rGO-WO3 Heterostructure: Synthesis, Characterization and Utilization as an Efficient Adsorbent for the Removal of Fluoroquinolone Antibiotic Levofloxacin in an Aqueous Phase
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of rGO-WO3 Heterostructure
2.3. Characterization
2.4. Adsorptive Removal of LVX Using rGO-WO3 Heterostructure
2.5. Application of the Prepared rGO-WO3 Heterostructure for Removal of LVX in Real Water Samples
2.6. Stability and Reusability of rGO-WO3 Adsorbent
3. Results and Discussion
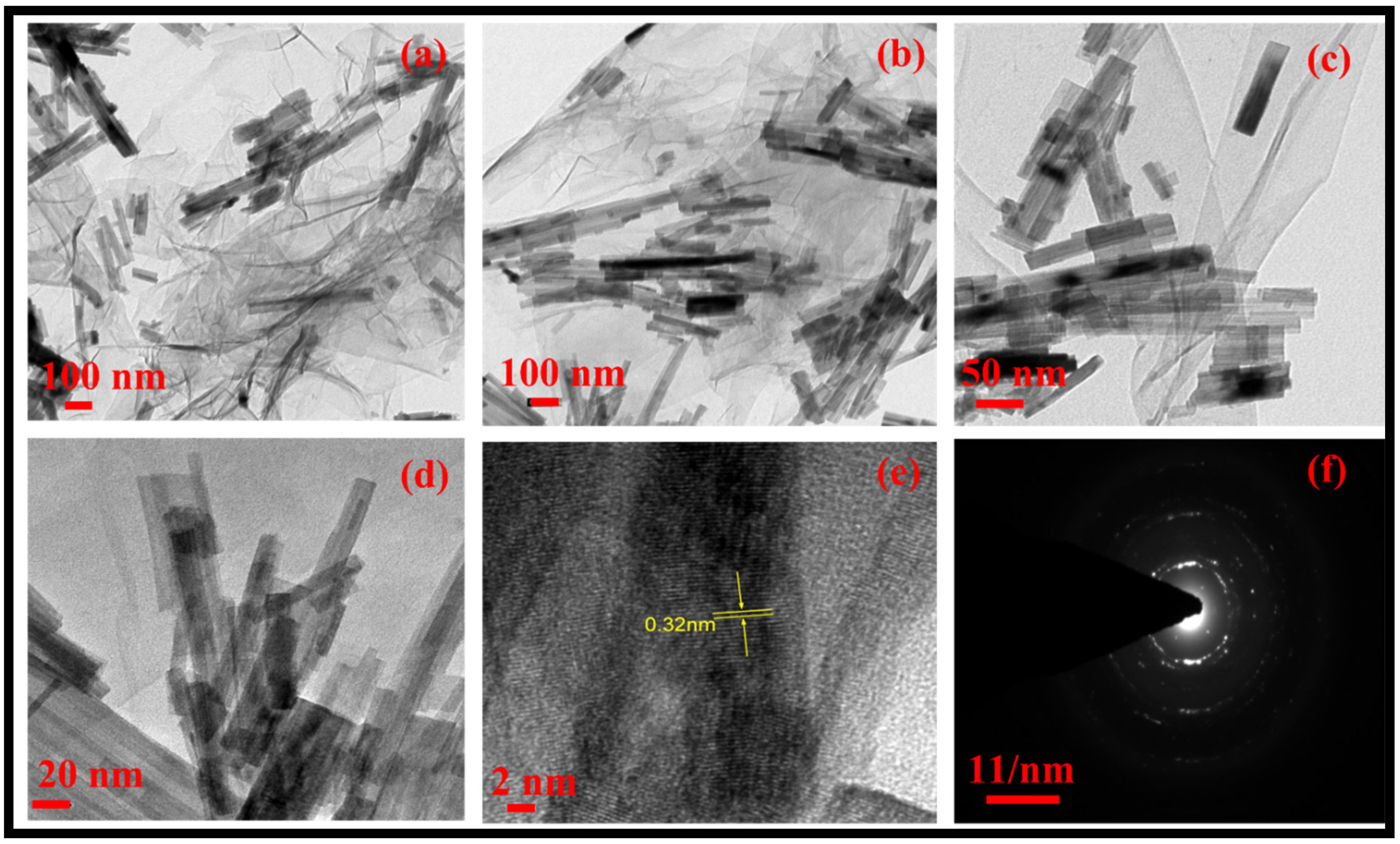
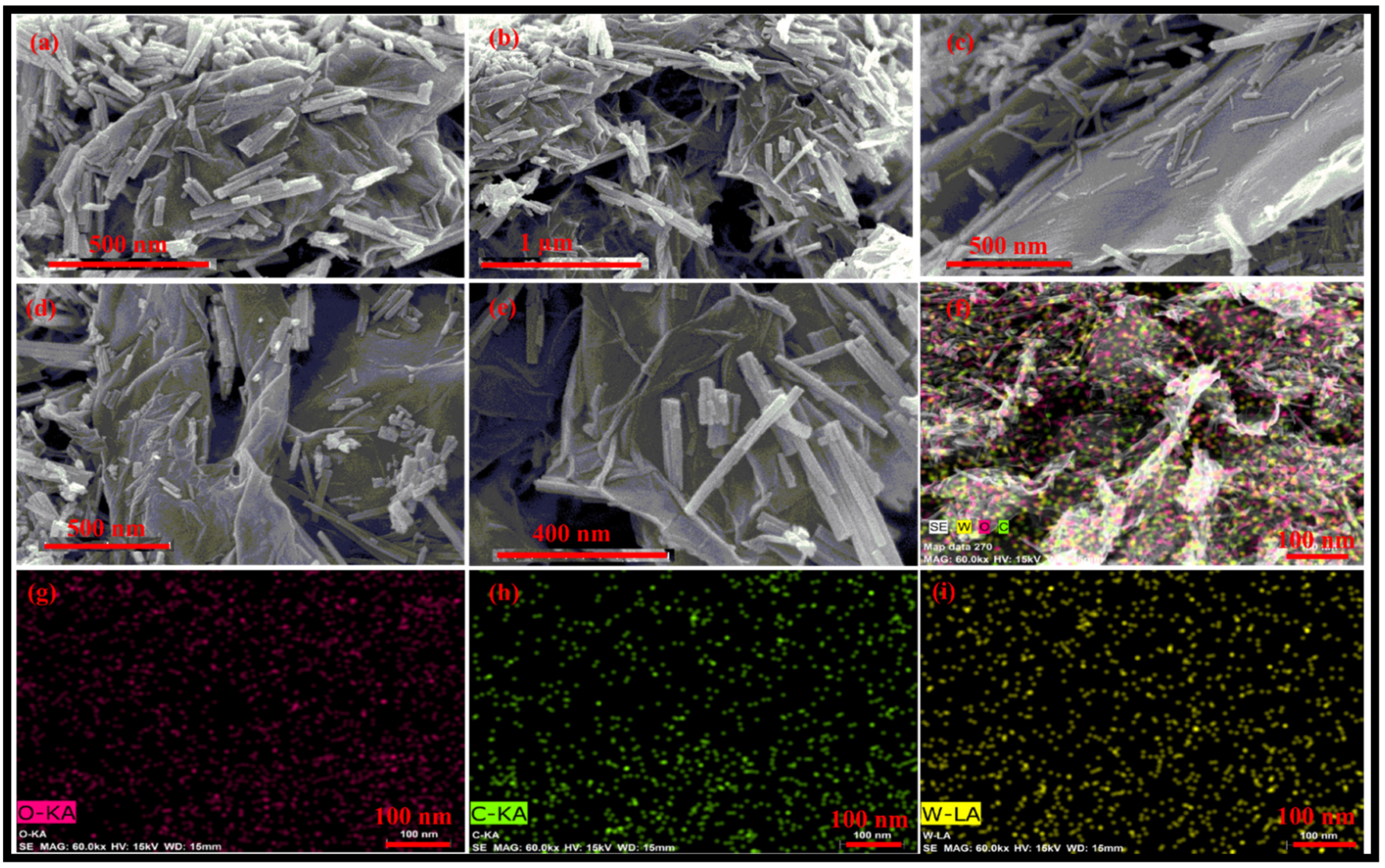
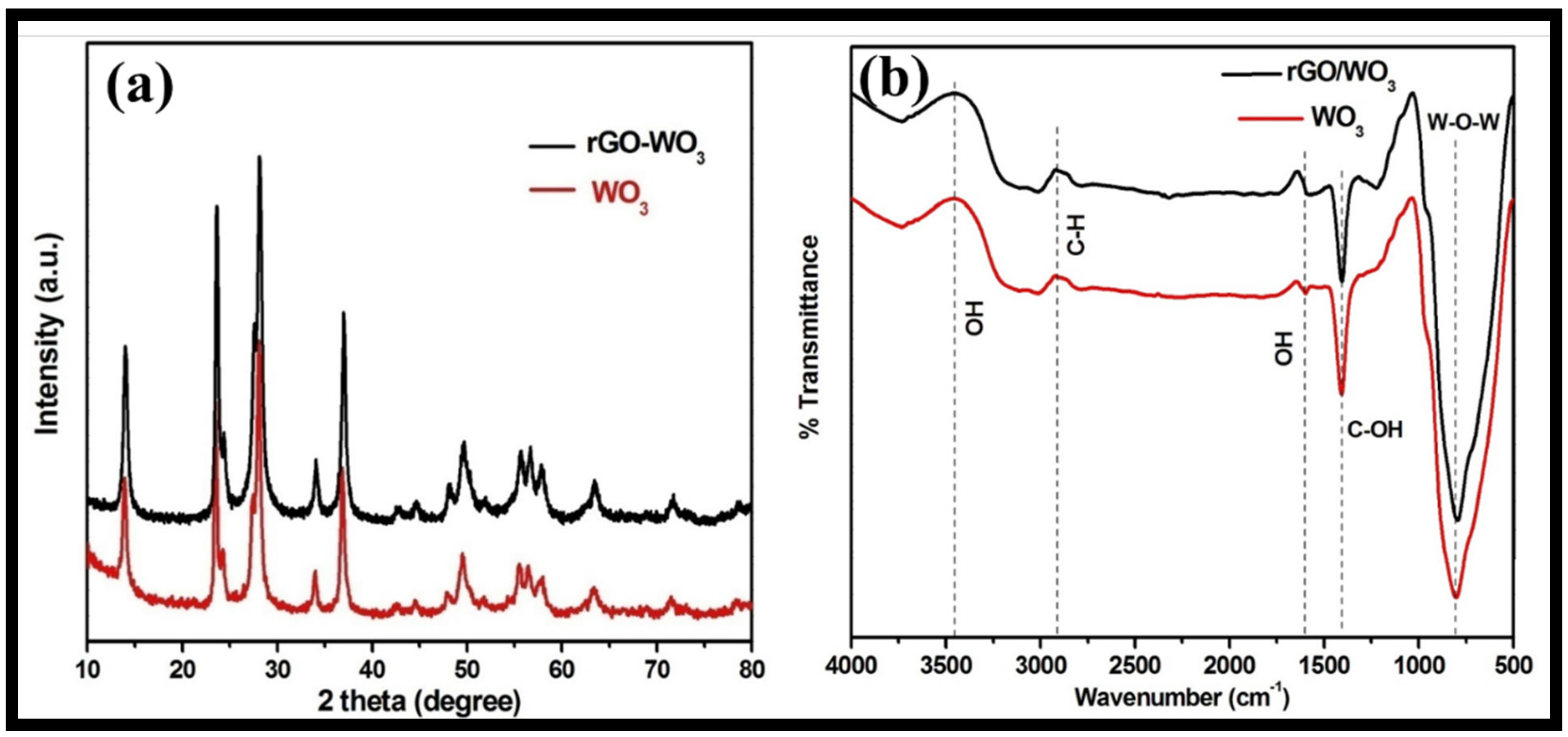
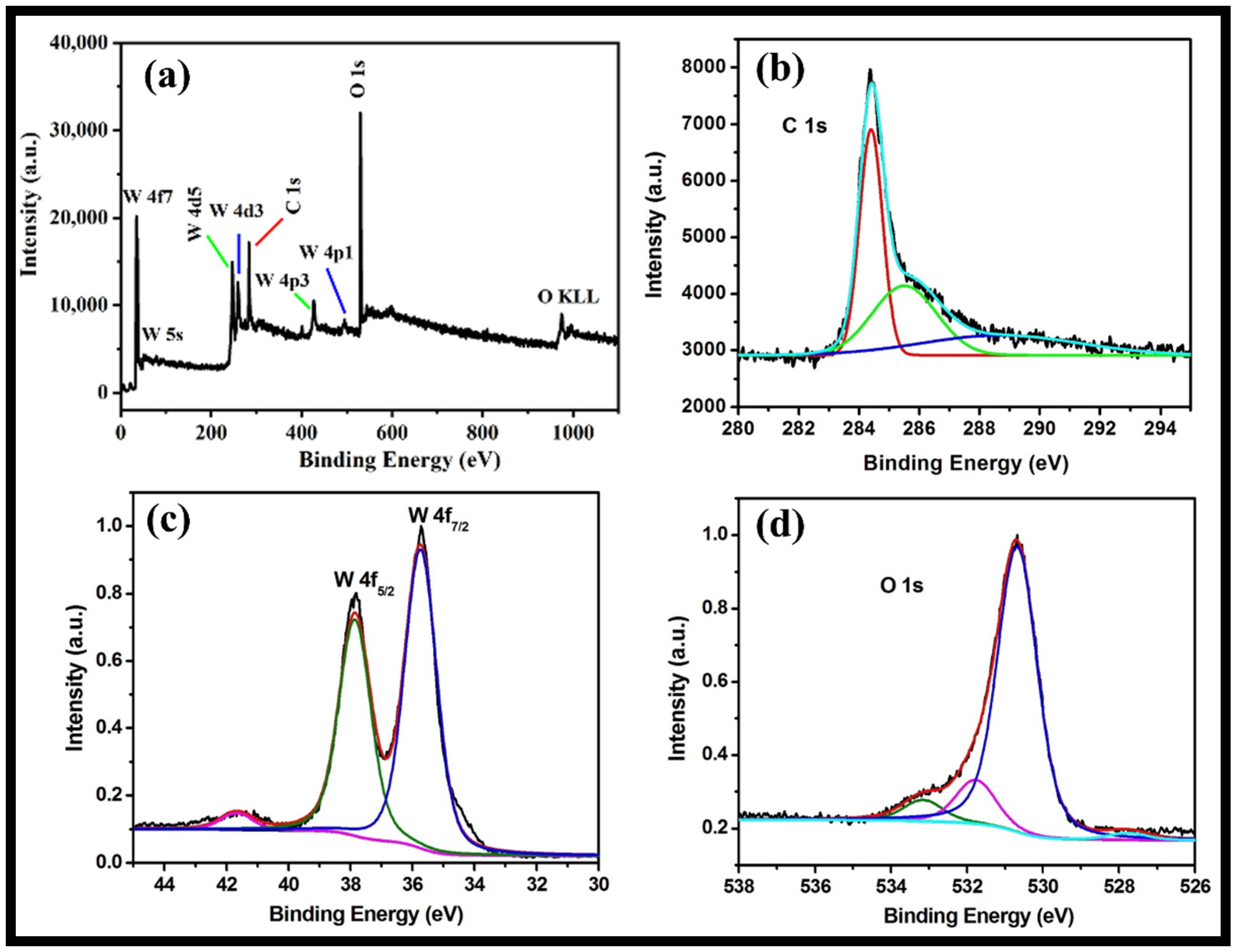
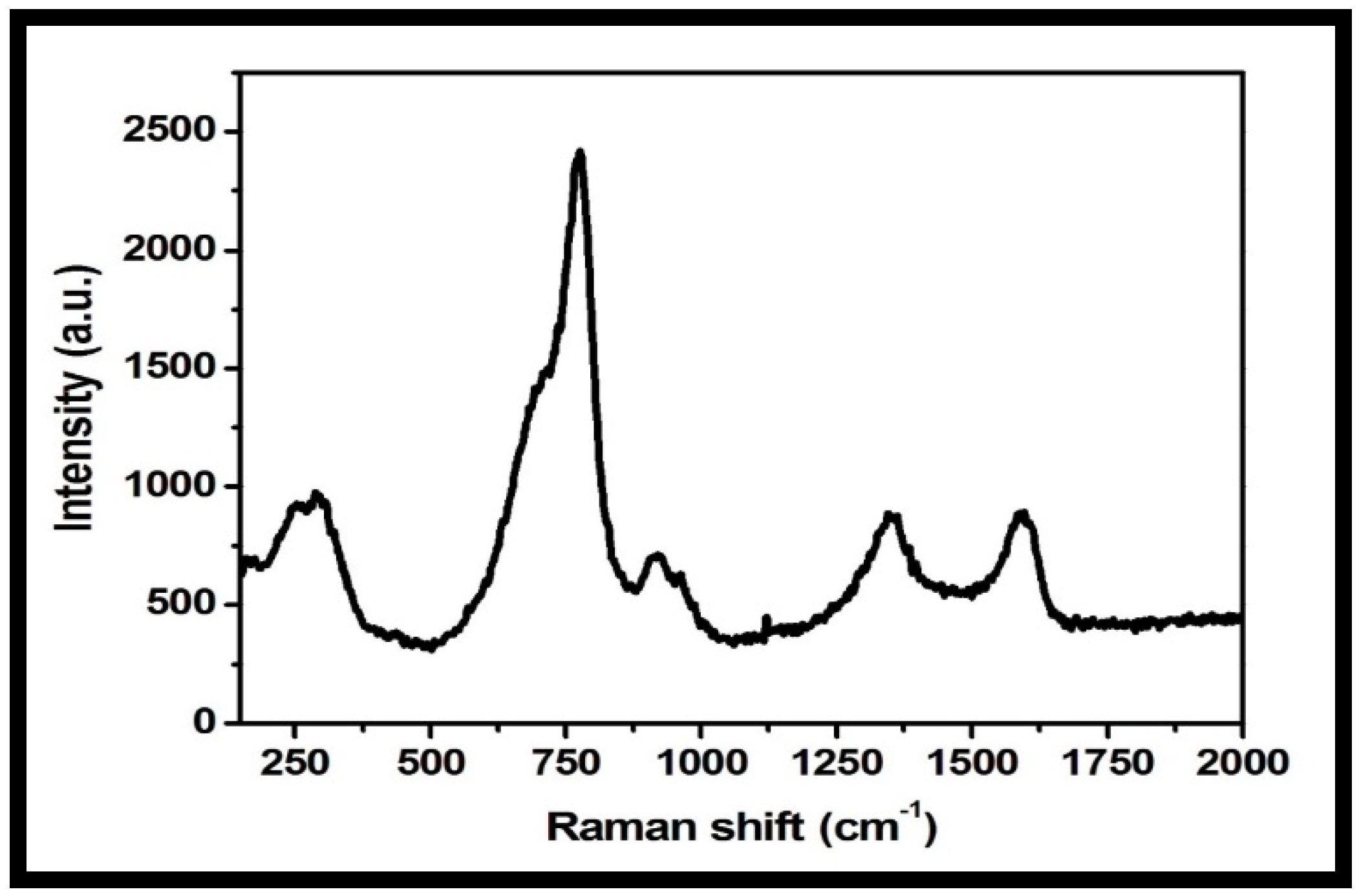
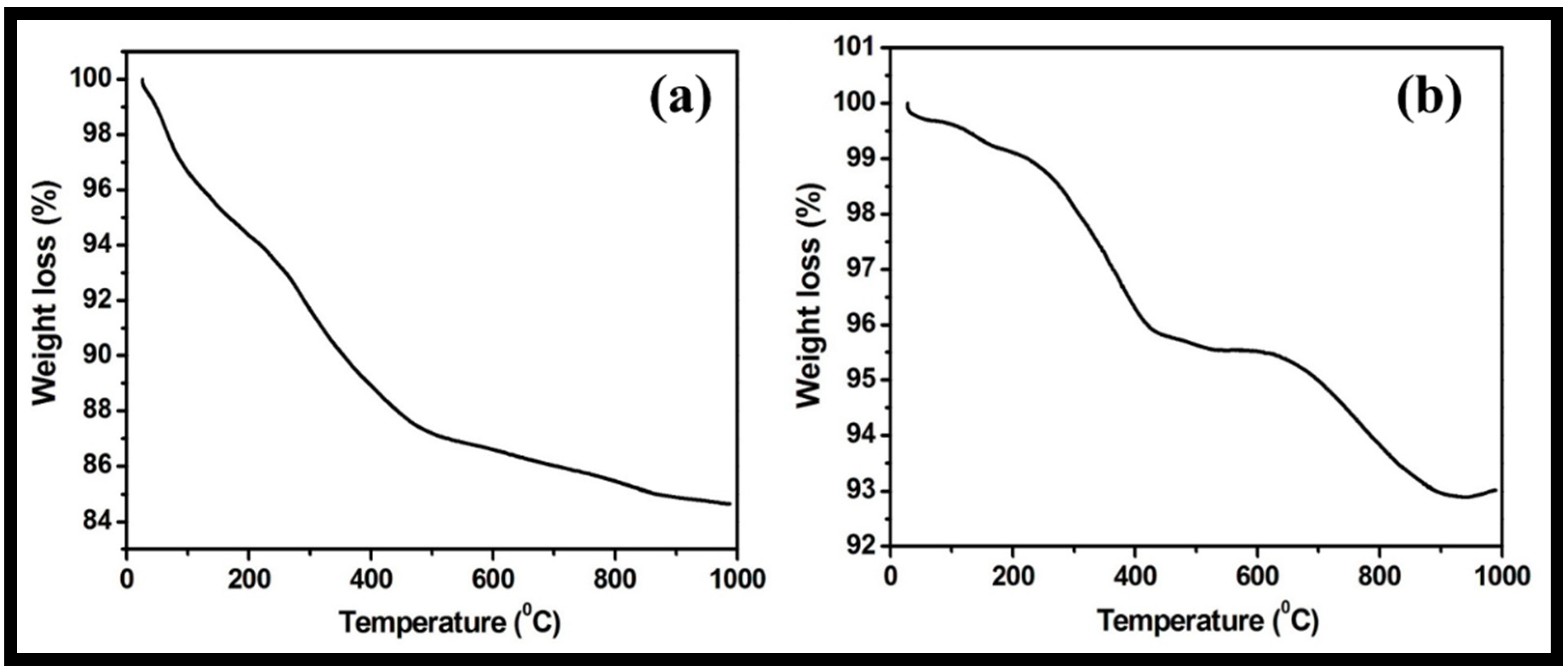
3.1. Morphological, Structural, Thermal and Optical Characterizations
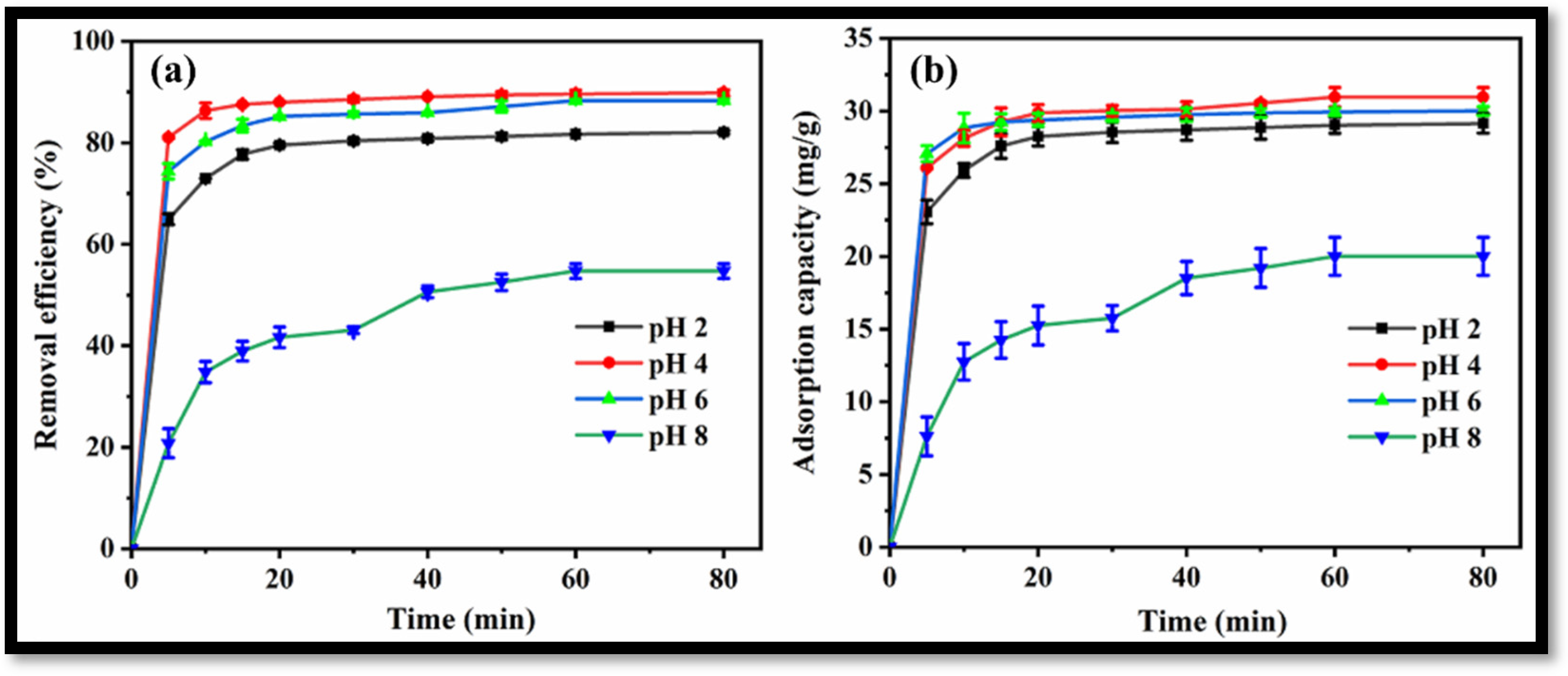
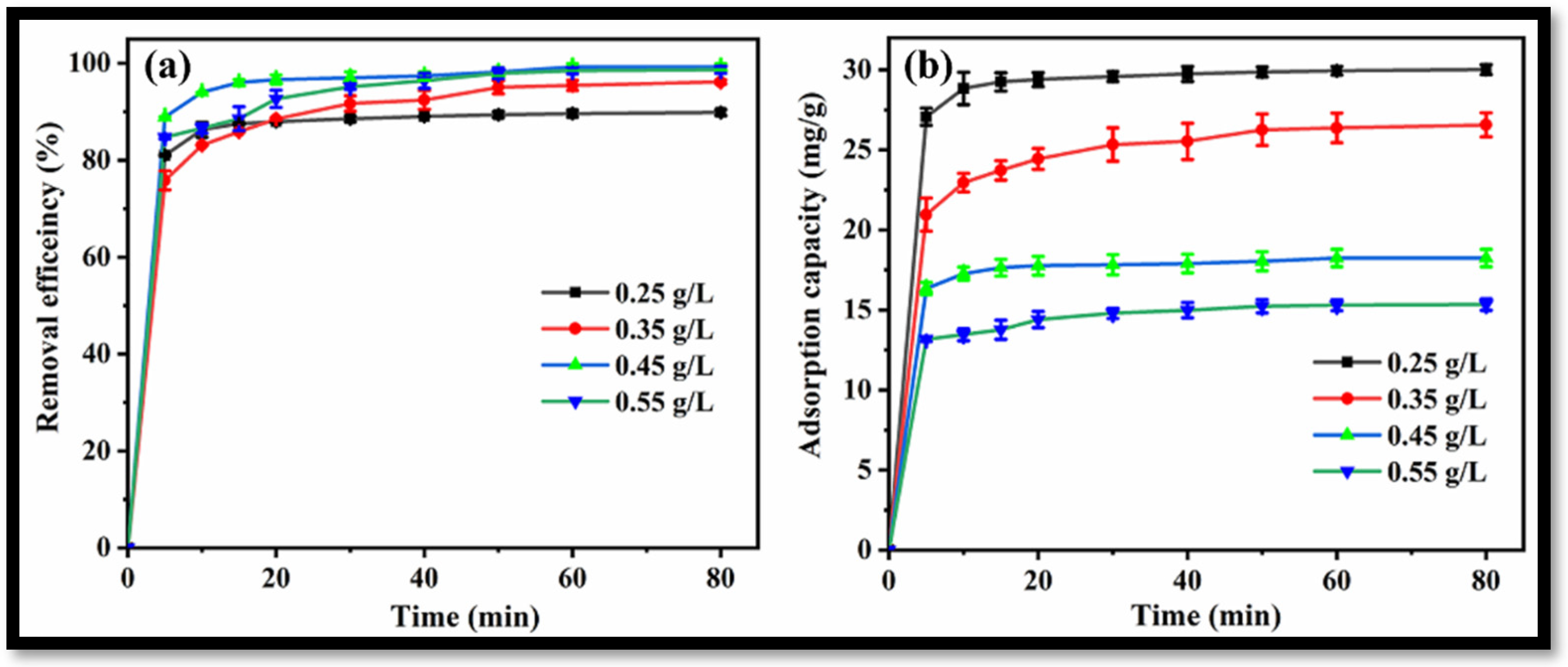
3.2. Utilization of the Synthesized rGO-WO3 Heterostructure as an Adsorbent for the Removal of LVX from Water
3.3. Application of the Prepared rGO-WO3 Heterostructure for Removal of LVX in Real Water Samples
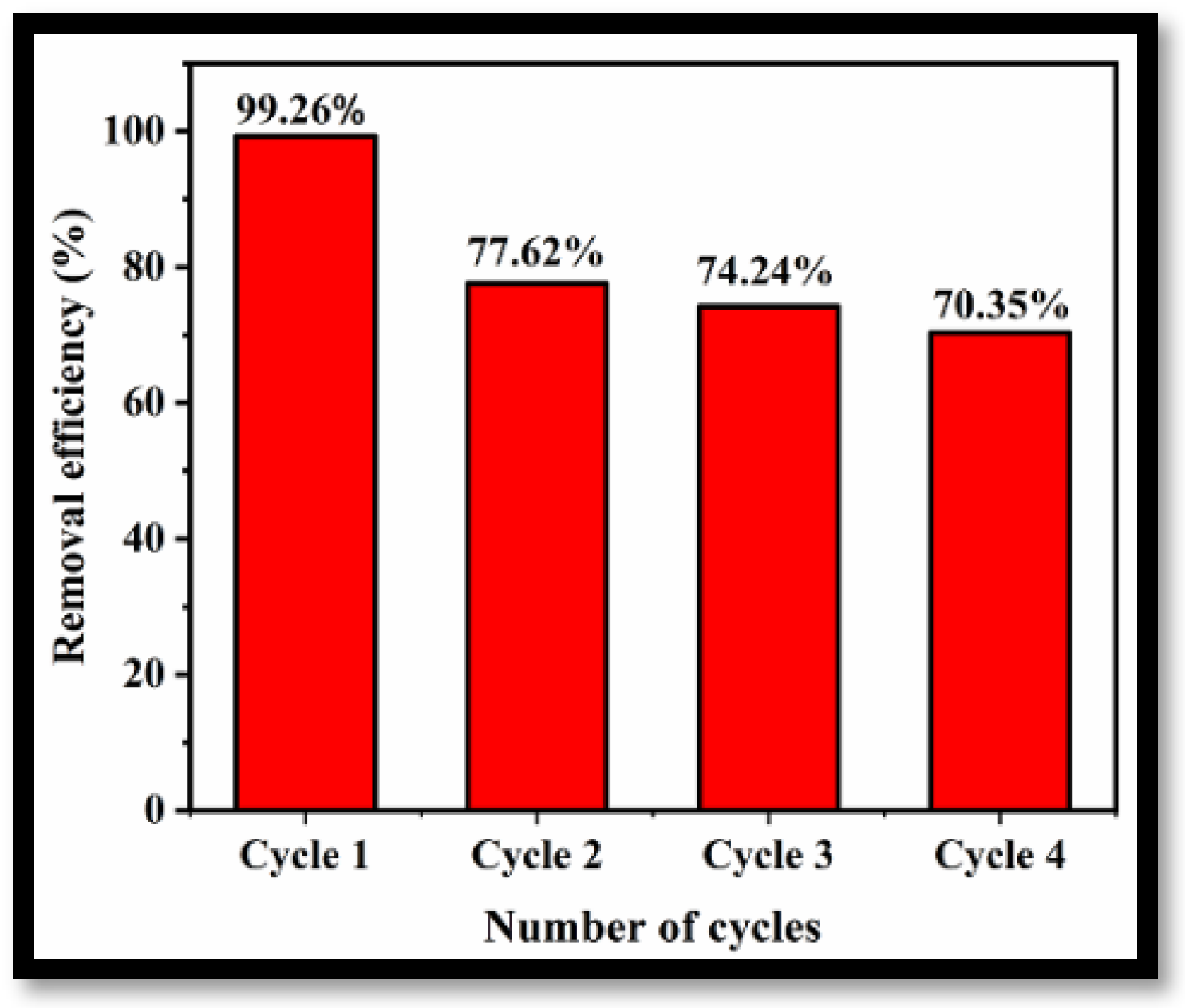
3.4. Stability and Reusability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasuhoglu, D.; Rodayan, A.; Berk, D.; Yargeau, V. Removal of the antibiotic levofloxacin (LEVO) in water by ozonation and TiO2 photocatalysis. Chem. Eng. J. 2012, 189–190, 41–48. [Google Scholar] [CrossRef]
- Ikehata, K.; Naghashkar, N.J.; El-Din, M.G. Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: A review. Ozone: Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef]
- Xekoukoulotakis, N.P.; Drosou, C.; Brebou, C.; Chatzisymeon, E.; Hapeshi, E.; Kassinos, D.F.; Mantzavinos, D. Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal. Today 2011, 161, 163–168. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Ozkal, C.B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D.; Meri, S. Removal of antibiotics in a parallel-plate thin-film-photocatalytic reactor: Process modeling and evolution of transformation by-products and toxicity. J. Environ. Sci. 2017, 60, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Najjar, N.H.E.; Deborde, M.; Journel, R.; Leitner, N.K.V. Aqueous chlorination of levofloxacin: Kinetic and mechanistic study, transformation product identification and toxicity. Water Res. 2013, 47, 121–129. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemoller, M. Titanium dioxide photocatalysis for pharmaceutical wastewater treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Irastorza, E.A.; Fasani, E.; Albini, A. Photolytic and photocatalytic degradation of fluoroquinolones in untreated river water under natural sunlight. Appl. Catal. B 2012, 119–120, 32–39. [Google Scholar] [CrossRef]
- Kaur, M.; Umar, A.; Mehta, S.K.; Kansal, S.K. Reduced graphene oxide-CdS heterostructure: An efficient fluorescent probe for the sensing of Ag(I) and sunset yellow and a visible-light responsive photocatalyst for the degradation of levofloxacin drug in aqueous phase. Appl. Catal. B 2019, 245, 143–158. [Google Scholar] [CrossRef]
- Kaur, M.; Mehta, S.K.; Kansal, S.K. Visible light driven photocatalytic degradation of ofloxacin and malachite green dye using cadmium sulphide nanoparticles. J. Environ. Chem. Eng. 2018, 6, 3631–3639. [Google Scholar] [CrossRef]
- Chen, Q.; Xin, Y.; Zhu, X. Au-Pd nanoparticles-decorated TiO2 nanobelts for photocatalytic degradation of antibiotic levofloxacin in aqueous solution. Electrochim. Acta 2015, 186, 34–42. [Google Scholar] [CrossRef]
- Olivares, C.A.; Esponda, S.M.; Ferrera, Z.S.; Rodríguez, J.J.S. Analytical tools employed to determine pharmaceutical compounds in wastewaters after application of advanced oxidation processes. Environ. Sci. Pollut. Res. 2016, 23, 24476–24494. [Google Scholar] [CrossRef]
- Nogueira, A.A.; Souza, B.M.; Dezotti, M.W.C.; Boaventura, R.A.R.; Vilar, V.J.P. Ferrioxalate complexes as strategy to drive a photo-Fenton reaction at mild pH conditions: A case study on levofloxacin oxidation. J. Photoch. Photobio. A 2017, 345, 109–123. [Google Scholar] [CrossRef]
- Doorslaer, X.V.; Dewulf, J.; Langenhove, H.V.; Demeestere, K. Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef]
- Li, W.; Guo, C.; Su, B.; Xu, J. Photodegradation of four fluoroquinolone compounds by titanium dioxide under simulated solar light irradiation. J. Chem. Technol. Biotechnol. 2012, 87, 643–650. [Google Scholar] [CrossRef]
- Epold, I.; Trapido, M.; Dulova, N. Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ion-activated persulfate and combined Fenton/persulfate systems. Chem. Eng. J. 2015, 279, 452–462. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Aceña, J.; Perez, S.; Petrović, M.; Zapata, A.; Barceló, D.; Malato, S.; Kassinos, D.F. Light-induced catalytic transformation of ofloxacin by solar Fenton in various water matrices at a pilot plant: Mineralization and characterization of major intermediate products. Sci. Total Environ. 2013, 461–462, 39–48. [Google Scholar] [CrossRef]
- Barhoumi, N.; Labiadh, L.; Oturan, M.A.; Oturan, N.; Gadri, A.; Ammara, S.; Brillas, E. Electrochemical mineralization of the antibiotic levofloxacin by electro-Fenton-pyrite process. Chemosphere 2015, 141, 250–257. [Google Scholar] [CrossRef]
- Ge, L.; Na, G.; Zhang, S.; Li, K.; Zhang, P.; Rena, H.; Yao, Z. New insights into the aquatic photochemistry of fluoroquinolone antibiotics: Direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity changes. Sci. Total Environ. 2015, 527–528, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.S.; Ribeiro, A.R.; Amorim, C.L.; Barreiro, J.C.; Cass, Q.B.; Castro, P.M.L.; Tiritan, M.E. Degradation of fluoroquinolone antibiotics and identification of metabolites/transformation products by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1333, 87–98. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Song, W.; Li, G.; Luo, H.; Cooper, W.J. Mechanistic considerations for the advanced oxidation treatment of fluoroquinolone pharmaceutical compounds using TiO2 heterogeneous catalysis. J. Phys. Chem. A 2010, 114, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Biodegradation of levofloxacin by an acclimated freshwater microalga. Chlorella Vulgaris. Chem. Eng. J. 2017, 313, 1251–1257. [Google Scholar] [CrossRef]
- Molinari, A.; Sarti, E.; Marchetti, N.; Pasti, L. Degradation of emerging concern contaminants in water by heterogeneous photocatalysis with Na4W10O32. Appl. Catal. B 2017, 203, 9–17. [Google Scholar] [CrossRef]
- Iwuozor, K.O. Prospects and challenges of using coagulation-flocculation method in the treatment of effluents. Adv. J. Chem. Sect. A 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Fang, F.; Qiao, L.L.; Ni, B.J.; Cao, J.S.; Yu, H.Q. Quantitative evaluation on the characteristics of activated sludge granules and flocs using a fuzzy entropy-based approach. Sci. Rep. 2017, 7, 42910. [Google Scholar] [CrossRef]
- Al-Abri, M.; Al-Ghafri, B.; Bora, T.; Dobretsov, S.; Dutta, J.; Castelletto, S.; Rosa, L.; Boretti, A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. NPJ Clean Water 2019, 2, 2. [Google Scholar] [CrossRef]
- Boddu, V.M.; Paul, T.; Page, M.A.; Byl, C.; Ward, L.; Ruan, J. Gray water recycle: Effect of pretreatment technologies on low pressure reverse osmosis treatment. J. Environ. Chem. Eng. 2016, 4, 4435–4443. [Google Scholar] [CrossRef]
- Esmaeili, H.; Foroutan, R. Investigation into ion exchange and adsorption methods for removing heavy metals from aqueous solutions. Int. J. Biol. Pharm. Allied Sci. 2015, 4, 620–629. [Google Scholar]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, L.; Dong, L.D.; Wang, J.; Sakiyama, H.; Muddassir, M.; Nezamzadeh-Ejhieh, A.; Liu, J. Series of highly stable Cd (II)-based MOFs as sensitive and selective sensors for detection of nitrofuran antibiotic. CrystEngComm 2021, 23, 8043–8052. [Google Scholar] [CrossRef]
- Wei, J.-H.; Yi, J.-W.; Han, M.-L.; Li, B.; Liu, S.; Wu, Y.-P.; Ma, L.-F.; Li, D.-S. A water-stable terbium (iii)–organic framework as a chemosensor for inorganic ions, nitro-containing compounds and antibiotics in aqueous solutions. Chem. Asian J. 2019, 14, 3694. [Google Scholar] [CrossRef]
- Wen, G.-X.; Han, M.-L.; Wu, X.-Q.; Wu, Y.-P.; Dong, W.-W.; Zhao, J.; Li, D.-S.; Ma, L.-F. A multi-responsive luminescent sensor based on a super-stable sandwich-type terbium(III)–organic framework. Dalton Trans. 2016, 45, 15492–15499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Fan, N.-N.; Han, M.-L.; Yang, G.-P.; Ma, L.-F. Porous Zn (II)-based metal–organic frameworks decorated with carboxylate groups exhibiting high gas adsorption and separation of organic dyes. Cryst. Growth Des. 2018, 18, 7114–7121. [Google Scholar] [CrossRef]
- Iqbal, Z.; Tanweer, M.S.; Alam, M. Recent advances in adsorptive removal of wastewater pollutants by chemically modified metal oxides: A review. J. Water Process. Eng. 2022, 46, 102641. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Adsorptive removal of sulfonamides, tetracyclines and quinolones from wastewater and water using carbon-based materials: Recent developments and future directions. J. Clean. Prod. 2022, 349, 131421. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 2017, 310, 389–398. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, V.; Bhattacharyya, K.; Krishnan, V. Synergetic effect of MoS2–RGO doping to enhance the photocatalytic performance of ZnO nanoparticles. New J. Chem. 2016, 40, 5185–5197. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Sun, D.D. Graphene oxide enwrapped Ag3PO4 composite: Towards a highly efficient and stable visible-light-induced photocatalyst for water purification. Catal. Sci. Technol. 2012, 2, 2525–2532. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Fabrication of WO3 nanorods on graphene nanosheets for improved visible light-induced photocapacitive and photocatalytic performance. RSC Adv. 2016, 6, 20824–20833. [Google Scholar] [CrossRef]
- Tie, L.; Yu, C.; Zhao, Y.; Chen, H.; Yang, S.; Sun, J.; Dong, S.; Sun, J. Fabrication of WO3 nanorods on reduced graphene oxide sheets with augmented visible light photocatalytic activity for efficient mineralization of dye. J. Alloy. Compd. 2018, 769, 83–91. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, F.; Goei, R.; Zhou, Y. Facile fabrication of RGO-WO3 composites for effective visible light photocatalytic degradation of sulfamethoxazole. Appl. Catal. B 2017, 207, 93–102. [Google Scholar] [CrossRef]
- Li, X.; Mu, W.; Xie, X.; Liu, B.; Tang, H.; Zhou, G.; Wei, H.; Jian, Y.; Luo, S. Strontium adsorption on tantalum-doped hexagonal tungsten oxide. J. Hazard. Mater. 2014, 264, 386–394. [Google Scholar] [CrossRef]
- Liu, B.; Mu, W.; Xie, X.; Li, X.; Wei, H.; Tan, Z.; Jian, Y.; Luo, S. Enhancing the adsorption capacity of Sr2+ and Cs+ onto hexagonal tungsten oxide by doped niobium. RSC Adv. 2015, 5, 15603–15611. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Wu, H.; Wei, Y. Adsorption behaviors of strontium using macroporous silica based hexagonal tungsten oxide. Sci. China Chem. 2016, 59, 601–608. [Google Scholar] [CrossRef]
- Ramar, V.; Balasubramanian, K. Charge transfer induced tunable band gap and enhanced saturable absorption behavior in rGO/WO3 composites. Appl. Phys. A 2018, 124, 779. [Google Scholar] [CrossRef]
- Kaur, M.; Mehta, S.K.; Kansal, S.K. A fluorescent probe based on nitrogen doped graphene quantum dots for turn off sensing of explosive and detrimental water pollutant, TNP in aqueous medium. Spectrochim. Acta Part A 2017, 180, 37–43. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Weon, S.; Moon, G.-H.; Kim, J.-H.; Choi, W. Robust co-catalytic performance of nanodiamonds loaded on wo3 for the decomposition of volatile organic compounds under visible light. ACS Catal. 2016, 6, 8350–8360. [Google Scholar] [CrossRef]
- Srivastava, S.; Jain, K.; Singh, V.N.; Singh, S.; Vijayan, N.; Dilawar, N.; Gupta, G.; Senguttuvan, T.D. Faster response of NO2 sensing in graphene-WO3 nanocomposites. Nanotechnology 2012, 23, 205501. [Google Scholar] [CrossRef]
- Prabhu, S.; Manikumar, S.; Cindrella, L.; Kwon, O.J. Charge transfer and intrinsic electronic properties of rGO-WO3 nanostructures for efficient photoelectrochemical and photocatalytic applications. Mater. Sci. Semicond. Process. 2018, 74, 136–146. [Google Scholar] [CrossRef]
- Weng, B.; Wu, J.; Zhang, N.; Xu, Y.-J. Observing the role of graphene in boosting the two-electron reduction of oxygen in graphene−wo3 nanorod photocatalysts. Langmuir 2014, 30, 5574–5584. [Google Scholar] [CrossRef]
- Hu, X.; Xu, P.; Gong, H.; Yin, G. Synthesis and characterization of wo3/graphene nanocomposites for enhanced photocatalytic activities by one-step in-situ hydrothermal reaction. Materials 2018, 11, 147. [Google Scholar] [CrossRef]
- Xing, L.L.; Huang, K.-J.; Fang, L.-X. Preparation of layered graphene and tungsten oxide hybrids for enhanced performance supercapacitors. Dalton Trans. 2016, 45, 17439–17446. [Google Scholar] [CrossRef] [PubMed]
- Adineh, E.; Rasuli, R. Facile synthesis of decorated graphene oxide sheets with WO3 nanoparticles. Appl. Phys. A 2015, 120, 1587–1592. [Google Scholar] [CrossRef]
- Ahmed, B.; Ojha, A.K.; Singh, A.; Hirsch, F.; Fischer, I.; Patrice, D.; Materny, A. Well-controlled in-situ growth of 2D WO3 rectangular sheets on reduced graphene oxide with strong photocatalytic and antibacterial properties. J. Hazard. Mater. 2018, 347, 266–278. [Google Scholar] [CrossRef]
- Kaur, J.; Anand, K.; Anand, K.; Singh, R.C. WO3 nanolamellae/reduced graphene oxide nanocomposites for highly sensitive and selective acetone sensing. J. Mater. Sci. 2018, 53, 12894–12907. [Google Scholar] [CrossRef]
- Shojaee, M.; Nasresfahani, S.; Sheikhi, M.H. Hydrothermally synthesized Pd-loaded SnO2/partially reduced graphene oxide nanocomposite for effective detection of carbon monoxide at room temperature. Sens. Actuators B 2018, 254, 457–467. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Huang, Y. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard Mater. 2017, 321, 711–719. [Google Scholar] [CrossRef]
- Yi, S.; Sun, Y.; Hu, X.; Xu, H.; Gao, B.; Wu, J. Porous nano-cerium oxide wood chip biochar composites for aqueous levofloxacin removal and sorption mechanism insights. Environ. Sci. Pollut. Res. 2018, 25, 25629–25637. [Google Scholar] [CrossRef]
- Chen, Y.; Lan, T.; Duan, L.; Wang, F.; Zhao, B.; Zhang, S.; Wei, W. Adsorptive removal and adsorption kinetics of fluoroquinolone by nano-hydroxyapatite. PLoS ONE 2015, 10, e0145025. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Metal organic framework (MOF) porous octahedral nanocrystals of Cu-BTC: Synthesis, properties and enhanced adsorption properties. Mater. Res. Bull. 2019, 109, 124–133. [Google Scholar] [CrossRef]
- Jin, T.; Yuan, W.; Xue, Y.; Wei, H.; Zhang, C.; Li, K. Co-modified MCM-41 as an effective adsorbent for levofloxacin removal from aqueous solution: Optimization of process parameters, isotherm, and thermodynamic studies. Environ. Sci. Pollut. Res. 2017, 24, 5238–5248. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Periasamy, V.; Sun, H.; Tade, M.O.; Wang, S. Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. J. Colloid Interface Sci. 2017, 500, 88–95. [Google Scholar] [CrossRef]
- Chi, X.; Zeng, L.; Du, Y.; Huang, J.; Kang, Y.; Luo, J.; Zhang, Q. Adsorption of levofloxacin on natural zeolite: Effects of ammonia nitrogen and humic acid. Water Sci. Technol. 2022, 85, 2944. [Google Scholar] [CrossRef]
- Jabari, M.H.A.; Sulaiman, S.; Ali, S.; Barakat, R.; Mubarak, A.; Khan, S.A. Adsorption study of levofloxacin on reusable magnetic nanoparticles: Kinetics and antibacterial activity. J. Mol. Liq. 2019, 291, 111249. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Jiang, H.; Lin, R.; Wang, Z.; Wu, J.; He, G.; Shearing, P.R.; Brett, D.J.L. ZIF-8-Derived Hollow Carbon for Efficient Adsorption of Antibiotics. Nanomaterials 2019, 9, 117. [Google Scholar] [CrossRef]
- Zhao, X.; Yi, S.; Dong, S.; Xu, H.; Sun, Y.; Hu, X. Removal of Levofloxacin from aqueous solution by Magnesium-impregnated Biochar: Batch and column experiments. Chem. Speciat. Bioavailab. 2018, 30, 68–75. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, C.; Wei, H.; Yuan, W.; Li, K. Adsorption of levofloxacin onto an iron-pillared montmorillonite (clay mineral): Kinetics, equilibrium and mechanism. Appl. Clay Sci. 2015, 118, 301–307. [Google Scholar] [CrossRef]
- Yi, S.; Gao, B.; Sun, Y.; Wu, J.; Shi, X.; Wu, B.; Hu, X. Removal of levofloxacin from aqueous solution using rice-husk and wood-chip biochars. Chemosphere 2016, 150, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hu, D.; Yang, L.Y.; Wang, N.; Wang, Y.G.; Ouyang, X.K. Efficient adsorption of levofloxacin from aqueous solution using calcium alginate/metal organic frameworks composite beads. J. Sol-Gel Sci. Technol. 2019, 91, 353–363. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, W.; Shi, J.; Zhu, S.; Yan, Y. Enhanced levofloxacin removal from water using zirconium (IV) loaded corn bracts. Environ. Sci. Pollut. Res. 2017, 24, 10685–10694. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, T.M.; Ahmed, M.J. Batch and fixed bed adsorption of levofloxacin on granular activated carbon from date (Phoenix dactylifera L.) stones by KOH chemical activation. Environ. Toxicol. Pharmacol. 2017, 50, 159–166. [Google Scholar] [CrossRef]
- Gopal, G.; Natarajan, C.; Mukherjee, A. Adsorptive removal of fluoroquinolone antibiotics using green synthesized and highly efficient Fe clay cellulose-acrylamide beads. Environ. Technol. Innov. 2022, 28, 102783. [Google Scholar] [CrossRef]
- Das, S.; Barui, A.; Adak, A. Montmorillonite impregnated electrospun cellulose acetate nanofiber sorptive membrane for ciprofloxacin removal from wastewater. J. Water Process. Eng. 2020, 37, 101497. [Google Scholar] [CrossRef]



| Kinetic Parameters | Concentration | ||||
|---|---|---|---|---|---|
| Pseudo-First-Order | 10 mg/L | 20 mg/L | 30 mg/L | 40 mg/L | 50 mg/L |
| R2 | 0.621 | 0.733 | 0.529 | 0.779 | 0.771 |
| k1 | −0.067 | −0.143 | −0.091 | −0.081 | −0.079 |
| qe(exp)(mg/g) | 3.25 | 8.66 | 6.59 | 16.04 | 19.43 |
| qe(cal)(mg/g) | 18.24 | 31.88 | 45.79 | 59.57 | 70.47 |
| Pseudo-second-order | |||||
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
| k2 | 0.101 | 0.079 | 0.069 | 0.018 | 0.018 |
| qe(exp)(mg/g) | 18.18 | 31.25 | 45.45 | 58.82 | 71.43 |
| qe(cal)(mg/g) | 18.24 | 31.88 | 45.79 | 59.57 | 70.47 |
| Intraparticle diffusion model | |||||
| R2 | 0.425 | 0.393 | 0.370 | 0.454 | 0.446 |
| k3 | 1.442 | 2.456 | 3.461 | 4.728 | 5.622 |
| cip | 8.813 | 15.917 | 23.389 | 27.467 | 33.160 |
| ΔG (kJ/mol) (303 K) | ΔG (kJ/mol) (308) K | ΔG (kJ/mol) (313 K) | ΔH (kJ/mol) | ΔS (kJ/mol.K) |
|---|---|---|---|---|
| −17.05743 | −15.18116 | −13.5691 | −110.975355 | −310.823546 |
| Langmuir | Freundlich | Redlich-Peterson | Jossen |
|---|---|---|---|
| R2 = 0.99033 | R2 = 0.96044 | R2 = 0.98928 | R2 = 0.93325 |
| qL (mg/g) = 73.05 | KF (mg/g) = 36.234 | β = 0.65575 | FJ = 0.03286 |
| b (L/mg) = 1.68 | n = 4.066 | A = 23.449 | HJ = 51.125 |
| Adsorbent | Adsorption Conditions | Specific Surface Area (m2/g) | Concentration (mg/L) | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|
| Natural zeolite | 0.5 g/L, 25 °C, pH 6.5, 180 rpm, 2 h | - | 5–50 | 22.17 | [66] |
| Magnetic nanoparticles | 1 g/L, 240 min, pH 6.5 | - | 2.5–20 | 6.848 | [67] |
| ZIF-8-derived hollow carbon | 5 mg, pH 7, 1000 rpm | 807.56 | 5–40 | 227.8 | [68] |
| WCM 315 | 2 g/L 24 h, 125 rpm | 2.70 | 15–150 | 7.38 | [69] |
| WCM 330 | 2 g/L 24 h, 125 rpm | 2.65 | 15–150 | 20.4 | [69] |
| WCM 615 | 2 g/L 24 h, 125 rpm | 266 | 15–150 | 14.8 | [69] |
| WCM 630 | 2 g/L 24 h, 125 rpm | 225 | 25–200 | 25.2 | [69] |
| Iron-pillared montmorillonite | 0.5 g/L, 48 h, 45 °C, pH 7 | 127 | 20–100 | 56.66 | [70] |
| Wood chip biochar | 10 g/L, 24 h, 30 °C, pH 6.5 | 312 | 30–200 | 7.72 | [71] |
| Rice husk biochar | 10 g/L, 24 h, 30 °C, pH 8.0 | 168 | 15–150 | 4.99 | [71] |
| UiO-66/CA | 0.32 g wet beads, pH 7, room temperature 150 rpm | 549.33 | 10–1000 | 86.43 | [72] |
| Zr-modified corn bracts | 2 g/L, pH 7, 240 rpm | 2.953 | 100–550 | 73.10 | [73] |
| WLC | 1 g/L, 24 h, 30 °C, pH 6.5, 125 rpm | - | 15–150 | 14.2 | [61] |
| WHC | 1 g/L, 24 h, 30 °C, pH 6.5, 125 rpm | - | 25–200 | 73.0 | [61] |
| Granular activated carbon | 0.15 g/L, 24 h, 30 °C, pH 9 | - | 50–250 | 100.38 | [74] |
| rGO/WO3 | 0.45 g/L, 80 min, 35 °C, pH 4, 200 rpm | 48.823 | 10–50 | 73.05 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, M.; Singh, S.; Mehta, S.K.; Kansal, S.K. rGO-WO3 Heterostructure: Synthesis, Characterization and Utilization as an Efficient Adsorbent for the Removal of Fluoroquinolone Antibiotic Levofloxacin in an Aqueous Phase. Molecules 2022, 27, 6956. https://doi.org/10.3390/molecules27206956
Kaur M, Singh S, Mehta SK, Kansal SK. rGO-WO3 Heterostructure: Synthesis, Characterization and Utilization as an Efficient Adsorbent for the Removal of Fluoroquinolone Antibiotic Levofloxacin in an Aqueous Phase. Molecules. 2022; 27(20):6956. https://doi.org/10.3390/molecules27206956
Chicago/Turabian StyleKaur, Manjot, Shafali Singh, Surinder Kumar Mehta, and Sushil Kumar Kansal. 2022. "rGO-WO3 Heterostructure: Synthesis, Characterization and Utilization as an Efficient Adsorbent for the Removal of Fluoroquinolone Antibiotic Levofloxacin in an Aqueous Phase" Molecules 27, no. 20: 6956. https://doi.org/10.3390/molecules27206956
APA StyleKaur, M., Singh, S., Mehta, S. K., & Kansal, S. K. (2022). rGO-WO3 Heterostructure: Synthesis, Characterization and Utilization as an Efficient Adsorbent for the Removal of Fluoroquinolone Antibiotic Levofloxacin in an Aqueous Phase. Molecules, 27(20), 6956. https://doi.org/10.3390/molecules27206956









