Spectroscopy; InTechOpen: Timisoara, Romania, 2012. [Google Scholar]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Ng, E.P. The Synthesis and Characterization of High Purity Mixed Microporous/Mesoporous Activated Carbon from Rice Husk Using Chemical Activation with NaOH and KOH. Microporous Mesoporous Mater. 2014, 197, 316–323. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American Lignocellulosic Biomass and Biochars in Terms of Their Candidacy for Alternate Renewable Fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Sharma, R.; Sarswat, A.; Pittman, C.U.; Mohan, D. Cadmium and Lead Remediation Using Magnetic and Non-Magnetic Sustainable Biosorbents Derived from Bauhinia Purpurea Pods. RSC Adv. 2017, 7, 8606–8624. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An Overview of the Organic and Inorganic Phase Composition of Biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Cheng, J.; Gu, J.J.; Tao, W.; Wang, P.; Liu, L.; Wang, C.Y.; Li, Y.K.; Feng, X.H.; Qiu, G.H.; Cao, F.F. Edible Fungus Slag Derived Nitrogen-Doped Hierarchical Porous Carbon as a High-Performance Adsorbent for Rapid Removal of Organic Pollutants from Water. Bioresour. Technol. 2019, 294, 122149. [Google Scholar] [CrossRef]
- Georgin, J.; Drumm, F.C.; Grassi, P.; Franco, D.; Allasia, D.; Dotto, G.L.; Caroline, F.; Patrícia, D.; Dison, G.; Guilherme, F.; et al. Potential of Araucaria Angustifolia Bark as Adsorbent to Remove Gentian Violet Dye from Aqueous Effluents. Water Sci. Technol. 2018, 78, 1693–1703. [Google Scholar] [CrossRef]
- Babas, H.; Khachani, M.; Warad, I.; Ajebli, S.; Guessous, A.; Guenbour, A.; Safi, Z.; Berisha, A.; Bellaouchou, A.; Abdelkader, Z.; et al. Sofosbuvir Adsorption onto Activated Carbon Derived from Argan Shell Residue: Optimization, Kinetic, Thermodynamic and Theoretical Approaches. J. Mol. Liq. 2022, 356, 119019. [Google Scholar] [CrossRef]
- Neolaka, Y.A.B.; Lawa, Y.; Naat, J.; Riwu, A.A.P.; Darmokoesoemo, H.; Widyaningrum, B.A.; Iqbal, M.; Kusuma, H.S. Indonesian Kesambi Wood (Schleichera Oleosa) Activated with Pyrolysis and H2SO4 Combination Methods to Produce Mesoporous Activated Carbon for Pb(II) Adsorption from Aqueous Solution. Environ. Technol. Innov. 2021, 24, 101997. [Google Scholar] [CrossRef]
- Jain, A.; Jayaraman, S.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal Pre-Treatment for Mesoporous Carbon Synthesis: Enhancement of Chemical Activation. J. Mater. Chem. A 2014, 2, 520–528. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Particle Technology Series; Springer: Dordrecht, The Netherlands, 2004; Volume 16, ISBN 978-90-481-6633-6. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Paredes-Laverde, M.; Salamanca, M.; Diaz-Corrales, J.D.; Flórez, E.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the Removal of an Anionic Dye in Textile Wastewaters by Adsorption on ZnCl2activated Carbons from Rice and Coffee Husk Wastes: A Combined Experimental and Theoretical Study. J. Environ. Chem. Eng. 2021, 9, 105685. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; Schnorr, C.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Rhoden, C.R.B.; Dotto, G.L. Microporous Activated Carbon from the Fruits of the Invasive Species Hovenia Dulcis to Remove the Herbicide Atrazine from Waters. J. Mol. Liq. 2022, 364, 120014. [Google Scholar] [CrossRef]
- Kerkhoff, C.M.; da Boit Martinello, K.; Franco, D.S.P.P.; Netto, M.S.; Georgin, J.; Foletto, E.L.; Piccilli, D.G.A.A.; Silva, L.F.O.O.; Dotto, G.L.; da Boit Martinello, K.; et al. Adsorption of Ketoprofen and Paracetamol and Treatment of a Synthetic Mixture by Novel Porous Carbon Derived from Butia Capitata Endocarp. J. Mol. Liq. 2021, 339, 117184. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Lima, E.C.; Dotto, G.L. Preparation of Activated Carbon from the Residues of the Mushroom (Agaricus Bisporus) Production Chain for the Adsorption of the 2,4-Dichlorophenoxyacetic Herbicide. J. Environ. Chem. Eng. 2021, 9, 106843. [Google Scholar] [CrossRef]
- Diel, J.C.; Franco, D.S.P.; Igansi, A.V.; Cadaval, T.R.S.; Pereira, H.A.; Nunes, I.D.S.; Basso, C.W.; Maria do Carmo, M.A.; Morais, J.; Pinto, D.; et al. Green Synthesis of Carbon Nanotubes Impregnated with Metallic Nanoparticles: Characterization and Application in Glyphosate Adsorption. Chemosphere 2021, 283, 131193. [Google Scholar] [CrossRef]
- Pedrosa, M.; Ribeiro, R.S.; Guerra-Rodríguez, S.; Rodríguez-Chueca, J.; Rodríguez, E.; Silva, A.M.T.; Ðolic, M.; Rita Lado Ribeiro, A. Spirulina-Based Carbon Bio-Sorbent for the Efficient Removal of Metoprolol, Diclofenac and Other Micropollutants from Wastewater. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100720. [Google Scholar] [CrossRef]
- Taoufik, N.; Elmchaouri, A.; Anouar, F.; Korili, S.A.; Gil, A. Improvement of the Adsorption Properties of an Activated Carbon Coated by Titanium Dioxide for the Removal of Emerging Contaminants. J. Water Process Eng. 2019, 31, 100876. [Google Scholar] [CrossRef]
- de Albuquerque, F.P.; de Oliveira, J.L.; Moschini-Carlos, V.; Fraceto, L.F. An Overview of the Potential Impacts of Atrazine in Aquatic Environments: Perspectives for Tailored Solutions Based on Nanotechnology. Sci. Total Environ. 2020, 700, 134868. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Shimizu, F.M.; Machado, S.A.S.; Oliveira, O.N. Selective and Sensitive Multiplexed Detection of Pesticides in Food Samples Using Wearable, Flexible Glove-Embedded Non-Enzymatic Sensors. Chem. Eng. J. 2021, 408, 127279. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, C.; Peng, F.; Hou, W.; Lin, X.; Wang, X. Adsorption Properties of Graphene Materials for Pesticides: Structure Effect. J. Mol. Liq. 2022, 364, 119967. [Google Scholar] [CrossRef]
- Trivedi, N.S.; Kharkar, R.A.; Mandavgane, S.A. Utilization of Cotton Plant Ash and Char for Removal of 2, 4-Dichlorophenoxyacetic Acid. Resour. Technol. 2016, 2, S39–S46. [Google Scholar] [CrossRef]
- Liu, M.W.; Liu, R.; Wu, H.Y.; Li, Y.Y.; Su, M.X.; Dong, M.N.; Zhang, W.; Qian, C.Y. Radix Puerariae Extracts Ameliorate Paraquat-Induced Pulmonary Fibrosis by Attenuating Follistatin-like 1 and Nuclear Factor Erythroid 2p45-Related Factor-2 Signalling Pathways through Downregulation of MiRNA-21 Expression. BMC Complement. Altern. Med. 2016, 16, 1–15. [Google Scholar] [CrossRef]
- Evy Alice Abigail, M.; Chidambaram, R. Rice Husk as a Low Cost Nanosorbent for 2,4-Dichlorophenoxyacetic Acid Removal from Aqueous Solutions. Ecol. Eng. 2016, 92, 97–105. [Google Scholar] [CrossRef]
- Hue, H.K.; Anh, L.V.; Thiep, T. Van Study of the Adsorption of 2,4-Dichlorophenoxyacetic Acid from the Aqueous Solution onto Carbon Nanotubes. Vietnam J. Chem. 2018, 56, 191–196. [Google Scholar] [CrossRef]
- Şahin, S.; Emik, S. Fast and Highly Efficient Removal of 2,4-D Using Amino-Functionalized Poly (Glycidyl Methacrylate) Adsorbent: Optimization, Equilibrium, Kinetic and Thermodynamic Studies. J. Mol. Liq. 2018, 260, 195–202. [Google Scholar] [CrossRef]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U.; Mohan, D.; Mlsna, T. Phenoxy Herbicide Removal from Aqueous Solutions Using Fast Pyrolysis Switchgrass Biochar. Chemosphere 2017, 174, 49–57. [Google Scholar] [CrossRef]
- Hameed, B.H.; Salman, J.M.; Ahmad, A.L. Adsorption Isotherm and Kinetic Modeling of 2,4-D Pesticide on Activated Carbon Derived from Date Stones. J. Hazard. Mater. 2009, 163, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the Nonlinear and Linear Forms of the van’t Hoff Equation for Calculation of Adsorption Thermodynamic Parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Ali, I.; Al-Othman, Z.A.; Alwarthan, A. Synthesis of Composite Iron Nano Adsorbent and Removal of Ibuprofen Drug Residue from Water. J. Mol. Liq. 2016, 219, 858–864. [Google Scholar] [CrossRef]
- Worch, E. Fixed-Bed Adsorption in Drinking Water Treatment: A Critical Review on Models and Parameter Estimation. J. Water Supply Res. Technol. 2008, 57, 171–183. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment: Fundamentals, Processes, and Modeling; De Gruyter: Dresden, Germany, 2012; ISBN 3110240238. [Google Scholar]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic Parameters of Liquid–Phase Adsorption Process Calculated from Different Equilibrium Constants Related to Adsorption Isotherms: A Comparison Study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Glueckauf, E. Theory of Chromatography. Part 10.—Formulæ for Diffusion into Spheres and Their Application to Chromatography. Trans. Faraday Soc. 1955, 51, 1540–1551. [Google Scholar] [CrossRef]





| Temperature (K) | ||||
|---|---|---|---|---|
| Model | 298 | 308 | 318 | 328 |
| Langmuir | ||||
| qmL (mg g−1) | 178.4 | 190.3 | 194.1 | 195.1 |
| KL (L mg−1) | 0.3657 | 0.5681 | 0.8954 | 2.4473 |
| R2 | 0.9836 | 0.9740 | 0.9648 | 0.9476 |
| R2adj | 0.9673 | 0.9479 | 0.9296 | 0.8952 |
| ARE (%) | 6.0592 | 7.6433 | 8.6760 | 9.9616 |
| MSR (mg g−1)2 | 121.5 | 230.9 | 339.8 | 548.3 |
| Freundlich | ||||
| KF ((mg g−1)(mg L−1)−1/nF) | 102.9 | 113.8 | 120.1 | 127.7 |
| 1/nF (dimensionless) | 0.1136 | 0.1136 | 0.1136 | 0.1136 |
| R2 | 0.9550 | 0.9534 | 0.9586 | 0.9737 |
| R2adj | 0.9100 | 0.9068 | 0.9173 | 0.9474 |
| ARE (%) | 11.31 | 12.48 | 12.19 | 10.12 |
| MSR (mg g−1)2 | 333.7 | 413.4 | 399.2 | 275.2 |
| Sips | ||||
| qmS (mg g−1) | 276.35 | 301.68 | 314.35 | 327.03 |
| KS (L mg−1)nS | 0.3234 | 0.3634 | 0.4098 | 0.5064 |
| nS (dimensionless) | 0.4046 | 0.3879 | 0.3636 | 0.3125 |
| R2 | 0.9993 | 0.9992 | 0.9988 | 0.9966 |
| R2adj | 0.9970 | 0.9968 | 0.9950 | 0.9863 |
| ARE (%) | 0.945 | 1.307 | 1.867 | 3.169 |
| MSR (mg g−1)2 | 8.242 | 10.58 | 17.98 | 53.70 |
| T (K) | Ke × 10−4 | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (kJ mol−1 K−1) |
|---|---|---|---|---|
| 298 | 1.35 | −23.58 | 16.16 | 0.1331 |
| 308 | 1.62 | −24.84 | ||
| 318 | 1.90 | −26.06 | ||
| 328 | 2.50 | −27.63 |
| Model | Initial Concentration (mg L−1) | ||
|---|---|---|---|
| 25 | 50 | 100 | |
| LDF-Sips | |||
| qpred (mg g−1) | 44.81 | 83.79 | 163.9 |
| kLDFx103 (s−1) | 7.78 | 13.61 | 31.36 |
| DS × 108 (cm2 s−1) | 8.497 | 14.85 | 34.23 |
| R2 | 0.9338 | 0.9795 | 0.9808 |
| ARE (%) | 16.78 | 14.24 | 15.03 |
| MSE (mg g−1)2 | 13.38 | 19.72 | 76.52 |
| qexp (mg g−1) | 38.79 | 80.58 | 169.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, R.; Schnorr, C.E.; Georgin, J.; Netto, M.S.; Franco, D.S.P.; Carissimi, E.; Wolff, D.; Silva, L.F.O.; Dotto, G.L. Transformation of Residual Açai Fruit (Euterpe oleracea) Seeds into Porous Adsorbent for Efficient Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide from Waters. Molecules 2022, 27, 7781. https://doi.org/10.3390/molecules27227781
Ramirez R, Schnorr CE, Georgin J, Netto MS, Franco DSP, Carissimi E, Wolff D, Silva LFO, Dotto GL. Transformation of Residual Açai Fruit (Euterpe oleracea) Seeds into Porous Adsorbent for Efficient Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide from Waters. Molecules. 2022; 27(22):7781. https://doi.org/10.3390/molecules27227781
Chicago/Turabian StyleRamirez, Rolando, Carlos Eduardo Schnorr, Jordana Georgin, Matias Schadeck Netto, Dison S. P. Franco, Elvis Carissimi, Delmira Wolff, Luis F. O. Silva, and Guilherme Luiz Dotto. 2022. "Transformation of Residual Açai Fruit (Euterpe oleracea) Seeds into Porous Adsorbent for Efficient Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide from Waters" Molecules 27, no. 22: 7781. https://doi.org/10.3390/molecules27227781
APA StyleRamirez, R., Schnorr, C. E., Georgin, J., Netto, M. S., Franco, D. S. P., Carissimi, E., Wolff, D., Silva, L. F. O., & Dotto, G. L. (2022). Transformation of Residual Açai Fruit (Euterpe oleracea) Seeds into Porous Adsorbent for Efficient Removal of 2,4-Dichlorophenoxyacetic Acid Herbicide from Waters. Molecules, 27(22), 7781. https://doi.org/10.3390/molecules27227781









