An Overview of Analytical Methods to Determine Pharmaceutical Active Compounds in Aquatic Organisms
Abstract
1. Introduction
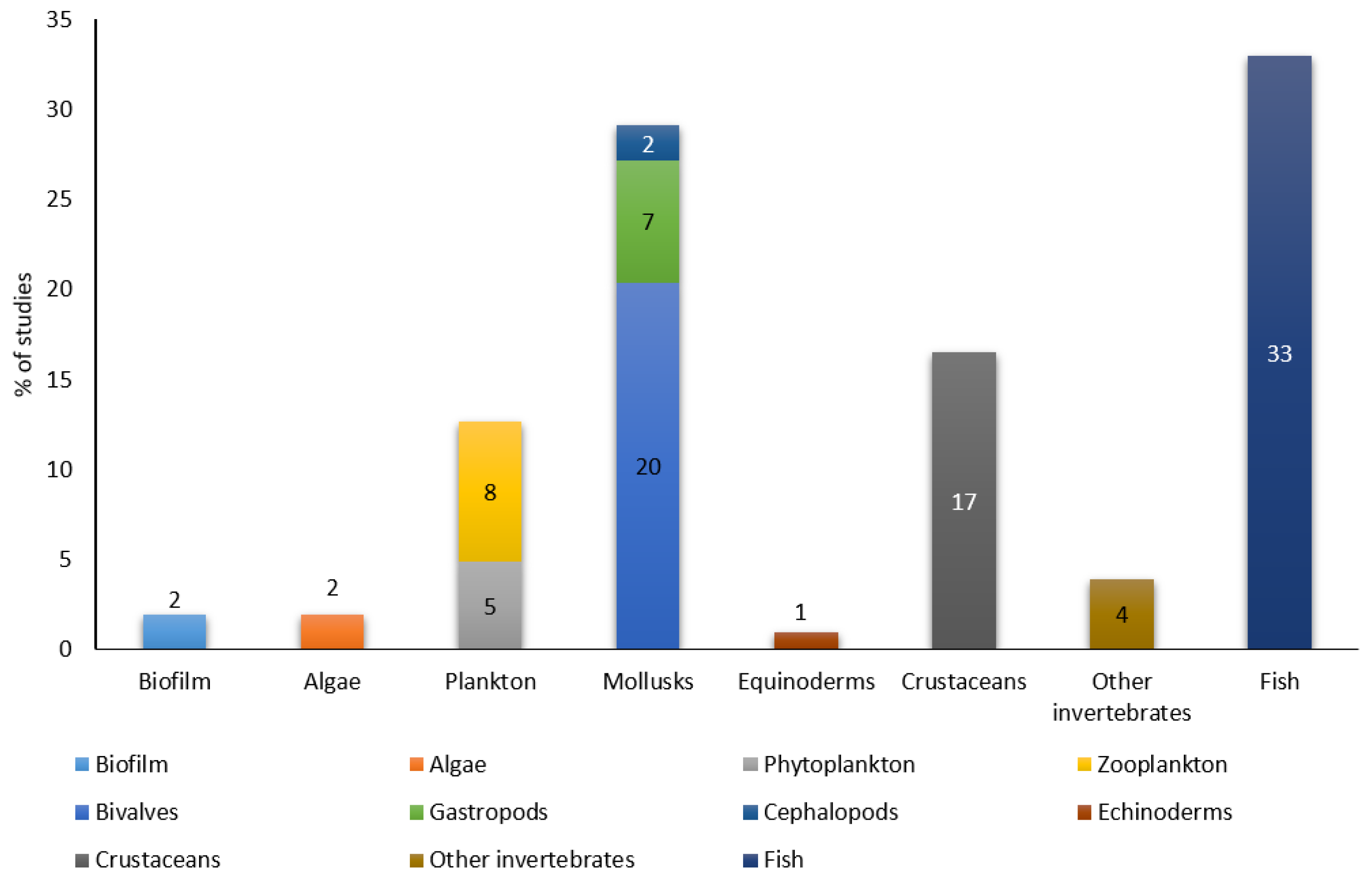
2. Multi-Level Biological Groups as Biomarkers of Exposure
2.1. Phytoplankton
2.2. Zooplankton
2.3. Benthos
2.4. Fish
3. Analytical Methodologies for the Determination of Pharmaceuticals in Biota Samples
3.1. Sample Collection
3.2. Sample Pretreatment
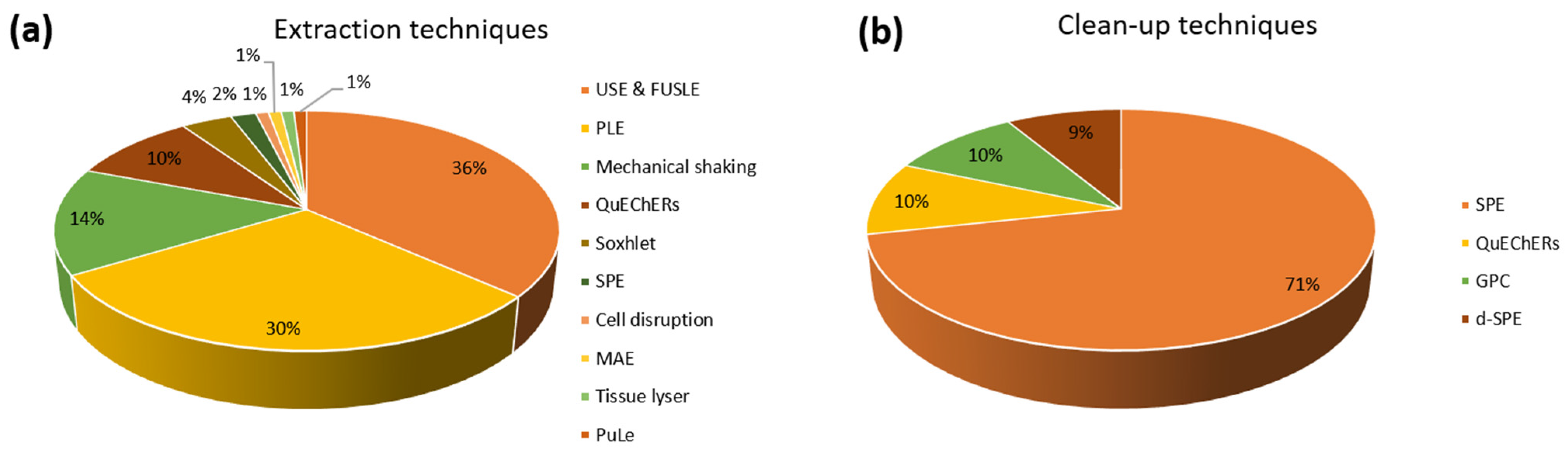
3.3. Sample Treatment (Extraction and/or Clean-Up)
3.3.1. Ultrasound USE and FUSLE
3.3.2. Pressurized Liquid Extraction
3.3.3. Microwave Assisted Extraction
3.3.4. Solid-Phase Extraction
3.3.5. Dispersive Solid Phase Extraction (dSPE)
3.3.6. Others
4. Instrumental Analysis
4.1. Liquid Chromatography
4.2. Detection Systems
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czeruckall, D.; et al. Human health and ocean pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar] [CrossRef] [PubMed]
- European Environmental Agency. Contaminants in Europe’s Seas Moving Towards a Clean, Non-Toxic Marine Environment. EEA Report 2019, 25/2018. Available online: https://www.eea.europa.eu/publications/contaminants-in-europes-seas (accessed on 3 November 2022).
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. Biomarkers based tools to asses environmental and chemical stressors in aquatic systems. Ecol. Indic. 2021, 122, 107207. [Google Scholar] [CrossRef]
- Zhang, C.; Barron, L.; Sturzenbaum, S. The transportation, transformation and (bio)accumulation of pharmaceuticals in the terrestrial exosystem. Sci. Total Environ. 2021, 781, 146684. [Google Scholar] [CrossRef] [PubMed]
- Vagi, M.C.; Petsas, A.S.; Kostopoulou, M.N. Potential effect of persistent organic contaminants on Marine Biota: A review on recent research. Water 2021, 13, 2488. [Google Scholar] [CrossRef]
- Arnold, K.E.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the environment: Assessing risks of pharmaceuticals to wildlife and ecosystems. Phil. Trans. R. Soc. B 2014, 369, 20130569. [Google Scholar] [CrossRef]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical pollution in aquatic environments: A concise review of environmental impacts and bioremediation systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef]
- Golbaz, S.; Yaghmaeian, K.; Isazadeh, S.; Zamanzadeh, M. Environmental risk assessment of multiclass pharmaceutical active compounds: Selection of high priority concern pharmaceuticals using entropy-utility functions. Environ. Sci. Pollut. Res. 2021, 28, 59745–59770. [Google Scholar] [CrossRef]
- Prionti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-disrupting compounds: An overview on their occurrence in the aquatic environment and human exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Ramírez-Morales, D.; Masís-Mora, M.; Montiel-Mora, J.R.; Cambronero-Heinrichs, J.C.; Pérez-Rojas, G.; Tormo-Budowski, R.; Méndez-Rivera, M.; Briceño-Guevara, S.; Gutiérrez-Quirós, J.A.; Arias-Mora, V.; et al. Multi-residue analysis of pharmaceuticals in water samples by liquid chromatography-mass spectrometry: Quality assessment and application to the risk assessment of urban-influenced surface waters in a metropolitan area of Central America. Process Saf. Environ. 2021, 153, 289–300. [Google Scholar] [CrossRef]
- Cravo, A.; Silva, S.; Rodrigues, J.; Cardoso, V.V.; Benoliel, M.J.; Correia, C.; Coelho, M.R.; Rosa, M.J.; Almeida, C.M.M. Understanding the bioaccumulation of pharmaceutical active compounds by clams Ruditapes decussatus exposed to a UWWTP discharge. Environ. Res. 2022, 208, 112632. [Google Scholar] [CrossRef]
- Blanco, G.; Junza, A.; Barrón, D. Occurrence of veterinary pharmaceuticals in golden eagle nestlings: Unnoticed scavenging on livestock carcasses and other potential exposure routes. Sci. Total Environ. 2017, 586, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Pan, C.-G.; Wang, Y.-H.; Xiao, S.-K.; Yu, K.-F. Antibiotics in a subtropical food web from the Beibu Gulf, South China: Occurrence, bioaccumulation and trophic transfer. Sci. Total Environ. 2021, 751, 141718. [Google Scholar] [CrossRef] [PubMed]
- Water Framework Directive (WFD) 2000/60/EC. Available online: https://www.eea.europa.eu/policy-documents/water-framework-directive-wfd-2000 (accessed on 27 September 2022).
- Marine Strategy Framework Directive 2008/56/EC. Available online: https://www.eea.europa.eu/policy-documents/2008-56-ec (accessed on 27 September 2022).
- European Union. Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. OJEU. 2022. 197/117. Available online: https://euroalert.net/en/oj/105661/commission-implementing-decision-eu-2022-1307-of-22-july-2022-establishing-a-watch-list-of-substances-for-union-wide-monitoring-in-the-field-of-water-policy-pursuant-to-directive-2008-105-ec-of-the-european-parliament-and-of-the-council-notified-under-document-c-2022-5098 (accessed on 3 November 2022).
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef]
- Huerta, B.; Rodríguez-Moraz, S.; Barceló, D. Pharmaceuticals in biota in the aquatic environment: Analytical methods and environmental implications. Anal. Bioanal. Chem. 2012, 404, 2611–2624. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.F.; Shugart, L.R. (Eds.) Biomarkers of Environmental Contamination, 1st ed.; Lewis Publishers: Boca Raton, FL, USA, 1990. [Google Scholar] [CrossRef]
- Vidal-Liñan, L.; Bellas, J.; Campillo, J.A.; Beiras, R. Integrated use of antioxidant enzymes in mussels, Mytilus galloprovincialis, for monitoring pollution in highly productive coastal areas of Galicia (NW Spain). Chemosphere 2010, 78, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Fuster, B.; Alomar, C.; Paniagua González, G.; Garcinuño Martínez, R.M.; Soliz Rojas, D.; Fernández Hernando, P. Assessing microplastic ingestion and occurrence of bisphenols and phthalates in bivalves, fish and holothurians from a Mediterranean marine protected area. Environ. Res. 2022, 214, 114034. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Pedà, C.; Compa, M.; Tsangaris, C.; Alomar, C.; Claro, F.; Ioakeimidis, C.; Galgani, F.; Hema, T.; Deudero, S.; et al. Bioindicators for monitoring marine litter ingestions and its impacts on Mediterranean biodiversity. Environ. Pollut. 2018, 237, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, L.; Etxebarria, N.; Martínez-Arkarazo, I.; Raposo, J.C.; Usobiaga, A.; Zuloaga, O.; Raingeard, D.; Cajaraville, M.P. Distribution of organic microcontaminants, butyltins, and metals in mussels from the Estuary of Bilbao. Arch. Environ. Contam. Toxicol. 2010, 59, 244–254. [Google Scholar] [CrossRef]
- Viñas, L.; Pérez-Fernández, B.; Soriano, J.A.; López, M.; Bargiela, J.; Alves, I. Limpet (Patella sp.) as a biomonitor for organic pollutants. A proxy for mussel? Mar. Pollut. Bull. 2018, 133, 271–280. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, T.; Zhang, R.; Wang, X.; Zhang, Q.; Wang, Q.; Dong, Z.; Zhao, J. Integrative assessment of biomarker responses in Mytilus galloprovincialis exposed to seawater acidification and copper ions. Sci. Total Environ. 2022, 851, 158146. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghosh, A.; Bhadury, P. Arsenic through aquatic trophic levels: Effects, tansformations and biomagnification—A concise review. Geosci. Lett. 2022, 9, 20. [Google Scholar] [CrossRef]
- Yang, H.; Lu, G.; Yan, Z.; Liu, J.; Dong, H.; Bao, X.; Zhang, X.; Sun, Y. Residues, bioaccumulation and trophic transfer of pharmaceuticals and personal care products in highly urbanizad rivers affected by water diversion. J. Hazard. Mater. 2020, 391, 122245. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, S.; Tai, Y.; Tam, N.F.; Su, L.; Shi, Y.; Luo, B.; Tao, R.; Yang, Y.; Zheng, X. Evaluation of factors influencing annual occurrence, bioaccumulation and biomagnification of antibiotics in planktonic food webs of a large subtropical river in South China. Water Res. 2020, 170, 115302. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Long, S.; Xiong, K.; Zhang, T. Antibiotic bioaccumulation in zooplankton from the Yelang Lake Reservoir of Anshun City, Southwest China. Pol. J. Environ. Stud. 2022, 31, 2367–2380. [Google Scholar] [CrossRef]
- Moreira, F.W.A.; Leite, M.G.P.; Fijaco, M.A.G.; Mendoça, F.P.C.; Campos, L.P.; Eskinazi-Sant’Anna, E.M. Assessing the impacts of mining activities on zooplankton functional diversity. Acta Limol. Bras. 2016, 28, 107. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, J.M.; Galloway, T.S. Microplastic ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, B.; Shu, F.; Chen, X.; Xu, N.; Ni, J. Bioaccumulation of 35 metal(loid)s in organis of a freshwater mussel (Hyriopsis cumingii) and environmental implications in Poyang Lake, China. Chemosphere 2022, 307, 136150. [Google Scholar] [CrossRef]
- Sharma, J.; Behera, P.K. Abundance & distribution of aquatic macro-invertebrate families of river Ganga and correlation with environmental parameters. Environ. Monit. Assess. 2022, 194, 546. [Google Scholar] [CrossRef]
- Grabicová, K.; Stanová, A.V.; Svecová, H.; Nováková, P.; Kodes, V.; Leontovycová, D.; Brooks, B.W.; Grabic, R. Invertebrates differentially bioaccumulate pharmaceuticals: Implications for routine biomonitoring. Environ. Pollut. 2022, 309, 119715. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Huerta, B.; Fernandez-Tejedor, M.; Rodríguez-Mozaz, S.; Barceló, D. Multi-residue method for the analysis of pharmaceuticals and some of their metabolites in bivalves. Talanta 2015, 136, 174–182. [Google Scholar] [CrossRef]
- Burket, S.R.; Sapozhnikova, Y.; Zheng, J.S.; Chung, S.S.; Brooks, B.W. At the Intersection of urbanization, water, and food security: Determination of select contaminants of emerging concern in mussels and oysters from Hong Kong. J. Agric. Food Chem. 2018, 66, 5009–5017. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Pharmaceuticals and personal care products in the marine environment around False Bay, Cape Town, South Africa: Occurrence and risk assessment study. Environ. Toxicol. Chem. 2022, 41, 614–634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Wang, W.-X.; Lv, Y.-J.; Mao, Z.-G.; Chen, C.; Wu, Q.L. Tissue concentrations, trophic transfer and human risks of antibiotics in freshwater food web in Lake Taihu, China. Ecotoxicol. Environ. Saf. 2020, 197, 110626. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hao, H.; Xu, N.; Liang, X.; Gao, D.; Xu, Y.; Gao, Y.; Tao, H.; Wong, M. Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: Occurrence, distribution, potential sources, and health risk assessment. Sci. Total Environ. 2019, 659, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Morcillo, S.; Rodríguez-Gil, J.L.; Fernández-Rubio, J.; Rodríguez-Mozaz, S.; Prado Míguez-Santiyán, M.; Valdes, M.E.; Barceló, D.; Valcárcel, Y. Presence of pharmaceutical compounds, levels of biochemical biomarkers in seafood tissues and risk assessment for human health: Results from a case study in North-Western Spain. Int. J. Hyg. Environ. Health 2020, 223, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.F.; Duarte, I.A.; Duarte, B.; Freitas, A.; Vila Pouca, A.S.; Barbosa, J.; Gillanders, B.M.; Reis-Santos, P. Environmental risk assessment and bioaccumulation of pharmaceuticals in a large urbanized estuary. Sci. Total Environ. 2021, 783, 147021. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.-R.; Liu, S.-S.; Zhou, G.-J.; Sun, K.-F.; Zhao, J.-L.; Ying, G.-G. Antibiotics in typical marine aquaculture farms sourrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure. Mar. Pollut. Bull. 2015, 90, 181–187. [Google Scholar] [CrossRef]
- Zheng, W.; Yoo, K.-H.; Choi, J.-M.; Park, D.-H.; Kim, S.-K.; Kang, Y.-S.; El-Aty, A.M.A.; Hacimüftüoglu, A.; Wang, J.; Shim, J.-H.; et al. Residual detection of naproxen, methyltestosterone and 17 α-hydroxyprogesterone caproate in aquatic products by simple liquid-liquid extraction method coupled with liquid Chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4396. [Google Scholar] [CrossRef]
- Ruan, Y.; Lin, H.; Zhang, X.; Wu, R.; Zhang, K.; Leung, K.M.Y.; Lam, J.C.W.; Lam, P.K.S. Enantiomer-specific bioaccumulation and distribution of chiral pharmaceuticals in a subtropical marine food web. J. Hazard. Mater. 2020, 394, 122589. [Google Scholar] [CrossRef]
- Mastángelo, M.M.; Valdés, M.E.; Eissa, B.; Ossana, N.A.; Barceló, D.; Sabater, S.; Rodríguez-Mozaz, S.; Giorgi, A.D.N. Occurrence and accumulation of pharmaceutical products in water and biota of urban lowland rivers. Sci. Total Environ. 2022, 828, 154303. [Google Scholar] [CrossRef]
- Pashaei, R.; Dzingeleviciene, R.; Abbasi, S.; Szultka-Mlynska, M.; Buszewski, B. Determination of 15 pharmaceutical residues in fish and shrimp tissues by high-performance liquid chromatography-tandem mass spectrometry. Environ. Mon. Assess. 2022, 194, 325. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.V.; Cunha, S.C.; Fogaça, F.H.S.; Alonso, M.B.; Torres, J.P.M.; Fernandes, J.O. Occurrence of pharmaceuticals in seafood from two Brazilian coastal areas: Implication for human risk assessment. Sci. Total Environ. 2022, 803, 149744. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, R.; Rodríguez-Mozaz, S.; Huerta, B.; Barceló, D.; León, V.M. Do pharmaceuticals bioaccumulate in marine molluscs and fish from a coastal lagoon? Environ. Res. 2016, 146, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Ojemaye, C.Y.; Petrik, L. Occurrences, levels and risk assessment studies of emerging pollutants (pharmaceuticals, perfluoroalkyl and endocrine disrupting compounds) in fish samples from Kalk Bay harbour, South Africa. Environ. Pollut. 2019, 252, 562–572. [Google Scholar] [CrossRef]
- Tanoue, R.; Nozaki, K.; Nomiyama, K.; Kunisue, T.; Tanabe, S. Rapid analysis of 65 pharmaceuticals and 7 personal care products in plasma and whole-body tissue samples of fish using acidic extraction, zirconia-coated silica cleanup, and liquid Chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1631, 461586. [Google Scholar] [CrossRef]
- Vitale, D.; Picó, Y.; Álvarez-Ruiz, R. Determination of organic pollutants in Anguilla anguilla by liquid Chromatography coupled with tandem mass spectrometry (LC-MS/MS). MEthodsX 2021, 8, 101342. [Google Scholar] [CrossRef]
- Ali, A.M.; Thorsen-Ronning, H.; Sydnes, L.K.; Alarif, W.M.; Kallenborn, R.; Al-Lihaibi, S.S. Detection of PPCPs in marine organisms from contaminated coastal waters of the Saudi Red Sea. Sci. Total Environ. 2018, 621, 654–662. [Google Scholar] [CrossRef]
- Rojo, M.; Álvarez-Muñoz, D.; Dománico, A.; Foti, R.; Rodriguez-Mozaz, S.; Barceló, D.; Carriquiriborde, P. Human pharmaceuticals in three major fish species from the Uruguay River (South America) with different feeding habits. Environ. Pollut. 2019, 252, 146–154. [Google Scholar] [CrossRef]
- Ruhí, A.; Acuña, V.; Barceló, D.; Huerta, B.; Mor, J.-R.; Rodríguez-Mozaz, S.; Sabater, S. Bioaccumulation and trophic magnification of pharmaceuticals endocrine disruptors in a Mediterranean river food web. Sci. Total Environ. 2016, 540, 250–259. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Hooda, P.S.; Swinden, J.; Barker, J.; Barton, S. Spatial (bio)accumulation of pharmaceuticals, illicit drugs, plasticisers, perfluorinated compounds and metabolites in river sediment, aquatic plants and benthic organisms. Environ. Pollut. 2018, 234, 864–875. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Yan, Z.; Liu, J.; Wang, P.; Wang, Y. Bioaccumulation and trophic transfer of pharmaceuticals in food webs from a large freshwater lake. Environ. Pollut. 2017, 222, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Lu, G.; Liu, J.; Yang, H.; Li, Y. Uptake, depuration and bioconcentration of two pharmaceuticals, roxithromycin and propranolol, in Daphnia magna. Ecotoxicol. Environ. Saf. 2016, 126, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jeon, J.; Hollender, J.; Yu, S.; Kim, S.D. Aqueous and dietary bioaccumulation of antibiotic tetracycline in D. magna and its multigenerational transfer. J. Hazard. Mater. 2014, 279, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Meyer, C.; Patrick, M.; Kosfeld, V.; Rüdel, H.; Koschorreck, J.; Hollender, J. Comprehensive screening of polar emerging organic contaminants including PFASs and evaluation of the trophic transfer behavior in a freshwater food web. Water Res. 2022, 218, 118514. [Google Scholar] [CrossRef] [PubMed]
- Danielle, G.; Fieu, M.; Joachim, S.; James-Casas, A.; Andres, S.; Baudoin, P.; Bonnard, M.; Bonnard, I.; Geffard, A.; Vulliet, E. Development of a multi-residue analysis of diclofenac and some transformation products in bivalves using QuEChERs extraction and liquid Chromatography-tandem mass spectrometry. Application to samples from mesocosm studies. Talanta 2016, 155, 1–7. [Google Scholar] [CrossRef]
- López-García, E.; Postigo, C.; López de Alda, M. Psychoactive substances in mussels: Analysis and occurrence assessment. Mar. Pollut. Bull. 2019, 146, 985–992. [Google Scholar] [CrossRef]
- McEneff, G.; Barron, L.; Kelleher, B.; Paull, B.; Quinn, B. The determination of pharmaceutical residues in cooked and uncooked marine bivalves using pressurized liquid extraction, solid-phase extraction and liquid Chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 9509–9521. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Boillot, C.; Fenet, H.; Chiron, S.; Casellas, C.; Gómez, E. Fast and easy extraction combined with high resolution-mass spectrometry for residue analysis of two anticonvulsants and their transformation products in marine mussels. J. Chromatogr. A 2013, 1305, 27–34. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Rodríguez-Mozaz, S.; Jacobs, S.; Serra-Compte, A.; Cáceres, N.; Sioen, I.; Verbeke, W.; Barbosa, V.; Ferrari, F.; Fernández-Tejedor, M.; et al. Pharmaceuticals and endocrine disruptors in raw and cooked seafood from European market: Concentrations and human exposure levels. Environ. Int. 2018, 119, 570–581. [Google Scholar] [CrossRef]
- Mijangos, L.; Ziarrusta, H.; Zabaleta, I.; Usobiaga, A.; Olivares, M.; Zuloaga, O.; Etxebarria, N.; Prieto, A. Multiresidue analytical method for the determination of 41 multiclass organic pollutants in mussel and fish tissues and biofluids by liquid Chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 493–506. [Google Scholar] [CrossRef]
- Núñez, M.; Borrull, F.; Pocurull, E.; Fontanals, N. Pressurized liquid extraction followed by liquid Chromatography with tandem mass spectrometry to determine pharmaceuticals in mussels. J. Sep. Sci. 2016, 39, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Albino, S.; Silva, S.; Cravo, A.; Cardoso, V.V.; Benoliel, M.J.; Almeida, C.M.M. Development of a multiresidue method for the determination of 24 pharmaceuticals in clams by QuEChERs and liquid Chromatography-triple quadrupole tandem mass spectrometry. Food. Anal. Methods 2019, 12, 838–851. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Zhang, N.; Xum, X.; Bao, T. Novel solid-phase extraction filter based on a zirconium meta-organic framework for determination of non-steroidal anti-inflammatory drugs residues. J. Chromatogr. A 2021, 1652, 462349. [Google Scholar] [CrossRef] [PubMed]
- Miossec, C.; Mille, T.; Lanceleur, L.; Monperrus, M. Simultaneous determination of 42 pharmaceuticals in seafood samples by solvent extraction coupled to liquid Chromatography-tandem mass spectrometry. Food Chem. 2020, 322, 126765. [Google Scholar] [CrossRef]
- Miller, T.H.; McEneff, G.L.; Brown, R.J.; Owen, S.F.; Bury, N.R.; Barron, L.P. Pharmaceuticals in the freshwater invertebrate, Gammarus pulex, determinet using pulverised liquid extraction, solid phase extraction and liquid Chromatograpgy-tandem mass spectrometry. Sci. Total Environ. 2015, 511, 153–160. [Google Scholar] [CrossRef]
- Rizzi, C.; Seveso, D.; Galli, P.; Villa, S. First record of emerging contaminants in sponges of an inhabited island in the Maldives. Mar. Pollut. Bull. 2020, 156, 111273. [Google Scholar] [CrossRef]
- Argüello-Pérez, M.A.; Ramírez-Ayala, E.; Mendoza-Pérez, J.A.; Monroy-Mendieta, M.M.; Vázquez-Guevara, M.; Lezama-Cervantes, C.; Godínez-Domínguez, E.; Silva-Bátiz, F.D.A.; Tintos-Gómez, A. Determination of the Bioaccumulative Potential Risk of emerging contaminants in fish muscle as an environmental quality indicator in Coastal Lagoons of he Central Mexican Pacific. Water 2020, 12, 22721. [Google Scholar] [CrossRef]
- Baesu, A.; Ballash, G.; Mollenkopf, D.; Wittum, T.; Sulliván, S.M.P.; Bayen, S. Suspect screening of pharmaceuticals in fish livers based on QuEChERs extraction coupled with high resolution mass spectrometry. Sci. Total Environ. 2021, 783, 146902. [Google Scholar] [CrossRef]
- Bobrowska-Korczak, B.; Stawarska, A.; Szterk, A.; Ofiara, K.; Czerwonka, M.; Giebultowicz, J. Determination of pharmaceuticals, heavy metals and oxysterols in fish muscle. Molecules 2021, 26, 1229. [Google Scholar] [CrossRef]
- Borik, A.; Stanová, A.V.; Brooks, B.W.; Grabicová, K.; Randák, T.; Grabic, R. Determination of citalopram in fish brain tissue: Benefits of coupling laser diode thermal desorption with low- and high-resolution mass spectrometry. Anal. Bioanal. Chem. 2021, 412, 4353–4361. [Google Scholar] [CrossRef]
- Boulard, L.; Parrhysius, P.; Jacobs, B.; Dierkes, G.; Wick, A.; Buchmeier, G.; Koschorreck, J.; Ternes, T.A. Development of an analytical method to quantify pharmaceuticals in fish tissues by liquid Chromatography-tandem mass spectrometry detection and application to environmental samples. J. Chromatogr. A 2020, 1633, 461612. [Google Scholar] [CrossRef] [PubMed]
- Danesaki, M.E.; Bletsou, A.A.; Koulis, G.A.; Thomaidis, N.S. Qualitative multiresidue screening method for 143 veterinary drugs and pharmaceuticals in milk and fish tissue using liquid Chromatography quadrupole-time-of-flght mass spectrometry. J. Agric. Food Chem. 2015, 63, 4493–4508. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, P.; Dai, X.; Hou, X.; Zhao, L. Trace determination of antibacterial pharmaceuticals in fishes by microwave-assisted extraction and solid-phase purification combined with dispersive liquid-liquid microextraction followed by ultra-high performance liquid Chromatography-tandem mass spectrometry. J. Chromatogr. B 2016, 1011, 136–144. [Google Scholar] [CrossRef]
- Kalogeropoulou, A.G.; Kosma, C.I.; Albanis, T.A. Simultaneous determinaton of pharmaceuticals and metabolites in fish tissue by QuEChERs extracion and UHPLC Q/Orbitrap MS analysis. Anal. Bioanal. Chem. 2021, 413, 7129–7140. [Google Scholar] [CrossRef] [PubMed]
- Wagil, M.; Kumirska, J.; Stolte, S.; Puckowskim, A.; Maszkowska, J.; Stepnowski, P.; Bialk-Bielinska, A. Development of sensitive and reliable LC-MS/MS methods for the determination of three fluoroquinolones in water and fish tissue samples and preliminary environmental risk assessment of their presence in two rivers in nothern Poland. Sci. Total Environ. 2014, 493, 1006–1013. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Kang, H.-S.; Cho, B.-H.; Oh, J.-H. Comparison of sample preparation and determination of 60 veterinary drugs residues in flatfish usin liquid Chromatography-tandem mass spectrometry. Molecules 2020, 25, 1206. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Zhang, X.; Chen, T.; Dong, D.; Hua, X.; Guo, Z. Enhanced bioaccumulation of fluorinated antibiotics in crucian carp (Carcassius carcassius): Influence of fluorine substituent. Sci. Total Environ. 2020, 748, 141567. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Chapter 1: Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds, 1st ed.; Domínguez, H., González-Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar] [CrossRef]
- Blanco-Zubiaguirre, L.; Arrieta, N.; Iturregui, A.; Martinez-Arkarazo, M.; Olivares, M.; Castro, K.; Olazabal, M.A.; Madariaga, J.M. Focused ultrasound solid-liquid extraction for the determination of organic biomarkers in beachrocks. Ultrason. Sonochem. 2015, 27, 430–439. [Google Scholar] [CrossRef]
- Martínez-Moral, M.P.; Tena, M.T. Focused ultrasound solid-liquid extraction and selective pressurized liquid extraction to determine bisphenol A and alkylphenols in sewage sludge by gas Chromatography-mass spectrometry. J. Sep. Sci. 2011, 34, 2513–2522. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Raisglid, M.E.; Burke, M.F. Fundamentals of solid phase extraction and its application to environmental analyses. Stud. Surf. Sci. Catal. 1999, 120, 37–75. [Google Scholar] [CrossRef]
- Anand, S.; Srivastava, P. Optimization strategies for purification of Mycophenolic Acid produced by Penicillium brevicompactum. Appl. Biochem. Biotechnol. 2020, 191, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Destendau, E.; Michel, T.; Elfakir, C. Chapter 4. Microwave-assisted extraction. In Natural Product Extraction: Principles and Applications, 2nd ed.; Rostagno, M.A., Prado, J.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 113–498. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Gil-Solana, R.; Rodriguez-Mozaz, S.; Diaz-Cruz, M.S.; Sunyer-Caldú, A.; Luarte, T.; Höfer, J.; Galbán-Malagón, C.; Gago-Ferrero, P. A protocol for wide-scope non-target analysis of contaminants in small amounts of biota using bead beating tissuelyser extraction and LC-HRMS. MethodsX 2021, 8, 101193. [Google Scholar] [CrossRef]
- Oluseyi, T.; Olayinka, K.; Alo, B.; Smith, R.M. Comparison of extraction and clean-up techniques for the determination of polycyclic aromatic hydrocarbons in contaminated soil samples. Afr. J. Environ. Sci. Technol. 2011, 5, 482–493. [Google Scholar]
- Islas, G.; Ibarra, I.S.; Hernandez, P.; Miranda, J.M.; Cepeda, A. Dispersive Solid Phase Extraction for the Analysis of Veterinary drugs applied to food samples: A review. Int. J. Anal. Chem. 2017, 2017, 8215271. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, G.G.; Rummler, M.; Nausch, I. Gel permeation Chromatography-high performance liquid Chromatography combination as an automated clean-up technique for the multiresidue analysis of fats. J. Chromatogr. A 1996, 737, 9–14. [Google Scholar] [CrossRef]
- Clarke, W. Mass spectrometry in the clinical laboratory: Determining the need and avoiding pitfalls. In Mass Spectrometry in the Clinical Laboratory, 1st ed.; Nair, H., Clarke, W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- González-Peña, O.I.; López-Zavala, M.A.; Cabral-Ruelas, H. Pharmaceuticasls market, consumption, trends and disease incidence are not driving the pharmaceutical research on water and wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef]
- Świacka, K.; Maculewicz, J.; Kowalska, D.; Caban, M.; Smolarz, K.; Świeżak, J. Presence of pharmaceuticals and their metabolites in wild-living aquatic organisms—Current state of knowledge. J. Hazard. Mater. 2022, 424, 127350. [Google Scholar] [CrossRef]
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| No data | Diclofenac, ibuprofen, 1-OH-ibuprofen, piroxicam, diltiazem, propyphenazone, sulfamethoxazole, verapamil, norverapamil, hydrochlorothiazide, bezafibrate, gemfibrozil, pravastatin, carbamazepine, acridone, 10,11-epoxy-CBZ, 2-OH-CBZ, citalopram, fluoxetine, paroxetine, venlafaxine, azaperone, dexamethasone, metoprolol, propanolol | 0.2 (d.w) | Freeze-dried, stored at −20 °C | PLE (citric buffer (pH 4)/ACN) | No data | UHPLC-MS/MS | No data | No data | [54] |
| Periphyton (No data) | Ethinylestradiol, acetaminophen, diclofenac | 0.67 (d.w) | Air dry, powdered | USE (ACN/ MeOH 1% acetic acid) | No data | HPLC-MS/MS | 62 | No data | [55] |
| (b) | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| Sea lettuce (Ulva sp.), Red algae (Gelidium pristoides), Hanginng wrack (Bifurcaria brassicaeformis), Strap caulerpa (Caulerpa filiformis), Slippery orbits (Aeodes orbotisa) | Phenytoin, lamivudine, acetaminophen, caffeine, sulfamethoxazole, diclofenac, carbamazepine | 10 (d.w) | Rinsed, deshelled and dissected. Freeze-dried | Soxhlet (MeOH/ Acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 96.1–100.5 | 0.62–1.05 ng L−1 | [37] |
| Water starwort (Callitriche sp.), Pondweed (Potamogeton sp.) | Ethinylestradiol, acetaminophen, diclofenac | 0.5 (d.w) | Air dry, powdered | USE (ACN/ MeOH 1% acetic acid) | No data | HPLC-MS/MS | 81 | No data | [55] |
| (c) | |||||||||
| Phytoplankton | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| No data | Roxithromycin, erythromycin, ofloxacin, norfloxaxin, ciprofloxacin, tetracycline, sulfamethoxazole, sulfadiazine, sulfaquinoxaline, ibuprofen, diclofenac, naproxen, bezafibrate, propranolol, ketoconazole, carbamazepine, caffeine, sertraline, fluoxetine, norfluoxetine, citalopram, paroxetine, venlafaxine, duloxetine, bupropion, amitriptyline, clozapine, fluvoxamine, quetiapine, aripiprazole, chlorpromazine | 0.5 (d.w) | Freeze-dried, homogenized, stored at −80 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 66–128 | 0.07–1.67 | [27] |
| No data | Sulfadiazine, suldapyridine, sulfacetamide, sulfamethazine, sulfamethoxazole, trimethoprim, norfloxacin, ciprofloxacin, ofloxacin, lomefloxacin, oxytetracycline, tetracycline, erythromycin, roxithromycin | 0.1–0.5 (d.w) | Freeze-dried, stored at −18 °C | QuEChERs (ACN, acetic acid, 0.1 M EDTA, NaCl, Na2SO4) | d-SPE: QuEChERs (ACN, PSA, C18, Na2SO4) | LC-MS/MS | 80.3–104.9 | 0.04–0.1 | [28] |
| Cyanobacteria (Microcystis aeruginosa), Chlorophyceae (Pediastrum spp. Crucigenia spp. Scenedesmus spp.), Sensu lato (Coscinodiscus spp., Cyclotella spp.), Diatoms (Melosira spp., Aulacoseira spp.), Dinophycaeae (Peridiniopsis spp.), Cryptophyceae (Cryptomonas), Chrysophyceae (Dinobryon spp.), Euglenoidea (Euglena spp.) | Sulfadiazine, sulfapyridine, sulfacetamide, sulfamethoxazole, sulfamethazine, trimethoprim, lomefloxacin, ciprofloxacin, norfloxacin, oxytetracycline, tetracycline, roxithromycin, dehydroerythromycin | 0.5–1.0 (d.w) | Frozen at −80 °C, stored in a vacuum desiccator | PLE (MeOH/ acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | No data | No data | [29] |
| Cyanobacteria (No data) | Sulfachlorpyridazine, sulfadiazine, sulfadoxine, sulfamerazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfapyridine, sulfaquinoxaline, sulfisoxazole, sulfathiazole, trimethoprim, chlortetracycline, doxycycline, oxytetracycline, tetracycline, ciprofloxacin, difloxacin, danofloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, sarafloxacin, azithromycin, clarithromycin, leucomycin, oleandomycin, roxithromycin, tylosin, salinomycin, monensin, florfenicol, chloramphenicol | 1 (d.w) | Washed (water), freeze-dried, stored at −20 °C | USE (MeOH, sodium acetate buffer pH 4) | SPE (SAX, HLB cartridges) | RRLC-MS/MS | 54.2–117 | 0.02–9.38 | [38] |
| Green algae (Chlorophyta), Diatoms (Bacillariophyta), Blue green algae (Cyanophyta) | Roxithromycin, erythromycin, ofloxacin, norfloxacin, ciprofloxacin, tetracycline, chloramphenicol, sulfamerazine and sulfadiazine, sulfamethoxazole, ibuprofen, diclofenac, naproxen and indomethacin, clofibric acid, gemfibrozil and bezafibrate, 17β-estradiol, 17α-ethynylestradiol, propranolol, carbamazepine, ketoconazole, sertraline | 0.25 (d.w) | Freeze-dried, ground, stored at −20 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | LC-MS/MS | 68–116 | 0.01–1.12 | [56] |
| Zooplankton | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| Water flea (Daphnia magna) | Roxithromycin, propanolol | Each sample point consisted by 10 daphnia individuals | Homogenized | Sonication (ACN) | SPE (HLB cartridges) | UHPLC-MS/MS | 83–106 | 0.2 | [57] |
| Water flea (Daphnia magna) | Tetracycline | 30 organisms | Homogenized | MeOH, formic acid, EDTA | No data | LC-MS/MS | 84.23 | 0.31 µg L−1 | [58] |
| No data | Sulfachlorpyridazine, sulfadiazine, sulfadoxine, sulfamerazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfapyridine, sulfaquinoxaline, sulfisoxazole, sulfathiazole, trimethoprim, chlortetracycline, doxycycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, sarafloxacin, azithromycin, clarithromycin, leucomycin, oleandomycin, roxithromycin, tylosin, salinomycin, monensin, florfenicol, chloramphenicol | 1 (d.w) | Washed (water), freeze-dried, stored at −20 °C | USE (MeOH, sodium acetate buffer pH 4) | SPE (SAX, HLB cartridges) | RRLC-MS/MS | 54.2–117 | 0.02–9.38 | [38] |
| No data | Roxithromycin, erythromycin, ofloxacin, norfloxaxin, ciprofloxacin, tetracycline, sulfamethoxazole, sulfadiazine, sulfaquinoxaline, ibuprofen, diclofenac, naproxen, bezafibrate, propranolol, ketoconazole, carbamazepine, caffeine, fluoxetine, norfluoxetine, citalopram, paroxetine, sertraline, venlafaxine, duloxetine, bupropion, amitriptyline, fluvoxamine, clozapine, quetiapine, aripiprazole, chlorpromazine | 0.5 (d.w) | Freeze-dried, homogenized, stored at −80 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 66–128 | 0.07–1.67 | [27] |
| Copepoda, Cladocera, Rotifera (No data) | Sulfadiazine, suldapyridine, sulfacetamide, sulfamethazine, sulfamethoxazole, trimethoprim, norfloxacin, ciprofloxacin, ofloxacin, lomefloxacin, oxytetracycline, tetracycline, erythromycin, roxithromycin | 0.1–0.5 (d.w) | Freeze-dried, stored at −18 °C | QuEChERs (ACN, acetic acid, 0.1 M EDTA, NaCl, Na2SO4) | d-SPE: QuEChERs (ACN, PSA, C18, Na2SO4) | LC-MS/MS | 81.1–100.7 | 0.01–0.12 | [28] |
| Copepoda, Cladocera, Rotifera (No data) | Roxithromycin, erythromycin, ofloxacin, norfloxacin, ciprofloxacin, tetracycline, chloramphenicol, sulfamerazine, sulfadiazine, sulfamethoxazole, ibuprofen, ketoconazole, diclofenac, naproxen, indomethacin, clofibric acid, gemfibrozil, bezafibrate, 17β-estradiol, sertraline, propranolol, 17α-ethynylestradiol, carbamazepine | 0.25 g (d.w) | Freeze-dried, ground, stored at −20 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | LC-MS/MS | 68–116 | 0.01–1.12 | [39] |
| Copepoda, Cladocera, Rotifera (No data) | Sulfadiazine, sulfapyridine, sulfacetamide, sulfamethoxazole, sulfamethazine, trimethoprim, lomefloxacin, ciprofloxacin, norfloxacin, oxytetracycline, tetracycline, dehydroerythromycin, roxithromycin | 0.5–1.0 (d.w) | Frozen at −80 °C, stored in a vacuum desiccator | PLE (MeOH/ acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | No data | No data | [29] |
| No data | Nicotine, haloperidol, pyremethamine | 0.14–0.2 (d.w) | Freeze-dried | USE (ACN, MeOH, H2O), vortex, USE | SPE (No data) | LC-HRMS/MS | 70–130 | 0.05–5.70 * | [59] |
| Green algae (Chlorophyta), Diatoms (Bacillariophyta), Blue green algae (Cyanophyta) | Roxithromycin, erythromycin, ofloxacin, norfloxacin, ciprofloxacin, tetracycline, chloramphenicol, ibuprofen, diclofenac, naproxen, indomethacin, clofibric acid, sulfamerazine, sulfadiazine, sulfamethoxazole, gemfibrozil, bezafibrate, propranolol, carbamazepine, sertraline, ketoconazole, 17β-estradiol, 17α-ethynylestradiol | 0.25 (d.w) | Freeze-dried, ground, stored at −20 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | LC-MS/MS | 68–116 | 0.01–1.12 | [56] |
| (d) | |||||||||
| Bivalves | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| Oysters (C. Gigas), Clams (C. gallina), Mussels (M. galloprovincialis) | Ronidazole, metronidazole, dimetridazole, sulfamethoxazole, N-acetyl-sulfamethoxazole, azithromycin, erythromycin, venlafaxine, O-desmethylvenlafaxine, carbamazepine, 10,11-Epoxycarbamazepine, citalopram,2-Hydroxycarbamazepine, alprazolam, codeine, phenazone, propyphenazone, piroxicam, azaperone, azaperol, diltiazem, hydrochlorthiazide, tamsulosin | 0.5 (d.w) | Shells removed, pooled for homogenizing, freeze-dried, ground and kept at −20 °C | PLE (MeOH/ H2O) | SPE (HLB cartridges) | UHPLC-MS/MS | 40–115 | 0.01–0.80 | [35] |
| Zebra mussels (Dreissena polymorpha) | Diclofenac | 0.1 (d.w) | Freeze-dried and grinded | QuEChERs (H2O, ACN, heptane, acetate salt, DMSO) | d-SPE: QuEChERs (acetate salt) | UHPLC-MS/MS | 73–117 | 0.02–1 | [60] |
| Mussels (Perna viridis), Oysters (Cassostrea hongkongensis) | Acetaminophen, amitrimtyline, aripiprazole, benzoylecgonine, buprenorphine, caffeine, carbamazepine, diclofenac, diltiazem, diphenydramine, fluoxetine, methylphenidate, norfluoxetine, promethazine, sertraline, amlodipine, desmethylsertraline, trimethoprim, erythromycin, sucralose, sulfamethoxazole | 1 (w.w) | Separated from their shells, homogenized and frozen at −20 °C | Mechanical shaking (0.1 M acetic acid, MeOH) | No data | LC-MS/MS | 80–120 | 0.01–0.75 | [36] |
| Mussel (Mytilus galloprovincialis) | Cocaine, benzoylecgonine, cocathylene, amphetamine, metamphetamine, MDMA, morphine, methadone, 6-monoacetylmorphine, EDDP, ketamine, lysergic acid diethylamide, A tetrahydrocannabinol, 11-hydroxy-A THC, 11-nor-9-carboxy-A THC, AH-7921, mephedrone, MDPV, caffeine, ephedrine, alprazolam, a-hydroxyalprazolam, midazolam, lormetazepam, a-hydroxymidazolam, diazepam, oxazepam, temazepam, citalopram, fluoxetine, sertraline, venlafaxine, zolpidem, chlorpromazine, hydroxyzine | 10 (w.w) | Homogenized | Manual shaking (ACN, MgSO4, NaCl, NaCitrate, DCS) | d-SPE: QuEChERs (PSA, C18, MgSO4) | LC-MS/MS | 77–118 | <2 | [61] |
| Mussel (Mytilus spp.) | Diclofenac, mefenamic acid, trimethoprim, carbamazepine, gemfibrozil | 1 (d.w) | Freeze-dried, ground | PLE (ACN/H2O), Al2O3 | SPE (Strata-X SPE cartridges) | LC-MS/MS | 83–94 | 4–29 * | [62] |
| Mussel (Mytilus galloprovincialis) | Carbamazepine, oxcarbazepine + non target compounds (caffeine, metoprolol, cotinine, ketoprofen) | 2 (d.w) | Freeze-dried | QuEChERs (ACN, Na2SO4, NaCl, Na3Cit: 2H2O, Na2HCit: 3H2O) | d-SPE: QuEChERs (Na2SO4, PSA, C18, formic acid) | LC-HRMS | 67–110 | 0.1–0.3 | [63] |
| Mussel (Mytilus galloprovincialis) | Diclofenac, diazepam, sotalol, carbamazepine, citalopram, venlafaxine, azithromycin, sulfamethoxazole | All edible meat (no data) | Pooled, homogenized, freeze-dried, kept at −20 °C | PLE (MeOH/ H2O) | SPE (HLB cartridges) | UHPLC-MS/MS | No data | 0.01–0.65 | [64] |
| Mussel (Mytilus galloprovincialis), Razor shell (Ensis siliqua), Cockle (cerastoderma edule) | Atenolol, metoprolol, nadolol, propanolol, sotalol, salbutamol, diazepam, carbamazepine, azaperol, azaperone, 10,11-epoxycarbamazepine, 2-OH-carbamazepine, citalopram, venlafaxine, alprazolam, chlorothiazide, codeine, phenazone, piroxicam, propyphenazone, ronidazole, dimetridazole, metronidazole, azithromycin, erythromycin | 0.5 (d.w) | Freeze-dried | PLE (MeOH/ H2O) | SPE (HLB cartridges) | UHPLC-MS/MS | No data | 0.01–2 | [40] |
| Mussel (Mytilus galloprovincialis) | Trimethoprim, ciprofloxacin, norfloxacin, sulfadiazine, sulfamethoxazole, amitriptyline, clomipramine, imipramine, nortriptyline, eprosartan, irbesartan, losartan, diclofenac, telmisartan, valsartan, propanolol, acetaminophen, ketoprofen, bezafibrate, clofibric acid, carbamazepine, phenytoin | 0.5 (d.w) | Freeze-dried, ground, homogenized | FUSLE (MeOH/ H2O) | SPE (HLB cartridges) | LC-MS/MS | 71–126 | 4–48 | [65] |
| Carib pointed-venus (Anomalocardia brasiliana), Blue Mussel (Mytilus edulis) | Bezafibrate, carbamazepine, chloramphenicol, diclofenac, 4′-Hydroxydiclofenac, furosemide, gemfibrozil, ibuprofen, indapamide, ketoprofen, naproxen, simvastatin | 0.5 (d.w) | Dissection to obtain the morphometric measures, freeze-dried | QuEChERs (ACN, formic acid, NH4Cl) | QuEChERs (MgSO4, Z-Sep) | HPLC-MS/MS | 77–126 | 0.002–1.09 | [47] |
| Limpets (Cymbula granatina and cymbula oculis), Sea snail (Oxystele sinensis and oxytele tigrina), Mussel (mytilus galloprovincialis) | Phenytoin, lamivudine, acetaminophen, caffeine, sulfamethoxazole, diclofenac, carbamazepine | 10 (d.w) | Rinsed, deshelled and dissected, freeze-dried | Soxhlet (MeOH/ Acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 96.1–100.5 | 0.62–1.05 ng L−1 | [37] |
| Oyster (Ostrea gigas), Scallop (Mimachlamys nobilis), Mussel (Mytilus edulis) | Sulfadiazine, sulfamerazine, sulfamethazine, trimethoprim, sulfamethoxazole, sulfathiazole, sulfapyridine, ciprofloxacin, norfloxacin, ofloxacin, tetracycline, flumequine, oxytetracycline, gemfibrozil isochlortetracycline, penicillin G sodium, cefotaxime, spectinomycin, roxithromycin, erythromycin, clarithromycin, thiamphenicol, chloramphenicol, paracetamol, naproxen, ibuprofen, ketoprofen, diclofenac acid, carbamazepine, diltiazem, diphenhydramine | 0.2 (d.w) | Freeze-dried, ground into powder, mixed | Sonication (ACN/H2O) | SPE (HLB cartridges) | UHPLC-MS/MS | 43–127 | 0.01–1.9 | [39] |
| Zebra mussels (Dreissena polymorpha) | Nicotine, haloperidol, pyremethamine | 0.14–0.2 (d.w) | Gut clearance, frozen, shelled, cryo-storage | USE (ACN, MeOH, H2O), vortex, USE | SPE (No data) | LC-HRMS/MS | 70–130 | 0.05–5.70 * | [59] |
| Mussels (Mytilus galloprovincialis, Mytilus edulis) | Salicylic acid, clofibric acid, ketoprofen, naproxen, bezafibrate, diclofenac, ibuprofen | 1 (d.w) | Lyophilized, homogenized | PLE (Ottawa sand, ultrapure water) | SPE (Oasis MAX cartridges) | LC-MS/MS | 61–90 | 2–50 | [66] |
| Clams (Ruditapes decussatus, ruditapes philippinarum) | Acetaminophen, clofibric acid, atenolol, bezafibrate, carbamazepine, cortisone, diclofenac, erythromycin, fluoxetine, ibuprofen, naproxen, propanolol, sulfadiazine, sulfapyridine, caffeine, sulfamethoxazole, testosterone, gestodene, metoprolol, diethylsilbestrol, estradiol, estriol, estrone, 17α-ethinylestradiol | 1 (w.w) | Depurated, frozen at −20 °C, homogenized before analysis | Manual shaking (ACN) | QuEChERs (Hexane) | LC-MS/MS | 35.2–118 | 0.35–5.86 | [67] |
| Mussel (Anodonta), Snail (Bellamya sp.), Bivalve (Corbiculidae) | Roxithromycin, erythromycin, ofloxacin, norfloxacin, ciprofloxacin, tetracycline, chloramphenicol, sulfamerazine and sulfadiazine, sulfamethoxazole, ibuprofen, diclofenac, naproxen and indomethacin, clofibric acid, gemfibrozil and bezafibrate, 17β-estradiol and 17α-ethynylestradiol, propranolol, carbamazepine, ketoconazole, sertraline | 0.5 (d.w) | Freeze-dried, ground, stored at −20 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | LC-MS/MS | 68–116 | 0.01–1.12 | [56] |
| Asian clam (Corbicula fluminea) | Sulfachlorpyridazine, sulfadiazine, sulfadoxine, sulfamerazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfapyridine, sulfaquinoxaline, sulfisoxazole, sulfathiazole, trimethoprim, chlortetracycline, doxycycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, sarafloxacin, azithromycin, clarithromycin, leucomycin, oleandomycin, roxithromycin, tylosin, salinomycin, monensin, florfenicol, chloramphenicol | 1 (d.w) | Washed (water), homogenized, freeze-dried, stored at −20 °C | USE (AcONa buffer/ MeOH) | SPE (SAX/PSA-HLB tandem cartridges) | RRLC-MS/MS | 47.9–136.7 | 0.01–1.99 | [38] |
| Mussel (Anodonta woodiana) | Roxithromycin, erythromycin, ofloxacin, norfloxaxin, ciprofloxacin, tetracycline, sulfamethoxazole, sulfadiazine, sulfaquinoxaline, ibuprofen, diclofenac, naproxen, bezafibrate, propranolol, ketoconazole, carbamazepine, caffeine, fluoxetine, norfluoxetine, citalopram, paroxetine, sertraline, venlafaxine, duloxetine, bupropion, amitriptyline, fluvoxamine, trihexylphenidyl, clozapine, quetiapine, aripiprazole, chlorpromazine | 0.5 (d.w) | Freeze-dried, homogenized, stored at −80 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 66–128 | 0.07–1.67 | [27] |
| Clam (Ruditapes decussatus), Cockle (Cerastodema glaucum), Noble pen shell (Pinna nobilis), Sea snail (Murex trunculus) | Diclofenac, codeine, carbamazepine, citalopram, diazepam, lorazepam, atenolol, sotalol, propanolol, nadolol, carazolol, hydrochlorothiazide, clopidogrel, salbutamol, levamisole | 1 (d.w) | Freeze-dried, milled | PLE (50 °C) | GPC, HPLC-DAD | UHPLC-MS/MS | <20–151.9 | 0.0004–6 | [48] |
| Pen shell (Atrina pectinate Linnaeus), Asian hard clam (Meretrix lusoria), Magallana rivularis (Crassostrea rivvularis Gould). | Sulfadiazine, sulfadimethoxine, sulfadoxine, sulfamerazine, sulfameter, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfapyridine, sulfaquinoxaline, sulfathiazole, sulfisoxazole, trimethoprim, chlortetracycline, doxycycline, methacycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, clarythromycin, erythromycin-H2O, leucomycin, roxithromycin, oleandomycin | 2 muscle (w.w) | Frozen, muscle dissected | USE (MeOH/ H2O 0.1 mol L−1 acetic acid) | SPE Cartridges (SAX/PSA and HLB cartridges) | LC-MS/MS | 50–150 | 0.05–9.06 | [42] |
| Clam (Anadara ferruginea) | Atenolol, metoprolol, venlafaxine, chloramphenicol | 2 (d.w) | Washed (water), dissected, homogenized, freeze-dried, stored at −50 °C | USE (MeOH/ H2O) | SPE (MCX cartridges) | LC-MS/MS | 68–96 | 0.05–0.25 | [44] |
| Gastropods | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| Snail (Bellamya aeruginosa) | Sulfachlorpyridazine, sulfadiazine, sulfadoxine, sulfamerazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfapyridine, sulfamonomethoxine, sulfaquinoxaline, sulfisoxazole, sulfathiazole, trimethoprim, chlortetracycline, doxycycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, sarafloxacin, azithromycin, leucomycin, clarithromycin, oleandomycin, roxithromycin, tylosin, salinomycin, monensin, florfenicol, chloramphenicol | 1 soft tissues (d.w) | Washed (water), homogenized, freeze-dried, stored at −20 °C | USE (AcONa buffer/ MeOH) | SPE (SAX/PSA−HLB tandem cartridges) | RRLC-MS/MS | 47.9–136.7 | 0.01–1.99 | [38] |
| Snail (Bellamya aeruginosa) | Roxithromycin, erythromycin, ofloxacin, norfloxaxin, ciprofloxacin, tetracycline, sulfamethoxazole, sulfadiazine, sulfaquinoxaline, ibuprofen, diclofenac, naproxen, bezafibrate, propranolol, ketoconazole, carbamazepine, caffeine, fluoxetine, norfluoxetine, citalopram, paroxetine, sertraline, venlafaxine, duloxetine, bupropion, amitriptyline, clozapine, fluvoxamine, trihexylphenidyl, quetiapine, aripiprazole, chlorpromazine | 0.5 (d.w) | Freeze-dried, homogenized, stored at −80 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 66–128 | 0.07–1.67 | [27] |
| Conch (Bufonaria perelegans) | Sulfadiazine, sulfamerazine, sulfamethazine, trimethoprim, sulfamethoxazole, sulfathiazole, sulfapyridine, ciprofloxacin, norfloxacin, ofloxacin, flumequine, tetracycline, oxytetracycline, isochlortetracycline, penicillin G sodium, cefotaxime sodium, spectinomycin, roxithromycin, erythromycin- H2O, clarithromycin, thiamphenicol, chloramphenicol, paracetamol, naproxen, ibuprofen, ketoprofen, diclofenac acid, carbamazepine, diltiazem, diphenhydramine, gemfibrozil | 0.2 (d.w) | Freeze-dried, ground into powder. The whole body was mixed | USE (ACN/H2O) | SPE (PRiME HLB cartridges) | UHPLC-MS/MS | 43–127 | 0.01–1.9 | [39] |
| Sea snail (Murex trunculus) | Diclofenac, codeine, carbamazepine, citalopram, diazepam, lorazepam, atenolol, sotalol, propanolol, nadolol, carazolol, hydrochlorothiazide, clopidogrel, salbutamol, levamisole | 1 (d.w) | Freeze-dried and milled | PLE (MeOH) | GPC, HPLC-DAD | UHPLC-MS/MS | <20–151.9 | 0.0004–6 | [48] |
| Snail (B. tentaculata) | Ethinylestradiol, acetaminophen, diclofenac | 0.35 (d.w) | Freeze-dried, powered | USE (ACN/MeOH 1% acetic acid) | No data | HPLC-MS/MS | 67 | No data | [55] |
| River limpet (Ancylus fluviatilis) | Diclofenac, ibuprofen, 1-OH-ibuprofen, piroxicam, acridone, propyphenazone, sulfamethoxazole, diltiazem, verapamil, norverapamil, hydrochlorothiazide, bezafibrate, gemfibrozil, pravastatin, carbamazepine, 10,11-epoxy-CBZ, 2-OH-CBZ, citalopram, fluoxetine, paroxetine, venlafaxine, azaperone, dexamethasone, metoprolol, propanolol | 0.1 (d.w) | Homogenized with a mortar, kept at 20 °C | USE (MeOH) | Protein Precipitation and Phospholipid Removal, PlateOSTRO™ plate | UHPLC-MS/MS | No data | No data | [54] |
| Turritella bacillum Murex trapa, Bufonaria rana (No data) | Atenolol, metoprolol, venlafaxine, chloramphenicol | 2 (d.w) | Washed (water), dissected, homogenized, freeze-dried, stored at −50 °C | USE (MeOH/ H2O) | SPE (MCX cartridges) | LC-MS/MS | 68–96 | 0.05–0.25 | [44] |
| (e) | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| Octopus (Octopus vulgaris) | Atenolol, metoprolol, nadolol, propanolol, sotalol, salbutamol, diazepam, carbamazepine, 10,11-epoxycarbamazepine, 2-OH-carbamazepine, citalopram, venlafaxine, alprazolam, azaperol, azaperone, hydrochlorothiazide, codeine, phenazone, propyphenazone, piroxicam, ronidazole, dimetridazole, metronidazole, azithromycin, erythromycin | 1 (d.w) | Freeze-dried | PLE (MeOH/ H2O) | GPC | UHPLC-MS/MS | No data | 0.02–0.3 | [40] |
| Sepia (Sepia indica), Octopus (Octopus rugosus), Octopus minor (Polypus variabilis), Urotheutis (Loligo oshimai) | Sulfamethazine, sulfapyridine, sulfathiazole, sulfanlamide, sulfadiazine, sulfadimethoxine, sulfamonomethoxin, sulfamerazine, sulfamethoxazole, norfloxacin, enoxacin, ofloxacin, ciprofloxacin, enrofloxacin, dehydrated erythromycin, clarithromycin, azithromycin, roxithromycin, florfenicol, chloramphenicol, trimethoprim, lincomycin | 5 (d.w) | Washed (water), dissected, homogenized, stored at −20 °C | USE (ACN, citric acid) | SPE (SAX-HLB cartridges) | UHPLC-MS/MS | 47.7–172.7 | 0.04–0.24 | [13] |
| (f) | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-up | Recovery (%) | LOD (ng g−1) | ||||||
| Starfish (Marthasterias glacialis), Sea urchins (parechinus angulosus) | Phenytoin, lamivudine, acetaminophen, caffeine, sulfamethoxazole, diclofenac, carbamazepine | 10 (d.w) | Rinsed, deshelled, dissected, freeze-dried | Soxhlet (MeOH/ Acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 96.1–100.5 | 0.62–1.05 ng L−1 | [37] |
| (g) | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| Barnacle (Balanus perforatus) | Atenolol, ranitidine, acetaminophen, caffeine, trimethoprim, atrazine, amitriptyline, carbamazepine, chloropheniramine malate, ciprofloxacin, diclofenac, fluoxetine, ibuprofen, metronidazole, sulfamethoxazole, warfarin, cephalexin. | 1 (d.w) | Dried, ground, pooled, homogenized, freeze-dried | USE (0.1 M acetic acid, MeOH) | SPE (Oasis MCX) | HPLC-MS/MS | 30–103 | 0.1–13 ng mL−1 | [52] |
| Shrimp (Caridea), Brown crab (Cancer pagurus) | Diclofenac, diazepam, sotalol, carbamazepine, citalopram, venlafaxine, azithromycin, sulfamethoxazole | All edible meat (no data) | Pooled, homogenized by grinding, freeze-dried, −20 °C | PLE (MeOH/ H2O) | SPE (HLB cartridges) | UHPLC-MS/MS | No data | 0.01–0.65 | [64] |
| Crabs (Calappa philargius), pen shell Atrina pectinate Linnaeus), shrimps (Fenneropenaeus penicillatus) | Sulfadiazine, sulfadimethoxine, sulfadoxine, sulfamerazine, sulfameter, sulfamethazine, sulfamethoxazole, sulfapyridine, sulfamonomethoxine, sulfaquinoxaline, sulfathiazole, sulfisoxazole, trimethoprim, chlortetracycline, doxycycline, methacycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, clarythromycin, erythromycin-H2O, leucomycin, roxithromycin, oleandomycin | 2 muscle (w.w) | Frozen and muscle dissected | USE (MeOH/ H2O 0.1 mol L−1 acetic acid | SPE (SAX/PSA, HLB cartridges) | LC-MS/MS | 50–150 | 0.05–9.06 | [42] |
| No data | Ketoprofen, naproxen, flurbiprofen, diclofenac sodium, ibuprofen | 5 muscle tissue (w.w) | Chopped into mince | USE (ACN) | SPE (CF@UiO−66 NH2) | UHPLC-PDA | 95–116.99 | 0.12–3.50 ng mL−1 | [68] |
| Goose Barnacle (Pollicipes,) Carb (Necora púber) | Atenolol, metoprolol, nadolol, propanolol, sotalol, salbutamol, diazepam, carbamazepine, 10,11-epoxycarbamazepine, 2-OH-carbamazepine, citalopram, venlafaxine, alprazolam, azaperone, azaperol, hydrochlorothiazide, codeine, phenazone, propyphenazone, piroxicam, ronidazole, dimetridazole, metronidazole, azithromycin, erythromycin | 1 (d.w) | Freeze-dried | PLE (MeOH) | GPC | UHPLC-MS/MS | No data | 0.03–0.09 | [40] |
| Shrimp (Palaemon serratus) | Metronidazole, acetaminophen, amoxicillin, acetazolamide, sulfadiazine, atenolol, caffeine, ampicillin, trimethoprim, norfloxacin, ofloxacin, ciprofloxacin, tetracycline, phenazone, metoprolol, spiramycin, azithromycin, sulfamethoxazole, oxolinic acid, erythromycin A, piperacillin, tylosine, cyclophosphamide, carbamazepine, flumequine, oxazepam, clarithromycin, roxithromycin, lorazepam, losartan, nordiazepam, josamycin, ketoprofen, 19-norethind-rone, amiodarone, hydrochlorothiazide, acetylsalicylic acid, niflumic acid, diclofenac, ibuprofen, gemfibrozil | 0.2 (d.w) | Separated abdomen muscle, freeze-dried | Mechanical shaking (MeOH 1% acetic acid) | No data | UHPLC-MS/MS | 26–132 | 0.1–40.2 * | [69] |
| Freshwater amphipod (Gammarus pulex) | Propanolol hydrochloride, ketoprofen, diclofenac salt, bezafibrate, warfarin, flurbiprofen, indomethacin, ibuprofen sodium salt, meclofenamic acid sodium salt, gemfibrozil, atenolol, sulfamethoxazole, sulfamethazine, furosemide, carbamazepone, nimesulide, (+-metoprolol) (+) tartrate, cimetidine, ranitidine, antipyrin, temazepam, diazepam, fluoxetine, nifedipine, mefenamic acid, trimethoprim, caffeine, naproxen | 0.1 (d.w) | Freeze-dried, pulverized | PuLE (ACN) | SPE (HLB cartridges) | LC-MS/MS | 41–89 | 1–13 | [70] |
| Green crab (Carcinus maenas) | Alprazolan, amoxicillin, atenolol, atorvastatin, azithromycin, bisoprolol, benzylpenicillin, bezafibrate, carbamazepine, carvedilol, cinoxacin, ciprofloxacin, ceftiofur, cephalexin, chlortetracycline, danofloxacin, diclofenac, doxicycline, enoxacin, enrofloxacin, epi-chlortetracycline, epi-tetracycline, erythromycin, epotetracycline, fenofibrate, flumequine, fluoxetine, furosemide, gabapentin, gemfibrozil, ibersartan, ibuprofen, indapamide, lorazepam, losartan, marbofloxacin, nalidixic acid, norfloxacin, nimesulide, ofloxacin, oxolinic acid, oxytetracycline, paracetamol, propanolol, sertraline, simvastatin, spiramycin, sulfachloropyridazine, sulfadiazine, sulfadimethoxine, sulfamethazine, sulfamethizole, sulfanilamide, sulfapyridine, sulfisomidine, sulfadoxine, sulfamethoxazole, sulfaquinoxaline, sulfathiazole, sulfisoxazole, tetracycline, tilmicosin, trimethoprim, venlafaxine, topiramate | 2 (w.w) | Homogenized | Mechanical shaking (ACN, EDTA) | No data | UHPLC-MS/MS | 79.2–109.5 | 0.59–4.11 | [41] |
| Shrimps: White vannamei prawn, Indian prawn, kiddi shrimp (No data) | Amoxicillin, azithromycin, caffeine, carbamazepine, ciprofloxacin, clarithromycin, diclofenac, erythromycin, furosemide, ketoprofen, ibuprofen, naproxen, sulfamethoxazole, tetracycline | 2 (w.w) | Abdomen muscle separated, cut into small parts, frozen at −20 °C | Mechanical shaking (ACN, 0.1 M EDTA, hexane) | No data | UHPLC-MS/MS | 81.2–99.4 | 0.017–1.371 | [46] |
| Shrimp (Harpiosquilla harpax), Crab (Charybdis japonica), Spear shrimp (Parapenaeopsis hardwickii), Giant tiger prawn (Penaeus monodon), Green mud crab (Scylla paramamosain), Prawn (Trachypenaeus sedili) | Sulfamethazine, sulfapyridine, sulfathiazole, sulfanilamide, sulfadiazine, sulfadimethoxine, sulfamonomethoxin, sulfamerazine, sulfamethoxazole, norfloxacin, enoxacin, ofloxacin, ciprofloxacin, enrofloxacin, dehydrated, erythromycin, clarithromycin, azithromycin, roxithromycin, florfenicol, chloramphenicol, trimethoprim, lincomycin | 5 (d.w) | Washed (water), dissected, homogenized, stored at −20 °C | USE (ACN, citric acid) | SPE (SAX-HLB cartridges) | UHPLC-MS/MS | 47.67–172.67 | 0.04–0.24 | [13] |
| Mud prawun (Meapenaeus ensis), Smoothshell shrimp (Parapenaeopsis tenella), Three-spot swimming crab (Portunus sanguinolentus), Jinga shrimp (Metapenaeus affinis), Robber harpiosquillid mantis shrimp (Harpiosquilla harpax) | Atenolol, metoprolol, venlafaxine, chloramphenicol | 2 (d.w) | Washed (water), dissected, homogenized, freeze-dried, stored at −50 °C | USE (MeOH/ H2O) | SPE (MCX cartridges) | LC-MS/MS | 68–96 | 0.05–0.25 | [44] |
| White shrimp (Exopalaemon modestus) Taihu shrimp (Macrobranchium nipponense) | Roxithromycin, erythromycin, ofloxacin, norfloxacin, ciprofloxacin, tetracycline, chloramphenicol, sulfamerazine, sulfadiazine, sulfamethoxazole, ibuprofen, diclofenac, naproxen and indomethacin, clofibric acid, gemfibrozil, bezafibrate, 17β-estradiol,17α-ethynylestradiol, propranolol, carbamazepine, ketoconazole, sertraline | 0.5 (d.w) | Separated muscle of shrimp. Freeze-dried, ground and stored at −20 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | LC-MS/MS | 68–116 | 0.01–1.12 | [56] |
| Water flea (Gammarus pulex) | Ethinylestradiol, acetaminophen, diclofenac | 0.34 (d.w) | Freeze-dry, powered | USE (ACN/ MeOH 1% acetic acid) | No data | HPLC-MS/MS | 67 | No data | [55] |
| Shrimps (Paranthura sp., Macrobrachium nipponense), Crab (Eriocheir sinensis) | Sulfachlorpyridazine, sulfadiazine, sulfadoxine, sulfamerazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfapyridine, sulfaquinoxaline, sulfisoxazole, sulfathiazole, trimethoprim, chlortetracycline, doxycycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, sarafloxacin, azithromycin, clarithromycin, leucomycin, oleandomycin, roxithromycin, tylosin, salinomycin, monensin, florfenicol, chloramphenicol | 1 (d.w) | Washed (water), homogenized, freeze-dried, stored at −20 °C | USE (sodium acetate buffer/ MeOH) | SPE (SAX/PSA−HLB tandem cartridges) | RRLC-MS/MS | 47.9–136.7 | 0.01–1.99 | [38] |
| Shrimp (Macrobranchium nipponense) | Roxithromycin, erythromycin, ofloxacin, norfloxaxin, ciprofloxacin, tetracycline, sulfadiazine, sulfamethoxazole, sulfaquinoxaline, naproxen, ibuprofen, diclofenac, bezafibrate, propranolol, ketoconazole, carbamazepine, caffeine, fluoxetine, norfluoxetine, citalopram, paroxetine, sertraline, venlafaxine, duloxetine, bupropion, amitriptyline, fluvoxamine, trihexylphenidyl, clozapine, quetiapine, aripiprazole, chlorpromazine | 0.5 (d.w) | Freeze-dried, homogenized, stored at −80 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 66–128 | 0.07–1.67 | [27] |
| Shrimps (No data) | Naproxen, methyltestosterone, 17α-hydroxyprogesterone caproate, progesterone | 2 (w.w) | Ground, homogenized | Manual shaking (ACN 0.1% acetic acid) | No data | LC-MS/MS | 68–117 | 1–2 | [43] |
| Crabs: Spectacled box crab (Calappa philargius). Shrimps: Redtail shrimpredtail prawn (Fenneropenaeus penicillatus) | Sulfadiazine, sulfadimethoxine, sulfadoxine, sulfamerazine, sulfameter, sulfamethazine, sulfamethoxazole, sulfapyridine, sulfamonomethoxine, sulfaquinoxaline, sulfathiazole, sulfisoxazole, trimethoprim, chlortetracycline, doxycycline, methacycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, clarythromycin, erythromycin- H2O, leucomycin, roxithromycin, oleandomycin | 2 (w.w) | Frozen and muscle dissected | USE (MeOH/ H2O, 0.1 M acetic acid) | SPE Cartridges (SAX/PSA, and HLB cartridges) | LC-MS/MS | 50–150 | 0.05–9.06 | [42] |
| (h) | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| Ragworm (Hedyste diversicolor) | Alprazolan, amoxicillin, atenolol, atorvastatin, azithromycin, bisoprolol, benzylpenicillin, bezafibrate, carbamazepine, carvedilol, cinoxacin, ciprofloxacin, ceftiofur, cephalexin, chlortetracycline, danofloxacin, diclofenac, doxicycline, enoxacin, enrofloxacin, fluoxetine, epi-chlortetracycline, epi-tetracycline, erythromycin, epotetracycline, fenofibrate, flumequine, furosemide, gabapentin, gemfibrozil, ibersartan, ibuprofen, indapamide, lorazepam, losartan, marbofloxacin, nalidixic acid, norfloxacin, nimesulide, ofloxacin, oxolinic acid, oxytetracycline, paracetamol, propanolol, sertraline, simvastatin, spiramycin, sulfachloropyridazine, sulfadiazine, sulfadimethoxine, sulfamethazine, sulfamethizole, sulfanilamide, sulfapyridine, sulfisomidine, sulfadoxine, sulfamethoxazole, sulfaquinoxaline, sulfathiazole, sulfisoxazole, tetraccline, tilmicosin, trimethoprim, tylosin venlafaxine, topiramate | 2.0 (w.w) | Homogenized | Mechanical shaking (ACN, EDTA) | No data | UHPLC-MS/MS | 79.2–109.5 | 0.59–4.11 | [41] |
| Polychaetas (Perinereis aibuhitensis, Notomastus latericeus, Sabella pavonina). Insecta (Chironomidae sp.). Worm (Limnodrilus hoffmeisteri) | Sulfachlorpyridazine, sulfadiazine, sulfadoxine, sulfamerazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfapyridine, sulfaquinoxaline, sulfisoxazole, sulfathiazole, trimethoprim, chlortetracycline, doxycycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, sarafloxacin, azithromycin, clarithromycin, leucomycin, oleandomycin, roxithromycin, tylosin, salinomycin, monensin, florfenicol, chloramphenicol | 1.0 (d.w) | Washed (water), homogenized, freeze-dried, stored at −20 °C | USE (sodium acetate buffer/ MeOH) | SPE (SAX/PSA−HLB tandem cartridges) | RRLC-MS/MS | 47.9–136.7 | 0.01–1.99 | [38] |
| Porifera: Sponge (Cf. Hyrtios) | Caffeine, fluoxetine, norfluoxetine | 0.25 (d.w) | Squeezed, wrapped in aluminium foil, and freeze-dried | USE (acidified methanol, acetonitrile/ methanol, acetonitrile) | SPE (HLB) | UHPLC-MS | 80 | 0.01–10 | [71] |
| Insecta (Hydropsyche sp., Phagocata vitta) | Diclofenac, ibuprofen, 1-OH-ibuprofen, piroxicam, propyphenazone, sulfamethoxazole, diltiazem, verapamil, norverapamil, hydrochlorothiazide, bezafibrate, gemfibrozil, pravastatin, carbamazepine, acridone, 10,11-epoxy-CBZ, 2-OH-CBZ, citalopram, fluoxetine, paroxetine, venlafaxine, dexamethasone, azaperone, metoprolol, propanolol | 0.1 (d.w) | Homogenized with a mortar, kept at 20 °C | USE (MeOH) | Protein precipitation and phospholipid removal, PlateOSTRO™ plate | UHPLC-MS/MS | No data | No data | [54] |
| (i) | |||||||||
| Specie | Pharmaceuticals | Type and Amount of Sample (g) | Pre-Treatment | Treatment | Analysis | Analytical Features | Ref. | ||
| Extraction Technique | Clean-Up | Recovery (%) | LOD (ng g−1) | ||||||
| Surgeonfish (Acanthurus xanthoperus), Smallmouth catfish (Ariopsis felis), Bull fish (Caranx caninus), Milkfish (Chanos chanos), Yellowfin mojarra (Gerres cinereus), Elongated grunt (Haemulopsis elongatus), Silk snapper (Lutjanus peru), White mullet (mugil curema), California halibut (Paralichthys californicus), Bigscale goatfish (Pseudupeneus grandisquamis), Peruvian moonfish (Selene peruvian), Common snook (Centropomus robalito), Reef Lizardfish (Synodus lacertinus), Striped bonito (Sarda orientalis) | Diclofenac, ibuprofen, ketorolac, naproxen | 25–30 (w.w) | Minced, homogenized | USE (No data) | No data | UHPLC-MS/MS | 92–95 | 0.97–23.1 | [72] |
| Black Crappie (Pomoxis nigromaculatus), Black Redhorse (Moxostoma duquesni), Bluegill (Lepomis macrochirus), Common Carp (Cyprinus carpio), Flathead Catfish (Pylodictis olivaris), Freshwater Drum (Aplodinotus grunniens), Gizzard Shad (Dorosoma cepedianum), Golden Redhorse (Moxostoma erythrurum), Hybrid White x Striped Bass (Morone chrysops x Morone saxatilis), Largemouth Bass (Micropterus salmoides), Mooneye (Hiodontidae), Nothern Hogsucker (Hypentelium nigricans), Quillback Carpsucker (Carpiodes cyprinus), River Carpsucker (Carpiodes carpio), Sauger (Sander canadensis), Saugeye (Sander canadensis x Sander vitreus), Silver Redhorse (Moxostoma anisurum), Smallmouth Bass (Micropetrus dolomieu), Smallmouth Buffalo (Ictiobus bubalus), Smallmouth Redhorse (Moxostoma breviceps), Spotted Sucker (Minytrema melanops), White Bass (Morone chrysops), White Crappie (Pomoxis annularis) | Tylosin, lincomycin, furazolidone, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfanilamide, cotinine, carbamazepine, acetaminophen, thiamphenicol, florfenicol, chloramphenicol, caffeine, trimethoprim, azithromycin, triclosan erythrohydrobupropion | 0.5 (w.w) | Homogenized | QuEChERs (ACN/H20 1% acetic acid, MgSO4, AcONa) | d-SPE: QuEChERs (MgSO4, PSA, C18) | UHPLC-MS/MS | 67–148 | 0.2–2.6 | [73] |
| Perch (Perca fluviatilis), Flounder (Platichthys flesus), Turbot (Scophthalmus maximus), Plaice (Pleuronectes platessa), Cod (Gadus morhua callarias), Bream (Abramis brama), Crucian (Carassius carassius) | Bisoprolol, carbamazepine, clarithromycin, erythromycin, fluoxetine, metronidazole, ofloxacin, promazine, sulfadimethoxine, thiabenzadole, tianeptine, acebutolol, 1-Naphthoxyacetic acid, amitriptyline, amlodipine, atenolol, azithromycin, bosentan, cefotaxime, chlorpromazine, chlortetracycline, clindamycin, clomipramine, codeine, desipramine, dextromethorphan, diclofenac, diltiazem, doxepin, drotaverine, duloxetine, enalapril, escitalopram, fenofibrate, fleroxacin, fluconazole, fluvoxamine, guaifenesin, imipramine, labetalol, losartan, levofloxacin, lincomycin, lomefloxacin, lovastatin, maprotiline, mebendazole, metformin, methoxyverapamil, metoprolol, mianserin, mirtazapine, moclobemide, morantel, mycophenolic acid, nalidixic acid, nifedipine, norfloxacin, nortriptyline, omeprazole, opipramol, oxymetazoline, oxytetracycline, pantoprazole, paroxetine, pefloxacin, piperacillin, propafenone, propanolol, protriptyline, pseudophedrine, quinapril, ramipril, ranitidine, roxithromycin, salbutamol, sotalol, sertraline, sulfadiazine, sulfamethazine, sulfamethoxazole, sulfanilamide, sulfathiazole, telmisartan, tetracycline, tiamulin, tianeptine, tolperisone, trazodone, trimethoprim, tylosin, valsartan, verapamil, xylometazoline | 0.05 (w.w) | Homogenized | Mechanical shaking (ACN 0.1% formic acid), frozen, centrifuged. Added ammonium acetate and stirred | d-SPE: C18 sorbent | LC-QTRAP | No data | 0.01–0.88 | [74] |
| Rainbow trout (Oncorhynchus mykis) | Citalopram | Brain tissue (no data) | Brain separated | TissueLyser II at 30 Hz for 10 min. (ACN:i-propanol 3:1 with 0.1% formic acid) | No data | LDTD- HRPS | 97–108 | 0.39 | [75] |
| Bream (no data) | Bezafibrate, carbamazepine, 2-hydroxicarbamazepine, 10,11-dihydroxy-10,11-dihydrocarbamazepine, cetirizine, citalopram, desmehylcitalopram, clopidogrel, diclofenac, diphenhydraine, fexodenadine, fluconazole, norfluoxetine, furosemide, hydrochlorothiazide, metoprolol, oxazepam, primidone, sertraline, sulfamethoxazole, trimethoprim, N-acetylsulfamethoxazole, telmisartan, tramadol, valsartan, venlafaxine, O-desmethylvenlafaxine. | 0.05 (fish liver), 0.1 for (fish fillet) (d.w) | Homogenized, lyophilized | Cell disruption (4 m/s for 40 s) | d-SPE: Silica gel | LC-MS/MS | 70–130 | 0.05–5.5 ng mL−1 * | [76] |
| Gilthead sea bream (Sparus aurata), Sea bass (Dicentrarchus labrax) | Ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, flumequine, marbofloxacin, norfloxacin, ofloxacin, oxolinic acid, sarafloxacin, chlortetracycline, doxycycline, minocycline, oxytetracycline, tetracycline, cefaclor, cefadroxil, cefalexin, cefapirin, ceftiofur, cefazolinamoxicillin, ampicillin, cloxacillin, dicloxacillin, oxacillin, penicillin G, penicillin V, azithromycin, clarithromycin, erythromycin- H2O, tiamulin, tilmicosin, dapsone, sulfachlorpyridazine, sulfaclozine, sulfadiazine, sulfadoxine, sulfadimethoxine, sulfadimidine, sulfaguanidine, sulfameter, sulfamerazine, sulfamethizole, sulfamethoxazole, sulfamethoxypuridazine, sulfaonomethoxine, sulfamoxole, sulfapyridine, sulfaquinoxaline, sulfathiazole, sulfisoxazole, carbadox, olaquindox, florfenicol, thiampenicol, baquiloprin, trimthoprim, lincomycin, novobiocin, rifaximin, albendazole, albendazole oxide, albendazole sulfone, febantel, dimetridazole, fenbendazole, flubendazole, morantel, levamisole, mebendazole, metronidazole, oxfendazole, piperazine, ronidazole, ternidazole, thiabenzadole, triclabendazole, arprinocid, clopidol, decoquinate, diaveridine, ethopabate, halofuginone, imidocarb, lasalocid, monensin, narasin, nigericin, robenidine, salinomycin, 5-hydroxyflunixin, aceclofenac, diclofenac, flunixin, ketoprofen, mefenamic acid, naproxen, meloxicam, niflumic acid, phenylbuntazone, tolfenamic acid, vedaprofen, cimaterol, clenbuterol, clenpenterol, mabuterol, ractopamine, salbutamol, terbutaline, betamethasone, cortisol, cortison, dexamethazone, methyl-thiouracil, methylprednisolone, progesteron, phenyl- thiouracil, propyl-thiouracil, ambroxol, atenolol, atorvastatin, caffeine, carbamazepine, cimetidine, gemfibrozil, haloperidol, indapamide, metformin, metoprolol, paracetamol, propranolol, ranitidine, simvastatin, theophyline, tramadol, triamterene, valsartan, bromhexine, chlorpromazine, colchicine, melamine, coumaphos | 1.0 (w.w) | Homogenized, stored at −20 °C | Ultrasonic bath (H2O containing 0.1% formic acid, 0.1% EDTA (w/v), MeOH, ACN). Precipitation of lipids and proteins | Hexane and further low temperature | UHPLC-MS/MS | No data | 20–200 | [77] |
| No data | Chloramphenicol, thiamphenicol, tinidazole, metronidazole, malachite green, crystal violet | 2.0 (d.w) | Cleaned, scaled and muscle tissue was taken. Homogenised, blotted dried, freeze at −20 °C | MAE (ACN) | SPE (Activated neutral alumina column), USE (ACN) and DLLME (H2O, CH2Cl2, ACN) | UHPLC-MS/MS | >87 | 4.54–101.3 pg kg−1 | [78] |
| Sea bream (Sparus aurata) | Erythromycin, N-acetyl sulfamethoxazole, sulfadiazine, sulfamethazine, sulfamethizole, sulfamethoxazole, sulfamethoxypyridazine, sulfapyridine, sulfaquinoxaline, sulfathiazole, trimethoprim, caffeine, paracetamol, phenazone, carbamazepine, carbamazepine-10,11- epoxide, citalopram, fluoxetine, N desmethyl sertraline, norfluoxetine, O desmethyl venlafaxine, sertraline, venlafaxine | 1.0 (w.w) | Filleted | QuEChERs (ACN, MgSO4, NaCl) | d-SPE: Z-Sep+ | UHPLC-MS/-MS | 62–107 | 0.5–19 * | [79] |
| Sonek (Thyrsites atun), Bonito (Sarda orientalis), Panga (Pachymetopon blochii), Hottentot (Pterogymnus laniarius) | Acetaminophen, caffeine, diclofenac, lamivudine, sulfamethoxazole, carbamazepine | 10 (d.w) | Dissection of different parts (fillet, gills, liver and intestine), freeze-dried and ground | Soxhlet (MeOH/ Acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 69.2–107.5 | 0.010–0.036 | [49] |
| Sabalo (Prochilodus lineatus), Boga (Megaleporinus obtusidens), Dorado (Salminus brasiliensis) | Atenolol, carazolol, metoprolol, nadolol, propanolol, sotalol, diazepam, lorazepam, carbamazepine, 10,11-epoxycarbamazepine, 2-hydroxycarbamazepine, venlafaxine, clopidogrel, salbutamol, codeine, diclofenac, hydrochlorothiazide | 1.0 (d.w) | Pooled, homogenized | PLE (MeOH) | GPC | UHPLC-MS/MS | 26–115 | 0.028–2.7 | [53] |
| Carps (Carassius), Japanese medakas (Oryzias latipes), Mosquitofish (Gambussia affinis) | Diclofenac, indomethacin, mefenamic acid, ibuprofen, bezafibrate, fenofibric acid, clofibric acid, gemfibrozil, diltiazem, amlodipine, propanolol, carvedilol, losartan, telmisartan, irbesartan, valsartan, rebamipide, cetirizine, diphenhydramine, chlorpheniramine, fexofenadine, epinastine, warfarin, tramadol, O-desmethyl tramadol, N-desmethyl tramadol, sertraline, norsertraline, fluoxetine, norfluoxetine, paroxetine, citalopram, venlafaxine, haloperidol, risperidone, quetiapine, chlorpromazine, aripiprazole, zotepine, phentyon, carbamazepine, clonazepam, diazepam, zolpidem, nitrazepam, oxazepam, flunitrazepam, lorazepam, alprazolam, etizolam, sulfapyridine, sulfamerazine, sulfisozole, sulfamethizole, sulfamethazine, sulfamonomethoxine, sulfamethoxazole, sulfadimethoxine, trimethoprim, lincomycin, fluconazole erythromycin, clarithromycin, rixothromycin, florfenicol | 200 µL plasma (Carassius carassius) and 0.1 g whole-body tissue (rest) | Homogenized | USE (MeOH/ ACN, and acetic acid- ammonium acetate buffer) | SPE (HybridSPE®-Phospholipid cartridge) | LC-MS/MS | 70–120 | 0.0077–0.93 ng mL−1 | [50] |
| European eel (Anguilla anguilla) | Acetaminophen, atenolol, caffeine, diclofenac, etoricoxib, ibuprofen, naproxen, salicylic acid, triclosan, vildagliptin) | 1.0 pool (w.w) | Pooled, chopped, and homogenized | d-SPE: QuEChERs (ACN, MgSO4, NaCl, DCS and TCD) | d-SPE: EMR-Lipid (MgSO4 and NaCl) | UHPLC-MS/MS | 70–120 | 1.4–12 | [51] |
| Rainbow trout (Oncorhynchus mykiss) | Enrofloxacin, norfloxacin, ciprofloxacin | 5.0 muscle (w.w) | Boned | SPE (0.1 M K2HPO4 (pH = 6.5)) | SPE (Strata XC cartridges) | LC-MS/MS | 91.1–108.9 | 3.3–3.6 | [80] |
| Nile Tilapia (Oreochromis niloticus), Milk fish (chanos chanos), Common silver biddy (gerres oyena), Golden snapper (lutjanus johni), Emperor fish (ethrinus nebulosus) | Atenolol, ranitidine, acetaminophen, caffeine, trimethoprim, atrazine, amitriptyline, carbamazepine, chloropheniramine malate, ciprofloxacin, diclofenac, fluoxetine, ibuprofen, metronidazole, sulfamethoxazole, warfarin, cephalexin. | 1.0 (d.w) | Filleted and cut into small sections and lyophilized. Pooled and homogenized | USE (0.1 M aqueous acetic acid/MeOH and NH4OH 0.1 M) | SPE (Oasis MCX cartridges) | HPLC-MS/MS | 30–103 | 0.1−13 ng mL−1 | [52] |
| Mackerel (Scomber scombrus), tuna (Thunnus thynnus), cod (Gadus morhua), perch (Perca fluviatilis), Pangas catfish (Pangasius pangasius), sole (Solea solea), seabream (Sparus aurata), plaice (Pleuronectes platessa), salmon (Salmonidae) | Diclofenac, diazepam, sotalol, carbamazepine, citalopram, venlafaxine, azithromycin, sulfamethoxazole | Fillet (no data) | Pooled, homogenized by grinding, freeze-dried, kept at −20 °C | PLE (MeOH) | GPC | UHPLC-MS/MS | No data | 0.01–0.65 | [64] |
| Rusell’s snapper (Lutjanus ruselli), Saddle tailed sea perch (Lutjanus erythopterus), Silverfish (Trachinotus ovatus) | Sulfadiazine, sulfadimethoxine, sulfadoxine, sulfamerazine, sulfameter, sulfamethazine, sulfamethoxazole, sulfapyridine, sulfamonomethoxine, sulfaquinoxaline, sulfathiazole, sulfisoxazole, trimethoprim, chlortetracycline, doxycycline, methacycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, difloxacin, enrofloxacin, fleroxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, clarythromycin, erythromycin, leucomycin, roxithromycin, oleandomycin | 2 (w.w) | Frozen, muscle dissected | USE: MeOH/H2O, 0.1 M acetic acid | SPE (SAX/PSA, HLB cartridges) | LC-MS/MS | 50–150 | 0.05–9.06 | [42] |
| No data | Ketoprofen, naproxen, flurbiprofen, diclofenac, ibuprofen | 5 (w.w) | Chopped into mince | USE (ACN) | SPE CF@UiO-66 NH2 | UHPLC- PDA | 95–116.99 | 0.12–3.50 ng mL−1 | [68] |
| European pilchardus (Sardina pilchardus) | Atenolol, metoprolol, nadolol, propanolol, sotalol, salbutamol, diazepam, carbamazepine, 10,11-epoxycarbamazepine, 2-OH-carbamazepine, citalopram, venlafaxine, alprazolam, azaperone, azaperol, hydrochlorothiazide, codeine, phenazone, propyphenazone, piroxicam, ronidazole, dimetridazole, metronidazole, azithromycin, erythromycin | 1 (d.w) | Freeze-dried | PLE (MeOH, 4 extraction cycles) | GPC | UHPLC-MS/MS | No data | 0.1–0.6 | [40] |
| Hake (Merluccius merluccius), Red mullet (Mullus surmuletus), Sole (Solea solea) | Metronidazole, acetaminophen, amoxicillin, acetazolamide, sulfadiazine, atenolol, caffeine, ampicillin, trimethoprim, norfloxacin, ofloxacin, ciprofloxacin, tetracycline, phenazone, metoprolol, spiramycin, azithromycin, sulfamethoxazole, oxolinic acid, erythromycin A, piperacillin, tylosine, cyclophosphamide, carbamazepine, flumequine, oxazepam, clarithromycin, roxithromycin, lorazepam, losartan, nordiazepam, josamycin, ketoprofen, 19-norethind-rone, amiodarone, hydrochlorothiazide, acetylsalicylic acid, niflumic acid, diclofenac, ibuprofen, gemfibrozil | 0.2 (d.w) | Separated white dorsal muscle, freeze-dried. | Mechanical shaking (MeOH, 1% acetic acid) | No data | UHPLC-MS/MS | 28–188 | 0.1–40.2 * | [69] |
| Sea bream (Sparus aurata) | Trimethoprim, ciprofloxacin, norfloxacin, sulfadiazine, sulfamethoxazole, amitriptyline, clomipramine, imipramine, nortriptyline, eprosartan, irbesartan, losartan, telmisartan, valsartan, propanolol, acetaminophen, diclofenac, ketoprofen, bezafibrate, clofibric acid, carbamazepine, phenytoin | 0.5 fish muscle and liver; 0.1 fish gills and brain (d.w) | Freeze-dried, ground, homogenized | FUSLE (MeOH/ H2O) | SPE (HLB cartridges) | LC-MS/MS | 71–126 | 4–48 | [79] |
| Mullet (Mugil spp., Mugil curema), Snook (Centropomus spp.) | Bezafibrate, carbamazepine, chloramphenicol, diclofenac, 4′-Hydroxydiclofenac, furosemide, gemfibrozil, ibuprofen, indapamide, ketoprofen, naproxen, simvastatin | 0.5 (d.w) | Dissection to obtain the morphometric measures, freeze-dried | QuEChERs (ACN, formic acid, NH4Cl) | d-SPE: QuEChERs (MgSO4, Z-Sep) | HPLC-MS/MS | 70–133 | 0.004–2.16 | [43] |
| Golden grey mullet (Liza aurata), Black goby (Gobius niger) | Diclofenac, codeine, carbamazepine, citalopram, diazepam, lorazepam, atenolol, sotalol, propanolol, nadolol, carazolol, hydrochlorothiazide, clopidogrel, salbutamol, levamisole | 1.0 golden grey mullet muscle and black goby; 0.5 liver golden grey mullet (d.w) | Freeze-dried, milled | PLE (MeOH) | GPC | UHPLC-MS/MS | <20–200 | 0.02–6.6 | [48] |
| Yellow grouper (Epinephelus awoara), Orbfish (Ephippus orbis), Topmouth culter (Culter alburnus) | Sulfadiazine, sulfamerazine, sulfamethazine, trimethoprim, sulfamethoxazole, sulfathiazole, sulfapyridine, ciprofloxacin, norfloxacin, ofloxacin, flumequine, tetracycline, penicillin G sodium, oxytetracycline, isochlortetracycline, cefotaxime sodium, spectinomycin, roxithromycin, erythromycin- H2O, clarithromycin, thiamphenicol, chloramphenicol, paracetamol, naproxen, ibuprofen, ketoprofen, diclofenac acid, diltiazem, carbamazepine, diphenhydramine, gemfibrozil | 0.2 (d.w) | Freeze-dried, ground into powder. Separation of back muscles and abdominal muscles | USE (ACN/H2O) | SPE (PRiME HLB cartridges) | UHPLC-MS/MS | 43–127 | 0.01–1.9 | [39] |
| Senegal seabram (Diplodus bellottii), European sea bass (Dicentrarchus labrax), Meagre (Argyrosomus regius), Lusitanian toadfish (Halobatrachus didactylus) | Alprazolan, amoxicillin, atenolol, atorvastatin, azithromycin, bisoprolol, benzylpenicillin, bezafibrate, carbamazepine, carvedilol, cinoxacin, ciprofloxacin, ceftiofur, cephalexin, chlortetracycline, danofloxacin, diclofenac, doxicycline, enoxacin, enrofloxacin, epi-chlortetracycline, epi-tetracycline, erythromycin, epotetracycline, fenofibrate, flumequine, fluoxetine, furosemide, gabapentin, gemfibrozil, ibersartan, ibuprofen, indapamide, lorazepam, losartan, marbofloxacin, nalidixic acid, norfloxacin, nimesulide, ofloxacin, oxolinic acid, oxytetracycline, paracetamol, propanolol, sertraline, simvastatin, spiramycin, sulfachloropyridazine, sulfadoxine, sulfadiazine, sulfadimethoxine, sulfamethazine, sulfamethizole, sulfanilamide, sulfapyridine, sulfisomidine, sulfamethoxazole, sulfaquinoxaline, sulfathiazole, sulfisoxazole, tetracycline, tilmicosin, trimethoprim, tylosin venlafaxine, topiramate | 2 (w.w) | Homogenized | Mechanical shaking (ACN, EDTA) | No data | UHPLC-MS/MS | 79.2–109.5 | 0.59–4.11 | [41] |
| White bream, roach, bleak, perch, asp, pike, pikeperch (No data) | Nicotine, haloperidol, pyremethamine | 0.14–0.2 (d.w) | Dissected into fillet and carcass, frozen | USE (ACN, MeOH, H2O) | SPE (No data) | LC-HRMS/MS | 70–130 | 0.05–5.7 * | [59] |
| Atlantic salmon, Atlantic sea wolf, rainbow trout, Atlantic cod (No data) | Amoxicillin, azithromycin, caffeine, carbamazepine, ciprofloxacin, clarithromycin, diclofenac, erythromycin, furosemide, ketoprofen, ibuprofen, naproxen, sulfamethoxazole, tetracycline | 2 (w.w) | Dorsal muscle separated, cut into small parts, frozen at −20 °C | Mechanical shaking (ACN, 0.1 M EDTA, hexane) | No data | UHPLC-MS/MS | 81.2–99.4 | 0.017–1.371 | [46] |
| Flatfish (No data) | Albendazole, 2-amino albendazole sulfone, albendazole sulfone, albendazole sulfoxide, febantel, fenbendazole, flubendazole, 2-amino flubendazole, oxfendazole, oxfendazole sulfone, oxibendazole, cefapirin, desacetylcefapirin, cefazoline, cefoperazone, halofuginone, azithromycin, tildipirosin, dimetridazole, ipronidazole, ipronidazole-OH, metronidazole, metronidazole-OH, tinidazole, ronidazole, dicloxacillin, nafcillin, oxacillin, penicillin V, 2-hydroxymethyl−1-methyl-5-nitromidazole, 4-methylaminoantipyrine, sarafloxacin, orbifloxacin, carbadox, quinoxaline-2-carboxylic acid, olaquindox, 3-methylquinoxaline-2-carboxilic acid, dapsone, N-acetyl dapsone, sulfapyridine, arprinocid, azaperol, azaperon, carazolol, caffeine, clenbuterol, clochicine, diphehydramine, flunixin, imidocarb, isometamidium, ketoprofen, loperamide, metoclopramide, nitroxynil, phenacetin, ractopamine, scopolamine, triamcinolone, valnemuline | 2 (w.w) | Homogenized, stored at −20 °C | Mechanical shaking (Water/ ACN) | d-SPE: C18 | UHPLC-MS/MS | 73.2–115 | 0.5–5 * | [81] |
| Goldsilk seabream (Acanthopagrus berda), Indo-Malaysian barracuda (Sphyraena jello), Pale-edged stingray (Dasyatis zugei), Japanese scaled sardine (Sardinella zunasi), Yellow seabream (Acanthopagrus latus), Spotted scat (Scatophagus argus), Dotted gizzard shad (Konosirus punctatus), Porgies (Acanthopagrus schleg), Grey large-eye bream (Gymnocranius griseus), Pompano (Trachinotus ovaus), Saddleback silver (Gerres limbatus), Asian seabasses (Lateolabrax maculatus), Silver sea bream (Rhabdosargus sarba), Rough flathead (Grammoplites scaber), Bloch’s gizzard shad (Nematalosa nasus), Gangetic anchovy (Thryssa mysiax), Japanese goatfosh (Upeaneus japonicus), Genus (Johnius fasciatus) | Sulfamethazine, sulfapyridine, sulfathiazole, sulfanilamide, sulfadiazine, sulfadimethoxine, sulfamonomethoxin, sulfamerazine, sulfamethoxazole, norfloxacin, enoxacin, ofloxacin, ciprofloxacin, enrofloxacin, dehydrated erythromycin, clarithromycin, azithromycin, roxithromycin, florfenicol, chloramphenicol, trimethoprim, lincomycin | 5 (d.w) | Washed (water), dissected, homogenized, stored at −20 °C | USE (ACN, citric acid) | SPE (SAX-HLB cartridges) | UHPLC-MS/MS | 47.67–172.67 | 0.04–0.24 | [13] |
| Eel, flatfish (No data) | Naproxen, methyltestosterone, 17α-hydroxyprogesterone caproate, progesterone | 2 (w.w) | Ground, homogenized | Manual shaking (ACN 0.1% acetic acid) | No data | LC-MS/MS | 68–117 | 1–2 | [43] |
| Silver carp (Hypophthalmichthys molitrix), Bighead carp (Aristichthys nobilis), Common carp (Cyprinus carpio), Goldfish (Carassius auratus), Common skygazer (Cultrichthys erythropterus), Topmouth culter (Culter alburnus), Japanese grenadier anchovy (Coilia ectenes taihuensis), Asian pencil halfbeak (Hyporhamphus intermedius), Clearhead icefish (Protosalanx hyalocranius), Common sawbelly (Hemiculter leucisculus), Bitterling (Rhodeus sinensis), River sand pond snakehead (Odontobutis potamophila), Yellow catfish (Pelteobagrus fulvidraco), Asian swamp eel (Monopterus albus) | Sulfachlorpyridazine, sulfadiazine, sulfadoxine, sulfamerazine, sulfadimethoxine, sulfamethazine, sulfamethoxazole, sulfamonomethoxine, sulfapyridine, sulfaquinoxaline, sulfisoxazole, sulfathiazole, trimethoprim, chlortetracycline, doxycycline, oxytetracycline, tetracycline, ciprofloxacin, danofloxacin, enrofloxacin, fleroxacin, difloxacin, lomefloxacin, marbofloxacin, norfloxacin, ofloxacin, pefloxacin, sarafloxacin, azithromycin, leucomycin, clarithromycin, oleandomycin, roxithromycin, tylosin, salinomycin, monensin, florfenicol, chloramphenicol | 0.2 liver; 1.0 muscle (d.w) | Washed (water), dissected, homogenized, freeze-dried, stored at −20 °C | No data | No data | RRLC-MS/MS | 37.6–135 | 0.01–1.99 | [38] |
| Grass carp (Ctenopharyngodon idellus), Silver carp (Hypophtha lmichthys molitrix), Common carp (Cyprinus carpio), Crucian carp (Carassius auratus), Bighead carp (Hypophthalmichthys nobilis), Whitebait (Reganisalanx brachyrostralis), Yellow catfish (Pelteobagrus fulvidraco), Catfish (Silurus asotus), Loach (Paramisgurnus dabryanus) | Roxithromycin, erythromycin, ofloxacin, norfloxaxin, ciprofloxacin, tetracycline, sulfamethoxazole, ibuprofen, sulfaquinoxaline, sulfadiazine, diclofenac, naproxen, bezafibrate, propranolol, ketoconazole, carbamazepine, caffeine, fluoxetine, norfluoxetine, citalopram, paroxetine, sertraline, venlafaxine, duloxetine, bupropion, amitriptyline, fluvoxamine, trihexylphenidyl, clozapine, quetiapine, aripiprazole, chlorpromazine | 0.5 (d.w) | Freeze-dried, homogenized, stored at −80 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | UHPLC-MS/MS | 66–128 | 0.07–1.67 | [27] |
| Red bigeye (Priacanthus macracanthus), Horn dragonet (Callionymus curvicornis), White-spotted spinefoot (Siganus canaliculatus), Silver jewfish (Pennahia argentata), Burrowing goby (Trypauchen vagina), Threadfin porgy (Evynnis cardinalis), Palad (Solea ovata), Anchovy (Thryssa kammalensis), Bony fishes (Johnius heterolepis), Japanese flathead (Inegocia japonica), Shortnose ponyfish (Leiognathus brevirostris), Big head croaker (Collichthys lucidus), Goatee croaker (Dendrophysa russelii), Yellow croaker (Larimichthys crocea), Largehead hairtail (Trichiurus lepturus) | Atenolol, metoprolol, venlafaxine, chloramphenicol | 2 (d.w) | Washed (water), dissected, homogenized, freeze-dried, stored at −50 °C | USE (MeOH/ H2O) | SPE (Oasis MCX cartridges) | LC-MS/MS | 68–96 | 0.05–0.25 | [44] |
| Silver carp (Hypophtha lmichtyts molitrix), Common carp (Cyprinus carpio), Crucian carp (Carassius auratus), Lake anchovy (Coilia extenes), whitebait (Reganisalanx brachyrostralis), Redfin culter (Cultrichthys erythropterus), Yellow catfish (Pelteobagrus fulvidraco) | Roxithromycin, erythromycin, ofloxacin, norfloxacin, ciprofloxacin, tetracycline, chloramphenicol, sulfamerazine and sulfadiazine, sulfamethoxazole, ibuprofen, diclofenac, naproxen, indomethacin, clofibric acid, gemfibrozil, bezafibrate, 17β-estradiol, 17α-ethynylestradiol, propranolol, carbamazepine, ketoconazole, sertraline | 0.5 (d.w) | Separation of liver, brain, gills, and muscle. Freeze-dried, ground, stored at −20 °C | PLE (MeOH/ acetone) | SPE (HLB cartridges) | LC-MS/MS | 68–116 | 0.01–1.12 | [56] |
| Crucian carp (Carcassius carcassius) | Florfenicol, thiamphenicol, ofloxacin, pipemidic acid | No data | Liver, muscle, gill and bile separated, washed with 0.15 M KCl, stored at −20 °C | USE (0.1 M AcONa, MeOH) | SPE (SAX/PSA-HLB tandem cartridges) | LC-MS/MS | 79.2–91.0 | 0.5–0.6 | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Regalado, M.d.C.; Martín-Pozo, L.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E.; Zafra-Gómez, A. An Overview of Analytical Methods to Determine Pharmaceutical Active Compounds in Aquatic Organisms. Molecules 2022, 27, 7569. https://doi.org/10.3390/molecules27217569
Gómez-Regalado MdC, Martín-Pozo L, Martín J, Santos JL, Aparicio I, Alonso E, Zafra-Gómez A. An Overview of Analytical Methods to Determine Pharmaceutical Active Compounds in Aquatic Organisms. Molecules. 2022; 27(21):7569. https://doi.org/10.3390/molecules27217569
Chicago/Turabian StyleGómez-Regalado, María del Carmen, Laura Martín-Pozo, Julia Martín, Juan Luis Santos, Irene Aparicio, Esteban Alonso, and Alberto Zafra-Gómez. 2022. "An Overview of Analytical Methods to Determine Pharmaceutical Active Compounds in Aquatic Organisms" Molecules 27, no. 21: 7569. https://doi.org/10.3390/molecules27217569
APA StyleGómez-Regalado, M. d. C., Martín-Pozo, L., Martín, J., Santos, J. L., Aparicio, I., Alonso, E., & Zafra-Gómez, A. (2022). An Overview of Analytical Methods to Determine Pharmaceutical Active Compounds in Aquatic Organisms. Molecules, 27(21), 7569. https://doi.org/10.3390/molecules27217569