Carnosine Potentiates Doxorubicin-Induced Cytotoxicity in Resistant NCI/ADR-RES Cells by Inhibiting P-Glycoprotein—In Silico and In Vitro Evidence
Abstract
1. Introduction
2. Results
2.1. MD Simulation and Docking Studies
2.1.1. Molecular Docking
2.1.2. The Root Mean Square Deviation (RMSD)
2.1.3. The Root Mean Square Fluctuation (RMSF)
2.1.4. The Number of Hydrogen Bonds (Hbonds)
2.1.5. The Length of Potential Bonding Interactions
2.2. Effect of Carnosine on Rhodamine-123 Efflux
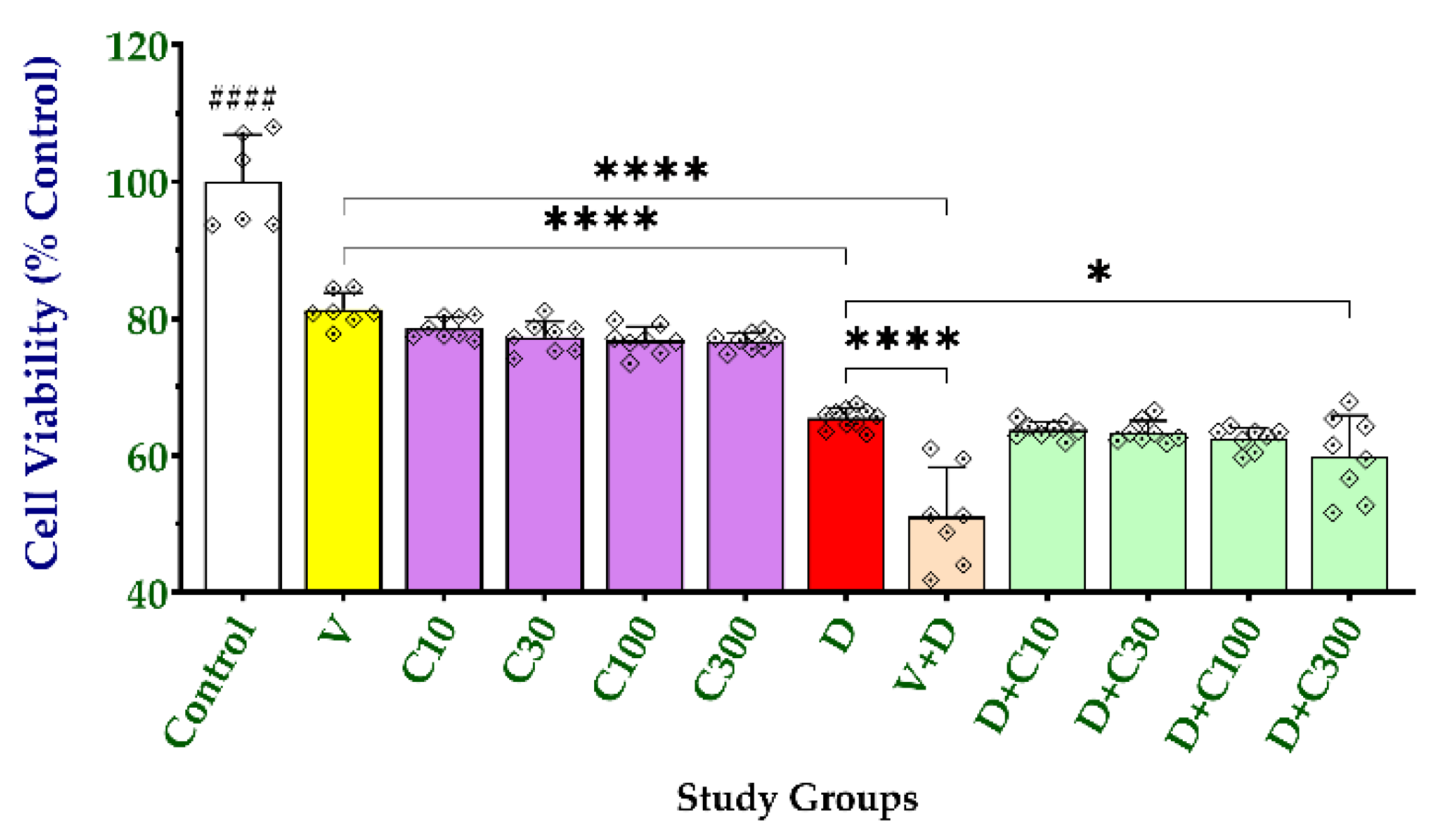
2.3. Effect of Carnosine on Cell Viability and Doxorubicin-Induced Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Protein Preparation, Docking, and MD Simulation
4.2. Rhodamine-123 Efflux Assay
4.3. Cell Viability and Cytotoxicity Assay
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; WHO: Geneva, Switzerland, 2020.
- Aziz, M.A.; Islam, M.S. The role of ABCB1 gene polymorphisms in steroid-resistant nephrotic syndrome: Evidence from a meta-analysis of steroid-receiving patients. J. Gene Med. 2022, 24, e3436. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.; Kumar, G. Cancer multidrug-resistance reversal by ABCB1 inhibition: A recent update. Eur. J. Med. Chem. 2022, 239, 114542. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, Y.S.; Spillane, A.J.; Jansson, P.J.; Sahni, S. Role of ABCB1 in mediating chemoresistance of triple-negative breast cancers. Biosci. Rep. 2021, 41, BSR20204092. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Frank, T. Teaching an old dog new tricks: Reactivated developmental signaling pathways regulate ABCB1 and chemoresistance in cancer. Cancer Drug Resist. (Alhambra, Calif.) 2021, 4, 424–452. [Google Scholar] [CrossRef]
- Morsy, M.A.; El-Sheikh, A.A.K.; Ibrahim, A.R.N.; Khedr, M.A.; Al-Taher, A.Y. In silico comparisons between natural inhibitors of ABCB1/P-glycoprotein to overcome doxorubicin-resistance in the NCI/ADR-RES cell line. Eur. J. Pharm. Sci. 2018, 112, 87–94. [Google Scholar] [CrossRef]
- Palmerini, E.; Meazza, C.; Tamburini, A.; Bisogno, G.; Ferraresi, V.; Asaftei, S.D.; Milano, G.M.; Coccoli, L.; Manzitti, C.; Luksch, R.; et al. Phase 2 study for nonmetastatic extremity high-grade osteosarcoma in pediatric and adolescent and young adult patients with a risk-adapted strategy based on ABCB1/P-glycoprotein expression: An Italian Sarcoma Group trial (ISG/OS-2). Cancer 2022, 128, 1958–1966. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Odone-Filho, V.; de Camargo, B.; Zerbini, M.C.; Fillipi, R.; Alencar, A.; Cristofani, L. P-glycoprotein expression, tumor weight, age, and relapse in patients with stage I and II favorable-histology Wilms’ tumor. Pediatr. Hematol. Oncol. 2011, 28, 194–202. [Google Scholar] [CrossRef]
- Babaer, D.; Amara, S.; Ivy, M.; Zhao, Y.; Lammers, P.E.; Titze, J.M.; Tiriveedhi, V. High salt induces P-glycoprotein mediated treatment resistance in breast cancer cells through store operated calcium influx. Oncotarget 2018, 9, 25193–25205. [Google Scholar] [CrossRef]
- Badowska-Kozakiewicz, A.M.; Sobol, M.; Patera, J. Expression of multidrug resistance protein P-glycoprotein in correlation with markers of hypoxia (HIF-1α, EPO, EPO-R) in invasive breast cancer with metastasis to lymph nodes. Arch. Med. Sci. AMS 2017, 13, 1303–1314. [Google Scholar] [CrossRef]
- James, A.D.; Leslie, T.K.; Kaggie, J.D.; Wiggins, L.; Patten, L.; Murphy O’Duinn, J.; Langer, S.; Labarthe, M.C.; Riemer, F.; Baxter, G.; et al. Sodium accumulation in breast cancer predicts malignancy and treatment response. Br. J. Cancer 2022, 127, 337–349. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Błaszczyk, M.; Środa-Pomianek, K.; Wesołowska, O. Isobavachalcone as an Active Membrane Perturbing Agent and Inhibitor of ABCB1 Multidrug Transporter. Molecules 2021, 26, 4637. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; El-Sheikh, A.A.; Ibrahim, A.R.; Venugopala, K.N.; Kandeel, M. In silico and in vitro identification of secoisolariciresinol as a re-sensitizer of P-glycoprotein-dependent doxorubicin-resistance NCI/ADR-RES cancer cells. PeerJ 2020, 8, e9163. [Google Scholar] [CrossRef] [PubMed]
- Grabarnick Portnoy, E.; Andriyanov, A.V.; Han, H.; Eyal, S.; Barenholz, Y. PEGylated Liposomes Remotely Loaded with the Combination of Doxorubicin, Quinine, and Indocyanine Green Enable Successful Treatment of Multidrug-Resistant Tumors. Pharmaceutics 2021, 13, 2181. [Google Scholar] [CrossRef]
- Laiolo, J.; Lanza, P.A.; Parravicini, O.; Barbieri, C.; Insuasty, D.; Cobo, J.; Vera, D.M.A.; Enriz, R.D.; Carpinella, M.C. Structure activity relationships and the binding mode of quinolinone-pyrimidine hybrids as reversal agents of multidrug resistance mediated by P-gp. Sci. Rep. 2021, 11, 16856. [Google Scholar] [CrossRef] [PubMed]
- Małek, A.; Taciak, B.; Sobczak, K.; Grzelak, A.; Wójcik, M.; Mieczkowski, J.; Lechowski, R.; Zabielska-Koczywąs, K.A. Enhanced Cytotoxic Effect of Doxorubicin Conjugated to Glutathione-Stabilized Gold Nanoparticles in Canine Osteosarcoma-In Vitro Studies. Molecules 2021, 26, 3487. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Kowal, J.; Broude, E.; Roninson, I.; Locher, K.P. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science 2019, 363, 753–756. [Google Scholar] [CrossRef]
- Seidel, E.C.; Birkemeyer, C.; Baran-Schmidt, R.; Meixensberger, J.; Oppermann, H.; Gaunitz, F. Viability of Glioblastoma Cells and Fibroblasts in the Presence of Imidazole-Containing Compounds. Int. J. Mol. Sci. 2022, 23, 5834. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Li, J.H.; Dong, C.D.; Chen, C.W.; Wu, C.C. Carnosine suppresses human colorectal cancer cell proliferation by inducing necroptosis and autophagy and reducing angiogenesis. Oncol. Lett. 2022, 23, 44. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. Defining the Drug-binding Site in the Human Multidrug Resistance P-glycoprotein Using a Methanethiosulfonate Analog of Verapamil, MTS-verapamil. J. Biol. Chem. 2001, 276, 14972–14979. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. Identification of Residues within the Drug-binding Domain of the Human Multidrug Resistance P-glycoprotein by Cysteine-scanning Mutagenesis and Reaction with Dibromobimane. J. Biol. Chem. 2000, 275, 39272–39278. [Google Scholar] [CrossRef]
- Domicevica, L.; Biggin, P.C. Homology modelling of human P-glycoprotein. Biochem. Soc. Trans. 2015, 43, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Paull, K.; Monks, A.; Hose, C.; Lee, J.S.; Weinstein, J.; Grever, M.; Bates, S.; Fojo, T. Generation of a drug resistance profile by quantitation of mdr-1/P-glycoprotein in the cell lines of the National Cancer Institute Anticancer Drug Screen. J. Clin. Investig. 1995, 95, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Paull, K.; Alvarez, M.; Hose, C.; Monks, A.; Grever, M.; Fojo, A.T.; Bates, S.E. Rhodamine efflux patterns predict P-glycoprotein substrates in the National Cancer Institute drug screen. Mol. Pharmacol. 1994, 46, 627–638. [Google Scholar] [PubMed]
- Kodan, A.; Futamata, R.; Kimura, Y.; Kioka, N.; Nakatsu, T.; Kato, H.; Ueda, K. ABCB1/MDR1/P-gp employs an ATP-dependent twist-and-squeeze mechanism to export hydrophobic drugs. FEBS Lett. 2021, 595, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef]
- Dantzic, D.; Noel, P.; Merien, F.; Liu, D.X.; Lu, J.; Han, H.; McKeage, M.J.; Li, Y. The Effects of Synthetically Modified Natural Compounds on ABC Transporters. Pharmaceutics 2018, 10, 127. [Google Scholar] [CrossRef]
- Gad, S.F.; Park, J.; Park, J.E.; Fetih, G.N.; Tous, S.S.; Lee, W.; Yeo, Y. Enhancing Docetaxel Delivery to Multidrug-Resistant Cancer Cells with Albumin-Coated Nanocrystals. Mol. Pharm. 2018. ahead of print. [Google Scholar] [CrossRef]
- Riganti, C.; Contino, M.; Guglielmo, S.; Perrone, M.G.; Salaroglio, I.C.; Milosevic, V.; Giampietro, R.; Leonetti, F.; Rolando, B.; Lazzarato, L.; et al. Design, Biological Evaluation, and Molecular Modeling of Tetrahydroisoquinoline Derivatives: Discovery of A Potent P-Glycoprotein Ligand Overcoming Multidrug Resistance in Cancer Stem Cells. J. Med. Chem. 2019, 62, 974–986. [Google Scholar] [CrossRef]
- Su, C.W.; Chiang, M.Y.; Lin, Y.L.; Tsai, N.M.; Chen, Y.P.; Li, W.M.; Hsu, C.H.; Chen, S.Y. Sodium Dodecyl Sulfate-Modified Doxorubicin-Loaded Chitosan-Lipid Nanocarrier with Multi Polysaccharide-Lecithin Nanoarchitecture for Augmented Bioavailability and Stability of Oral Administration In Vitro and In Vivo. J. Biomed. Nanotechnol. 2016, 12, 962–972. [Google Scholar] [CrossRef]
- Kaehler, M.; Cascorbi, I. Pharmacogenomics of Impaired Tyrosine Kinase Inhibitor Response: Lessons Learned From Chronic Myelogenous Leukemia. Front. Pharmacol. 2021, 12, 696960. [Google Scholar] [CrossRef]
- Tan, T.; Li, J.; Luo, R.; Wang, R.; Yin, L.; Liu, M.; Zeng, Y.; Zeng, Z.; Xie, T. Recent Advances in Understanding the Mechanisms of Elemene in Reversing Drug Resistance in Tumor Cells: A Review. Molecules 2021, 26, 5792. [Google Scholar] [CrossRef] [PubMed]
- Riehle, R.; Pattni, B.; Jhaveri, A.; Kulkarni, A.; Thakur, G.; Degterev, A.; Torchilin, V. Combination Nanopreparations of a Novel Proapoptotic Drug—NCL-240, TRAIL and siRNA. Pharm. Res. 2016, 33, 1587–1601. [Google Scholar] [CrossRef] [PubMed]






| Docking Score | Ligand Efficiency | Hbond | Lipo Score | Ecoul | |
|---|---|---|---|---|---|
| Carnosine | −5.54 | −0.32 | −0.547 | −0.079 | −22.234 |
| Verapamil | −7.38 | −0.22 | −0.64 | −3.86 | −6.18 |
| Co-crystalized ligand | −9.1 | −0.157 | −0.219 | −4.169 | −6.169 |
| Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|
| Carnosine | 1 | 8 | 4.45 | 1.01 |
| Verapamil | 0 | 5 | 2.98 | 1.23 |
| Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|
| Carnosine | 1.51 | 5.49 | 2.9 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morsy, M.A.; Kandeel, M.; Ibrahim, A.R.N.; Abdel-Gaber, S.A.; Jacob, S.; Venugopala, K.N.; Shinu, P.; El-Daly, M. Carnosine Potentiates Doxorubicin-Induced Cytotoxicity in Resistant NCI/ADR-RES Cells by Inhibiting P-Glycoprotein—In Silico and In Vitro Evidence. Molecules 2022, 27, 7383. https://doi.org/10.3390/molecules27217383
Morsy MA, Kandeel M, Ibrahim ARN, Abdel-Gaber SA, Jacob S, Venugopala KN, Shinu P, El-Daly M. Carnosine Potentiates Doxorubicin-Induced Cytotoxicity in Resistant NCI/ADR-RES Cells by Inhibiting P-Glycoprotein—In Silico and In Vitro Evidence. Molecules. 2022; 27(21):7383. https://doi.org/10.3390/molecules27217383
Chicago/Turabian StyleMorsy, Mohamed A., Mahmoud Kandeel, Ahmed R. N. Ibrahim, Seham A. Abdel-Gaber, Shery Jacob, Katharigatta N. Venugopala, Pottathil Shinu, and Mahmoud El-Daly. 2022. "Carnosine Potentiates Doxorubicin-Induced Cytotoxicity in Resistant NCI/ADR-RES Cells by Inhibiting P-Glycoprotein—In Silico and In Vitro Evidence" Molecules 27, no. 21: 7383. https://doi.org/10.3390/molecules27217383
APA StyleMorsy, M. A., Kandeel, M., Ibrahim, A. R. N., Abdel-Gaber, S. A., Jacob, S., Venugopala, K. N., Shinu, P., & El-Daly, M. (2022). Carnosine Potentiates Doxorubicin-Induced Cytotoxicity in Resistant NCI/ADR-RES Cells by Inhibiting P-Glycoprotein—In Silico and In Vitro Evidence. Molecules, 27(21), 7383. https://doi.org/10.3390/molecules27217383









