Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits
Abstract
1. Introduction
2. Catechins: Biosynthesis and Mechanism of Action
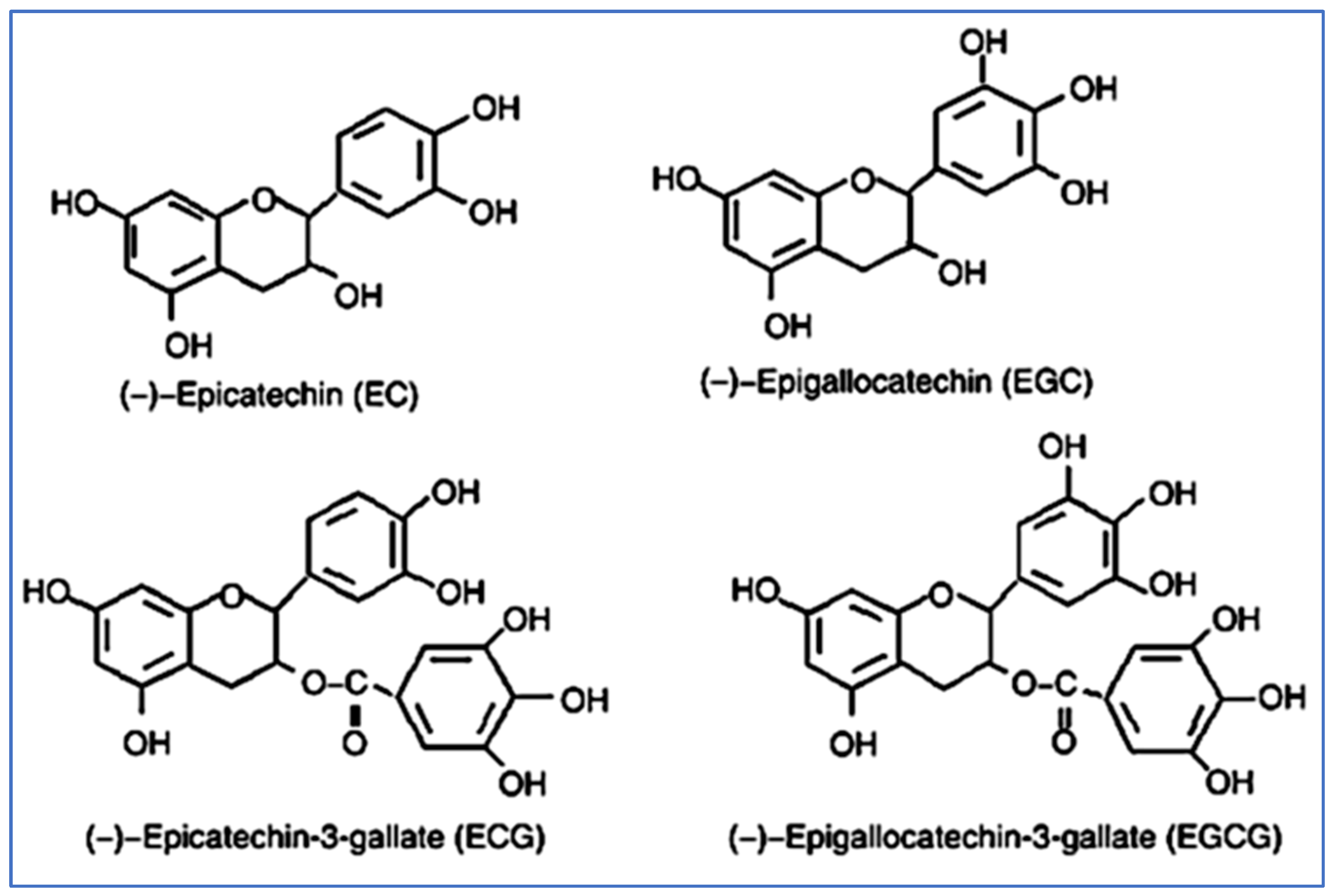
2.1. Synthesis and Structure of Catechins
2.2. Physical and Chemical Properties of Catechins
2.3. Bioavailability of Catechin
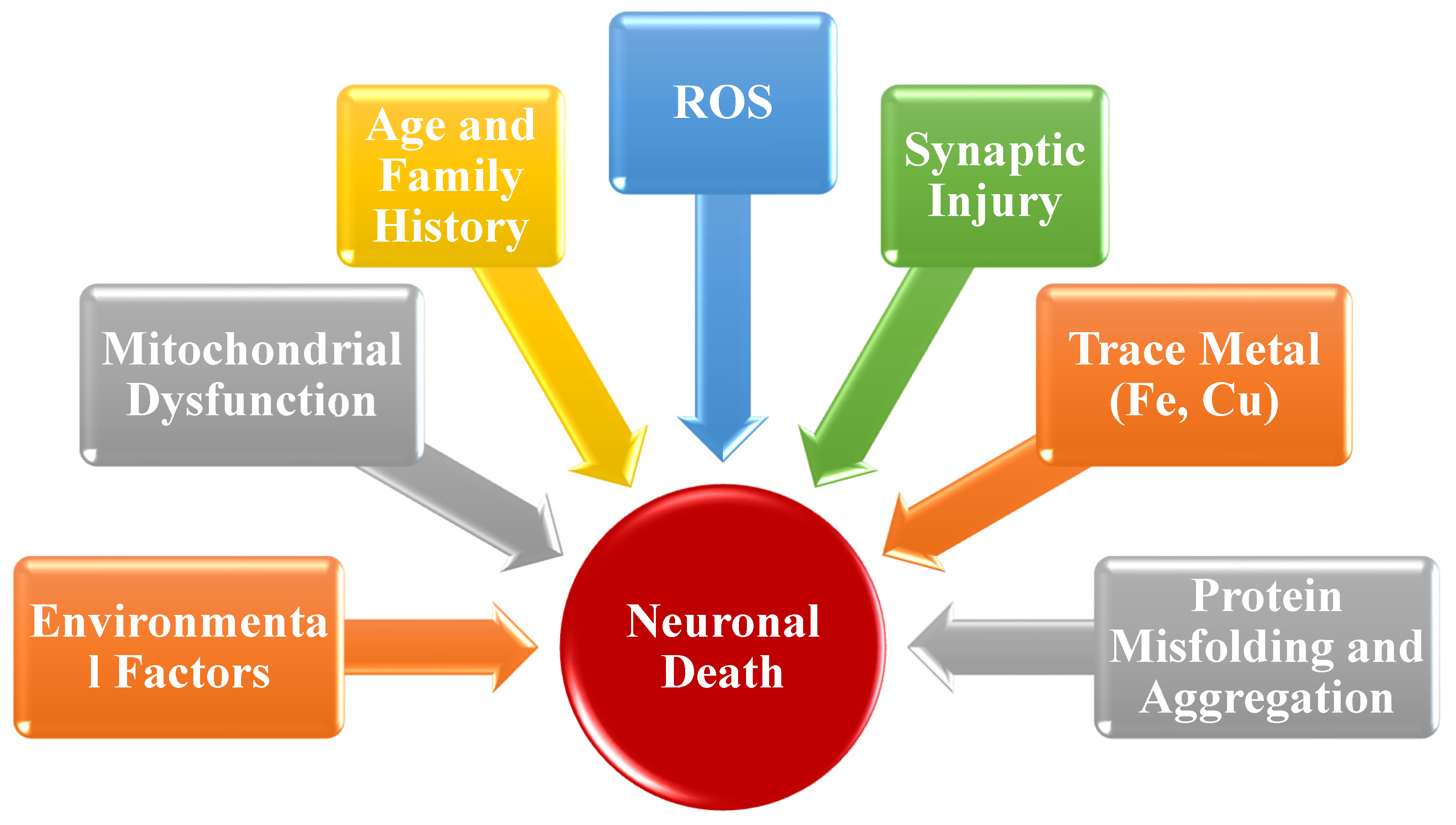
2.4. Catechins and Neurodegeneration
2.4.1. Anti-Inflammatory and Antioxidant Activity
2.4.2. Autophagic and Neuritogenic Activity
2.4.3. Dual-Specific Tyrosine Phosphorylation Regulated Kinase 1a Inhibition
2.5. The Role of Catechins in Various Neurodegenerative Disorders
2.5.1. Alzheimer’s Disease (AD)
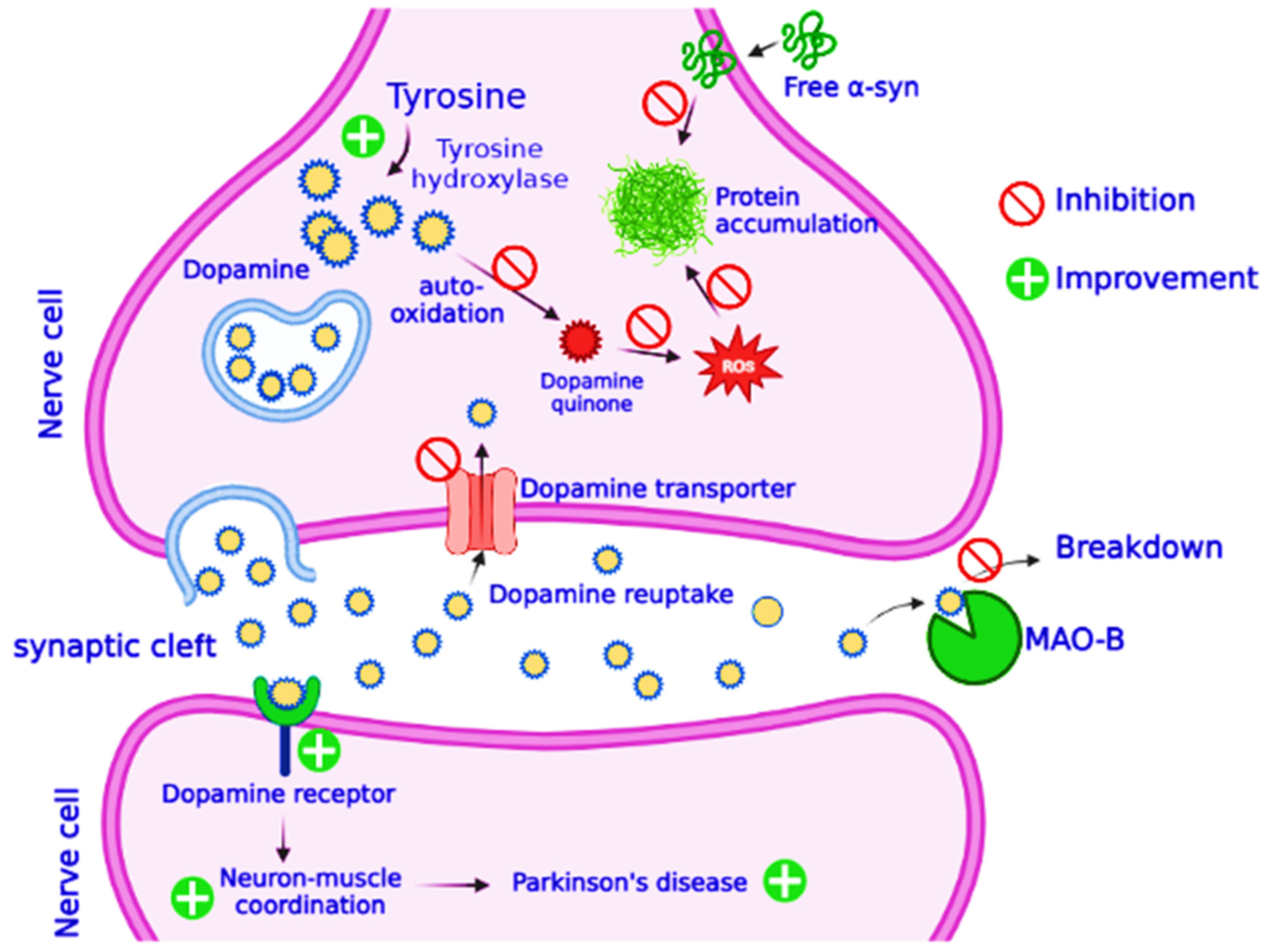
2.5.2. Parkinson’s Disease
2.5.3. Huntington’s Disease (HD)
2.5.4. Multiple Sclerosis (MS)
2.5.5. Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
2.5.6. Fetal Alcohol Spectrum Disorders (FASD)
2.5.7. Down Syndrome (DS)
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonçalves, P.B.; Sodero, A.C.R.; Cordeiro, Y. Green Tea Epigallocatechin-3-gallate (EGCG) Targeting Protein Misfolding in Drug Discovery for Neurodegenerative Diseases. Biomolecules 2021, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Sheikh, S.; Haque, E.; Mir, S.S. Neurodegenerative diseases: Multifactorial conformational diseases and their therapeutic interventions. J. Neurodegener. Dis. 2013, 2013, 563481. [Google Scholar] [CrossRef]
- Gribkoff, V.K.; Kaczmarek, L.K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 2017, 120, 11–19. [Google Scholar] [CrossRef]
- Danon, J.J.; Reekie, T.A.; Kassiou, M.J.T.i.C. Challenges and opportunities in central nervous system drug discovery. Trends Chem. 2019, 1, 612–624. [Google Scholar] [CrossRef]
- Mallucci, G.R.; Klenerman, D.; Rubinsztein, D.C.J.A.R.o.C.; Biology, D. Developing therapies for neurodegenerative disorders: Insights from protein aggregation and cellular stress responses. Annu. Rev. Cell Dev. Biol. 2020, 36, 165–189. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K.; Research, D.T.; Interventions, C. Alzheimer’s disease drug development pipeline: 2020. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12050. [Google Scholar] [CrossRef]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S.J.A.i.n. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. Int. Rev. J. 2015, 6, 64–72. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frölich, L.; Jack, C.R.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P.J.A.s.r.; et al. Drug development in Alzheimer’s disease: The path to 2025. Alzheimers Res. Ther. 2016, 8, 39. [Google Scholar] [CrossRef]
- Bokuchava, M.A.; Skobeleva, N.I.; Sanderson, G.W. Nutrition. The biochemistry and technology of tea manufacture. Crit. Rev. Food Sci. Nutr. 1980, 12, 303–370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Meng, X.; Gan, R.-Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Venkata, S.P.C.; Indra, P. The aroma, taste, color and bioactive constituents of tea. J. Med. Plants Res. 2011, 5, 2110–2124. [Google Scholar]
- Samanta, S. Potential bioactive components and health promotional benefits of tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Zhao, C.-N.; Xu, X.-Y.; Gan, R.-Y.; Cao, S.-Y.; Liu, Q.; Shang, A.; Mao, Q.-Q.; Li, H.-B.J.A. Phytochemical composition and antioxidant capacity of 30 Chinese teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Liu, M.; Tian, H.-l.; Wu, J.-H.; Cang, R.-R.; Wang, R.-X.; Qi, X.-H.; Xu, Q.; Chen, X.-H.J.H.r. Relationship between gene expression and the accumulation of catechin during spring and autumn in tea plants (Camellia sinensis L.). Hortic. Res. 2015, 2, 15011. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Li, B.-Y.; Gan, R.-Y.; Mao, Q.-Q.; Wang, Y.-F.; Shang, A.; Meng, J.-M.; Xu, X.-Y.; Wei, X.-L.; Li, H.-B.J.F. The in vivo antioxidant and hepatoprotective actions of selected Chinese teas. Foods 2020, 9, 262. [Google Scholar] [CrossRef]
- Yokogoshi, H. 22 Green Tea in the Protection against Neurodegeneration. In Health Benefits of Green Tea: An Evidence-Based Approach; Nova Science Publishers, Inc. Hauppauge: New York, NY, USA, 2017; p. 185. [Google Scholar]
- Suzuki, T.; Miyoshi, N.; Hayakawa, S.; Imai, S.; Isemura, M.; Nakamura, Y. Health benefits of tea consumption. In Beverage Impacts on Health and Nutrition; Springer: Berlin/Heidelberg, Germany, 2016; pp. 49–67. [Google Scholar]
- Miyoshi, N.; Pervin, M.; Suzuki, T.; Unno, K.; Isemura, M.; Nakamura, Y.J.B.T. Therapy. Green tea catechins for well-being and therapy: Prospects and opportunities. Bot. Targets Ther. 2015, 5, 85–96. [Google Scholar]
- Cao, S.-Y.; Zhao, C.-N.; Gan, R.-Y.; Xu, X.-Y.; Wei, X.-L.; Corke, H.; Atanasov, A.G.; Li, H.-B. Effects and mechanisms of tea and its bioactive compounds for the prevention and treatment of cardiovascular diseases: An updated review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Zhao, C.-N.; Cao, S.-Y.; Tang, G.-Y.; Gan, R.-Y.; Li, H.-B. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, C.; Cao, S.; Gan, Y.; Sun, H.; Gong, Y.; Yang, H.; Yin, X.; Lu, Z. Tea consumption and the risk of depression: A meta-analysis of observational studies. Aust. N. Z. J. Psychiatry 2015, 49, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-L.; Shi, H.-S.; Wei, Y.-M.; Wang, S.-J.; Sun, C.-Y.; Ding, Z.-B.; Lu, L. Green tea polyphenols produce antidepressant-like effects in adult mice. Pharmacol. Res. 2012, 65, 74–80. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Wang, Z.-S.; Ma, Y.-X.; Zhang, W.; Lu, J.-L.; Liang, Y.-R.; Zheng, X.-Q. Neuroprotective effects and mechanisms of tea bioactive components in neurodegenerative diseases. Molecules 2018, 23, 512. [Google Scholar] [CrossRef]
- Walker, J.M.; Klakotskaia, D.; Ajit, D.; Weisman, G.A.; Wood, W.G.; Sun, G.Y.; Serfozo, P.; Simonyi, A.; Schachtman, T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 2015, 44, 561–572. [Google Scholar] [CrossRef]
- Ettcheto, M.; Cano, A.; Manzine, P.R.; Busquets, O.; Verdaguer, E.; Castro-Torres, R.D.; García, M.L.; Beas-Zarate, C.; Olloquequi, J.; Auladell, C. Epigallocatechin-3-Gallate (EGCG) improves cognitive deficits aggravated by an obesogenic diet through modulation of unfolded protein response in APPswe/PS1dE9 mice. Mol. Neurobiol. 2020, 57, 1814–1827. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Wang, M.-H.; Chang, K.-C.; Soung, H.-S.; Fang, C.-H.; Lin, Y.-W.; Li, K.-Y.; Yang, C.-C.; Tsai, C.-C. Protective effect of (−) epigallocatechin-3-gallate on rotenone-induced parkinsonism-like symptoms in rats. Neurotox. Res. 2020, 37, 669–682. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Wu, X.; Zhu, C. A dual-inhibitor system for the effective antifibrillation of Aβ40 peptides by biodegradable EGCG–Fe (iii)/PVP nanoparticles. J. Mater. Chem. B 2019, 7, 1292–1299. [Google Scholar] [CrossRef]
- Singh, N.A.; Bhardwaj, V.; Ravi, C.; Ramesh, N.; Mandal, A.K.A.; Khan, Z.A. EGCG nanoparticles attenuate aluminum chloride induced neurobehavioral deficits, beta amyloid and tau pathology in a rat model of Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y. Green Tea: Health Benefits and Applications; CRC press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Ninomiya, M. Chemical and physicochemical properties of green tea polyphenols. In Chemistry and Applications of Green Tea; Yamamoto, T., Juneja, L.R., Chu, D.C., Kim, M., Eds.; ORC: New York, NY, USA, 1997; pp. 23–35. [Google Scholar]
- Masukawa, Y.; Matsui, Y.; Shimizu, N.; Kondou, N.; Endou, H.; Kuzukawa, M.; Hase, T. Determination of green tea catechins in human plasma using liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. B 2006, 834, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.O.; Pang, C.C. Pharmacokinetics and disposition of green tea catechins. In Pharmacokinetics and Adverse Effects of Drugs: Mechanisms and Risks Factors; IntechOpen: London, UK, 2018; Volume 17. [Google Scholar]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.; Roach, P.D. Extraction and isolation of catechins from tea. J. Sep. Sci. 2010, 33, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xue, S.J.; Kakuda, Y. Green tea induced thermogenesis controlling body weight. In Tea and Tea Products: Chemistry and Health-Promoting Properties; Ho, C.T., Lin, J.-K., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 221–232. [Google Scholar]
- Balentine, D.A.; Harbowy, M.E.; Graham, H.N. Tea: The Plant and its Manufacture; Chemistry and Consumpatin of the Beverage. In Caffeine; CRC Press: Boca Raton, FL, USA, 2019; pp. 35–72. [Google Scholar]
- Vuong, Q.V.; Stathopoulos, C.E.; Nguyen, M.H.; Golding, J.B.; Roach, P.D. Isolation of green tea catechins and their utilization in the food industry. Food Rev. Int. 2011, 27, 227–247. [Google Scholar] [CrossRef]
- Khanongnuch, C.; Unban, K.; Kanpiengjai, A.; Saenjum, C. Recent research advances and ethno-botanical history of miang, a traditional fermented tea (Camellia sinensis var. assamica) of northern Thailand. J. Ethn. Foods 2017, 4, 135–144. [Google Scholar] [CrossRef]
- Penders, M.H.; Jones, D.P.; Needham, D.; Pelan, E.G. Mechanistic study of equilibrium and kinetic behaviour of tea cream formation. Food Hydrocoll. 1998, 12, 9–15. [Google Scholar] [CrossRef]
- Zijp, I.M.; Korver, O.; Tijburg, L.B. Effect of tea and other dietary factors on iron absorption. Crit. Rev. Food Sci. Nutr. 2000, 40, 371–398. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green tea (Camellia sinensis): A review of its phytochemistry, pharmacology, and toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Subramanian, N.; Venkatesh, P.; Ganguli, S.; Sinkar, V.P. Role of polyphenol oxidase and peroxidase in the generation of black tea theaflavins. J. Agric. Food Chem. 1999, 47, 2571–2578. [Google Scholar] [CrossRef]
- Gulati, A.; Rawat, R.; Singh, B.; Ravindranath, S.D. Application of microwave energy in the manufacture of enhanced-quality green tea. J. Agric. Food Chem. 2003, 51, 4764–4768. [Google Scholar] [CrossRef]
- Yeasmen, N.; Orsat, V. Maximization of the recovery of phenolic compounds from sugar maple leaves. Biomass Convers. Biorefinery 2022, 1–16. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta 1996, 1304, 210–222. [Google Scholar] [CrossRef]
- Terao, J.; Piskula, M.; Yao, Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 1994, 308, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef]
- Lu, H.; Meng, X.; Yang, C.S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab. Dispos. Biol. Fate Chem. 2003, 31, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Scholl, C.; Lepper, A.; Lehr, T.; Hanke, N.; Schneider, K.L.; Brockmöller, J.; Seufferlein, T.; Stingl, J.C. Population nutrikinetics of green tea extract. PLoS ONE 2018, 13, e0193074. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Mauer, L.J. Degradation kinetics of catechins in green tea powder: Effects of temperature and relative humidity. J. Agric. Food Chem. 2011, 59, 6082–6090. [Google Scholar] [CrossRef]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Shirai, N.; Suzuki, H. Effects of simultaneous intakes of fish oil and green tea extracts on plasma, glucose, insulin, C-peptide, and adiponectin and on liver lipid concentrations in mice fed low- and high-fat diets. Ann. Nutr. Metab. 2008, 52, 241–249. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Hou, H.; Zhu, Y.; Salemi, J.; Ruscin, A.; Shytle, R.D.; Tan, J. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci. Lett. 2010, 471, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Fernández, V.; Almeida Toledano, L.; Pizarro, N.; Navarro-Tapia, E.; Gómez-Roig, M.D.; de la Torre, R.; García-Algar, Ó. Bioavailability of Epigallocatechin Gallate Administered With Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef] [PubMed]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef]
- Weinreb, O.; Mandel, S.; Amit, T.; Youdim, M.B.H. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004, 15, 506–516. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van Den Berg, D.-j.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Green tea extract-biomembrane interaction study: The role of its two major components, (−)-epigallocatechin gallate and (−)-epigallocatechin. Biochim. Biophys. Acta (BBA)—Biomembr. 2021, 1863, 183476. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 6626. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-Z.; Wang, Z.-J.; Bai, F.; Qin, X.-J.; Cao, J.; Lv, J.-Y.; Zhang, M.-S. Epigallocatechin-3-Gallate Protects HUVECs from PM2.5-Induced Oxidative Stress Injury by Activating Critical Antioxidant Pathways. Molecules 2015, 20, 6626–6639. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Intrieri, M.; D’Agata, V.; Mangano, N.G.; Oriani, G.; Ontario, M.L.; Scapagnini, G. The Major Green Tea Polyphenol, (-)-Epigallocatechin-3-Gallate, Induces Heme Oxygenase in Rat Neurons and Acts as an Effective Neuroprotective Agent against Oxidative Stress. J. Am. Coll. Nutr. 2009, 28, 492S–499S. [Google Scholar] [CrossRef]
- Degan, D.; Ornello, R.; Tiseo, C.; Carolei, A.; Sacco, S.; Pistoia, F. The Role of Inflammation in Neurological Disorders. Curr. Pharm. Des. 2018, 24, 1485–1501. [Google Scholar] [CrossRef] [PubMed]
- Marinovic, M.P.; Morandi, A.C.; Otton, R. Green tea catechins alone or in combination alter functional parameters of human neutrophils via suppressing the activation of TLR-4/NFκB p65 signal pathway. Toxicol. Vitr. 2015, 29, 1766–1778. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Barro, L.; Tsai, S.-T.; Feng, T.-W.; Wu, X.-Y.; Chao, C.-W.; Yu, R.-S.; Chin, T.-Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Zhou, H.-Y.; Gui, Y.-R.; Yang, Y.-H.; Wu, M.-J.; Xiao, Y.-F.; Shang, J.-T.; Long, G.-F.; Shu, X.-J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020, 40, 18–27. [Google Scholar] [CrossRef]
- Urrutia, P.; Aguirre, P.; Esparza, A.; Tapia, V.; Mena, N.P.; Arredondo, M.; González-Billault, C.; Núñez, M.T. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 2013, 126, 541–549. [Google Scholar] [CrossRef]
- Sheelakumari, R.; Kesavadas, C.; Varghese, T.; Sreedharan, R.M.; Thomas, B.; Verghese, J.; Mathuranath, P.S. Assessment of Iron Deposition in the Brain in Frontotemporal Dementia and Its Correlation with Behavioral Traits. Am. J. Neuroradiol. 2017, 38, 1953. [Google Scholar] [CrossRef]
- Mandel, S.A.; Avramovich-Tirosh, Y.; Reznichenko, L.; Zheng, H.; Weinreb, O.; Amit, T.; Youdim, M.B.H. Multifunctional Activities of Green Tea Catechins in Neuroprotection. Neurosignals 2005, 14, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood–Brain Barrier Permeability of Green Tea Catechin Metabolites and their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61, 1700294. [Google Scholar] [CrossRef] [PubMed]
- Gundimeda, U.; McNeill, T.H.; Fan, T.K.; Deng, R.; Rayudu, D.; Chen, Z.; Cadenas, E.; Gopalakrishna, R. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: Role of 67-kDa laminin receptor and hydrogen peroxide. Biochem. Biophys. Res. Commun. 2014, 445, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.; Cortés, N.; Pastor, G.; Gonzalez, A.; Ramos-Escobar, N.; Pastene, E.; Rojo, L.E.; Maccioni, R.B. N-Acetyl Cysteine and Catechin-Derived Polyphenols: A Path Toward Multi-Target Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 75, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Zhang, X.; Kang, X.; Li, Y.; Zhang, W.; Chen, Y.; Liu, Y.; Wang, W.; Ge, M.; et al. Exposure to PM2.5 aggravates Parkinson’s disease via inhibition of autophagy and mitophagy pathway. Toxicology 2021, 456, 152770. [Google Scholar] [CrossRef]
- Sato, S.; Uchihara, T.; Fukuda, T.; Noda, S.; Kondo, H.; Saiki, S.; Komatsu, M.; Uchiyama, Y.; Tanaka, K.; Hattori, N. Loss of autophagy in dopaminergic neurons causes Lewy pathology and motor dysfunction in aged mice. Sci. Rep. 2018, 8, 2813. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Holczer, M.; Besze, B.; Zámbó, V.; Csala, M.; Bánhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef]
- Khalil, H.; Tazi, M.; Caution, K.; Ahmed, A.; Kanneganti, A.; Assani, K.; Kopp, B.; Marsh, C.; Dakhlallah, D.; Amer, A.O. Aging is associated with hypermethylation of autophagy genes in macrophages. Epigenetics 2016, 11, 381–388. [Google Scholar] [CrossRef]
- Gu, H.-F.; Nie, Y.-X.; Tong, Q.-Z.; Tang, Y.-L.; Zeng, Y.; Jing, K.-Q.; Zheng, X.-L.; Liao, D.-F. Epigallocatechin-3-Gallate Attenuates Impairment of Learning and Memory in Chronic Unpredictable Mild Stress-Treated Rats by Restoring Hippocampal Autophagic Flux. PLoS ONE 2014, 9, e112683. [Google Scholar] [CrossRef]
- Choi, C.; Song, H.-D.; Son, Y.; Cho, Y.K.; Ahn, S.-Y.; Jung, Y.-S.; Yoon, Y.C.; Kwon, S.W.; Lee, Y.-H. Epigallocatechin-3-Gallate Reduces Visceral Adiposity Partly through the Regulation of Beclin1-Dependent Autophagy in White Adipose Tissues. Nutrients 2020, 12, 3072. [Google Scholar] [CrossRef] [PubMed]
- Reznichenko, L.; Amit, T.; Youdim, M.B.H.; Mandel, S. Green tea polyphenol (–)-epigallocatechin-3-gallate induces neurorescue of long-term serum-deprived PC12 cells and promotes neurite outgrowth. J. Neurochem. 2005, 93, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.A.; Amit, T.; Weinreb, O.; Reznichenko, L.; Youdim, M.B. Simultaneous manipulation of multiple brain targets by green tea catechins: A potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci. Ther. 2008, 14, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, F.J.; Hämmerle, B. MNB/DYRK1A as a multiple regulator of neuronal development. FEBS J. 2011, 278, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Moroy, G.; Paul, J.-L.; Rebillat, A.-S.; Dierssen, M.; de la Torre, R.; Cieuta-Walti, C.; Dairou, J.; Janel, N. Molecular rescue of Dyrk1A overexpression alterations in mice with Fontup® dietary supplement: Role of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1404. [Google Scholar] [CrossRef] [PubMed]
- George, R.C.; Lew, J.; Graves, D.J. Interaction of cinnamaldehyde and epicatechin with tau: Implications of beneficial effects in modulating Alzheimer’s disease pathogenesis. J. Alzheimer’s Dis. JAD 2013, 36, 21–40. [Google Scholar] [CrossRef]
- Beasley, M.; Stonebraker, A.R.; Hasan, I.; Kapp, K.L.; Liang, B.J.; Agarwal, G.; Groover, S.; Sedighi, F.; Legleiter, J. Lipid Membranes Influence the Ability of Small Molecules To Inhibit Huntingtin Fibrillization. Biochemistry 2019, 58, 4361–4373. [Google Scholar] [CrossRef]
- Teixeira, M.D.; Souza, C.M.; Menezes, A.P.; Carmo, M.R.; Fonteles, A.A.; Gurgel, J.P.; Lima, F.A.; Viana, G.S.; Andrade, G.M. Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol. Biochem. Behav. 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Al-Amri, J.S.; Hagras, M.M.; Mohamed, I.M. Effect of epigallocatechin-3-gallate on inflammatory mediators release in LPS-induced Parkinson’s disease in rats. Indian J. Exp. Biol. 2013, 51, 357–362. [Google Scholar]
- Jelenković, A.; Jovanović, M.D.; Stevanović, I.; Petronijević, N.; Bokonjić, D.; Zivković, J.; Igić, R. Influence of the green tea leaf extract on neurotoxicity of aluminium chloride in rats. Phytother. Res. PTR 2014, 28, 82–87. [Google Scholar] [CrossRef]
- Herges, K.; Millward, J.M.; Hentschel, N.; Infante-Duarte, C.; Aktas, O.; Zipp, F. Neuroprotective effect of combination therapy of glatiramer acetate and epigallocatechin-3-gallate in neuroinflammation. PLoS ONE 2011, 6, e25456. [Google Scholar] [CrossRef] [PubMed]
- Semnani, M.; Mashayekhi, F.; Azarnia, M.; Salehi, Z. Effects of green tea epigallocatechin-3-gallate on the proteolipid protein and oligodendrocyte transcription factor 1 messenger RNA gene expression in a mouse model of multiple sclerosis. Folia Neuropathol. 2017, 55, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Dér, N.P.; Zsindely, N.; Bodai, L. Green tea infusion alleviates neurodegeneration induced by mutant Huntingtin in Drosophila. Nutr. Neurosci. 2020, 23, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mähler, A.; Steiniger, J.; Bock, M.; Klug, L.; Parreidt, N.; Lorenz, M.; Zimmermann, B.F.; Krannich, A.; Paul, F.; Boschmann, M. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2015, 101, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Lovera, J.; Ramos, A.; Devier, D.; Garrison, V.; Kovner, B.; Reza, T.; Koop, D.; Rooney, W.; Foundas, A.; Bourdette, D. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: Phase I single group and phase II randomized placebo-controlled studies. J. Neurol. Sci. 2015, 358, 46–52. [Google Scholar] [CrossRef]
- de la Torre, R.; de Sola, S.; Hernandez, G.; Farré, M.; Pujol, J.; Rodriguez, J.; Espadaler, J.M.; Langohr, K.; Cuenca-Royo, A.; Principe, A.; et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 801–810. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Takuma, N.; Kawasaki, Y.; Harada, S.; Nakase, J.; Ukawa, Y.; Sagesaka, Y.M. Effects of green tea consumption on cognitive dysfunction in an elderly population: A randomized placebo-controlled study. Nutr. J. 2016, 15, 49. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef]
- Hong, M.; Yu, J.; Wang, X.; Zhan, S.; Wu, Z.; Zhang, X. Tea Polyphenols as Prospective Natural Attenuators of Brain Aging. Nutrients 2022, 14, 3012. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H. Clinical benefits of green tea consumption for cognitive dysfunction. PharmaNutrition 2015, 3, 136–145. [Google Scholar] [CrossRef]
- Ali, B.; Jamal, Q.M.; Shams, S.; Al-Wabel, N.A.; Siddiqui, M.U.; Alzohairy, M.A.; Al Karaawi, M.A.; Kesari, K.K.; Mushtaq, G.; Kamal, M.A. In Silico Analysis of Green Tea Polyphenols as Inhibitors of AChE and BChE Enzymes in Alzheimer’s Disease Treatment. CNS Neurol. Disord. Drug Targets 2016, 15, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Grant, M.M.; Aldred, S. Oxidative stress in vascular dementia and Alzheimer’s disease: A common pathology. J. Alzheimer’s Dis. JAD 2009, 17, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: Lights and shadows. Ann. New York Acad. Sci. 2008, 1147, 70–78. [Google Scholar] [CrossRef]
- Haque, A.M.; Hashimoto, M.; Katakura, M.; Hara, Y.; Shido, O. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J. Nutr. Biochem. 2008, 19, 619–626. [Google Scholar] [CrossRef]
- Biasibetti, R.; Tramontina, A.C.; Costa, A.P.; Dutra, M.F.; Quincozes-Santos, A.; Nardin, P.; Bernardi, C.L.; Wartchow, K.M.; Lunardi, P.S.; Gonçalves, C.A. Green tea (-)epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav. Brain Res. 2013, 236, 186–193. [Google Scholar] [CrossRef]
- Sang, S.; Tian, S.; Wang, H.; Stark, R.E.; Rosen, R.T.; Yang, C.S.; Ho, C.-T. Chemical studies of the antioxidant mechanism of tea catechins: Radical reaction products of epicatechin with peroxyl radicals. Bioorg. Med. Chem. 2003, 11, 3371–3378. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Mandel, S.; Youdim, M.B. Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: A reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009, 4, 283–296. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Morales, I.; Guzmán-Martínez, L.; Cerda-Troncoso, C.; Farías, G.A.; Maccioni, R.B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, D.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J. Nutr. Biochem. 2013, 24, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Hsieh, M.T.; Wu, C.R.; Wood, W.G.; Chen, Y.F. Green Tea Extract Ameliorates Learning and Memory Deficits in Ischemic Rats via Its Active Component Polyphenol Epigallocatechin-3-gallate by Modulation of Oxidative Stress and Neuroinflammation. Evid.-Based Complement. Altern. Med. eCAM 2012, 2012, 163106. [Google Scholar] [CrossRef]
- Berra, E.; Municio, M.M.; Sanz, L.; Frutos, S.; Diaz-Meco, M.T.; Moscat, J. Positioning atypical protein kinase C isoforms in the UV-induced apoptotic signaling cascade. Mol. Cell. Biol. 1997, 17, 4346–4354. [Google Scholar] [CrossRef]
- Alkon, D.L.; Sun, M.K.; Nelson, T.J. PKC signaling deficits: A mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol. Sci. 2007, 28, 51–60. [Google Scholar] [CrossRef]
- Levites, Y.; Amit, T.; Mandel, S.; Youdim, M.B. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 952–954. [Google Scholar] [CrossRef]
- Kaur, T.; Pathak, C.M.; Pandhi, P.; Khanduja, K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008, 67, 25–30. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, M.; Kim, S.; Kim, M.; Chung, J.H. Effects of green tea polyphenol on cognitive and acetylcholinesterase activities. Biosci. Biotechnol. Biochem. 2004, 68, 1977–1979. [Google Scholar] [CrossRef]
- Srividhya, R.; Gayathri, R.; Kalaiselvi, P. Impact of epigallo catechin-3-gallate on acetylcholine-acetylcholine esterase cycle in aged rat brain. Neurochem. Int. 2012, 60, 517–522. [Google Scholar] [CrossRef]
- Ghiglieri, V.; Calabrese, V.; Calabresi, P. Alpha-synuclein: From early synaptic dysfunction to neurodegeneration. Front. Neurol. 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Südhof, T.C. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J. Neurosci. 2015, 35, 5221–5232. [Google Scholar] [CrossRef]
- Parker, W.D., Jr.; Boyson, S.J.; Parks, J.K. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1989, 26, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998, 4, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
- Ni, A.; Ernst, C. Evidence That Substantia Nigra Pars Compacta Dopaminergic Neurons Are Selectively Vulnerable to Oxidative Stress Because They Are Highly Metabolically Active. Front. Cell. Neurosci. 2022, 16, 826193. [Google Scholar] [CrossRef]
- Barber, T.R.; Klein, J.C.; Mackay, C.E.; Hu, M.T. Neuroimaging in pre-motor Parkinson’s disease. NeuroImage Clin. 2017, 15, 215–227. [Google Scholar] [CrossRef]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Parkinson’s Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef]
- Salari, S.; Bagheri, M. In vivo, in vitro and pharmacologic models of Parkinson’s disease. Physiol. Res. 2019, 68, 17–24. [Google Scholar] [CrossRef]
- Chen, M.; Wang, T.; Yue, F.; Li, X.; Wang, P.; Li, Y.; Chan, P.; Yu, S. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience 2015, 286, 383–392. [Google Scholar] [CrossRef]
- Levites, Y.; Weinreb, O.; Maor, G.; Youdim, M.B.; Mandel, S. Green tea polyphenol (–)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001, 78, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Maor, G.; Youdim, M.B.H. Iron and α-synuclein in the substantia nigra of MPTP-treated mice. J. Mol. Neurosci. 2004, 24, 401–416. [Google Scholar] [CrossRef]
- Kalfon, L.; Youdim, M.B.; Mandel, S.A. Green tea polyphenol (–)-epigallocatechin-3-gallate promotes the rapid protein kinase C-and proteasome-mediated degradation of Bad: Implications for neuroprotection. J. Neurochem. 2007, 100, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 2929–2938. [Google Scholar] [CrossRef]
- Pan, T.; Fei, J.; Zhou, X.; Jankovic, J.; Le, W. Effects of green tea polyphenols on dopamine uptake and on MPP+-induced dopamine neuron injury. Life Sci. 2003, 72, 1073–1083. [Google Scholar] [CrossRef]
- Kang, K.S.; Wen, Y.; Yamabe, N.; Fukui, M.; Bishop, S.C.; Zhu, B.T. Dual beneficial effects of (-)-epigallocatechin-3-gallate on levodopa methylation and hippocampal neurodegeneration: In vitro and in vivo studies. PLoS ONE 2010, 5, e11951. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-M.; Wang, S.-W.; Ho, S.-C.; Tang, Y.-L. Protective effect of green tea (-)-epigallocatechin-3-gallate against the monoamine oxidase B enzyme activity increase in adult rat brains. Nutrition 2010, 26, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Batista-Nascimento, L.; Pimentel, C.; Andrade Menezes, R.; Rodrigues-Pousada, C. Iron and neurodegeneration: From cellular homeostasis to disease. Oxidative Med. Cell. Longev. 2012, 2012, 128647. [Google Scholar] [CrossRef]
- Harjes, P.; Wanker, E.E. The hunt for huntingtin function: Interaction partners tell many different stories. Trends Biochem. Sci. 2003, 28, 425–433. [Google Scholar] [CrossRef]
- Rosenblatt, A. Neuropsychiatry of Huntington’s disease. Dialogues Clin. Neurosci. 2007, 9, 191–197. [Google Scholar] [CrossRef]
- Savani, A.A.; Login, I.S. Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology 2007, 68, 797. [Google Scholar] [CrossRef] [PubMed]
- Wyant, K.J.; Ridder, A.J.; Dayalu, P. Huntington’s Disease-Update on Treatments. Curr. Neurol. Neurosci. Rep. 2017, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Duennwald, M.; Markovic, P.; Wacker, J.L.; Engemann, S.; Roark, M.; Legleiter, J.; Marsh, J.L.; Thompson, L.M.; Lindquist, S.; et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum. Mol. Genet. 2006, 15, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Tundis, R.; Belwal, T.; Devkota, H.P.; Daglia, M.; Cetin, Z.; Saygili, E.I.; Campos, M.d.G.; Capanoglu, E.J.E. Dietary flavonoids in the management of huntington’s disease: Mechanism and clinical perspective. eFood 2020, 1, 38–52. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef]
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72 (Suppl. S1), 1–5. [Google Scholar] [CrossRef]
- Rae-Grant, A.; Day, G.S.; Marrie, R.A.; Rabinstein, A.; Cree, B.A.C.; Gronseth, G.S.; Haboubi, M.; Halper, J.; Hosey, J.P.; Jones, D.E.; et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 777–788. [Google Scholar] [CrossRef]
- Semnani, M.-R.; Mashayekhi, F.; Azarnia, M.; Salehi, Z. Effects of green tea epigallocatechin-3-gallate (EGCG) on proteolipid protein (PLP) and oligodendrocyte transcription factor 1 (Olig1) expression in the cerebral cortex of cuprizone induced multiple sclerosis mice; a western blot study. Casp. J. Neurol. Sci. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Bellmann-Strobl, J.; Paul, F.; Wuerfel, J.; Dörr, J.; Infante-Duarte, C.; Heidrich, E.; Körtgen, B.; Brandt, A.; Pfüller, C.; Radbruch, H.; et al. Epigallocatechin Gallate in Relapsing-Remitting Multiple Sclerosis: A Randomized, Placebo-Controlled Trial. Neurol.(R) Neuroimmunol. Neuroinflamm. 2021, 8, e981. [Google Scholar] [CrossRef]
- Mossakowski, A.A.; Pohlan, J.; Bremer, D.; Lindquist, R.; Millward, J.M.; Bock, M.; Pollok, K.; Mothes, R.; Viohl, L.; Radbruch, M.; et al. Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol. 2015, 130, 799–814. [Google Scholar] [CrossRef]
- Rust, R.; Chien, C.; Scheel, M.; Brandt, A.U.; Dörr, J.; Wuerfel, J.; Klumbies, K.; Zimmermann, H.; Lorenz, M.; Wernecke, K.D.; et al. Epigallocatechin Gallate in Progressive MS: A Randomized, Placebo-Controlled Trial. Neurol.(R) Neuroimmunol. Neuroinflamm. 2021, 8, e964. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Regodón Wallin, A.; Samuelsson, K.; Press, R.; Zachau, A.; Ronnevi, L.O.; Kierkegaard, M.; Andersen, P.M.; Hillert, J.; Fang, F.; et al. The Swedish motor neuron disease quality registry. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Greaves, C.V.; Rohrer, J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Couratier, P.; Corcia, P.; Lautrette, G.; Nicol, M.; Marin, B. ALS and frontotemporal dementia belong to a common disease spectrum. Rev. Neurol. 2017, 173, 273–279. [Google Scholar] [CrossRef]
- Koh, S.H.; Kwon, H.; Kim, K.S.; Kim, J.; Kim, M.H.; Yu, H.J.; Kim, M.; Lee, K.W.; Do, B.R.; Jung, H.K.; et al. Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicology 2004, 202, 213–225. [Google Scholar] [CrossRef]
- Koh, S.H.; Lee, S.M.; Kim, H.Y.; Lee, K.Y.; Lee, Y.J.; Kim, H.T.; Kim, J.; Kim, M.H.; Hwang, M.S.; Song, C.; et al. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci. Lett. 2006, 395, 103–107. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, S.; Li, X.; Luo, G.; Li, L.; Le, W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem. Res. 2006, 31, 1263–1269. [Google Scholar] [CrossRef]
- Che, F.; Wang, G.; Yu, J.; Wang, X.; Lu, Y.; Fu, Q.; Su, Q.; Jiang, J.; Du, Y. Effects of epigallocatechin-3-gallate on iron metabolism in spinal cord motor neurons. Mol. Med. Rep. 2017, 16, 3010–3014. [Google Scholar] [CrossRef]
- Taniguchi, S.; Suzuki, N.; Masuda, M.; Hisanaga, S.; Iwatsubo, T.; Goedert, M.; Hasegawa, M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005, 280, 7614–7623. [Google Scholar] [CrossRef]
- Almeida, L.; Andreu-Fernández, V.; Navarro-Tapia, E.; Aras-López, R.; Serra-Delgado, M.; Martínez, L.; García-Algar, O.; Gómez-Roig, M.D. Murine Models for the Study of Fetal Alcohol Spectrum Disorders: An Overview. Front. Pediatrics 2020, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.G.; Kril, J.J.; Holloway, R.L. Brain shrinkage in chronic alcoholics: A pathological study. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Harwood, D.G.; Barker, W.W.; Loewenstein, D.A.; Ownby, R.L.; St George-Hyslop, P.; Mullan, M.; Duara, R. A cross-ethnic analysis of risk factors for AD in white Hispanics and white non-Hispanics. Neurology 1999, 52, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, E.B.; McLean, T.; Tong, M.; de la Monte, S.M. Progressive white matter atrophy with altered lipid profiles is partially reversed by short-term abstinence in an experimental model of alcohol-related neurodegeneration. Alcohol 2017, 65, 51–62. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef]
- Heaton, M.B.; Paiva, M.; Madorsky, I.; Mayer, J.; Moore, D.B. Effects of ethanol on neurotrophic factors, apoptosis-related proteins, endogenous antioxidants, and reactive oxygen species in neonatal striatum: Relationship to periods of vulnerability. Dev. Brain Res. 2003, 140, 237–252. [Google Scholar] [CrossRef]
- Joya, X.; Garcia-Algar, O.; Salat-Batlle, J.; Pujades, C.; Vall, O. Advances in the development of novel antioxidant therapies as an approach for fetal alcohol syndrome prevention. Birth Defects Res. Part A Clin. Mol. Teratol. 2015, 103, 163–177. [Google Scholar] [CrossRef]
- Pei, J.; Baugh, L.; Andrew, G.; Rasmussen, C. Intervention recommendations and subsequent access to services following clinical assessment for fetal alcohol spectrum disorders. Res. Dev. Disabil. 2017, 60, 176–186. [Google Scholar] [CrossRef]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Choy, K.W.; Pang, C.P.; Rogers, M.S. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2007, 22, 280–287. [Google Scholar] [CrossRef]
- Tiwari, V.; Kuhad, A.; Chopra, K. Epigallocatechin-3-gallate ameliorates alcohol-induced cognitive dysfunctions and apoptotic neurodegeneration in the developing rat brain. Int. J. Neuropsychopharmacol. 2010, 13, 1053–1066. [Google Scholar] [CrossRef]
- Long, L.; Li, Y.; Wang, Y.D.; He, Q.Y.; Li, M.; Cai, X.D.; Peng, K.; Li, X.P.; Xie, D.; Wen, Y.L.; et al. The preventive effect of oral EGCG in a fetal alcohol spectrum disorder mouse model. Alcohol. Clin. Exp. Res. 2010, 34, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Toledano, L.; Andreu-Fernández, V.; Aras-López, R.; García-Algar, Ó.; Martínez, L.; Gómez-Roig, M.D. Epigallocatechin Gallate Ameliorates the Effects of Prenatal Alcohol Exposure in a Fetal Alcohol Spectrum Disorder-Like Mouse Model. Int. J. Mol. Sci. 2021, 22, 715. [Google Scholar] [CrossRef] [PubMed]
- Antonio, A.M.; Druse, M.J. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008, 1204, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Shim, J.Y.; Zhu, B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005, 68, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Dierssen, M. Down syndrome: The brain in trisomic mode. Nat. Rev. Neurosci. 2012, 13, 844–858. [Google Scholar] [CrossRef]
- Lott, I.T.; Dierssen, M. Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol. 2010, 9, 623–633. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Wyganowska-Świątkowska, M.; Matthews-Kozanecka, M.; Matthews-Brzozowska, T.; Skrzypczak-Jankun, E.; Jankun, J. Can EGCG Alleviate Symptoms of Down Syndrome by Altering Proteolytic Activity? Int. J. Mol. Sci. 2018, 19, 248. [Google Scholar] [CrossRef]
- Souchet, B.; Guedj, F.; Penke-Verdier, Z.; Daubigney, F.; Duchon, A.; Herault, Y.; Bizot, J.C.; Janel, N.; Créau, N.; Delatour, B.; et al. Pharmacological correction of excitation/inhibition imbalance in Down syndrome mouse models. Front. Behav. Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef]
- Abunofal, O.; Mohan, C. Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review. Medicines 2022, 9, 20. [Google Scholar] [CrossRef]
- Catuara-Solarz, S.; Espinosa-Carrasco, J.; Erb, I.; Langohr, K.; Gonzalez, J.R.; Notredame, C.; Dierssen, M. Combined Treatment with Environmental Enrichment and (-)-Epigallocatechin-3-Gallate Ameliorates Learning Deficits and Hippocampal Alterations in a Mouse Model of Down Syndrome. eNeuro 2016, 3, e0103. [Google Scholar] [CrossRef] [PubMed]
- Catuara-Solarz, S.; Espinosa-Carrasco, J.; Erb, I.; Langohr, K.; Notredame, C.; Gonzalez, J.R.; Dierssen, M. Principal Component Analysis of the Effects of Environmental Enrichment and (-)-epigallocatechin-3-gallate on Age-Associated Learning Deficits in a Mouse Model of Down Syndrome. Front. Behav. Neurosci. 2015, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- De Toma, I.; Ortega, M.; Aloy, P.; Sabidó, E.; Dierssen, M. DYRK1A Overexpression Alters Cognition and Neural-Related Proteomic Pathways in the Hippocampus That Are Rescued by Green Tea Extract and/or Environmental Enrichment. Front. Mol. Neurosci. 2019, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- De Toma, I.; Ortega, M.; Catuara-Solarz, S.; Sierra, C.; Sabidó, E.; Dierssen, M. Re-establishment of the epigenetic state and rescue of kinome deregulation in Ts65Dn mice upon treatment with green tea extract and environmental enrichment. Sci. Rep. 2020, 10, 16023. [Google Scholar] [CrossRef]
- Valenti, D.; De Rasmo, D.; Signorile, A.; Rossi, L.; de Bari, L.; Scala, I.; Granese, B.; Papa, S.; Vacca, R.A. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. Biochim. Biophys. Acta 2013, 1832, 542–552. [Google Scholar] [CrossRef]
- Valenti, D.; de Bari, L.; de Rasmo, D.; Signorile, A.; Henrion-Caude, A.; Contestabile, A.; Vacca, R.A. The polyphenols resveratrol and epigallocatechin-3-gallate restore the severe impairment of mitochondria in hippocampal progenitor cells from a Down syndrome mouse model. Biochim. Biophys. Acta 2016, 1862, 1093–1104. [Google Scholar] [CrossRef]
- Stagni, F.; Giacomini, A.; Emili, M.; Trazzi, S.; Guidi, S.; Sassi, M.; Ciani, E.; Rimondini, R.; Bartesaghi, R. Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome. Neuroscience 2016, 333, 277–301. [Google Scholar] [CrossRef]


| Objective | Experimental Model | Disease | Outcome | References |
|---|---|---|---|---|
| EGCG | In vitro | Alzheimer’s | Inhibition of Tau aggregation and oxidation | [94] |
| EGCG | In vitro | Huntington disease | Inhibitory effect on htt aggregation | [95] |
| CAT | Rat model | Parkinson disease | Improve rotational behavior, locomotion, and memory | [96,97] |
| EGCG | Rat model | Alzheimer’s | Decrease oxidative stress and improve cholinergic synaptic and mitochondrial functions | [98] |
| EGCG | Mice model | Multiple sclerosis | Decrease onset of disease and clinical severity. Reduce inflammatory infiltrates. Increase Olig 1 expression | [99,100] |
| Green tea | Drosophila model | Huntington disease | Green tea consumption may modulate symptoms | [101] |
| EGCG | Human studies | Multiple Sclerosis | Improve muscle metabolism, counteract NOX overactivation, decrease plasma NAA levels | [102,103] |
| EGCG | Human studies | Down syndrome | Improve visual recognition memory | [104] |
| EGCG | Human studies | Alzheimer’s | Low prevalence of cognitive impairment, decrease oxidative stress, and lipid peroxidation | [105,106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, O.; Dalhat, M.H.; Altamimi, A.S.A.; Rasool, R.; Alzarea, S.I.; Almalki, W.H.; Murtaza, B.N.; Iftikhar, S.; Nadeem, S.; Nadeem, M.S.; et al. Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits. Molecules 2022, 27, 7604. https://doi.org/10.3390/molecules27217604
Afzal O, Dalhat MH, Altamimi ASA, Rasool R, Alzarea SI, Almalki WH, Murtaza BN, Iftikhar S, Nadeem S, Nadeem MS, et al. Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits. Molecules. 2022; 27(21):7604. https://doi.org/10.3390/molecules27217604
Chicago/Turabian StyleAfzal, Obaid, Mahmood Hassan Dalhat, Abdulmalik S. A. Altamimi, Rabia Rasool, Sami I. Alzarea, Waleed Hassan Almalki, Bibi Nazia Murtaza, Saima Iftikhar, Shamaila Nadeem, Muhammad Shahid Nadeem, and et al. 2022. "Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits" Molecules 27, no. 21: 7604. https://doi.org/10.3390/molecules27217604
APA StyleAfzal, O., Dalhat, M. H., Altamimi, A. S. A., Rasool, R., Alzarea, S. I., Almalki, W. H., Murtaza, B. N., Iftikhar, S., Nadeem, S., Nadeem, M. S., & Kazmi, I. (2022). Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits. Molecules, 27(21), 7604. https://doi.org/10.3390/molecules27217604







