High Catalytic Efficiency of a Nanosized Copper-Based Catalyst for Automotives: A Physicochemical Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of the Catalyst
2.1.1. XRF Analysis
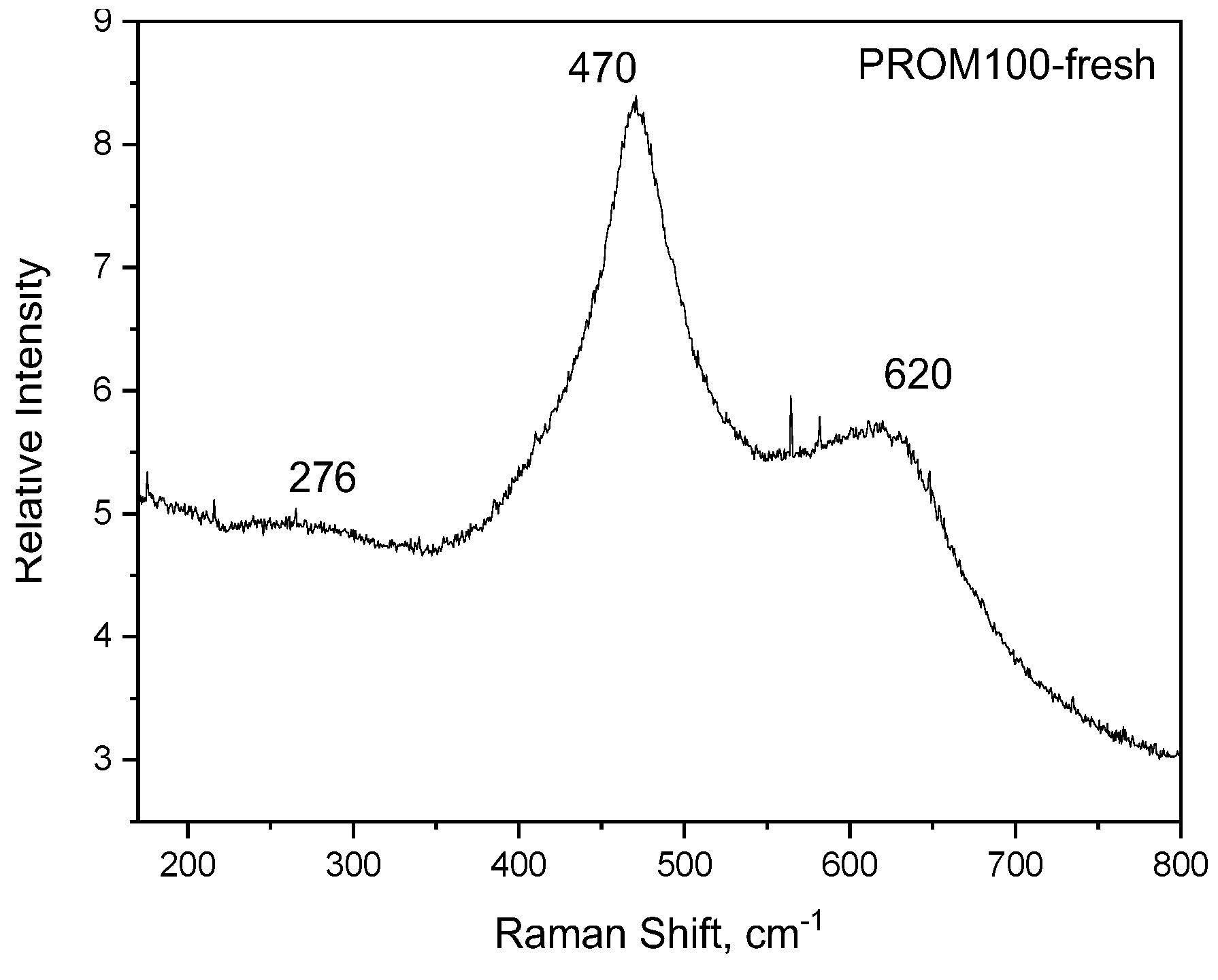
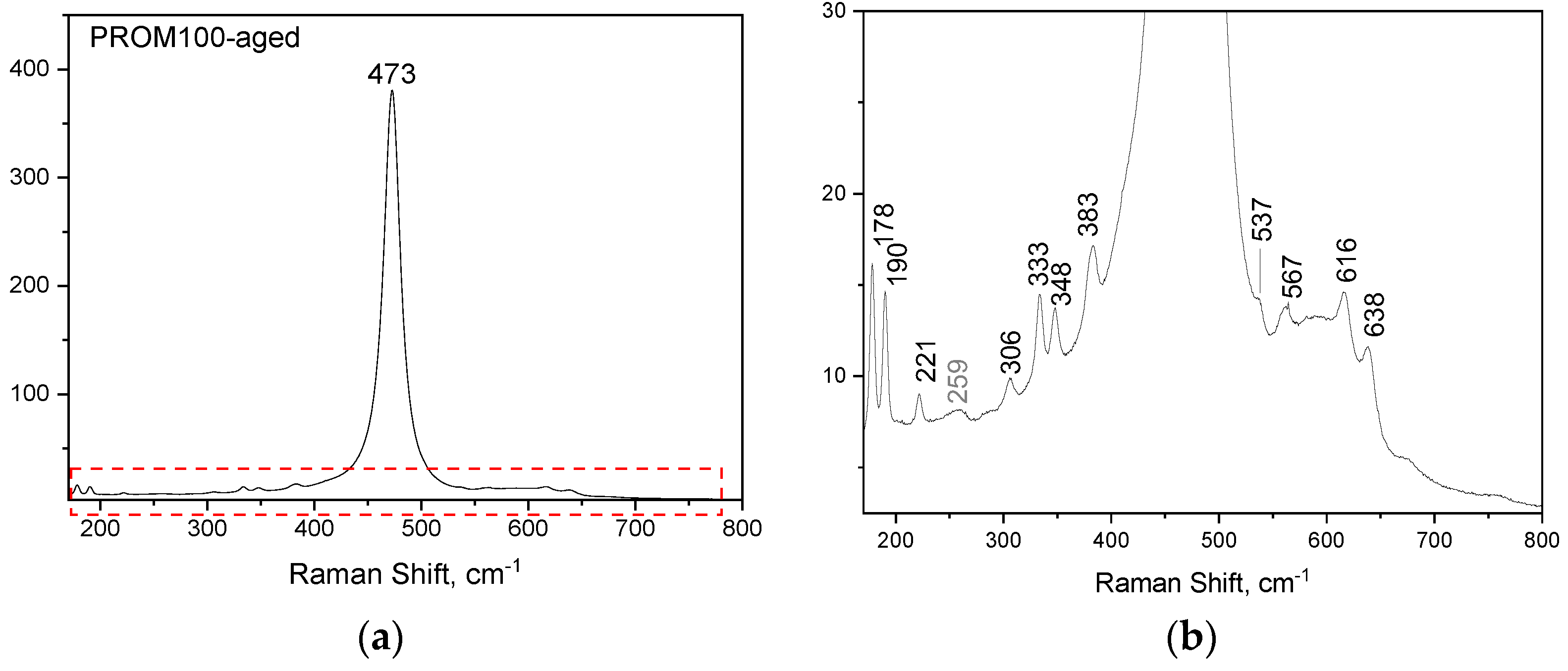
2.1.2. Raman Characterization
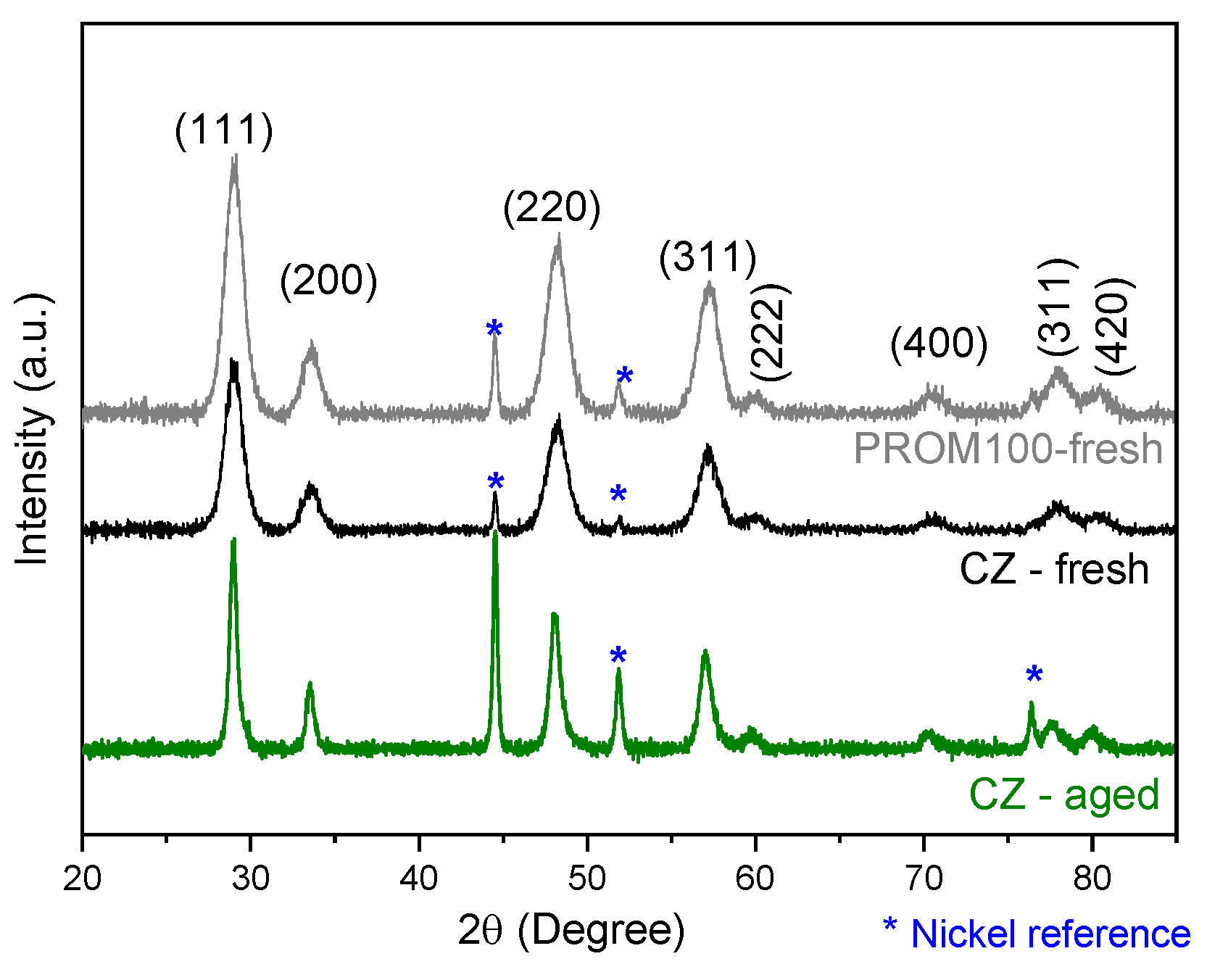
2.1.3. XRD Characterization
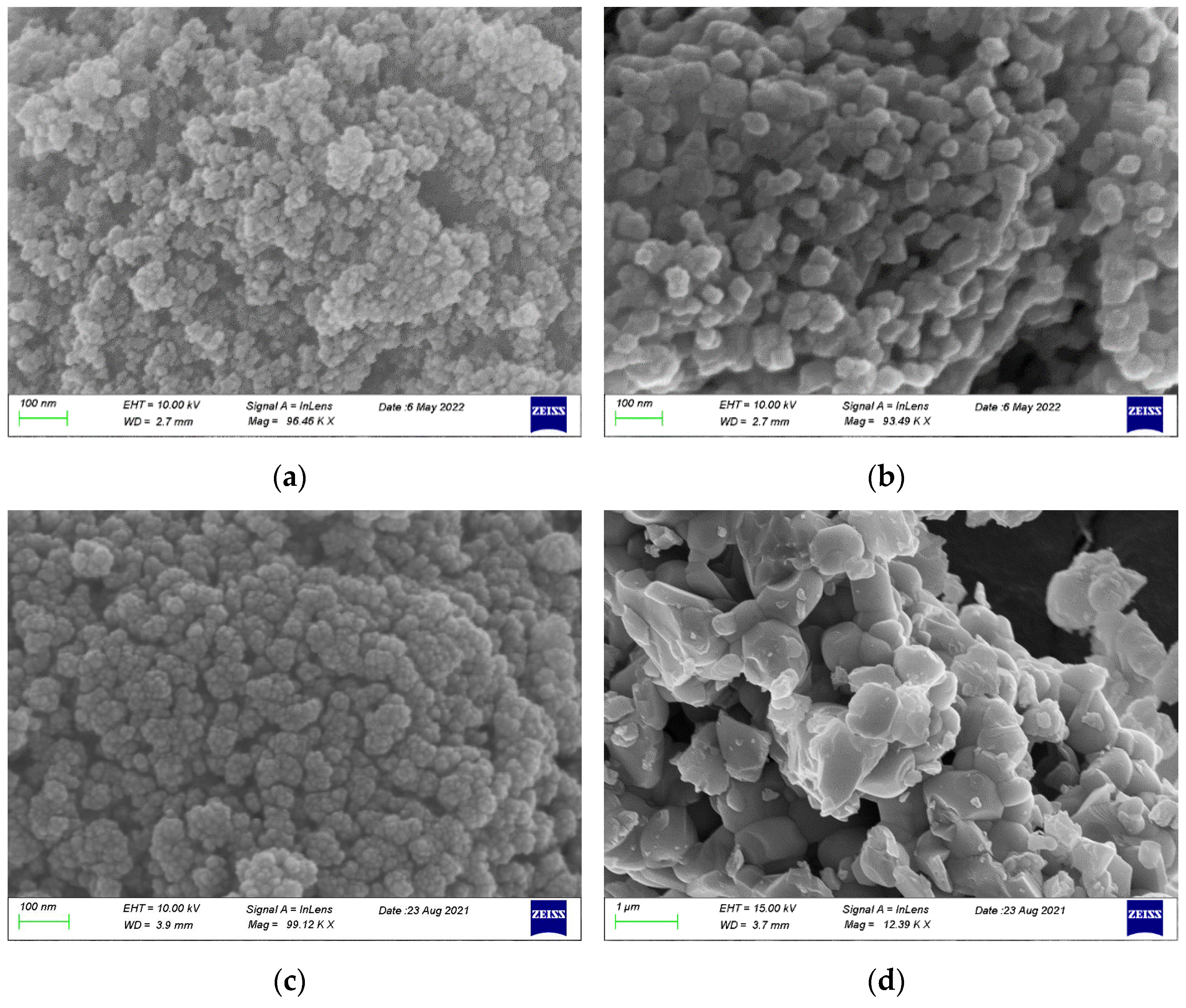
2.1.4. SEM-EDX Characterization
2.1.5. BET Characterization
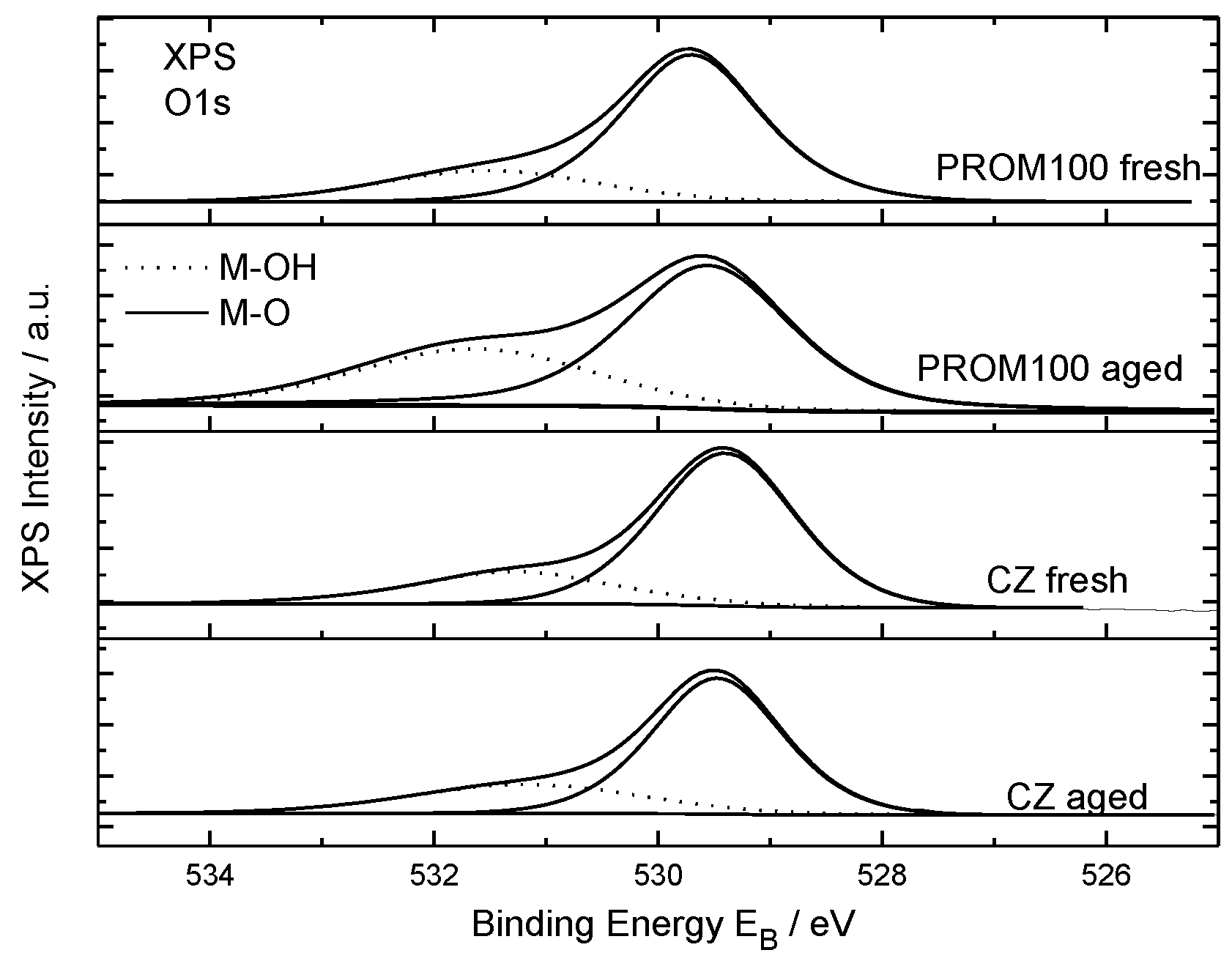
2.1.6. XPS Characterization
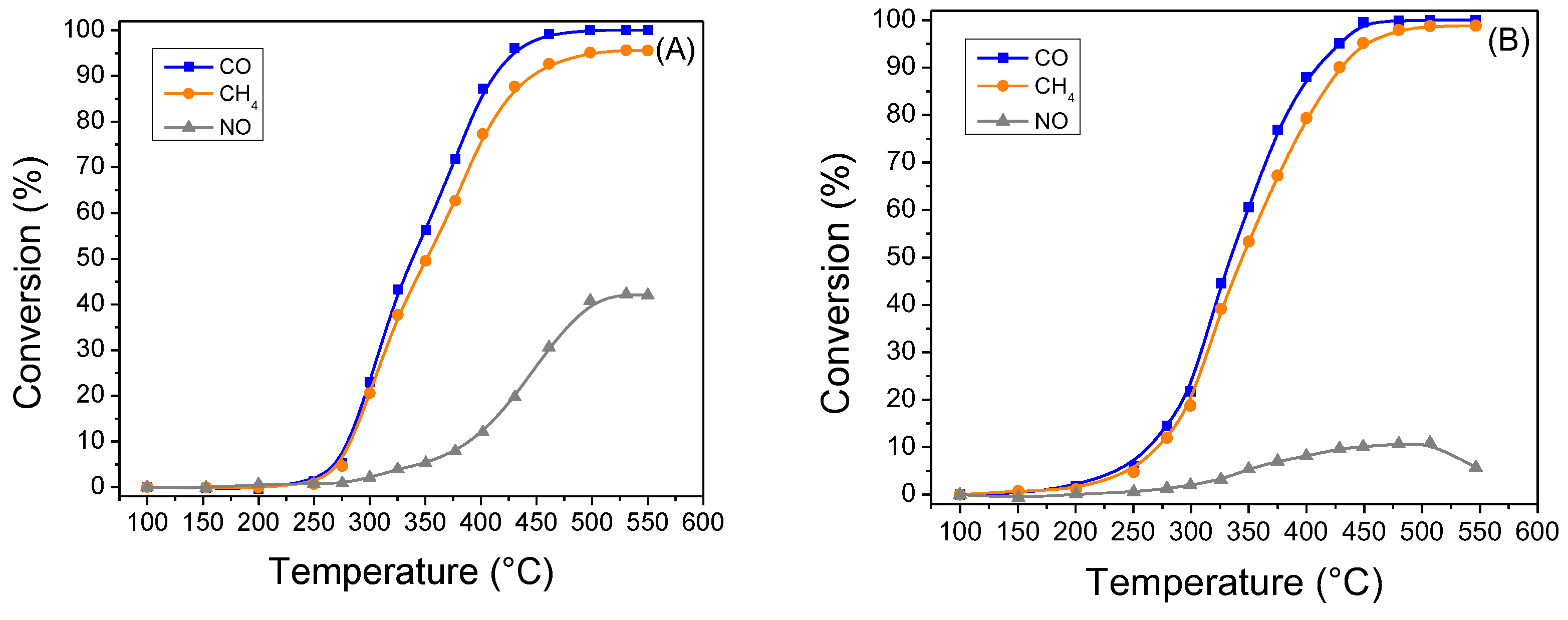
2.2. Catalytic Activity Tests
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Materials Characterization
3.3. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heck, R.M.; Farrauto, R.J. Automobile exhaust catalysts. Appl. Catal. A Gen. 2001, 221, 443–457. [Google Scholar] [CrossRef]
- Lyu, P.; Wang, P.; Liu, Y.; Wang, Y. Review of the studies on emission evaluation approaches for operating vehicles. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 493–509. [Google Scholar] [CrossRef]
- Dey, S.; Mehta, N.S. Automobile pollution control using catalysis. Resour. Environ. Sustain. 2020, 2, 100006. [Google Scholar] [CrossRef]
- Aminzadegan, S.; Shahriari, M.; Mehranfar, F.; Abramović, B. Factors affecting the emission of pollutants in different types of transportation: A literature review. Energy Rep. 2022, 8, 2508–2529. [Google Scholar] [CrossRef]
- Johnson, T.; Joshi, A. Review of Vehicle Engine Efficiency and Emissions. SAE Int. J. Engines 2018, 11, 1307–1330. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Rood, S.; Eslava, S.; Manigrasso, A.; Bannister, C. Recent advances in gasoline three-way catalyst formulation: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2020, 234, 936–949. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H. Monoliths: A Review of the Basics, Preparation Methods and Their Relevance to Oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Bickel, J.; Odendall, B.; Eigenberger, G.; Nieken, U. Oxygen storage dominated three-way catalyst modeling for fresh catalysts. Chem. Eng. Sci. 2017, 160, 34–53. [Google Scholar] [CrossRef]
- Lin, S.; Yang, L.; Yang, X.; Zhou, R. Redox properties and metal–support interaction of Pd/Ce0.67Zr0.33O2–Al2O3 catalyst for CO, HC and NOx elimination. Appl. Surf. Sci. 2014, 305, 642–649. [Google Scholar] [CrossRef]
- Huang, C.; Shan, W.; Lian, Z.; Zhang, Y.; He, H. Recent advances in three-way catalysts of natural gas vehicles. Catal. Sci. Technol. 2020, 10, 6407–6419. [Google Scholar] [CrossRef]
- Russell, A.; Epling, W.S. Diesel Oxidation Catalysts. Catal. Rev. 2011, 53, 337–423. [Google Scholar] [CrossRef]
- Kasab, J.; Strzelec, A. Automotive Emissions Regulations and Exhaust Aftertreatment Systems; SAE: Warrendale, PA, USA, 2020. [Google Scholar]
- Lambert, C.K. Current state of the art and future needs for automotive exhaust catalysis. Nat. Catal. 2019, 2, 554–557. [Google Scholar] [CrossRef]
- Cooper, J.; Beecham, J. A Study of Platinum Group Metals in Three-Way Autocatalysts. Platin. Met. Rev. 2013, 57, 281–288. [Google Scholar] [CrossRef]
- Mazzone, S.; Leishman, C.; Zhang, G.; García-García, F.R. A compact non-PGM catalytic hollow fibre converter for on-board hydrogen production. Sustain. Energy Fuels 2022, 6, 1554–1567. [Google Scholar] [CrossRef]
- Nakayama, H.; Kanno, Y.; Nagata, M.; Zheng, X. Development of TWC and PGM Free Catalyst Combination as Gasoline Exhaust Aftertreatment. SAE Int. J. Engines 2016, 9, 2194–2200. [Google Scholar] [CrossRef]
- Hirakawa, T.; Shimokawa, Y.; Tokuzumi, W.; Sato, T.; Tsushida, M.; Yoshida, H.; Ohyama, J.; Machida, M. Multicomponent 3d Transition-Metal Nanoparticles as Catalysts Free of Pd, Pt, or Rh for Automotive Three-Way Catalytic Converters. ACS Appl. Nano Mater. 2020, 3, 9097–9107. [Google Scholar] [CrossRef]
- Yakoumis, I. PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part I: Synthesis and Characterization. Materials 2021, 14, 622. [Google Scholar] [CrossRef]
- Yakoumis, I.; Polyzou, Ε.; Moschovi, A.M. PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part II: Catalytic Efficiency an Endurance as Compared with Original Catalysts. Materials 2021, 14, 2226. [Google Scholar] [CrossRef]
- Mrabet, D.; Abassi, A.; Cherizol, R.; Do, T.-O. One-pot solvothermal synthesis of mixed Cu-Ce-Ox nanocatalysts and their catalytic activity for low temperature CO oxidation. Appl. Catal. A Gen. 2012, 447–448, 60–66. [Google Scholar] [CrossRef]
- Mota, N.; Alvarez-Galván, M.C.; Navarro, R.M.; Al-Zahrani, S.M.; Goguet, A.; Daly, H.; Zhang, W.; Trunschke, A.; Schlögl, R.; Fierro, J.L.G. Insights on the role of Ru substitution in the properties of LaCoO3-based oxides as catalysts precursors for the oxidative reforming of diesel fuel. Appl. Catal. B Environ. 2012, 113–114, 271–280. [Google Scholar] [CrossRef]
- Choudhury, P. An Innovative Approach For Emission Control Using Copper Plate Catalytic Converter. Int. J. Adv. Sci. Eng. Technol. 2014, 3, 19–23, ISSN 2319-5924. [Google Scholar]
- Amin, C.M.; Rathod, P.P.; Goswami, J.J. Copper based catalytic converter. Int. J. Eng. Res. Technol. (IJERT) 2012, 1, 1–6. [Google Scholar]
- Yakoumis, I. Copper and Noble Metal Polymetallic Catalysts for Engine Exhaust Gas Treatment. European Patent EP3569309, 20 November 2019. [Google Scholar]
- Sun, S.; Jin, C.; He, W.; Li, G.; Zhu, H.; Huang, J. A review on management of waste three-way catalysts and strategies for recovery of platinum group metals from them. J. Environ. Manag. 2022, 305, 114383. [Google Scholar] [CrossRef]
- Nicol, G.; Goosey, E.; Yıldız, D.Ş.; Loving, E.; Nguyen, V.T.; Riaño, S.; Yakoumis, I.; Martinez, A.M.; Siriwardana, A.; Unzurrunzaga, A.; et al. Platinum Group Metals Recovery Using Secondary Raw Materials (PLATIRUS): Project Overview with a Focus on Processing Spent Autocatalyst. Johns. Matthey Technol. Rev. 2021, 65, 127–147. [Google Scholar] [CrossRef]
- Abdolmaleki, S.; Najafi, G.; Ghobadian, B.; Zakeri, A.; Nejat, M. Comparison of Aged and fresh Automotive Three-Way Catalyst in Driving Cycle. Int. J. Engine Res. 2019, 57, 75–83. [Google Scholar]
- Uy, D.; O’Neill, A.E.; Xu, L.; Weber, W.H.; McCabe, R.W. Observation of cerium phosphate in aged automotive catalysts using Raman spectroscopy. Appl. Catal. B Environ. 2003, 41, 269–278. [Google Scholar] [CrossRef]
- Winkler, A.; Ferri, D.; Hauert, R. Influence of aging effects on the conversion efficiency of automotive exhaust gas catalysts. Catal. Today 2010, 155, 140–146. [Google Scholar] [CrossRef]
- Matam, S.K.; Otal, E.H.; Aguirre, M.H.; Winkler, A.; Ulrich, A.; Rentsch, D.; Weidenkaff, A.; Ferri, D. Thermal and chemical aging of model three-way catalyst Pd/Al2O3 and its impact on the conversion of CNG vehicle exhaust. Catal. Today 2012, 184, 237–244. [Google Scholar] [CrossRef]
- Giuliano, M.; Ricchiardi, G.; Damin, A.; Sgroi, M.; Nicol, G.; Parussa, F. Thermal ageing effects in a commercial three-way catalyst: Physical characterization of washcoat and active metal evolution. Int. J. Automot. Technol. 2020, 21, 329–337. [Google Scholar] [CrossRef]
- Giuliano, M.; Ricchiardi, G.; Valsania, M.C.; Parussa, F.; Nicol, G.; Sgroi, M. Characterization of Vehicle and Laboratory Aged Commercial Three Way Catalyst: A Morphological and Functional Correlation between Real and Simulated Ageing. Int. J. Automot. Technol. 2021, 22, 131–139. [Google Scholar] [CrossRef]
- Moschovi, A.M.; Giuliano, M.; Kourtelesis, M.; Nicol, G.; Polyzou, E.; Parussa, F.; Yakoumis, I.; Sgroi, M.F. First of Its Kind Automotive Catalyst Prepared by Recycled PGMs-Catalytic Performance. Catalysts 2021, 11, 942. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Martínez-Arias, A.; Fernández-García, M. Metal–promoter interface in Pd/(Ce,Zr)Ox/Al2O3 catalysts: Effect of thermal aging. J. Catal. 2004, 221, 148–161. [Google Scholar] [CrossRef]
- Dai, H.; Ye, T.; Wang, K.; Zhang, M.; Wu, L.-M.; Ouyang, G. Enhanced Performance and Stability of a Trimetallic CuZnY/SiBEA Catalyst in Ethanol to Butadiene Reaction by Introducing Copper to Optimize Acid/Base Ratio. Catalysts 2022, 12, 1147. [Google Scholar] [CrossRef]
- Pothu, R.; Challa, P.; Rajesh, R.; Boddula, R.; Balaga, R.; Balla, P.; Perugopu, V.; Radwan, A.B.; Abdullah, A.M.; Al-Qahtani, N. Vapour-Phase Selective Hydrogenation of γ-Valerolactone to 2-Methyltetrahydrofuran Biofuel over Silica-Supported Copper Catalysts. Nanomaterials 2022, 12, 3414. [Google Scholar] [CrossRef]
- Ágoston, Á.; Balog, Á.; Narbutas, Š.; Dékány, I.; Janovák, L. Optimization of Plasmonic Copper Content at Copper-Modified Strontium Titanate (Cu-SrTiO3): Synthesis, Characterization, Photocatalytic Activity. Catalysts 2022, 12, 1041. [Google Scholar] [CrossRef]
- Liu, G.; Xia, N.; Tian, L.; Sun, Z.; Liu, L. Progress in the Development of Biosensors Based on Peptide–Copper Coordination Interaction. Biosensors 2022, 12, 809. [Google Scholar] [CrossRef]
- Martin, L.; Arranz, J.L.; Prieto, O.; Trujillano, R.; Holgado, M.J.; Galán, M.A.; Rives, V. Simulation three-way catalyst ageing: Analysis of two conventional catalyst. Appl. Catal. B Environ. 2003, 44, 41–52. [Google Scholar] [CrossRef]
- Zheng, T.; He, J.; Zhao, Y.; Xia, W.; He, J. Precious metal-support interaction in automotive exhaust catalysts. J. Rare Earths 2014, 32, 97–107. [Google Scholar] [CrossRef]
- Keramidas, V.G.; White, W.B. Raman spectra of oxides with the fluorite structure. J. Chem. Phys. 1973, 59, 1561–1562. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhu, X.; Hensen, E.J.M.; Lefferts, L.; Mojet, B.L. Defect Chemistry of Ceria Nanorods. J. Phys. Chem. C 2014, 118, 4131–4142. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. In situ Raman analyses of the soot oxidation reaction over nanostructured ceria-based catalysts. Sci. Rep. 2019, 9, 3875. [Google Scholar] [CrossRef] [PubMed]
- Lamas, D.G.; Lascalea, G.E.; Juárez, R.E.; Djurado, E.; Pérez, L.; Walsöe de Reca, N.E. Metastable forms of the tetragonal phase in compositionally homogeneous, nanocrystalline zirconia–ceria powders synthesised by gel-combustion. J. Mater. Chem. 2003, 13, 904–910. [Google Scholar] [CrossRef]
- Pezzotti, G.; Porporati, A.A. Raman spectroscopic analysis of phase-transformation and stress patterns in zirconia hip joints. J. Biomed. Opt. 2004, 9, 372–384. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.-C. Raman and X-ray Photoelectron Spectroscopy Study of CeO2−ZrO2 and V2O5/CeO2−ZrO2 Catalysts. Langmuir 2003, 19, 3025–3030. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Periyasamy, K.; Aswathy, V.T.; Ashok kumar, V.; Manikandan, M.; Shukla, R.; Tyagi, A.K.; Raja, T. An efficient robust fluorite CeZrO4−δ oxide catalyst for the eco-benign synthesis of styrene. RSC Adv. 2015, 5, 3619–3626. [Google Scholar] [CrossRef]
- Nagai, Y.; Yamamoto, T.; Tanaka, T.; Yoshida, S.; Nonaka, T.; Okamoto, T.; Suda, A.; Sugiura, M. XAFS and XRD Analysis of Ceria–Zirconia Oxygen Storage Promoters for Automotive Catalysts. Top. Catal. 2008, 47, 137–147. [Google Scholar] [CrossRef]
- Di Monte, R.; Fornasiero, P.; Desinan, S.; Kašpar, J.; Gatica, J.M.; Calvino, J.J.; Fonda, E. Thermal Stabilization of CexZr1-xO2 Oxygen Storage Promoters by Addition of Al2O3: Effect of Thermal Aging on Textural, Structural, and Morphological Properties. Chem. Mater. 2004, 16, 4273–4285. [Google Scholar] [CrossRef]
- Gong, J.; Wang, D.; Li, J.; Kamasamudram, K.; Currier, N.; Yezerets, A. An experimental and kinetic modeling study of aging impact on surface and subsurface oxygen storage in three-way catalysts. Catal. Today 2019, 320, 51–60. [Google Scholar] [CrossRef]
- Bunluesin, T.; Gorte, R.J.; Graham, G.W. CO oxidation for the characterization of reducibility in oxygen storage components of three-way automotive catalysts. Appl. Catal. B Environ. 1997, 14, 105–115. [Google Scholar] [CrossRef]
- Giuliano, M.; Valsania, M.C.; Ticali, P.; Sartoretti, E.; Morandi, S.; Bensaid, S.; Ricchiardi, G.; Sgroi, M. Characterization of the Evolution of Noble Metal Particles in a Commercial Three-Way Catalyst: Correlation between Real and Simulated Ageing. Catalysts 2021, 11, 247. [Google Scholar] [CrossRef]
- Neofytidis, C.; Ioannidou, E.; Sygellou, L.; Kollia, M.; Niakolas, D.K. Affecting the H2O electrolysis process in SOECs through modification of NiO/GDC; experimental case of Au-Mo-Ni synergy. J. Catal. 2019, 373, 260–275. [Google Scholar] [CrossRef]
- Kroner, A.B.; Newton, M.A.; Tromp, M.; Russell, A.E.; Dent, A.J.; Evans, J. Structural Characterization of Alumina-Supported Rh Catalysts: Effects of Ceriation and Zirconiation by using Metal–Organic Precursors. ChemPhysChem 2013, 14, 3606–3617. [Google Scholar] [CrossRef]
- Huo, C.; Yang, H. Preparation and enhanced photocatalytic activity of Pd–CuO/palygorskite nanocomposites. Appl. Clay Sci. 2013, 74, 87–94. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Fernández-García, M.; Gálvez, O.; Coronado, J.M.; Anderson, J.A.; Conesa, J.C.; Soria, J.; Munuera, G. Comparative Study on Redox Properties and Catalytic Behavior for CO Oxidation of CuO/CeO2 and CuO/ZrCeO4 Catalysts. J. Catal. 2000, 195, 207–216. [Google Scholar] [CrossRef]
- Yang, Z.; He, B.; Lu, Z.; Hermansson, K. Physisorbed, Chemisorbed, and Oxidized CO on Highly Active Cu−CeO2(111). J. Phys. Chem. C 2010, 114, 4486–4494. [Google Scholar] [CrossRef]
- Jia, A.-P.; Jiang, S.-Y.; Lu, J.-Q.; Luo, M.-F. Study of Catalytic Activity at the CuO−CeO2 Interface for CO Oxidation. J. Phys. Chem. C 2010, 114, 21605–21610. [Google Scholar] [CrossRef]
- Christou, S.Y.; Bradshaw, H.; Butler, C.; Darab, J.; Efstathiou, A.M. Effect of Thermal Aging on the Transient Kinetics of Oxygen Storage and Release of Commercial Ce x Zr1−x O2-based Solids. Top. Catal. 2009, 52, 2013–2018. [Google Scholar] [CrossRef]
- Daley, R.A.; Christou, S.Y.; Efstathiou, A.M.; Anderson, J.A. Influence of oxychlorination treatments on the redox and oxygen storage and release properties of thermally aged Pd-Rh/CexZr1−xO2/Al2O3 model three-way catalysts. Appl. Catal. B Environ. 2005, 60, 117–127. [Google Scholar] [CrossRef]
- Kang, S.B.; Han, S.J.; Nam, S.B.; Nam, I.-S.; Cho, B.K.; Kim, C.H.; Oh, S.H. Effect of Aging Atmosphere on Thermal Sintering of Modern Commercial TWCs. Top. Catal. 2013, 56, 298–305. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Xu, W.; Li, X.; Wang, W.; Zhang, L.; Li, Y.; Peng, Z.; Yang, F.; Liu, Z. Nature of Active Sites on Cu–CeO2 Catalysts Activated by High-Temperature Thermal Aging. ACS Catal. 2020, 10, 12385–12392. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirakawa, T.; Oyama, H.; Nakashima, R.; Hinokuma, S.; Machida, M. Effect of Thermal Aging on Local Structure and Three-Way Catalysis of Cu/Al2O3. J. Phys. Chem. C 2019, 123, 10469–10476. [Google Scholar] [CrossRef]
- Graham, G.W.; Jen, H.W.; Chun, W.; McCabe, R.W. High-Temperature-Aging-Induced Encapsulation of Metal Particles by Support Materials: Comparative Results for Pt, Pd, and Rh on Cerium–Zirconium Mixed Oxides. J. Catal. 1999, 182, 228–233. [Google Scholar] [CrossRef]
- Graham, G.W.; Jen, H.W.; Chun, W.; McCabe, R.W. Encapsulation of Pd particles by ceria-zirconia mixed oxides. Catal. Lett. 1997, 44, 185–187. [Google Scholar] [CrossRef]
- Graham, G.W.; O’Neill, A.E.; Chen, A.E. Pd encapsulation in automotive exhaust-gas catalysts. Appl. Catal. A Gen. 2003, 252, 437–445. [Google Scholar] [CrossRef]
- Dalianis, G.; Nanaki, E.; Xydis, G.; Zervas, E. New Aspects to Greenhouse Gas Mitigation Policies for Low Carbon Cities. Energies 2016, 9, 128. [Google Scholar] [CrossRef]




| Expected (wt.%) | Expected (wt.%) | |||||
|---|---|---|---|---|---|---|
| Samples | Cu | Pd | Rh | Cu | Pd | Rh |
| PROM100-fresh | 1.41 | 0.42 | 0.07 | 1.45 | 0.48 | 0.07 |
| PROM100 CZ-aged | 0.86 | 0.37 | 0.06 | |||
| Sample | Surface Area m2/g | Volume-Specific Surface Area 1 m2/cm3 |
|---|---|---|
| CZ-fresh | 91 | 594 |
| CZ-aged | 16 | 104 |
| PROM100-fresh | 81 | 528 |
| PROM100-aged | 1 | 6 |
| % Atomic Concentration (±0.5) (Atomic Ratio) | % at. Ce4+ | % at. Osurf./O | ||||||
|---|---|---|---|---|---|---|---|---|
| Samples | Ce | Zr | O | Pd | Cu | Cu:Pd | ||
| CZ-fresh | 23.3 (0.70) | 12.3 (0.37) | 64.4 (1.93) | - | - | 76.8% | 23.5 | |
| CZ-aged | 28.0 (0.84) | 11.5 (0.35) | 60.5 (1.82) | - | - | 73.7% | 26.7 | |
| PROM100 CZ-fresh | 23.7 (0.76) | 9.3 (0.30) | 60.5 (1.94) | 1.16 | 5.5 | 4.7 | 86.4% | 28.2 |
| PROM100 CZ-aged | 17.7 (0.57) | 10.0 (0.32) | 65.8 (2.11) | 0.17 | 6.4 | 37.6 | 84.7% | 30.8 |
| Sample | Rich-Burn Conditions (λ ≈ 0.99) | Lean-Burn Conditions (λ ≈ 1.03) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO Oxidation | CH4 Oxidation | NO Reduction | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||
| T50 (°C) | Maximum Efficiency (%) | T50 (°C) | Maximum Efficiency (%) | T50 (°C) | Maximum Efficiency (%) | T50 (°C) | Maximum Efficiency (%) | T50 (°C) | Maximum Efficiency (%) | T50 (°C) | Maximum Efficiency (%) | |
| PROM100-fresh | 162 | 100 | 161 | 100 | 282 | 96 | 158 | 100 | 162 | 93 | - | 8 |
| PROM100-aged | 338 | 100 | 351 | 96 | - | 42 | 334 | 100 | 344 | 99 | - | 11 |
| Technique | |||||||
|---|---|---|---|---|---|---|---|
| Sample | XRF | Raman | XRD | SEM | SBET | XPS | Cata. Activity * Max. Efficiency (λ ≈ 0.99) |
| CZ-fresh | Ce1-x ZrxO2 | Ce0.75Zr0.25O2 | Aggregates of nano-particles (<100 nm) | Ce0.70Zr0.37O1.93 | |||
| CZ-aged | Ce1-x ZrxO2 | Ce0.75Zr0.25O2 (narrow peaks) | Bigger particles (micro size) | No-nano | Ce0.84Zr0.35O1.82 | ||
| PROM100-fresh | Cu: 1.41% Pd: 0.42% | Ce1-x ZrxO2 | Ce0.75Zr0.25O2 (broad peaks) | Aggregates of nano-particles (<100 nm) | Ce0.76Zr0.30O1.94 Cu:Pd = 4.7 | CO and CH4: 100% NO: 96% | |
| PROM100-aged | Cu: 0.86% Pd: 0.37% | -high increase in Ce-O bonds -presence ZrO2 monoclinic -contribution ZrO2 tetragonal | -CeO2 -ZrO2 monoclinic and tetragonal (narrow peaks) | Bigger particles (micro size) | No-nano | Ce0.57Zr0.32O2.1 Cu:Pd = 37.6 | CO and CH4: ~100% NO:42% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto Beobide, A.; Moschovi, A.M.; Mathioudakis, G.N.; Kourtelesis, M.; Lada, Z.G.; Andrikopoulos, K.S.; Sygellou, L.; Dracopoulos, V.; Yakoumis, I.; Voyiatzis, G.A. High Catalytic Efficiency of a Nanosized Copper-Based Catalyst for Automotives: A Physicochemical Characterization. Molecules 2022, 27, 7402. https://doi.org/10.3390/molecules27217402
Soto Beobide A, Moschovi AM, Mathioudakis GN, Kourtelesis M, Lada ZG, Andrikopoulos KS, Sygellou L, Dracopoulos V, Yakoumis I, Voyiatzis GA. High Catalytic Efficiency of a Nanosized Copper-Based Catalyst for Automotives: A Physicochemical Characterization. Molecules. 2022; 27(21):7402. https://doi.org/10.3390/molecules27217402
Chicago/Turabian StyleSoto Beobide, Amaia, Anastasia M. Moschovi, Georgios N. Mathioudakis, Marios Kourtelesis, Zoi G. Lada, Konstantinos S. Andrikopoulos, Labrini Sygellou, Vassilios Dracopoulos, Iakovos Yakoumis, and George A. Voyiatzis. 2022. "High Catalytic Efficiency of a Nanosized Copper-Based Catalyst for Automotives: A Physicochemical Characterization" Molecules 27, no. 21: 7402. https://doi.org/10.3390/molecules27217402
APA StyleSoto Beobide, A., Moschovi, A. M., Mathioudakis, G. N., Kourtelesis, M., Lada, Z. G., Andrikopoulos, K. S., Sygellou, L., Dracopoulos, V., Yakoumis, I., & Voyiatzis, G. A. (2022). High Catalytic Efficiency of a Nanosized Copper-Based Catalyst for Automotives: A Physicochemical Characterization. Molecules, 27(21), 7402. https://doi.org/10.3390/molecules27217402








