Enrichment of Quercetin from Winemaking Residual Diatomaceous Earth via a Tailor-Made Imprinted Adsorbent
Abstract
1. Introduction
2. Results and Discussion
2.1. Rationale for MIP Synthesis
- YM is the mass fraction of FMs and the crosslinker in the solution (%);
- YI is the mole fraction of initiator relative to the FMs and the crosslinker (%);
- YCL is the mole fraction of the crosslinker in the mixture of FMs/crosslinker (%);
- YFM/T is the mole ratio of the FM to the template molecule.
FTIR Characterization
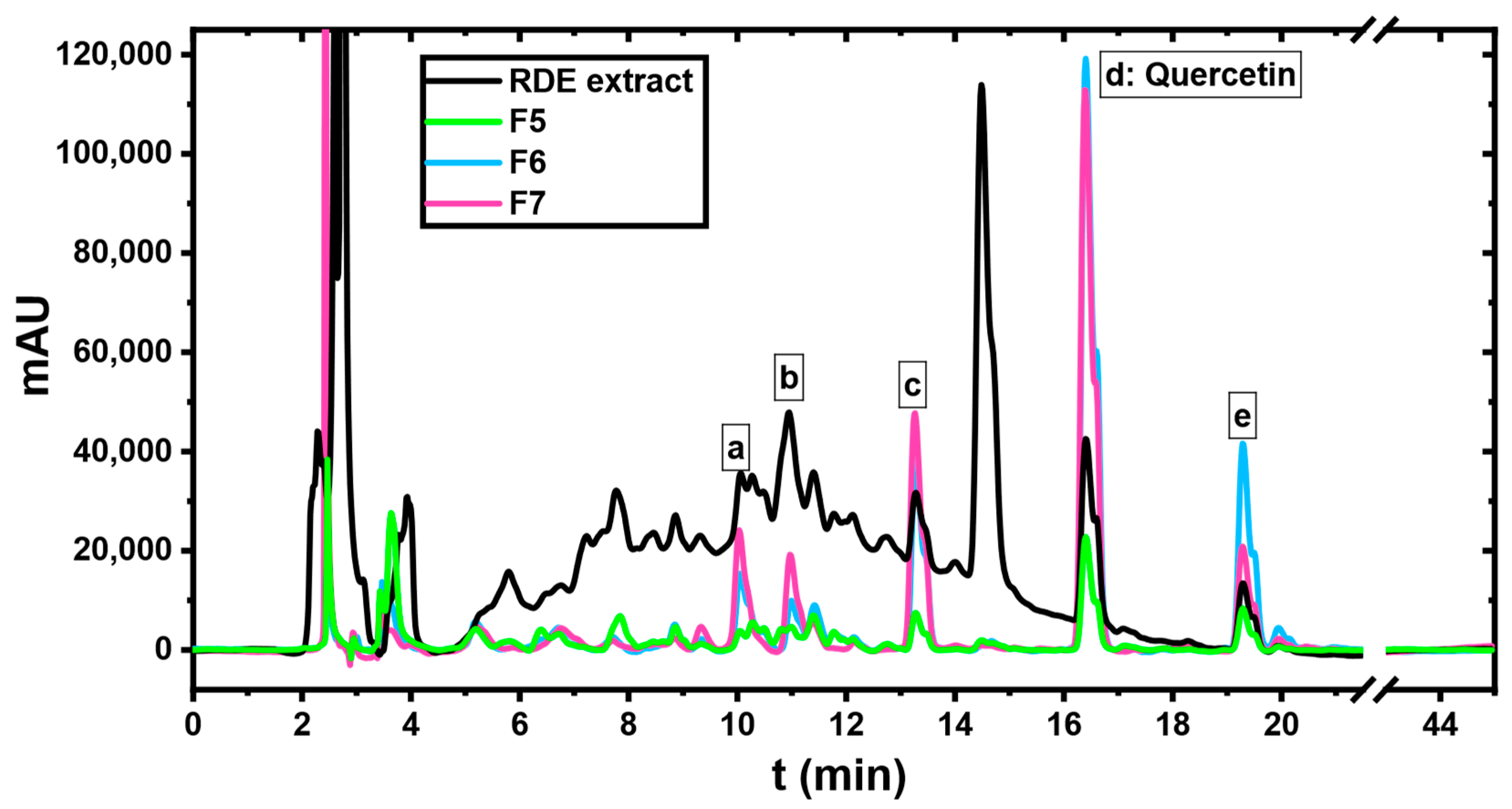
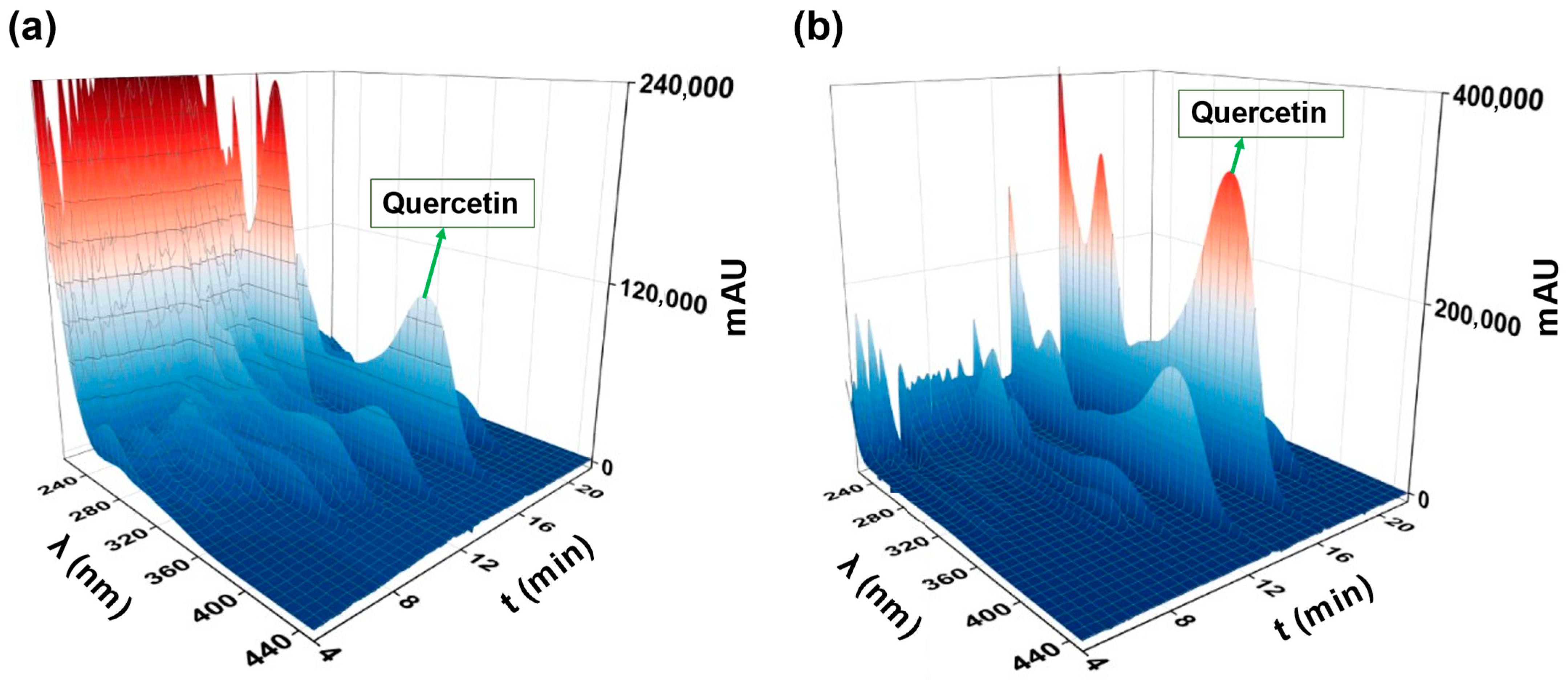
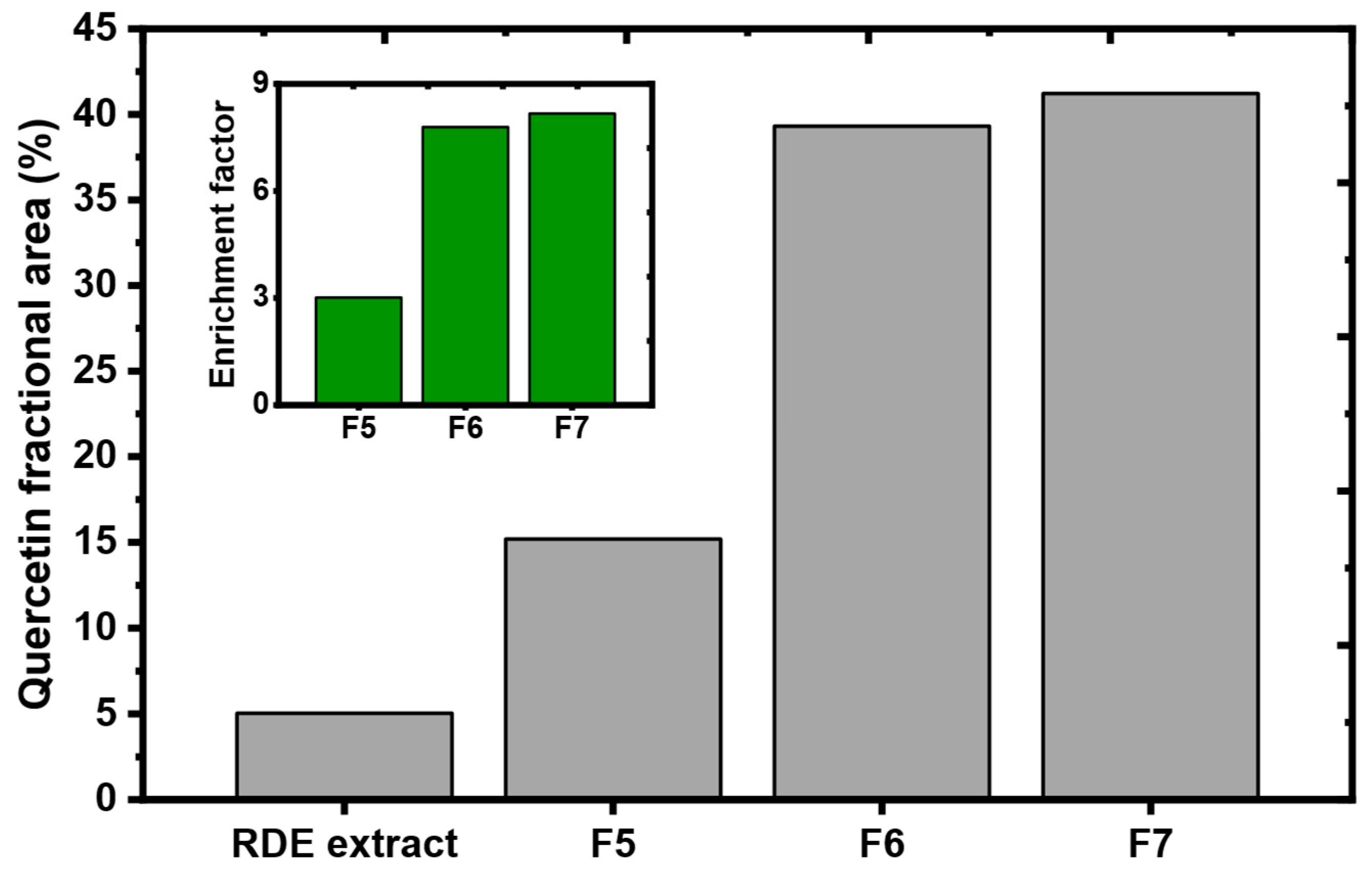
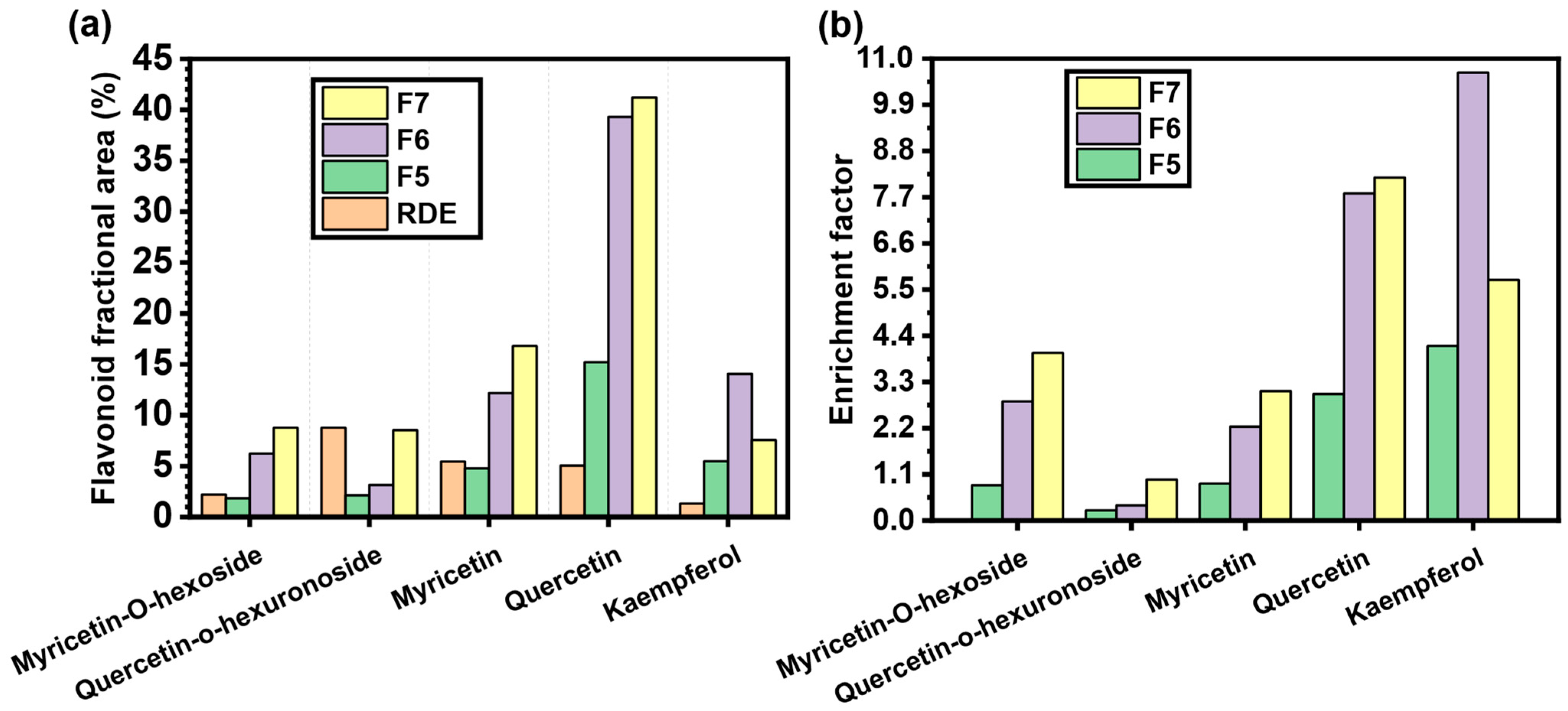
2.2. Screening of the Potential Adsorbent for RDE Treatment
2.3. Residual Diatomaceous Earth Treatment with MQ3
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of 4VAN-BTPI Monomer
3.3. Synthesis of Quercetin-Imprinted Polymers
3.4. FTIR
3.5. Batch Adsorption of Polyphenols
3.6. Extraction of Diatomaceous Earth
3.7. RDE Extract Treatment by a MIP
- 100% Water: F1;
- Water/MeOH 80/20 (v/v): F2;
- Water/MeOH 60/40 (v/v): F3;
- Water/MeOH 40/60 (v/v): F4;
- Water/MeOH 20/80 (v/v): F5;
- 100% MeOH F6;
- MeOH/AcOH 90/10 (v/v): F7.
3.8. UHPLC-DAD Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- McGovern, P.E. Ancient Wine: The Search for the Origins of Viniculture; Princeton University Press: Princeton, NJ, USA, 2019; pp. 64–78. [Google Scholar]
- OIV. Available online: https://www.oiv.int/ (accessed on 3 July 2022).
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess 2018, 5, 46. [Google Scholar] [CrossRef]
- Bolzonella, D.; Papa, M.; Da Ros, C.; Muthukumar, L.A.; Rosso, D. Winery wastewater treatment: A critical overview of advanced biological processes. Crit. Rev. Biotechnol. 2019, 39, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Nanni, A.; Parisi, M.; Colonna, M. Wine By-Products as Raw Materials for the Production of Biopolymers and of Natural Reinforcing Fillers: A Critical Review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.É.; Belyakov, A.V. Diatomite and its applications. Glas Ceram. 2008, 65, 48–51. [Google Scholar] [CrossRef]
- Reka, A.A.; Pavlovski, B.; Fazlija, E.; Berisha, A.; Pacarizi, M.; Daghmehchi, M.; Sacalis, C.; Jovanovski, G.; Makreski, P.; Oral, A. Diatomaceous Earth: Characterization, thermal modification, and application. Open. Chem. 2021, 19, 451–461. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food. Sci. Food. Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S29–S45. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Abadi, R.E.N.; Kazemipour, N.; Pahlevanneshan, Z.; Beheshti, S. Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP. Sci. Rep. 2019, 9, 6876. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xia, P.; Wang, S.; Liang, J.; Sun, Y.; Yue, P.; Gao, X. Packaging films formulated with gelatin and anthocyanins nanocomplexes: Physical properties, antioxidant activity and its application for olive oil protection. Food Hydrocoll. 2019, 96, 617–624. [Google Scholar] [CrossRef]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the physical properties and biological activity of chitosan films grafted with gallic acid and caffeic acid: A comparison study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, B.; Cheng, D.; Li, J.; Huang, F.; Lu, Y. Dyeing characteristics and functionability of tussah silk fabric with oak bark extract. Text Res. J. 2017, 87, 1806–1817. [Google Scholar] [CrossRef]
- Kelly, N.P.; Kelly, A.L.; O’Mahony, J.A. Strategies for enrichment and purification of polyphenols from fruit-based materials. Trends Food Sci. Technol. 2019, 83, 248–258. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Spigno, G.; Amendola, D.; Dahmoune, F.; Jauregi, P. Colloidal gas aphrons based separation process for the purification and fractionation of natural phenolic extracts. Food Bioprod. Process. 2015, 94, 434–442. [Google Scholar] [CrossRef]
- Noriega, D.; Zuñiga, M.E.; Soto, C.; MohdMaidin, N.; Michael, N.; Jauregi, P. Colloidal Gas Aphrons separation to obtain polyphenol rich fractions from artichoke agro-industrial discards. Food Bioprod. Process. 2018, 110, 50–59. [Google Scholar] [CrossRef]
- Pinelli, D.; Bacca, A.E.M.; Kaushik, A.; Basu, S.; Nocentini, M.; Bertin, L.; Frascari, D. Batch and Continuous Flow Adsorption of Phenolic Compounds from Olive Mill Wastewater: A Comparison between Nonionic and Ion Exchange Resins. Int. J. Chem. Eng. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Kalecki, J.; Iskierko, Z.; Cieplak, M.; Sharma, P.S. Oriented Immobilization of Protein Templates: A New Trend in Surface Imprinting. ACS Sens. 2020, 5, 3710–3720. [Google Scholar] [CrossRef]
- Gomes, C.P.; Dias, R.C.S.; Rui, M. Preparation of Molecularly Imprinted Adsorbents with Improved Retention Capability of Polyphenols and Their Application in Continuous Separation Processes. Chromatographia 2019, 82, 893–916. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Mora-Granados, M.; González-Gómez, D.; Jeong, J.S.; Gallego-Picó, A. A Molecularly Imprinted Polymer for Selective Extraction of Phenolic Acids from Human Urine. Appl. Sci. 2021, 11, 1577. [Google Scholar] [CrossRef]
- El-Schich, Z.; Abdullah, M.; Shinde, S.; Dizeyi, N.; Rosén, A.; Sellergren, B.; Wingren, A.G. Different expression levels of glycans on leukemic cells—a novel screening method with molecularly imprinted polymers (MIP) targeting sialic acid. Tumor. Biol. 2016, 37, 13763–13768. [Google Scholar] [CrossRef]
- Didaskalou, C.; Buyuktiryaki, S.; Kecili, R.; Fonte, C.P.; Szekely, G. Valorisation of agricultural waste with an adsorption/nanofiltration hybrid process: From materials to sustainable process design. Green Chem. 2017, 19, 3116–3125. [Google Scholar] [CrossRef]
- Oliveira, D.; Freitas, A.; Kadhirvel, P.; Dias, R.C.S.; Costa, M.R.P.F.N. Development of high performance and facile to pack molecularly imprinted particles for aqueous applications. Biochem. Eng. J. 2016, 111, 87–99. [Google Scholar] [CrossRef]
- Sigma Aldrich. Available online: https://www.sigmaaldrich.com/PT/en (accessed on 12 July 2022).
- Gomes, C.P.; Franco, V.; Dias, R.C.S.; Costa, M.R.P.F.N. Processing of Onion Skin Extracts with Quercetin-Molecularly Imprinted Adsorbents Working at a Wide Range of Water Content. Chromatographia 2020, 83, 1539–1551. [Google Scholar] [CrossRef]
- Gomes, C.; Sadoyan, G.; Dias, R.; Costa, M. Development of Molecularly Imprinted Polymers to Target Polyphenols Present in Plant Extracts. Processes 2017, 5, 72. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Hall, A.J.; Manesiotis, P.; Emgenbroich, M.; Quaglia, M.; De Lorenzi, E.; Sellergren, B. Urea Host Monomers for Stoichiometric Molecular Imprinting of Oxyanions. J. Org. Chem. 2005, 70, 1732–1736. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]

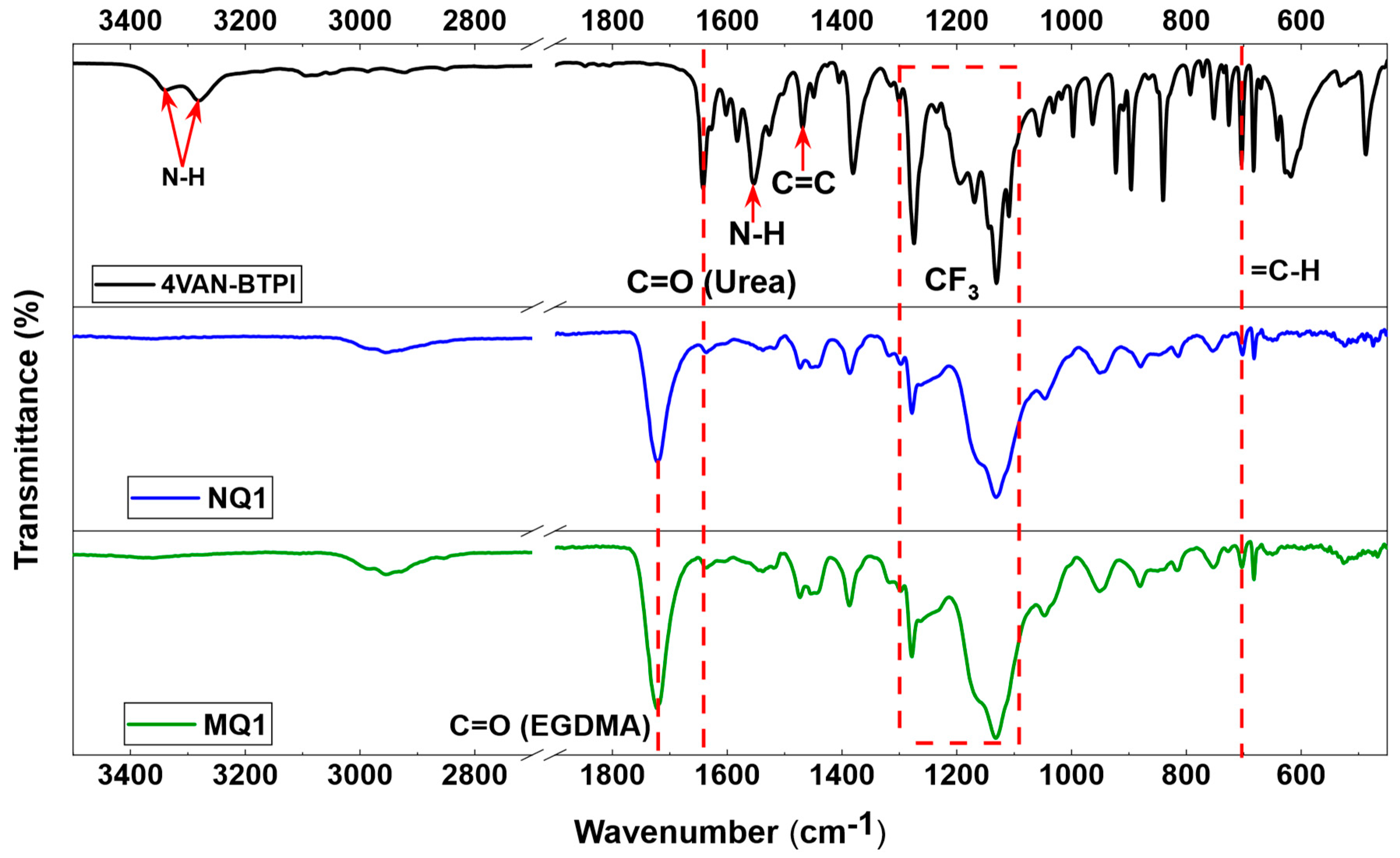
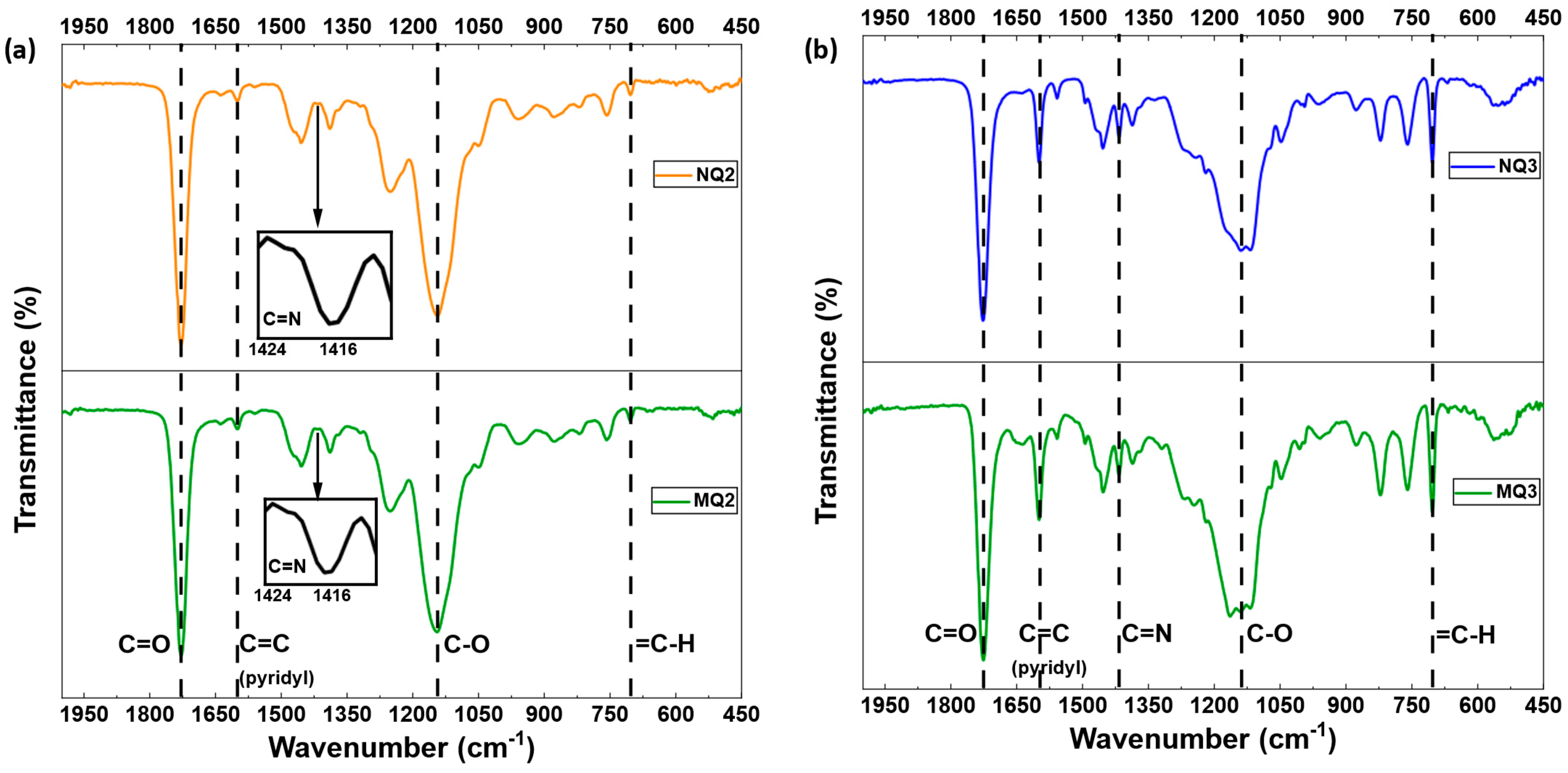
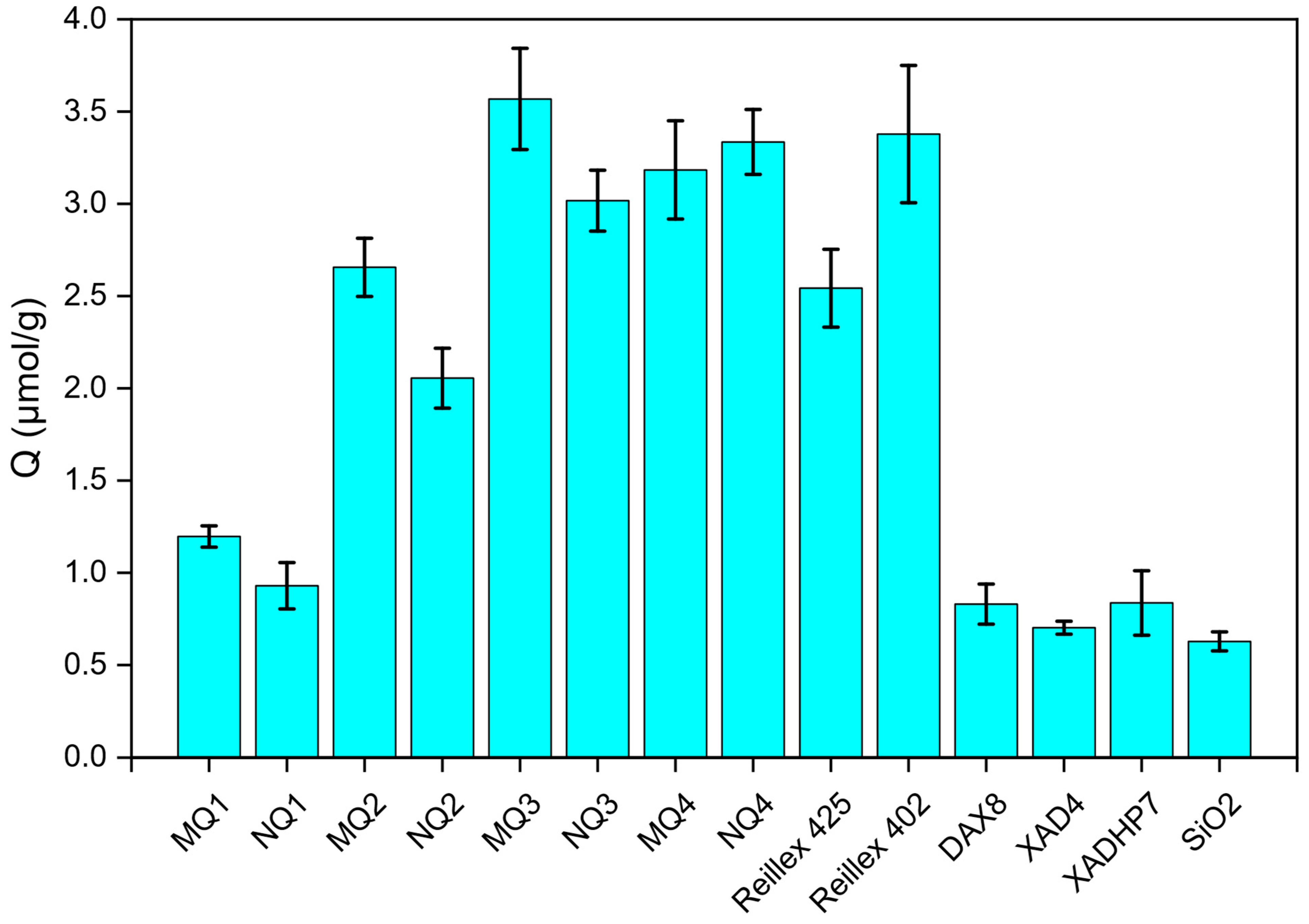
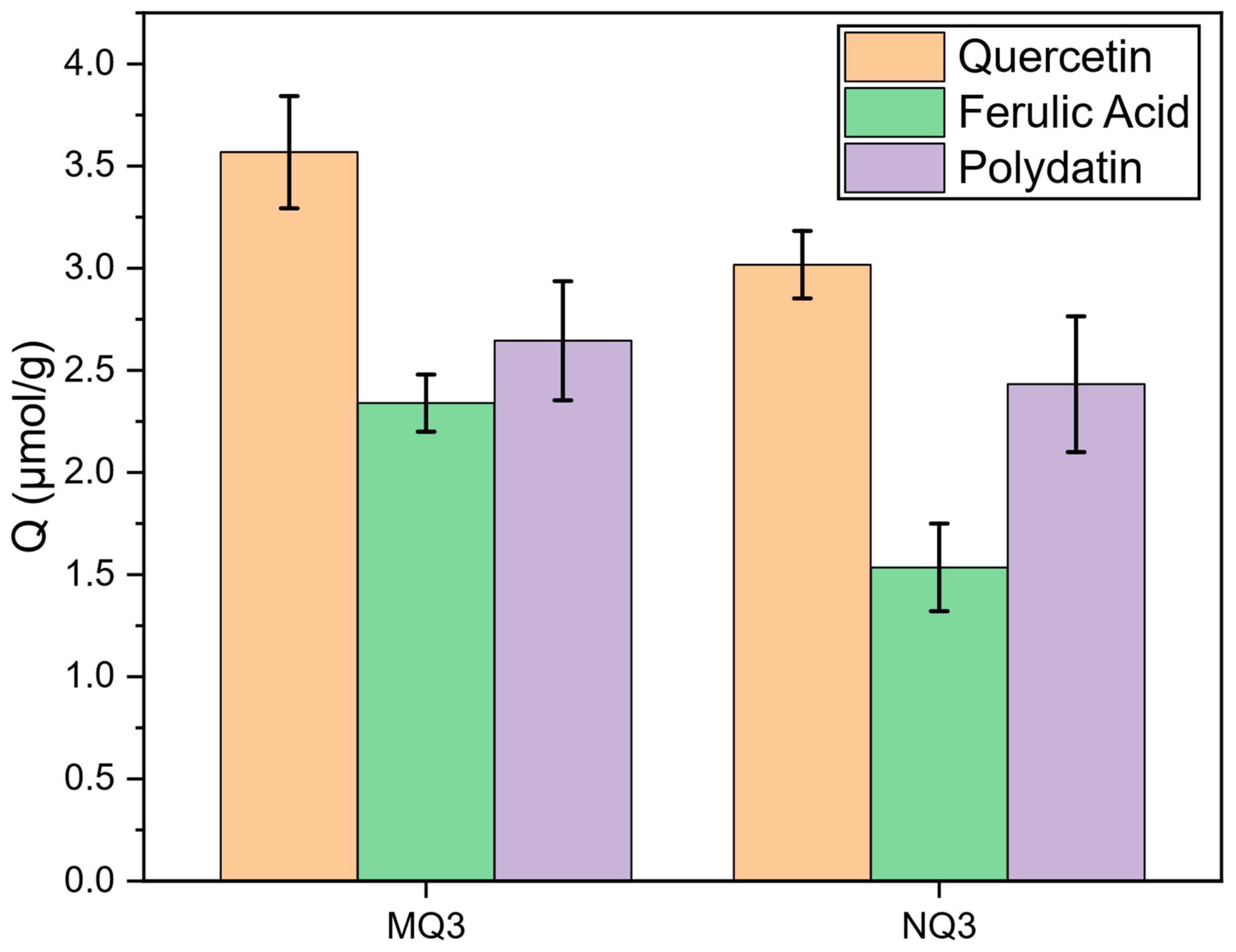


| Material | Template | FM1 | FM2 | Solvent (v/v) | YM | YI | YCL | YFM1/T | YFM2/T | Yield (%) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MQ1 NQ1 | Quercetin N/A | 4VAN-BTPI | STY | ACN/DMF 85/15 | 23.97 | 5.32 | 83.33 | 2 N/A | 1 N/A | 81.73 86.70 | Bulky material |
| MQ2 | Quercetin | 4VP | STY | ACN/DMF 85/15 | 21.46 | 5.32 | 83.33 | 2 | 1 | 95.9 | Bulky material |
| NQ2 | N/A | N/A | N/A | 97.9 | |||||||
| MQ3 | Quercetin | 4VP | STY | ACN/DMF 85/15 | 6.00 | 5.22 | 40 | 2 | 1 | 68.6 | Particle powder |
| NQ3 | N/A | N/A | N/A | 74.3 | |||||||
| MQ4 | Quercetin | 4VP | STY | ACN/DMF 70/30 | 21.76 | 5.33 | 40 | 2 | 1 | 96.1 | Bulky material |
| NQ4 | N/A | N/A | N/A | 96.8 |
| Peak | Retention Time (min) | Tentative Identification |
|---|---|---|
| a | 10.06 | Myricetin-O-hexoside |
| b | 10.9 | Quercetin-o-hexuronoside |
| c | 13.3 | Myricetin |
| d | 16.4 | Quercetin |
| e | 19.3 | Kaempferol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bzainia, A.; Dias, R.C.S.; Costa, M.R.P.F.N. Enrichment of Quercetin from Winemaking Residual Diatomaceous Earth via a Tailor-Made Imprinted Adsorbent. Molecules 2022, 27, 6406. https://doi.org/10.3390/molecules27196406
Bzainia A, Dias RCS, Costa MRPFN. Enrichment of Quercetin from Winemaking Residual Diatomaceous Earth via a Tailor-Made Imprinted Adsorbent. Molecules. 2022; 27(19):6406. https://doi.org/10.3390/molecules27196406
Chicago/Turabian StyleBzainia, Amir, Rolando C. S. Dias, and Mário Rui P. F. N. Costa. 2022. "Enrichment of Quercetin from Winemaking Residual Diatomaceous Earth via a Tailor-Made Imprinted Adsorbent" Molecules 27, no. 19: 6406. https://doi.org/10.3390/molecules27196406
APA StyleBzainia, A., Dias, R. C. S., & Costa, M. R. P. F. N. (2022). Enrichment of Quercetin from Winemaking Residual Diatomaceous Earth via a Tailor-Made Imprinted Adsorbent. Molecules, 27(19), 6406. https://doi.org/10.3390/molecules27196406






