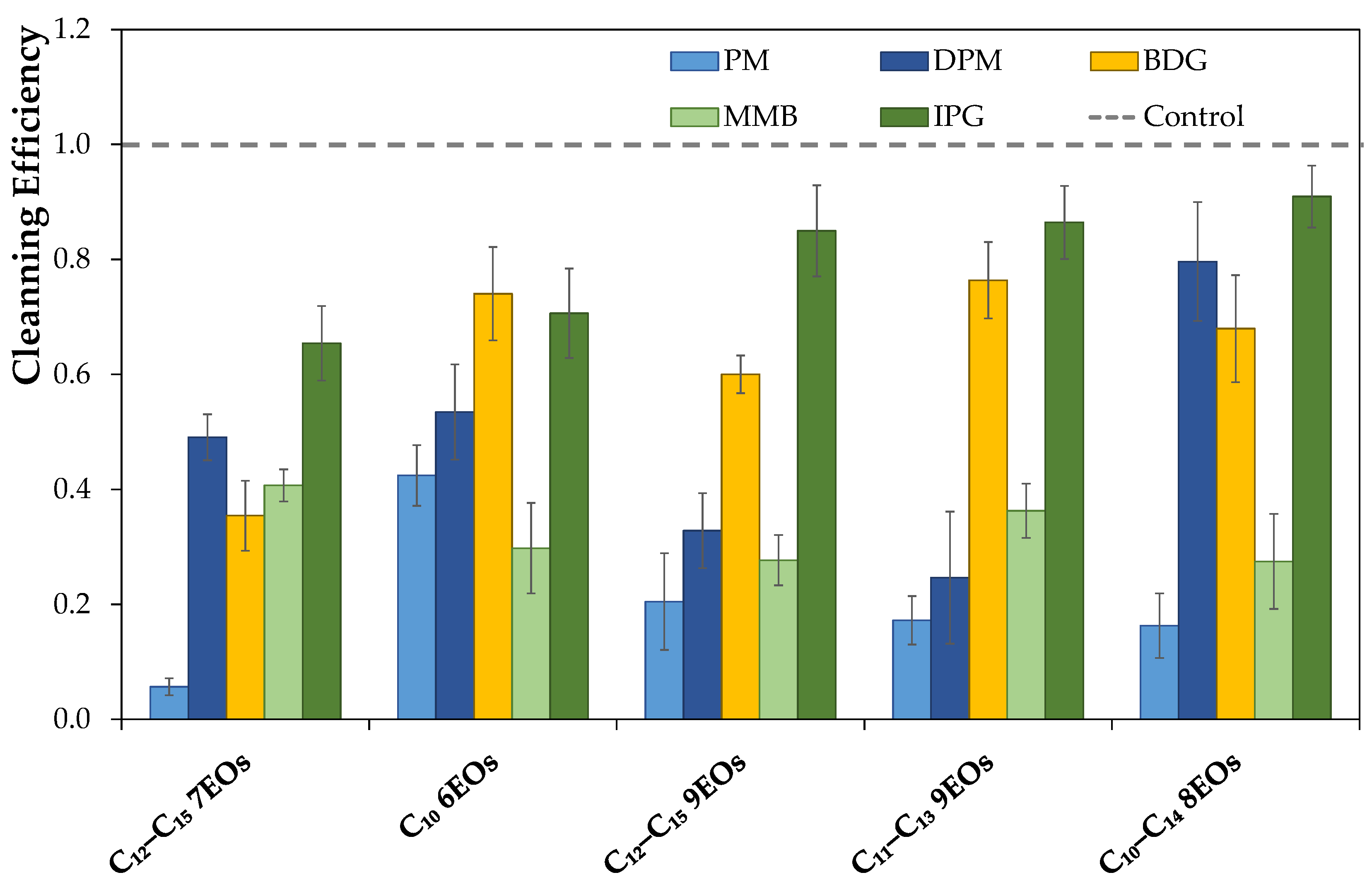1. Introduction
Soiling and cleaning are ubiquitous in the food sector, from the domestic kitchen to large-scale factories, where grease and fatty soils, especially carbonized ones, are difficult to remove [
1]. Fat-based soils are usually present as an emulsion, and generally they can be rinsed with hot water above the soil melting point. However, more abrasive detergents are usually required for carbonized residues that are more difficult to remove. These detergents are usually a complex mixture of surfactants, organic solvents, phosphate-based scouring, and alkaline agents (emulsifying/saponifying compounds), which results in an alkaline pH [
2]. The use of these compounds and the alkaline medium (pH > 11) in degreaser formulations ensures high efficiency in removing grease, fats, and carbonized food-derived soils [
3]. Nevertheless, some of these compounds create risks for human health and environmental pollution [
4,
5]. Therefore, the development of eco-friendly degreasers (without phosphates and pH < 11) is essential, while maintaining their cleaning efficiency. For that purpose, it is necessary to consider the raw materials used in the degreaser and their function in the formulation [
6]. Traditionally, two of the most significant chemicals in degreasers are solvents and surfactants.
The solvents are directly involved in the removal of resins, waxes, adhesives, greases, and paints [
7]. They are used in the cleaning industry and are petroleum derivates, including alcohols, amides, amines, esters, glycols, glycol ethers, and hydrocarbons [
8,
9,
10]. Nowadays, there is a demand for biobased solvents, i.e., solvents from renewable sources, such as carbohydrates, carbohydrate polymers, proteins, alkaloids, plant oils, and animal fats to replace petroleum-based ones [
7,
11]. Companies, such as Solvay, Vertec Biosolvents, and AstroBio, have commercial biosolvents for application in, among others, cosmetics and cleaning products.
Regarding the surfactants, they are present in a wide range of products, such as personal care, lubricants, and detergent formulations, and they are the active ingredient responsible for most of the cleaning power [
12,
13]. Their main function is lowering a liquid’s surface tension, allowing it to spread over the surface easily, and they adsorb onto the soil, allowing them to remove the soil from the surface into the bulk liquid [
14]. It is important to note that after the soil removal, it should be stabilized and suspended (via emulsification and dispersion) in the wash liquor to be rinsed via mechanical agitation [
15]. When considering the design of new formulations, surfactant properties, such as molecular weight, critical micelle concentration (CMC), and hydrophilic–lipophilic balance (
HLB) for the nonionic, must be considered. The surfactants’ molecular weight is an important feature to determine their toxicity and biodegradability [
16], mainly due to their alkyl chain length. Generally, surfactants with a low molecular weight have high biodegradability and low toxicity [
17].
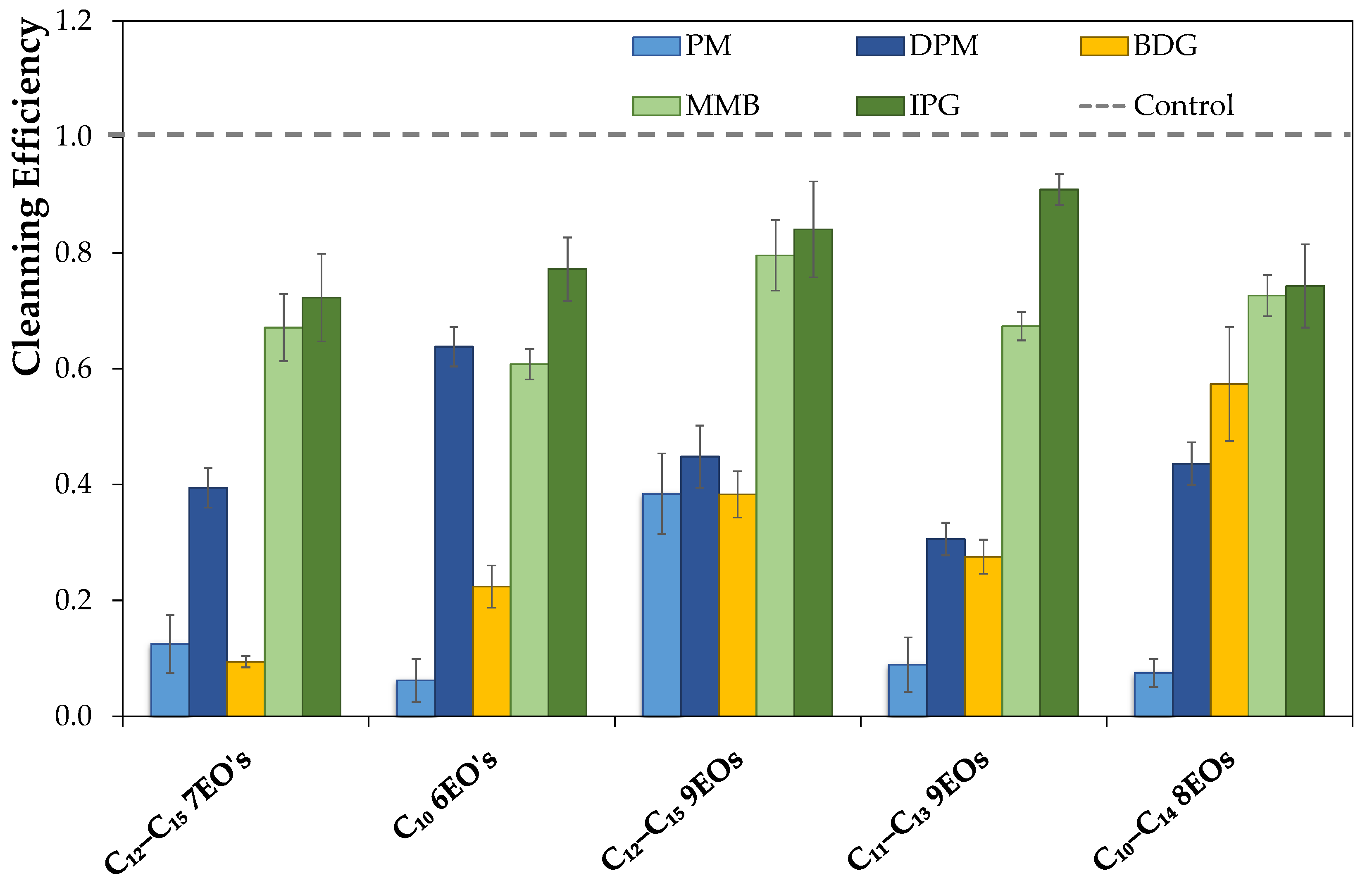
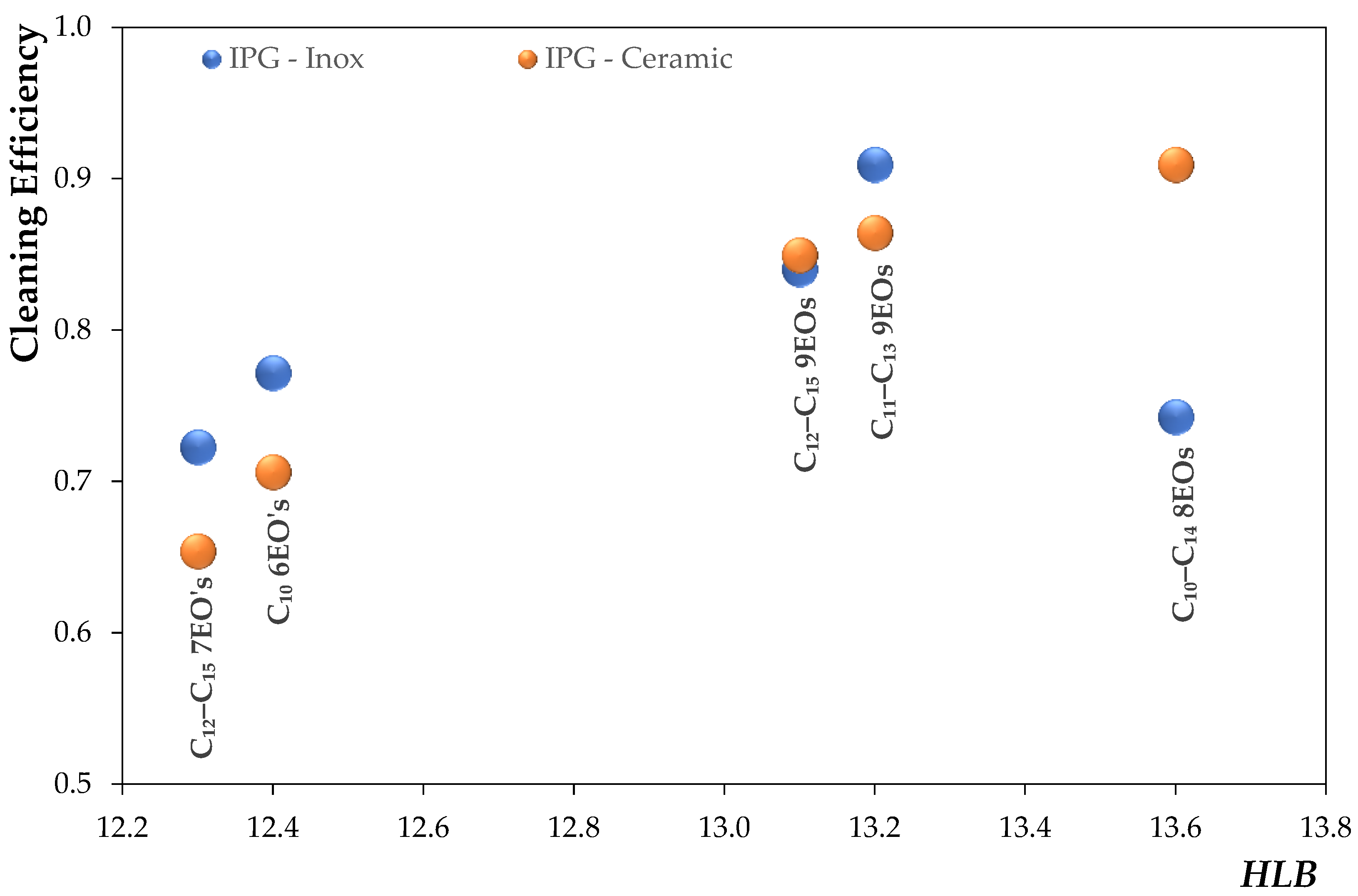
Aiming at developing an eco-friendly and a safer degreaser for users (pH below 11 and without phosphates) to remove a carbonized soil, a series of simple formulations composed of only one solvent, one surfactant (nonionic or ionic), and water were investigated. For that purpose, first the soil used was characterized by Fourier Transform Infrared—Attenuated Total Reflection (FTIR–ATR), and its surface energy was determined for the two surfaces (ceramic and stainless steel) used in this work. Then, a screening of the individual combinations of 5 wt% solvents and 5 wt% of surfactants was performed using abrasion tests on ceramic and stainless steel surfaces in an attempt to understand which are most effective in removing a particular carbonized soil. In addition to using commercially available nonionic surfactants, a new class of ionic surfactants based on long alkyl trimethylammonium carboxylate was also synthesized. Finally, the best formulations for cleaning carbonized soil were optimized for both surfaces using a mixture design, i.e., to find the optimal percentage of each component (solvent, surfactant, and water) in the formulation for the best cleaning performance. The gathered results show the possibility of having a more benign degreaser for both the user and the environment, with a low pH and phosphate-free, without compromising the market demands regarding the cleaning efficiency of carbonized soil.
3. Materials and Methods
3.1. Materials
In this work, the solvents used to test new formulations, namely propylene glycol methyl ether (PM), dipropylene glycol methyl ether (DPM, 1-((1-methoxypropan-2-yl)oxy)propan-2-ol), diethylene glycol butyl ether (BDG), and 3-methoxy-3-methyl-1-butanol (MMB) were supplied by Kuraray CO (Tokyo, Japan), while isopropylene glycerol (IPG) was kindly provided by Solvay (Paris, France). The nonionic surfactants used were C10 6EOs, C11–C13 9EOs, and C12–C15 7EOs that were kindly supplied by Sasol (Milan, Italy) and KAO (Barcelona, Spain), C12–C15 9EOs that was kindly provided by Evonik (Essen, Germany), and C10–C14 8EOs, that was kindly provided by Huntsman (Freeport, TX, USA). In order to synthesize the new ionic surfactants, the starting materials octyltrimethylammonium bromide (98.0%, from TCI, Tokyo, Japan), decyltrimethylammonium bromide (98.0%, from TCI, Tokyo, Japan), decyltrimethylammonium chloride (98.0%, from TCI, Tokyo, Japan), dodecyltrimethylammonium bromide (99.0%, from Alfa Aesar, Kandel, Germany), sodium octanoate (98.0%, from Acros Organics, India), sodium decanoate (98.0%, from Sigma, USA), and sodium dodecanoate (98.0%, from Acros Organics, China) were used.
The soil used in the abrasion tests comprised lard, vegetable oil, and sugar powder (all acquired in a local supermarket, Portugal); paraffin wax (purum, from Aldrich, Germany), carbon black (from Kremer Pigmente, Aichstetten, Germany), and albumin powder from egg (from Acros Organics, Poland). All new formulations were compared with the commercial detergent KH7 degreaser from the company KH Lloreda (details in Suplementary Materials), purchased in a local supermarket, hereafter denominated as control.
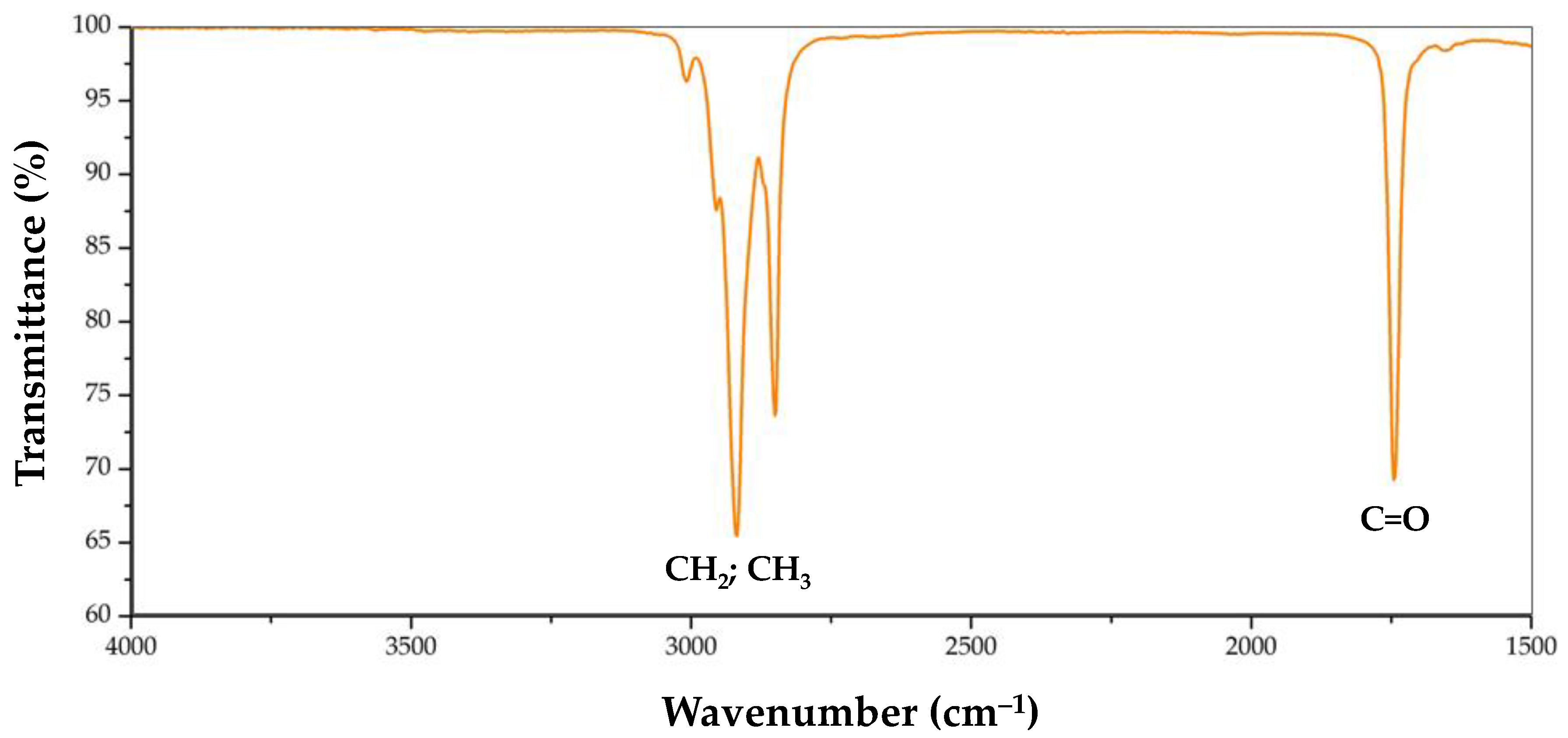
3.2. FTIR-ATR
The soil FTIR (Fourier-transform infrared) spectra were taken with a PIKE MIRacle MB3000 FTIR System spectrometer equipped with an ATR (attenuated total reflection) cell with a ZnSe crystal. The resolution was 4 cm−1 after 40 scans, and the spectra were collected from 4000 to 600 cm−1.
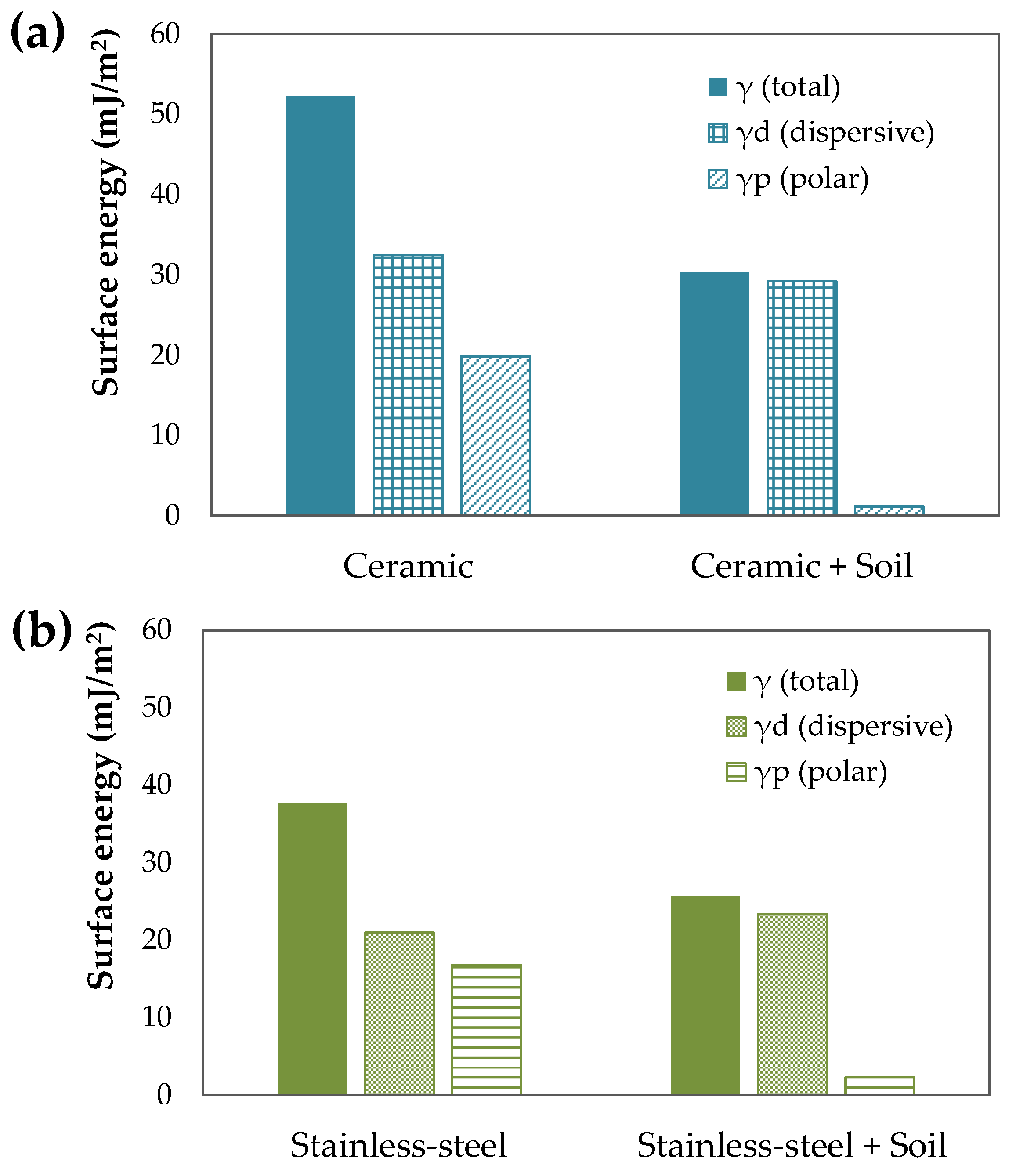
3.3. Surface Energy and Contact Angle Measurements
For the soil characterization, contact angle and surface energy were measured. Contact angle measurements of the liquids water (MilliQ grade), formamide (Sigma, 99% purity GC), and diiodomethane (Aldrich, 99% purity GC) were carried out using the sessile drop method with the Contact Angle System OCA 20 (DataPhysics Instruments GmbH, Germany) at room temperature. Drop volumes of (1 ± 0.01) µL were obtained using a Hamilton DS 500/GT syringe connected to a Teflon-coated needle placed inside an aluminum chamber. The reported contact angles of each standard were an average of at least ten independent measurements.
The surface energy can be used to describe the wettability/permeability. The total surface energy (
) of the soil, ceramic, and stainless steel was obtained by the Owens–Wendt–Rabel–Kaelble (OWRK) model [
18], and the corresponding polar (
) and dispersive (
) components by measuring contact angles (
θ), liquid-probe surface tension (
Table 1) data and Equations (1) and (2):
where
is the total surface tension of the liquid (mN/m);
θ is the contact angle (radians), and
and
are the dispersive components, and
and
are the polar components of solid and liquid surface energies, respectively. Plotting the right-hand side of Equation (2) as a function of
enables the calculation of
and
from the parameters of the linear regression (Equation (2)).
3.4. Synthesis of Ionic Surfactants
The synthesis of new ionic surfactants consists of a salt metathesis reaction described in
Scheme 1. Chloride or bromide salt and the corresponding sodium salt were used to synthesize these ionic compounds in the stoichiometry of 1:1. The reaction occured in water, under stirring for at least 12 hours. Then, the water was removed in a moderate vacuum (0.1 Pa) for at least 48 hours, and the surfactant structures were checked by
1H and
13C Nuclear Magnetic Ressonance (NMR) spectroscopy (
cf Figure S6 from Supplementary Materials). This is were the different combinations of the sodium and chloride or bromide salts were performed, resulting in the surfactants octyltrimethilammonium octanoate ([N
1118][C
8O
2]), octyltrimethilammonium decanoate ([N
1118][C
10O
2]), octyltrimethilammonium dodecanoate ([N
1118][C
12O
2]), decyltrimethilammonium octanoate ([N
11110][C
8O
2]), decyltrimethilammonium decanoate ([N
11110][C
10O
2]), decyltrimethilammonium dodecanoate ([N
11110][C
12O
2]), dodeciltrimethilammonium octanoate ([N
11112][C
8O
2]), dodeciltrimethilammonium decanoate ([N
11112][C
10O
2]), and dodeciltrimethilammonium dodecanoate ([N
11112][C
12O
2]). Moreover, the surfactant was used without a purification step to remove the sodium chloride or bromide salt produced as a secondary product from the synthesis.
3.5. New Formulations
After the synthesis of surfactants, different combinations between the solvents and surfactants were tested for new formulations. The chemical structures of solvents and surfactants used are presented in
Figure 10. The composition of new formulations comprised 5 wt% solvent, 5 wt% surfactant, and 90 wt% distilled water. The pH of each formulation was measured by a SevenExcellence pH meter from Mettler Toledo
™ (Columbus, OH, USA).
3.6. Wet Abrasion Scrub Test
To test the new formulations, an abrasion test was conducted using a Sheen
® 903 Wet Abrasion Scrub Tester against a soil previously applied to the ceramic and stainless steel surface. The soil used, adapted from the literature [
20], comprised lard, paraffin, carbon black, vegetable oil, sugar powder, and albumin powder at different proportions. For the soil preparation, lard, paraffin, and vegetable oil were first mixed under stirring at 90 °C, then, the carbon black and the albumin were added. Next, the temperature was increased up to 110 °C, and the sugar powder was added. Subsequently, the heat was turned off while stirring it until the mixture cooled down to room temperature, and was afterward stored in a humidity-controlled chamber for three days before use. The soil was then applied to the different surfaces using a stencil, which guaranteed an even application. Then, the surfaces were cooked at 185 °C for 15 minutes, and after that, stored in a humidity-controlled chamber overnight.
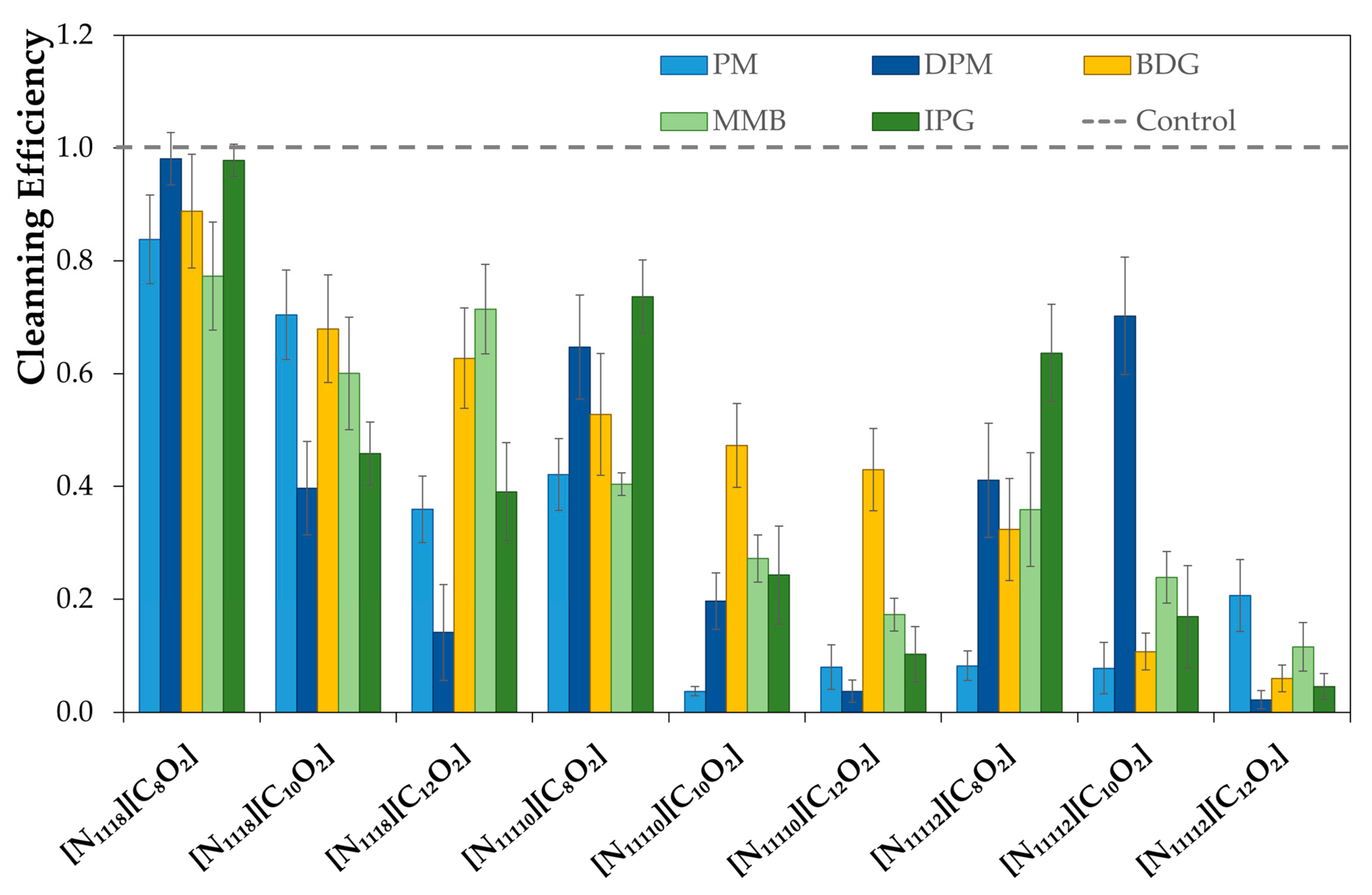
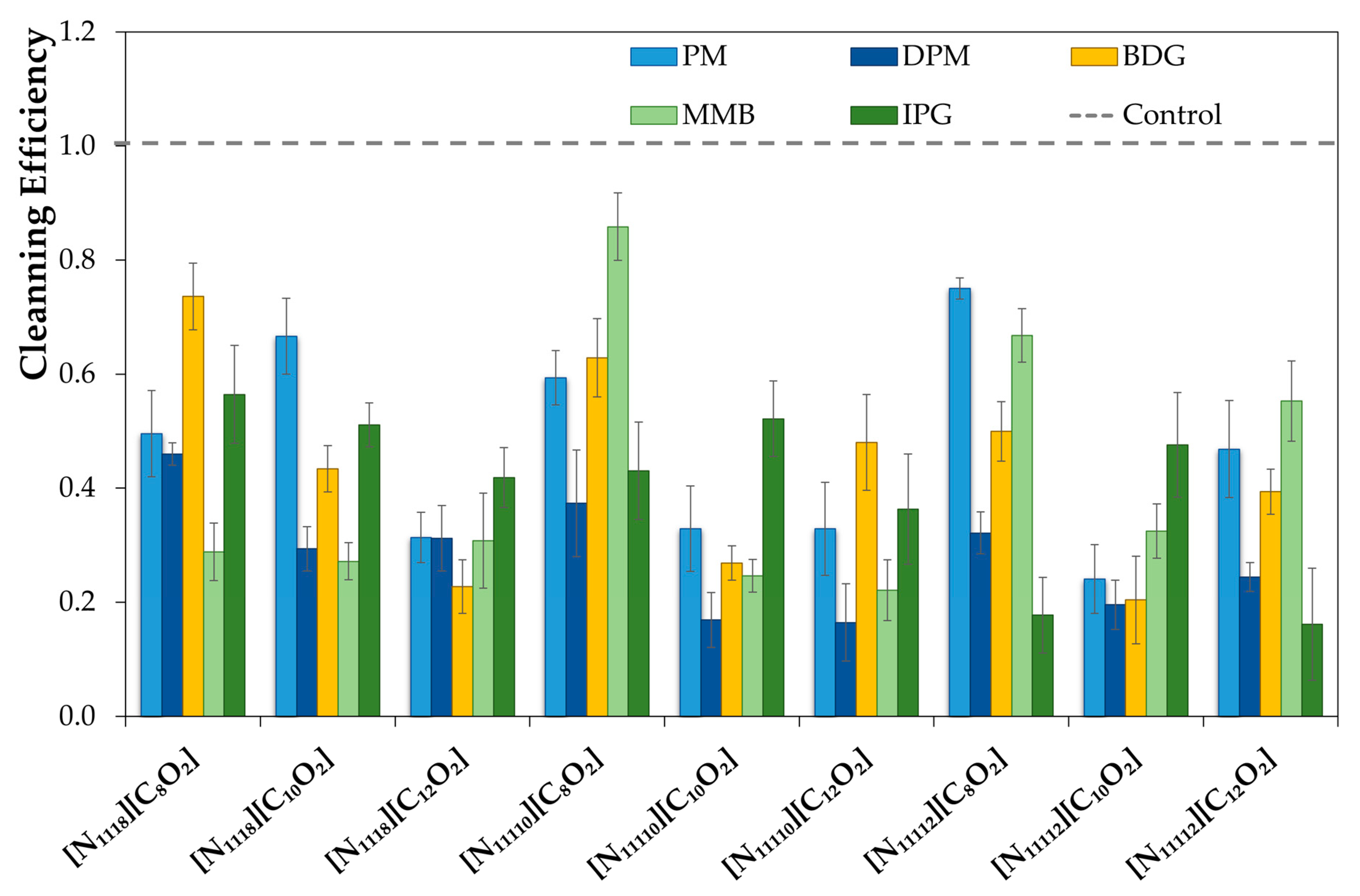
To test the formulations, the surfaces and sponges (with 15 g of formulation) were placed in the Sheen® 903 Wet Abrasion Scrub Tester. A few swipes (back and forward movement is one swipe) were determined as the minimum number required to remove 70% of the soil from the surface using a control formulation. Then, the tests with new formulations were performed with the same number of swipes.
In order to analyze the cleaning potential of each formulation in the ceramic tiles, a black and white photograph was taken before and after the cleaning procedure, using GenoSmart2 Image Capture equipment from VWR. To quantify the soil removal in each tile, a Matlab routine was used to analyze the photograph on a scale of gray. From it, a histogram considering the number of pixels for each gray tone was plotted, and the results were analyzed taking into consideration the mean (
µ) of the histogram, which is given by the following equation:
where
f(
k) is the histogram;
k is the bin index (gray level), and
K is the number of gray levels. The difference in the means of the distribution of the histogram representing the pictures before and after cleaning (in absolute values) gave the cleaning performance of each formulation. Another method was used for the stainless steel surfaces since the contrast of soil and surfaces was not adequate to apply the process previously described. Therefore, a weighting method was used to quantify the cleaning efficiency by weighting the surface before and after the cleaning procedure, in which the cleaning efficiency was determined by the soil mass removed. Each formulation was tested with at least five surfaces for both methods, and the cleaning value obtained was the average of those values. Finally, all the results were normalized with the control value and have the standard deviations presented as error bars in the data analysis.
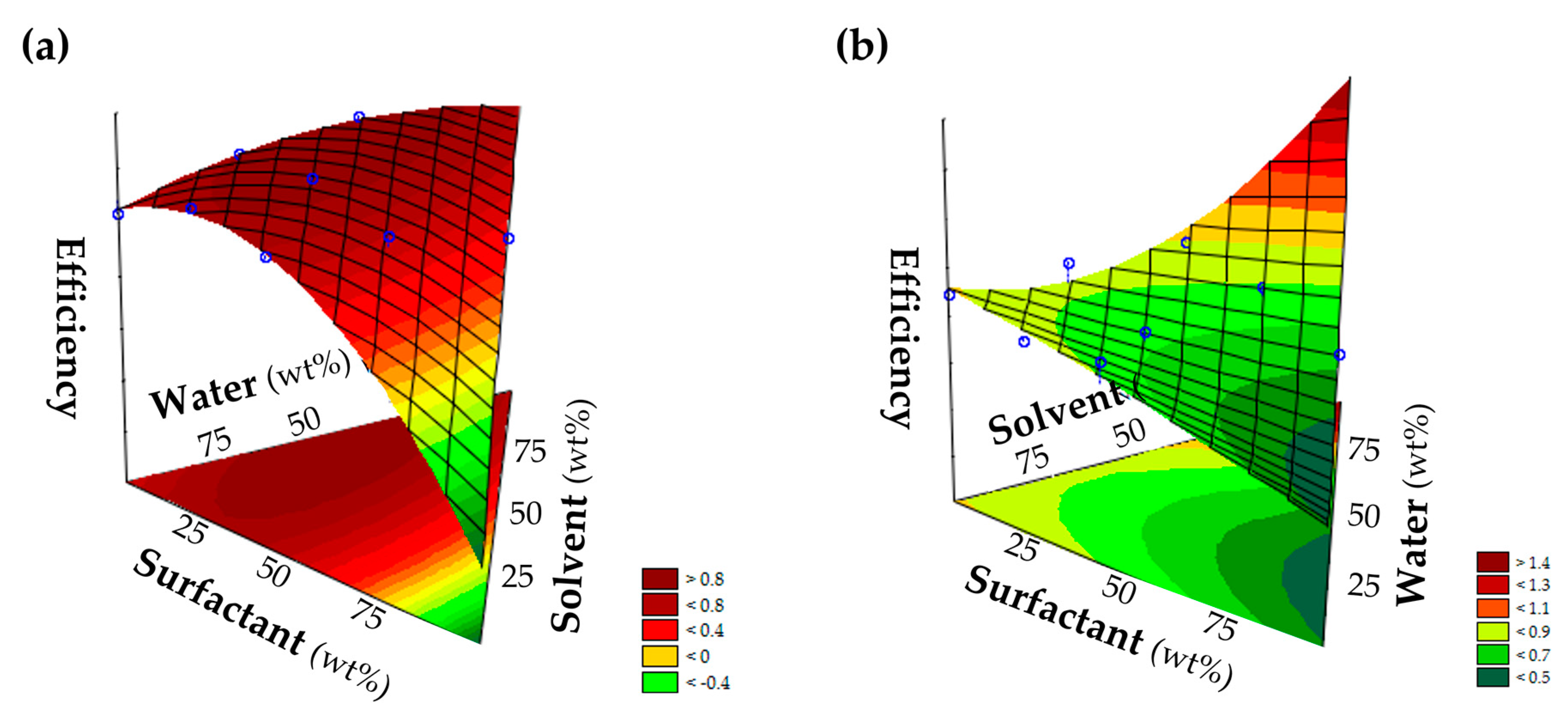
3.7. Mixture Design
Mixture design is a tool to study synergistic and antagonistic effects among components and determine the optimal formulation composition, through experimental planning [
21]. This study aimed to obtain the optimal concentration of the formulation components (independent variables) to reach the maximum cleaning efficiency (dependent variable). In this work, the formulations were a mixture of three components (surfactant, solvent, and water) with concentrations ranging from 3 wt% to 10 wt% of surfactant, 3 wt% to 13 wt% of solvent, and from 77 wt% to 94 wt% of water. For each formulation, nine experiments were developed, and the concentrations applied are provided in the
Supplementary Materials (Table S3). To design this mixture, the statistic and data analysis software JMP was used. The obtained results were statistically analyzed with a confidence level of 95% and subjected to analysis of variance (ANOVA) and regression analysis using the Statistica 7.0 software (StatSoft, Tulsa, OK, USA). The response surface of the concentrations was generated from adjusted models, and the optimal conditions could be determined through their analysis.
4. Conclusions
In this work, we attempted to develop new detergent formulations that could clean a carbonized soil from ceramics and stainless steel surfaces with a pH much lower than the current pH that most of the available commercial products use, which constitutes a risk for the user and the environment. A screening of a large number of combinations of solvents and ionic and nonionic surfactants was carried, and the formulations IPG + C11–C13 9EOs and BDG + [N1118][C8O2] stood out. The formulation composition was then optimized by experimental design, and the results show a cleaning efficiency equal or slightly higher than the control. For the formulation composed of IPG and C11–C13 9EOs, the optimal composition was 10–15 wt% solvent and 3–5 wt% surfactant, presenting a pH of around 4, while the formulation with BDG and [N1118][C8O2] showed an optimal composition of 13 wt% solvent and 7–8 wt% surfactant, with a pH of approximately 8. Moreover, nonionic surfactants showed better efficiencies in removing the carbonized soil from ceramic surface (cleaning efficiency of 1.00), and ionic surfactants showed better efficiencies on stainless steel surfaces (cleaning efficiency of 1.04). The use of IPG as a solvent is a more sustainable approach to degreaser formulations since it is a biobased solvent derived from renewable sources. This work shows that it is possible to develop simple formulations with environmentally friendly and sustainable compounds, with lower pH values. The results presented here produce new perspectives on using more sustainable compounds in the development of degreaser formulations while removing the necessity of using phosphates and high alkaline conditions.