N-Hydroxy-N-Propargylamide Derivatives of Ferulic Acid: Inhibitors of Cholinesterases and Monoamine Oxidases
Abstract
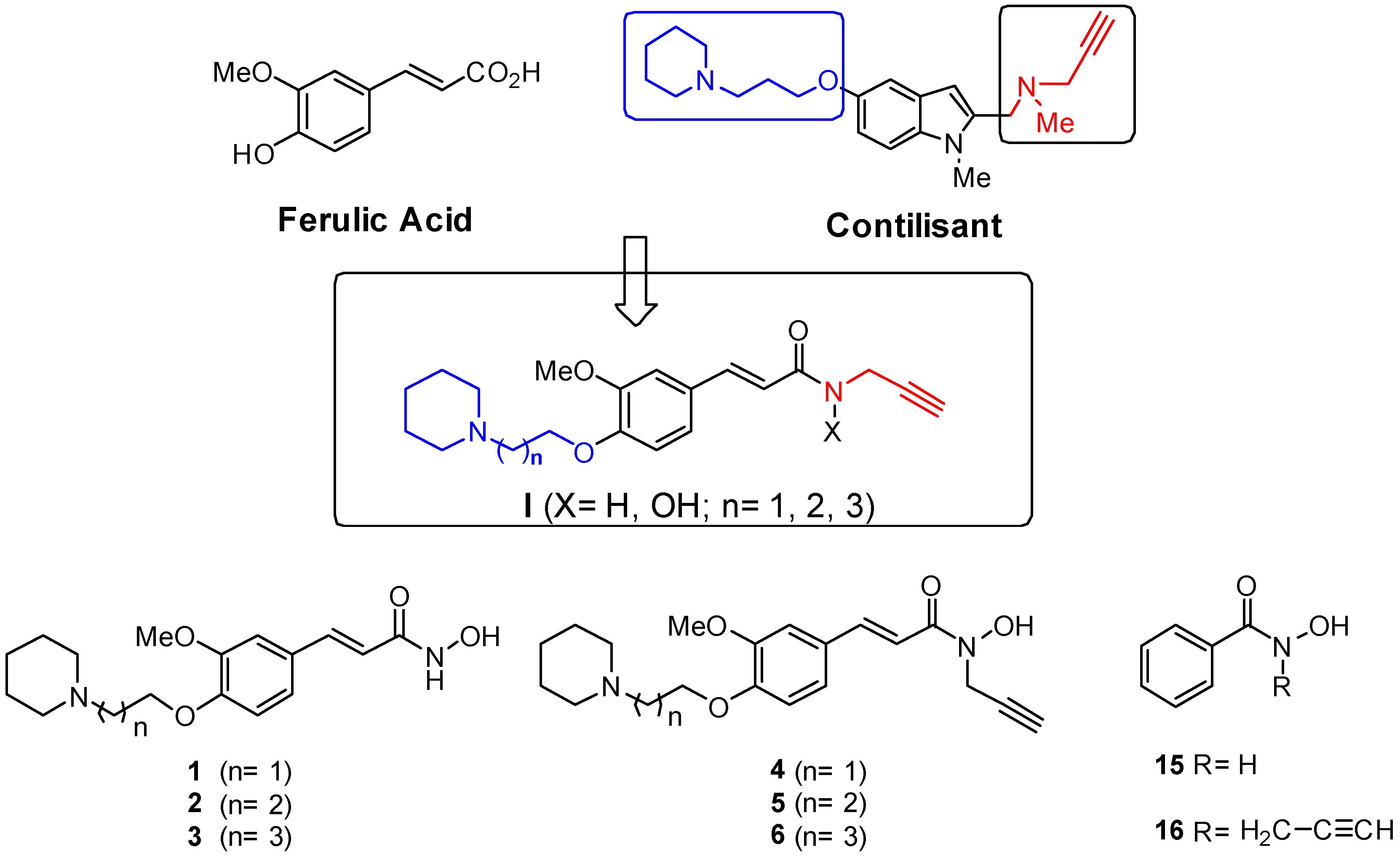
1. Introduction
2. Results
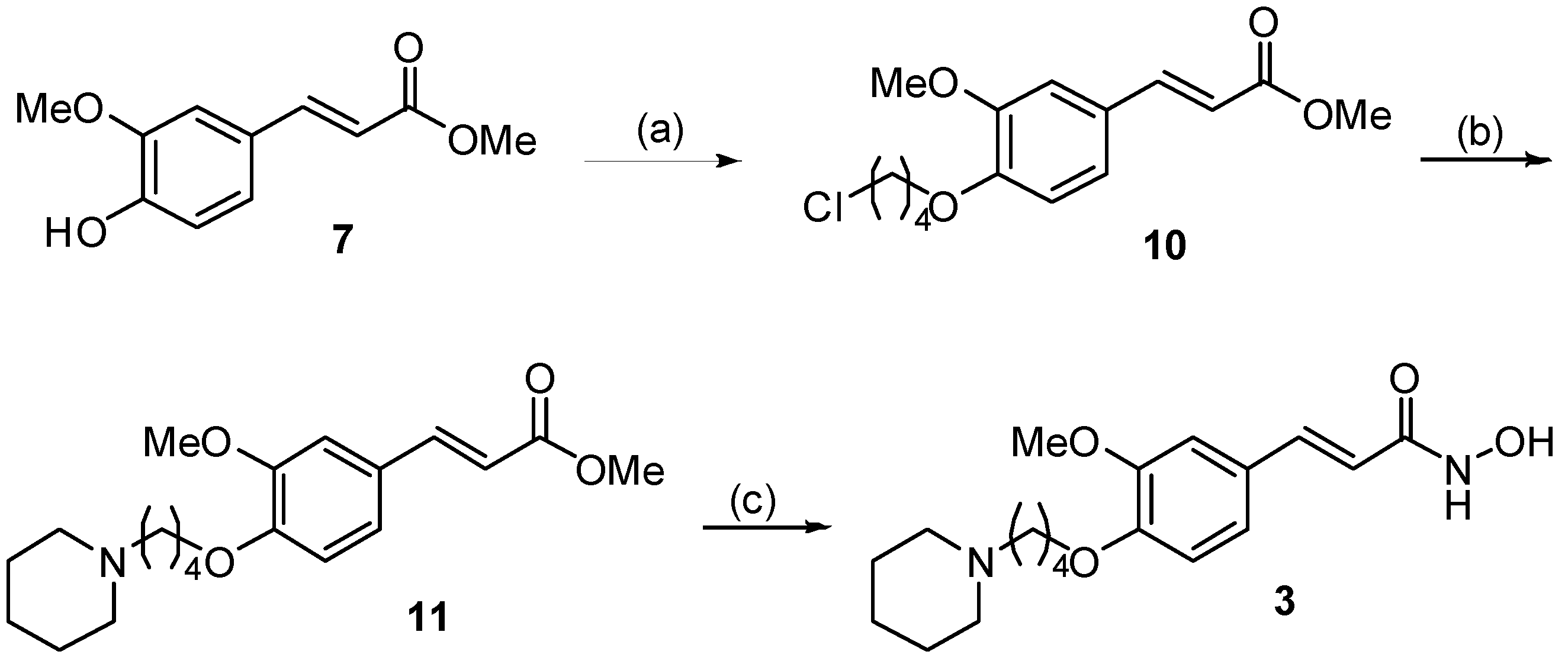
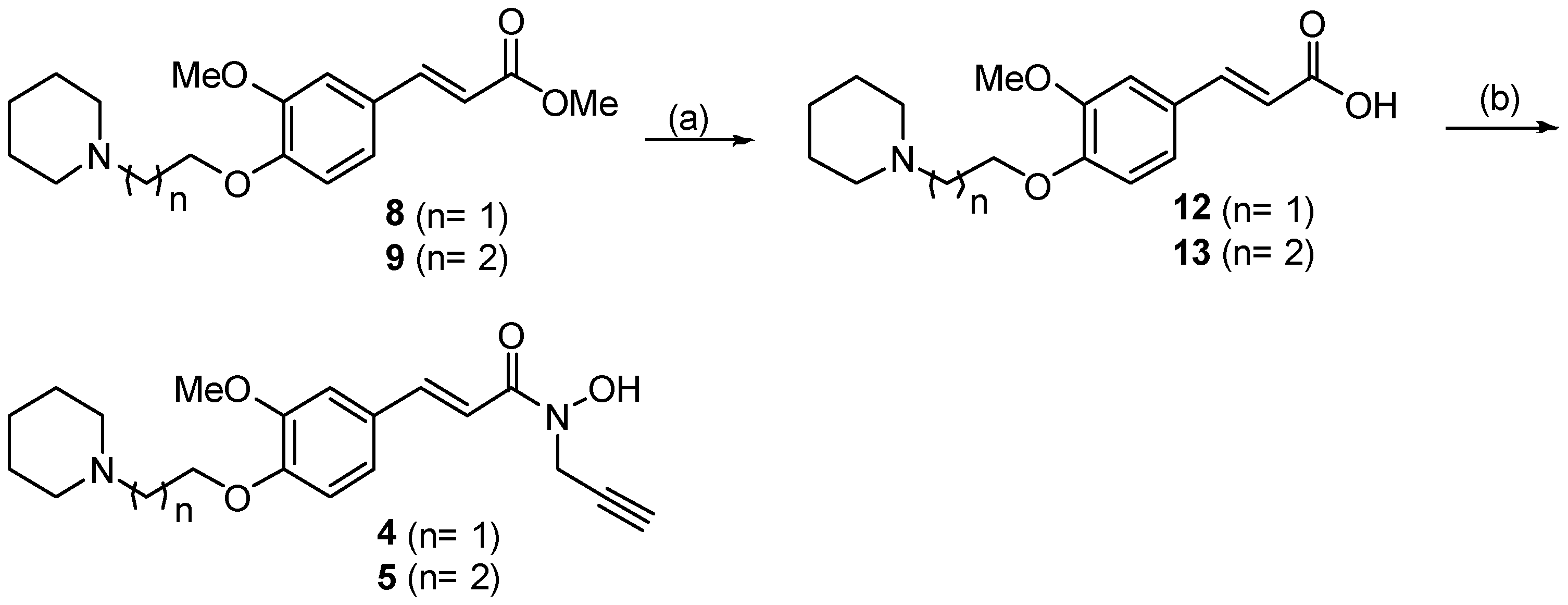
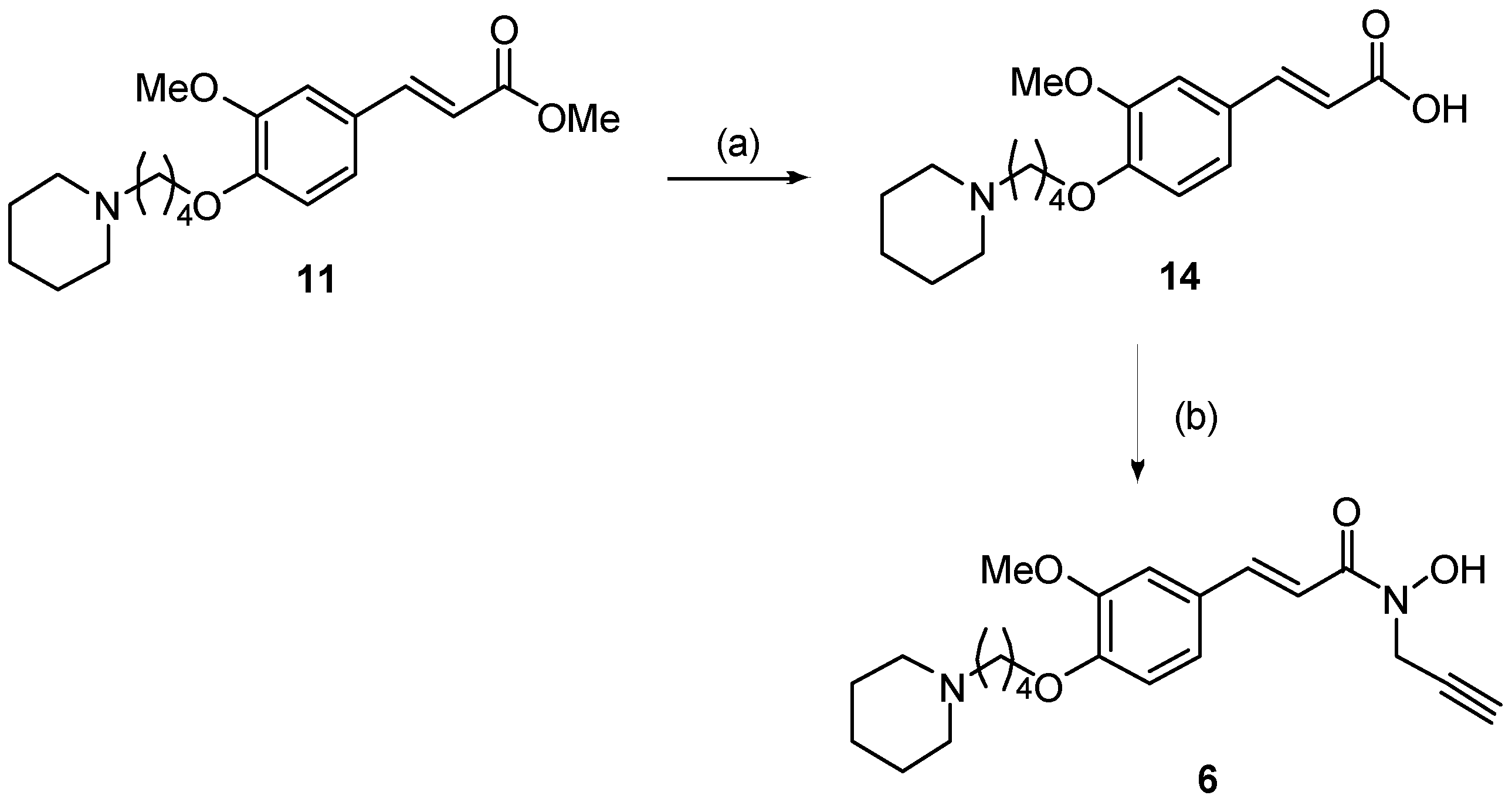
2.1. Synthesis
2.2. Biological Evaluation: Inhibition of hChEs/hMAOs
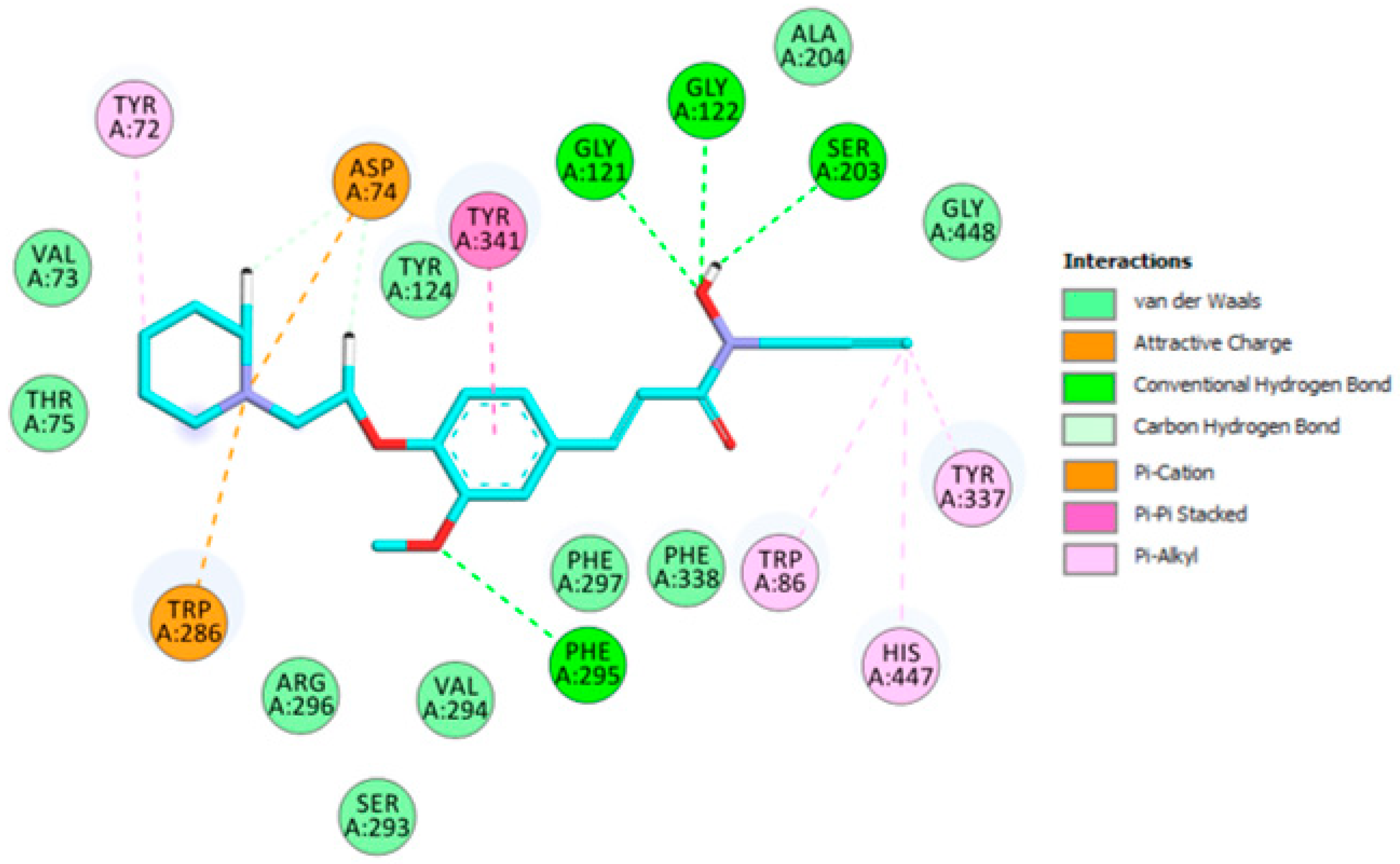
2.3. Molecular Modeling
2.4. ADME Virtual Profile for Compound 4
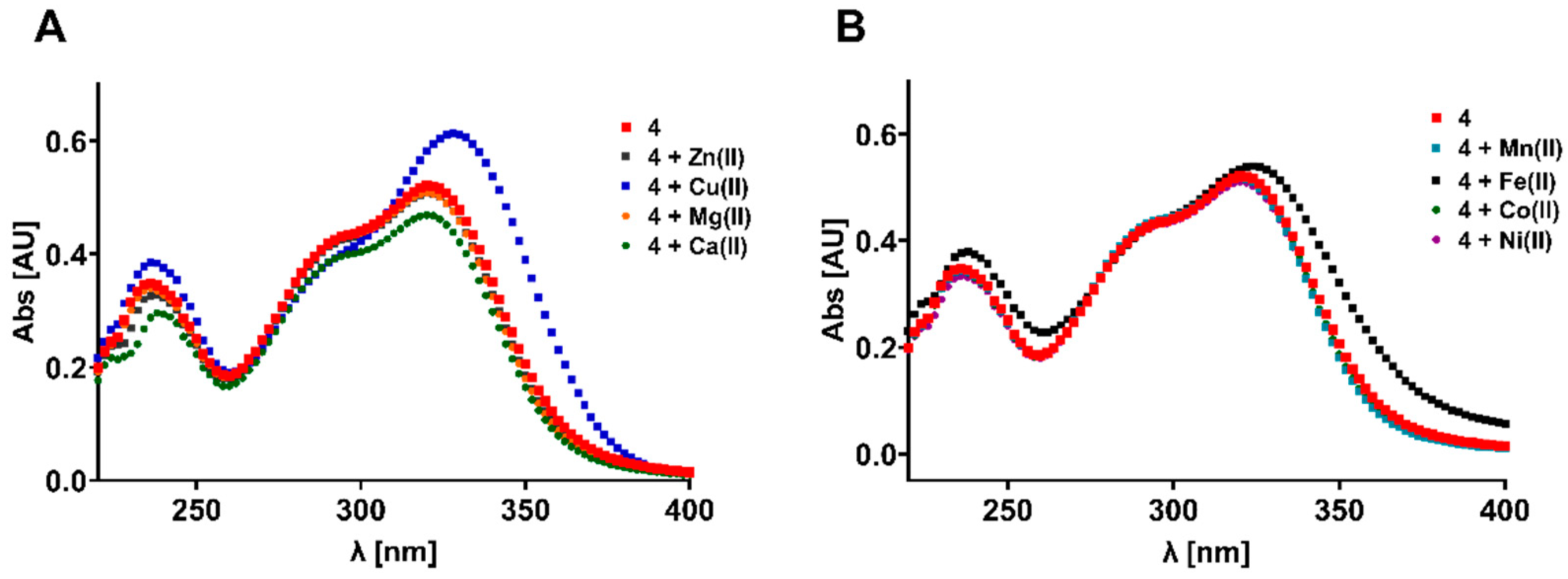
2.5. Antioxidative and Metal Chelating Properties
3. Materials and Methods
3.1. Synthesis of Compounds 1–6
3.2. Biological Evaluation: Inhibition of hChEs and hMAOs
3.3. Molecular Modeling
3.4. DPPH Radical-Scavenging Assay
3.5. Metal Chelation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ahmad, H.; Ahmad, S.; Ali, M.; Latif, A.; Shah, S.A.A.; Naz, H.; ur Rahman, N.; Shaheen, F.; Wadood, A.; Khan, H.U.; et al. Norditerpenoid alkaloids of Delphinium denudatum as cholinesterase inhibitors. Bioorg. Chem. 2018, 78, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.D.; Holtzman, D.M. TREM2 Function in Alzheimer’s disease and neurodegeneration. ACS Chem. Neurosci. 2016, 7, 420–427. [Google Scholar] [CrossRef]
- Bouza, C.; Martínez-Alés, G.; López-Cuadrado, T. Effect of dementia on the incidence, short-term outcomes, and resource utilization of invasive mechanical ventilation in the elderly: A nationwide population-based study. Crit. Care 2019, 23, 291. [Google Scholar] [CrossRef] [PubMed]
- Frölich, L. The cholinergic pathology in Alzheimer’s disease-discrepancies between clinical experience and pathophysiological findings. J. Neural Transm. 2002, 109, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Tumiatti, V.; Minarini, A.; Bolognesi, M.L.; Milelli, A.; Rosini, M.; Melchiorre, C. Tacrine derivatives and Alzheimer’s disease. Curr. Med. Chem. 2010, 17, 1825–1838. [Google Scholar] [CrossRef]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative stress in Alzheimer’s disease: Are we connecting the dots? J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef]
- Bush, A.I. Drug development based on the metals hypothesis of Alzheimer’s disease. J. Alzheimers Dis. 2008, 15, 223–240. [Google Scholar] [CrossRef]
- Bond, M.; Rogers, G.; Peters, J.; Anderson, R.; Hoyle, M.; Miners, A.; Moxham, T.; Davis, S.; Thokala, P.; Wailoo, A.; et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease (review of Technology Appraisal No. 111): A systematic review and economic model. Health Technol. Assess. 2012, 16, 1–470. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, M.; García, A.G.; Marco-Contelles, J.; López, M.G. An update on the pharmacology of galantamine. Expert Opin. Investig. Drugs 2007, 16, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Wirth, Y.; Goebel, C. Memantine in patients with moderate to severe Alzheimer’s disease: Meta-analyses using realistic definitions of response. Dement. Geriatr. Cogn. Disord. 2014, 37, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.N.; Raghanti, M.A. The role of monoamine oxidase Enzymes in the pathophysiology of neurological disorders. J. Chem. Neuroanat. 2021, 114, 101957. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Hoda, N. A Comprehensive review of monoamine oxidase inhibitors as anti-Alzheimer’s disease agents: A review. Eur. J. Med. Chem. 2020, 206, 112787. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and treatment of Parkinson disease: A review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.-H.; Park, J.-H.; Song, H.J.; Choi, J.W.; Kim, S.; Jang, B.K.; Yoon, H.H.; Heo, J.Y.; Lee, H.; An, H.; et al. KDS2010, a newly developed reversible MAO-B inhibitor, as an effective therapeutic candidate for Parkinson’s disease. Neurotherapeutics 2021, 18, 1729–1747. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; de los Ríos, C.; Bolea, I.; Chioua, M.; Iriepa, I.; Moraleda, I.; Bartolini, M.; Andrisano, V.; Gálvez, E.; Valderas, C.; et al. Multipotent MAO and cholinesterase inhibitors for the treatment of Alzheimer’s disease: Synthesis, pharmacological analysis and molecular modeling of heterocyclic substituted alkyl and cycloalkyl propargyl amine. Eur. J. Med. Chem. 2012, 52, 251–262. [Google Scholar] [CrossRef]
- Bautista-Aguilera, O.M.; Budni, J.; Mina, F.; Medeiros, E.B.; Deuther-Conrad, W.; Entrena, J.M.; Moraleda, I.; Iriepa, I.; Lòpez-Munoz, F.; Marco-Contelles, J. Contilisant, a tetratarget small molecule for Alzheimer’s disease therapy combining cholinesterase, monoamine oxidase inhibition, and H3R antagonism with S1R agonism profile. J. Med. Chem. 2018, 61, 6937–6943. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Combes, J.; Woolley, J.M.; Rodrigues, N.d.N.; Mention, M.M.; Stavros, V.G.; Allais, F. From biomass-derived p-hydroxycinnamic acids to novel sustainable and non-toxic phenolics-based UV-filters: A multidisciplinary journey. Front. Chem. 2022, 10, 886367. [Google Scholar] [CrossRef]
- Flourat, A.L.; Combes, J.; Bailly-Maitre-Grand, C.; Magnien, K.; Haudrechy, A.; Renault, J.-H.; Allais, F. Accessing p-hydroxycinnamic acids: Chemical synthesis, biomass recovery, or engineered microbial production? ChemSusChem 2021, 14, 118–129. [Google Scholar] [CrossRef]
- Singh, Y.P.; Rai, H.; Singh, G.; Singh, G.K.; Mishra, S.; Kumar, S.; Srikrishna, S.; Modi, G. A review on ferulic acid and analogs based scaffolds for the management of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 215, 113278. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Devi, K.P.; Malar, D.S.; Sureda, A.; Daglia, M.; Nabavi, S.M. Ferulic acid and Alzheimer’s disease: Promises and pitfalls. Mini Rev. Med. Chem. 2015, 15, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.; Chen, H.; Bian, X.; Yang, G. A new series of salicylic acid derivatives as non-saccharide α-glucosidase inhibitors and antioxidants. Biol. Pharm. Bull. 2019, 42, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Temming, R.P.; Eggermont, L.; van Eldijk, M.B.; van Hest, J.C.M.; van Delft, F.L. N-terminal dual protein functionalization by strain-promoted alkyne-nitrone cycloaddition. Org. Biomol. Chem. 2013, 11, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; De Palma, A.; Giangregorio, N.; Miniero, D.V.; Pesce, P.; Nicolotti, O.; Campagna, F.; Altomare, C.D.; Catto, M. Mannich base approach to 5-methoxyisatin 3-(4-isopropylphenyl)hydrazone: A water-soluble prodrug for a multitarget inhibition of cholinesterases, beta-amyloid fibrillization and oligomer-induced cytotoxicity. Eur. J. Pharm. Sci. 2017, 109, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Duffy, E.; Jorgensen, W. Prediction of properties from simulations: Free energies of solvation in hexadecane, octanol, and water. J. Am. Chem. Soc. 2000, 122, 2878–2888. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from Monte Carlo simulations. Bioorg. Med. Chem. Lett. 2000, 10, 1155–1158. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchís, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andrés-Lacueva, C.; Somogyvari, M.; Soti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dement. 2022, 8, e12295. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Mehndiratta, S.; Nepali, K.; Satapathy, M.K. Hydroxamic acids as potent antioxidants and their methods of evaluation. In Oxidative Stress: Diagnostic Methods and Applications in Medical Science; Kumar Maurya, P.K., Chandra, P., Eds.; Springer: Singapore, 2017; Chapter 5; pp. 97–112. [Google Scholar]
- Yanovsky, I.; Finkin-Groner, E.; Zaikin, A.; Lerman, L.; Shalom, H.; Zeeli, S.; Weill, T.; Ginsburg, I.; Nudelman, A.; Weinstock, M. Carbamate derivatives of indolines as cholinesterase inhibitors and antioxidants for the treatment of alzheimer’s disease. J. Med. Chem. 2012, 55, 10700–10715. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pachauri, V.; Flora, S.J.S. Advances in multi-functional ligands and the need for metal-related pharmacology for the management of alzheimer disease. Front. Pharmacol. 2018, 9, 1247. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, H.J.; Kim, K.Y.; Nam, G.; Chae, J.; Lim, M.H. Mechanistic approaches for chemically modifying the coordination sphere of copper-amyloid-β complexes. Proc. Natl. Acad. Sci. USA 2020, 117, 5160–5167. [Google Scholar] [CrossRef] [PubMed]
- Poreddy, A.R.; Schall, O.F.; Osiek, T.A.; Wheatley, J.R.; Beusen, D.D.; Marshall, G.R.; Slomczynska, U. Hydroxamate-based iron chelators: Combinatorial syntheses of desferrioxamine b analogs and evaluation of binding affinities. J. Comb. Chem. 2004, 6, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Iacobazzi, R.M.; Catto, M.; Rullo, M.; Farina, R.; Denora, N.; Cellamare, S.; Altomare, C.D. Investigating alkyl nitrates as nitric oxide releasing precursors of multitarget acetylcholinesterase-monoamine oxidase B inhibitors. Eur. J. Med. Chem. 2019, 161, 292–309. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L., III; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comp. Chem. 2009, 30, 1545–1615. [Google Scholar] [CrossRef]
- Chioua, M.; Buzzi, E.; Moraleda, I.; Iriepa, I.; Maj, M.; Wnorowski, A.; Giovannini, C.; Tramarin, A.; Portali, F.; Ismaili, L.; et al. Tacripyrimidines, the first tacrine-dihydropyrimidine hybrids, as multi-target-directed ligands for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 155, 839–846. [Google Scholar] [CrossRef]
- Diez-Iriepa, D.; Iriepa, I.; Knez, D.; Gobec, S.; de los Ríos, C.; Bravo, I.; López-Muñoz, F.; Marco-Contelles, J.; Hadjipavlou-Litina, D. Polyfunctionalized α-phenyl-tert-buty(benzyl)lnitrones: Multifunctional antioxidants for stroke treatment. Antioxidants 2022, 11, 1735. [Google Scholar] [CrossRef]
- Knez, D.; Brus, B.; Coquelle, N.; Sosic, I.; Sink, R.; Brazzolotto, X.; Mravljak, J.; Colletier, J.-P.; Gobec, S. Structure-based development of nitroxoline derivatives as potential multifunctional anti-Alzheimer agents. Bioorg. Med. Chem. 2015, 23, 4442–4444. [Google Scholar] [CrossRef] [PubMed]


| Compound | hAChE | hBChE | hMAO-A | hMAO-B | |
|---|---|---|---|---|---|
| % @ 10 µM | IC50 (µM) | % @ 10 µM | |||
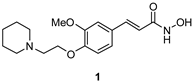 | 51 ± 2 | 18 ± 4 | 20 ± 5 | 26 ± 5 | |
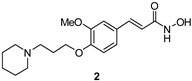 | 42 ± 2 | ni | 15 ± 5 | 21 ± 4 | |
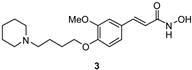 | 6.58 ± 0.27 | ni | 5 ± 3 | 11 ± 4 | |
 | 2.63 ± 0.57 | 38 ± 2 | 26 ± 5 | 20 ± 4 | |
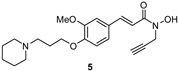 | 41 ± 4 | ni | 23 ± 6 | 16 ± 5 | |
 | 7.20 ± 0.54 | ni | ni | ni | |
 | 58 ± 5 | ni | 11 ± 3 | 16 ± 3 | |
 | 40 ± 4 | ni | 14 ± 4 | 16 ± 5 | |
| CNS | MW | SASA | Volume | donorHB | accptHB | QPlogPo/w | QPlogS |
| 0 | 358.436 | 746.494 | 1264.897 | 1.500 | 7.700 | 2.888 | −3.982 |
| QPPCaco | QPlogBB | metab | % HOA | PSA | ROF | ROT | |
| 261.504 | −0.895 | 4 | 87.1240 | 67.634 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Aguilera, Ó.M.; Alonso, J.M.; Catto, M.; Iriepa, I.; Knez, D.; Gobec, S.; Marco-Contelles, J. N-Hydroxy-N-Propargylamide Derivatives of Ferulic Acid: Inhibitors of Cholinesterases and Monoamine Oxidases. Molecules 2022, 27, 7437. https://doi.org/10.3390/molecules27217437
Bautista-Aguilera ÓM, Alonso JM, Catto M, Iriepa I, Knez D, Gobec S, Marco-Contelles J. N-Hydroxy-N-Propargylamide Derivatives of Ferulic Acid: Inhibitors of Cholinesterases and Monoamine Oxidases. Molecules. 2022; 27(21):7437. https://doi.org/10.3390/molecules27217437
Chicago/Turabian StyleBautista-Aguilera, Óscar M., José M. Alonso, Marco Catto, Isabel Iriepa, Damijan Knez, Stanislav Gobec, and José Marco-Contelles. 2022. "N-Hydroxy-N-Propargylamide Derivatives of Ferulic Acid: Inhibitors of Cholinesterases and Monoamine Oxidases" Molecules 27, no. 21: 7437. https://doi.org/10.3390/molecules27217437
APA StyleBautista-Aguilera, Ó. M., Alonso, J. M., Catto, M., Iriepa, I., Knez, D., Gobec, S., & Marco-Contelles, J. (2022). N-Hydroxy-N-Propargylamide Derivatives of Ferulic Acid: Inhibitors of Cholinesterases and Monoamine Oxidases. Molecules, 27(21), 7437. https://doi.org/10.3390/molecules27217437









