Toward Depth-Resolved Analysis of Plant Metabolites by Nanospray Desorption Electrospray Ionization Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
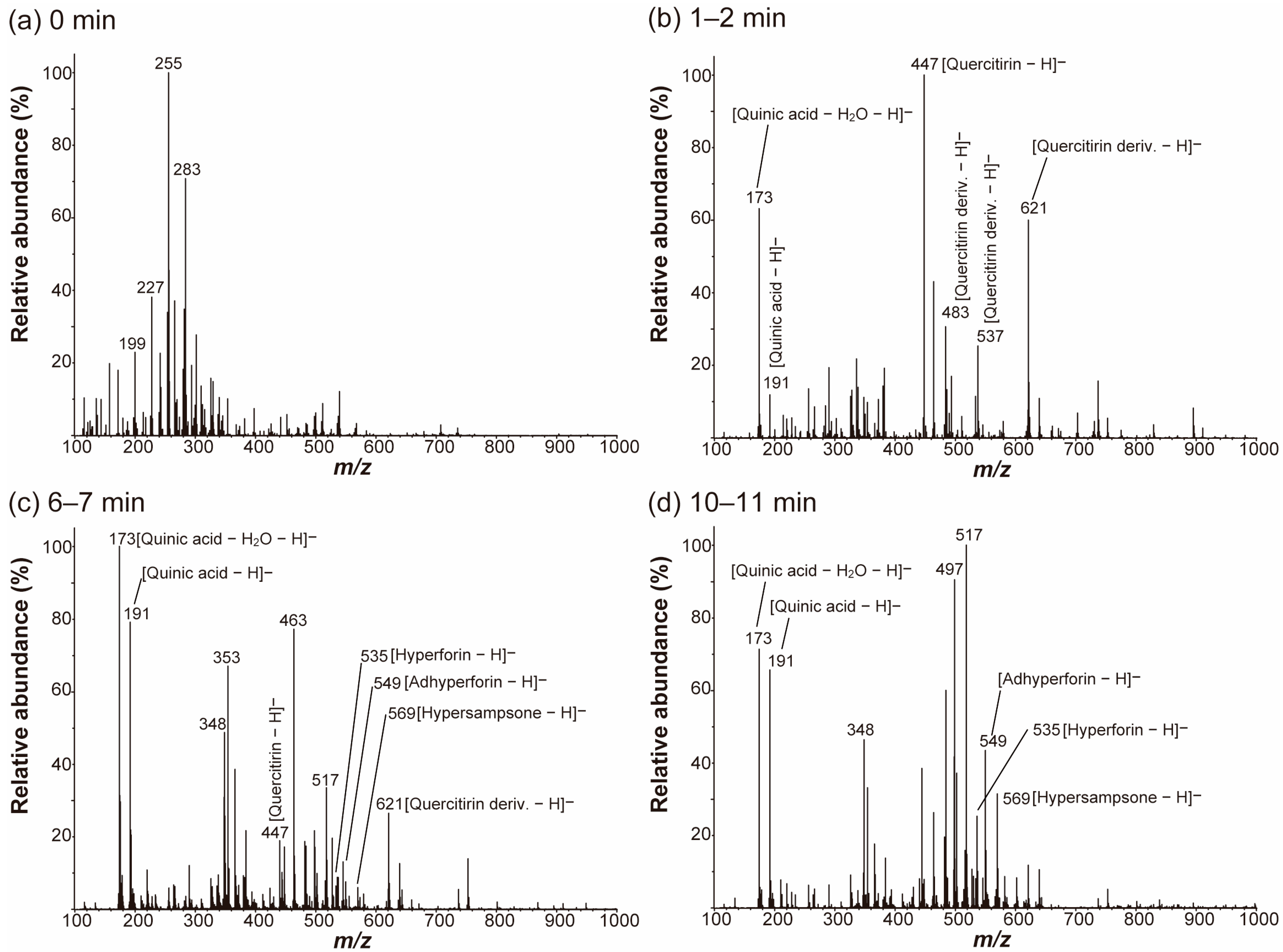
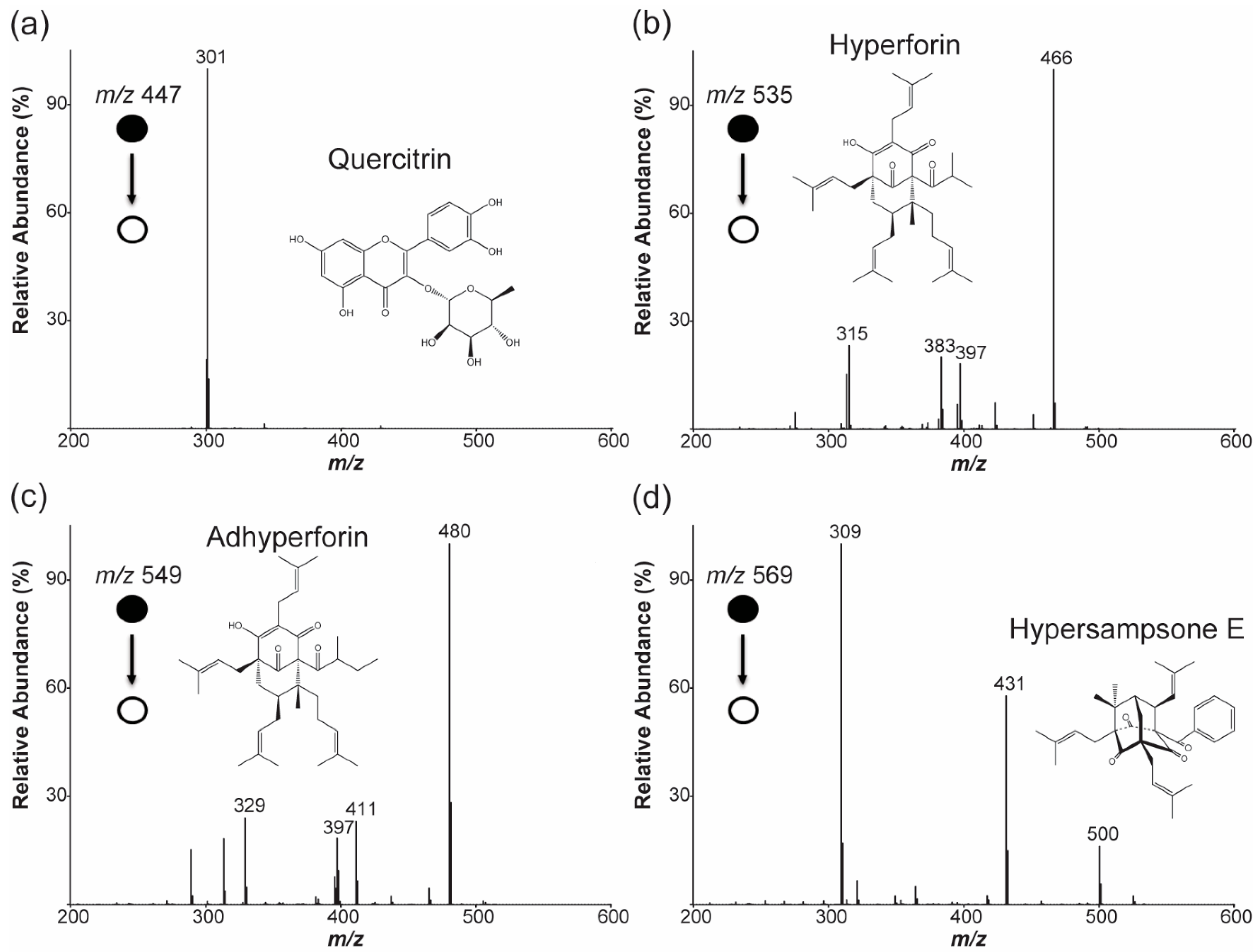
2.1. Metabolite Fingerprinting of a Hypericum Raw Leaf Surface by Nano-DESI MS
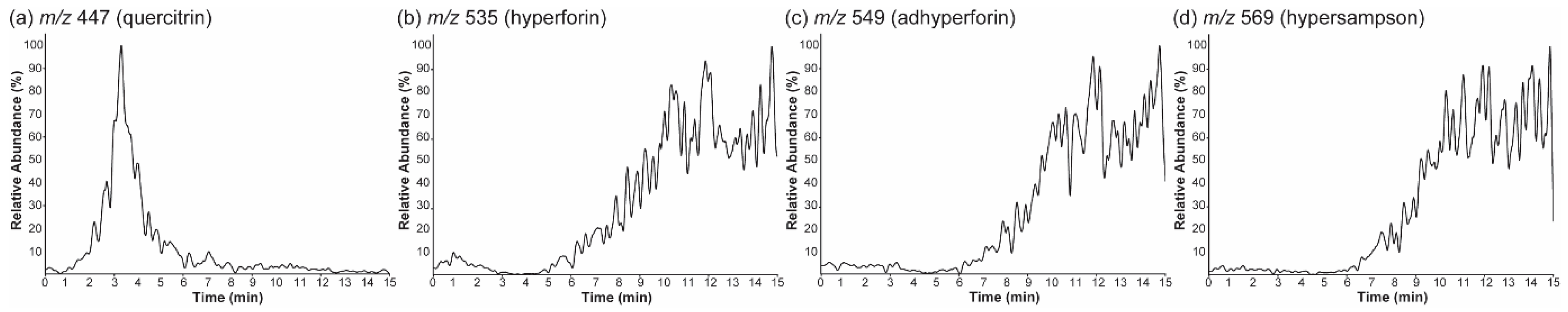
2.2. Selected Ion Monitoring and Pseudo-Selected Reaction Monitoring for the Major Ions
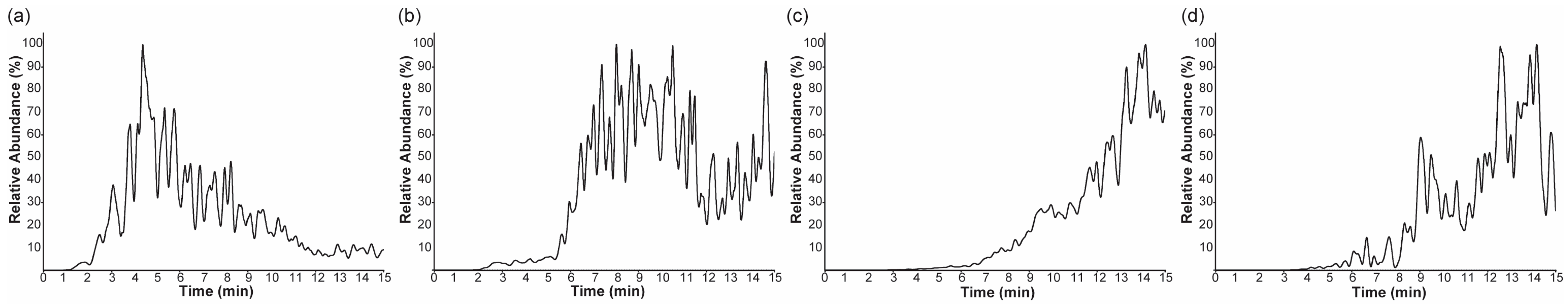
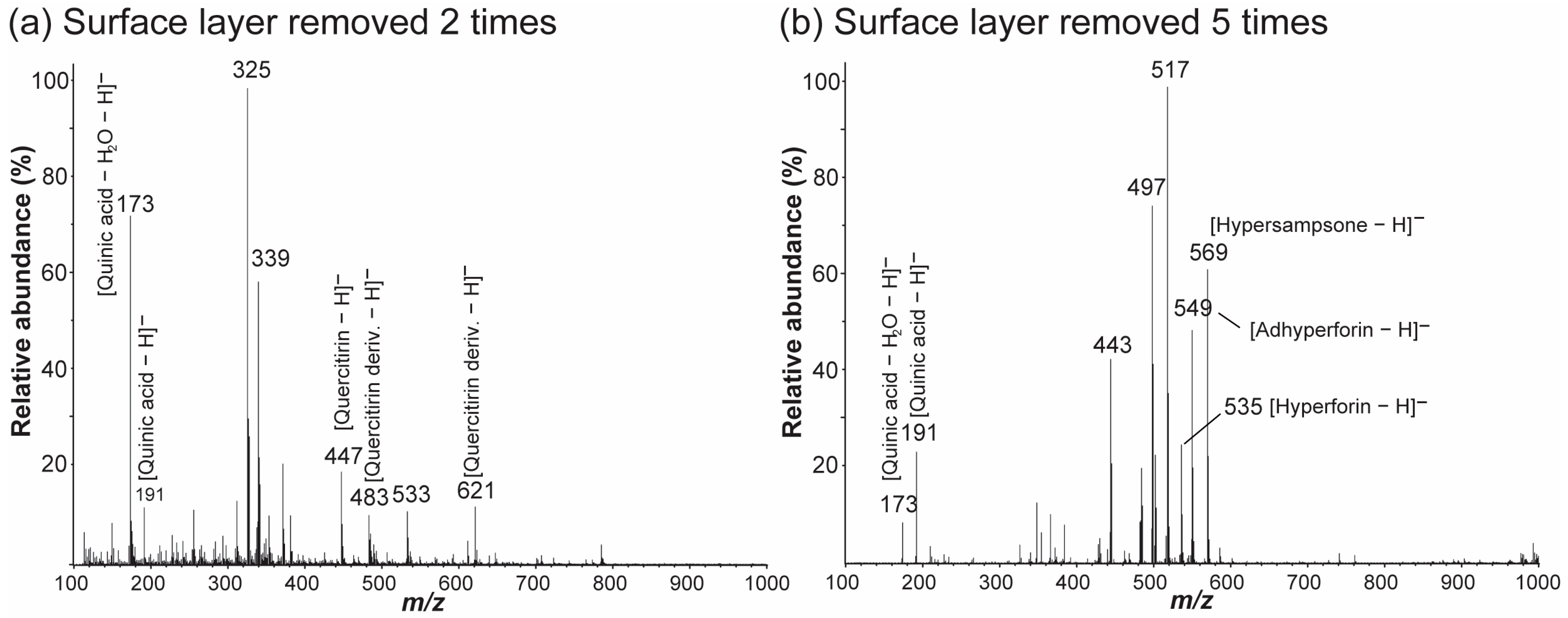
2.3. Nano-DESI MS of the Surface-Layer-Removed Hypericum Leaves
3. Materials and Methods
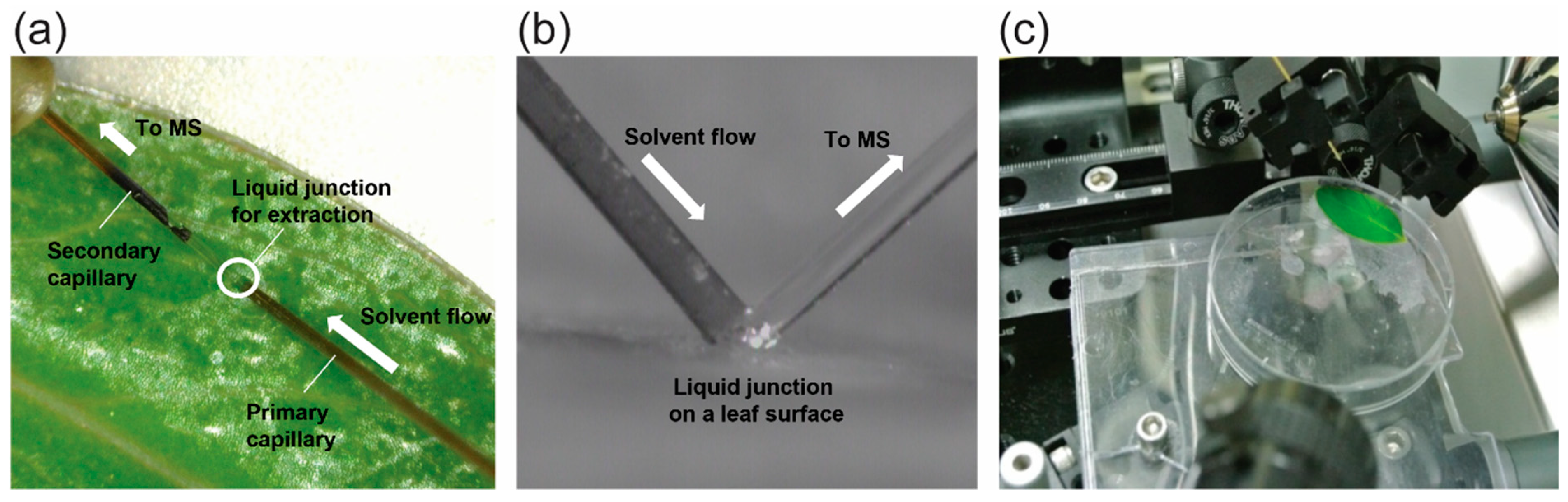
3.1. Nano-DESI MS
3.1.1. Nano-DESI Probe
3.1.2. Mass Spectrometry
3.2. Plant Sample Preparation for Nano-DESI MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.A.; Cha, S. Ambient desorption/ionization mass spectrometry for direct solid material analysis. TrAC Trends Anal. Chem. 2021, 144, 116420. [Google Scholar] [CrossRef]
- Roach, P.J.; Laskin, J.; Laskin, A. Nanospray desorption electrospray ionization: An ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst 2010, 135, 2233–2236. [Google Scholar] [CrossRef] [PubMed]
- Eckert, P.A.; Roach, P.J.; Laskin, A.; Laskin, J. Chemical Characterization of Crude Petroleum Using Nanospray Desorption Electrospray Ionization Coupled with High-Resolution Mass Spectrometry. Anal. Chem. 2012, 84, 1517–1525. [Google Scholar] [CrossRef]
- Liu, P.; Lanekoff, I.T.; Laskin, J.; Dewald, H.D.; Chen, H. Study of Electrochemical Reactions Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2012, 84, 5737–5743. [Google Scholar] [CrossRef]
- Watrous, J.; Roach, P.; Alexandrov, T.; Heath, B.S.; Yang, J.Y.; Kersten, R.D.; van der Voort, M.; Pogliano, K.; Gross, H.; Raaijmakers, J.M.; et al. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. USA 2012, 109, E1743–E1752. [Google Scholar] [CrossRef]
- Lanekoff, I.; Geydebrekht, O.; Pinchuk, G.E.; Konopka, A.E.; Laskin, J. Spatially resolved analysis of glycolipids and metabolites in living Synechococcus sp. PCC 7002 using nanospray desorption electrospray ionization. Analyst 2013, 138, 1971–1978. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Chou, P.-T.; Zare, R.N. Imaging of Proteins in Tissue Samples Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2015, 87, 11171–11175. [Google Scholar] [CrossRef]
- Lanekoff, I.; Burnum-Johnson, K.; Thomas, M.; Cha, J.; Dey, S.K.; Yang, P.; Conaway, M.C.P.; Laskin, J. Three-dimensional imaging of lipids and metabolites in tissues by nanospray desorption electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2063–2071. [Google Scholar] [CrossRef]
- Roach, P.J.; Laskin, J.; Laskin, A. Molecular Characterization of Organic Aerosols Using Nanospray-Desorption/Electrospray Ionization-Mass Spectrometry. Anal. Chem. 2010, 82, 7979–7986. [Google Scholar] [CrossRef]
- Lanekoff, I.; Heath, B.S.; Liyu, A.; Thomas, M.; Carson, J.P.; Laskin, J. Automated Platform for High-Resolution Tissue Imaging Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2012, 84, 8351–8356. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.N.; Sontag, R.L.; Carson, J.P.; Corley, R.A.; Ansong, C.; Laskin, J. Towards High-Resolution Tissue Imaging Using Nanospray Desorption Electrospray Ionization Mass Spectrometry Coupled to Shear Force Microscopy. J. Am. Soc. Mass Spectrom. 2018, 29, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Venter, A.; Sojka, P.E.; Cooks, R.G. Droplet Dynamics and Ionization Mechanisms in Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2006, 78, 8549–8555. [Google Scholar] [CrossRef]
- Lee, G.; Cha, S. Depth-Dependent Chemical Analysis of Handwriting by Nanospray Desorption Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2021, 32, 315–321. [Google Scholar] [CrossRef]
- Buchberger, A.R.; Delaney, K.; Johnson, J.; Jillian, J. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem. 2018, 90, 240–265. [Google Scholar] [CrossRef]
- Boughton, B.A.; Thinagaran, D.; Sarabia, D.; Bacic, A.; Roessner, U. Mass spectrometry imaging for plant biology: A review. Phytochem. Rev. 2016, 15, 445–488. [Google Scholar] [CrossRef]
- Dunham, S.J.B.; Ellis, J.F.; Li, B.; Sweedler, J.V. Mass Spectrometry Imaging of Complex Microbial Communities. Acc. Chem. Res. 2017, 50, 96–104. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Zaima, N.; Yukihiro, Y. Application of Mass Spectrometry Imaging for Visualizing Food Components. Foods 2020, 9, 575. [Google Scholar] [CrossRef]
- Vos, D.R.N.; Ellis, S.R.; Balluff, B.; Heeren, R.M.A. Experimental and Data Analysis Considerations for Three-Dimensional Mass Spectrometry Imaging in Biomedical Research. Mol. Imaging Biol. 2021, 23, 149–159. [Google Scholar] [CrossRef]
- Seeley, E.; Caprioli, R.M. 3D Imaging by Mass Spectrometry: A New Frontier. Anal. Chem. 2012, 84, 2105–2110. [Google Scholar] [CrossRef]
- Nemes, P.; Barton, A.A.; Vertes, A. Three-Dimensional Imaging of Metabolites in Tissues under Ambient Conditions by Laser Ablation Electrospray Ionization Mass Spectrometry. Anal. Chem. 2009, 81, 6668–6675. [Google Scholar] [CrossRef] [PubMed]
- Nemes, P.; Barton, A.A.; Li, Y.; Vertes, A. Ambient Molecular Imaging and Depth Profiling of Live Tissue by Infrared Laser Ablation Electrospray Ionization Mass Spectrometry. Anal. Chem. 2008, 80, 4575–4582. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.A.; Clemens, S.; Zachgo, S.; Giblin, E.M.; Taylor, D.C.; Kunst, L. CUT1, an Arabidopsis Gene Required for Cuticular Wax Biosynthesis and Pollen Fertility, Encodes a Very-Long-Chain Fatty Acid Condensing Enzyme. Plant Cell 1999, 11, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Razeq, F.M.; Kosma, D.K.; Rowland, O.; Molina, I. Extracellular lipids of Camelina sativa: Characterization of chloroform-extractable waxes from aerial and subterranean surfaces. Phytochemistry 2014, 106, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hvattum, E.; Ekeberg, D. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef]
- Porzel, A.; Farag, M.A.; Mülbradt, J.; Wessjohann, L.A. Metabolite profiling and fingerprinting of Hypericum species: A comparison of MS and NMR metabolomics. Metabolomics 2014, 10, 574–588. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 1–30. [Google Scholar] [CrossRef]
- Franklin, G.; Dias, A. Chlorogenic acid participates in the regulation of shoot, root and root hair development in Hypericum perforatum. Plant Physiol. Biochem. 2011, 49, 835–842. [Google Scholar] [CrossRef]
- Huang, H.-S.; Liaw, E.-T. Extraction Optimization of Flavonoids from Hypericum formosanum and Matrix Metalloproteinase-1 Inhibitory Activity. Molecules 2017, 22, 2172. [Google Scholar] [CrossRef]
- Camuesco, D.; Comalada, M.; Rodriguez-Cabezas, M.E.; Nieto, A.; Lorente, M.D.; Concha, A.; Zarzuelo, A.; Gálvez, J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2004, 143, 908–918. [Google Scholar] [CrossRef]
- Choi, S.-J.; Tai, B.H.; Cuong, N.M.; Kim, Y.-H.; Jang, H.-D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Truong, V.-L.; Ko, S.-Y.; Jun, M.; Jeong, W.-S. Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation. Nutrients 2016, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-M.; Ham, Y.-M.; Yoon, W.-J.; Roh, S.W.; Jeon, Y.-J.; Oda, T.; Kang, S.-M.; Kang, M.-C.; Kim, E.-A.; Kim, D.; et al. Quercitrin protects against ultraviolet B-induced cell death in vitro and in an in vivo zebrafish model. J. Photochem. Photobiol. B Biol. 2012, 114, 126–131. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Son, Y.-O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef]
- Müller, W.E. Current St. John’s wort research from mode of action to clinical efficacy. Pharmacol. Res. 2003, 47, 101–109. [Google Scholar] [CrossRef]
- Schempp, C.M.; Pelz, K.; Wittmer, A.; Schöpf, E.; Simon, J.C. Antibacterial activity of hyperforin from St John’s wort, against multiresistant Staphylococcus aureus and gram-positive bacteria. Lancet 1999, 353, 2129. [Google Scholar] [CrossRef]
- Schempp, C.M.; Kirkin, V.; Simon-Haarhaus, B.; Kersten, A.; Kiss, J.; Termeer, C.C.; Gilb, B.; Kaufmann, T.; Borner, C.; Sleeman, J.P.; et al. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s wort that acts by induction of apoptosis. Oncogene 2002, 21, 1242–1250. [Google Scholar] [CrossRef]
- Medina, M.A.; Martínez-Poveda, B.; Amores-Sánchez, M.I.; Quesada, A.R. Hyperforin: More than an antidepressant bioactive compound? Life Sci. 2006, 79, 105–111. [Google Scholar] [CrossRef]
- Zanoli, P. Role of Hyperforin in the Pharmacological Activities of St. John’s Wort. CNS Drug Rev. 2004, 10, 203–218. [Google Scholar] [CrossRef]
- Brechner, M.L.; Albright, L.D.; Weston, L. Effects of UV-B on Secondary Metabolites of St. John’s Wort (Hypericum perforatum L.) Grown in Controlled Environments. Photochem. Photobiol. 2011, 87, 680–684. [Google Scholar] [CrossRef]
- Rizzo, P.; Altschmied, L.; Ravindran, B.M.; Rutten, T.; D’Auria, J.C. The biochemical and genetic basis for the biosynthesis of bioactive compounds in Hypericum perforatum L., one of the largest medicinal crops in europe. Genes 2020, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Schäffer, S. Chemical Composition of the Prunus laurocerasus Leaf Surface. Dynamic Changes of the Epicuticular Wax Film during Leaf Development. Plant Physiol. 2001, 126, 1725–1737. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, S.; Jun, G.; Park, Y.; An, S.J.; Lee, D. Toward Depth-Resolved Analysis of Plant Metabolites by Nanospray Desorption Electrospray Ionization Mass Spectrometry. Molecules 2022, 27, 7582. https://doi.org/10.3390/molecules27217582
Cha S, Jun G, Park Y, An SJ, Lee D. Toward Depth-Resolved Analysis of Plant Metabolites by Nanospray Desorption Electrospray Ionization Mass Spectrometry. Molecules. 2022; 27(21):7582. https://doi.org/10.3390/molecules27217582
Chicago/Turabian StyleCha, Sangwon, Gyouwoong Jun, Yougyeong Park, Sung Jun An, and Donghoon Lee. 2022. "Toward Depth-Resolved Analysis of Plant Metabolites by Nanospray Desorption Electrospray Ionization Mass Spectrometry" Molecules 27, no. 21: 7582. https://doi.org/10.3390/molecules27217582
APA StyleCha, S., Jun, G., Park, Y., An, S. J., & Lee, D. (2022). Toward Depth-Resolved Analysis of Plant Metabolites by Nanospray Desorption Electrospray Ionization Mass Spectrometry. Molecules, 27(21), 7582. https://doi.org/10.3390/molecules27217582





