The Changes in Cyanobacterial Concentration of β-Methylamino-L-Alanine during a Bloom Event
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Cyanobacteria Samples
4.2. Chemicals & Standards
4.3. Sample Preparation
4.3.1. Cell Lysis
4.3.2. Hydrolysis
4.3.3. Derivatisation
4.4. Sample Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Pichel, F.; Belnap, J.; Neuer, S.; Schanz, F. Estimates of global cyanobacterial biomass and its distribution. Algol. Stud. 2003, 109, 213. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Hardwick, L.; Dorani, F. Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. J. Plankton Res. 2011, 33, 229–241. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H. Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater–marine continuum. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Berlin/Heidelberg, Germany, 2008; pp. 217–237. [Google Scholar]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef]
- Banack, S.A.; Murch, S.J.; Cox, P.A. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J. Ethnopharmacol. 2006, 106, 97–104. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Violi, J.P.; Facey, J.A.; Mitrovic, S.M.; Colville, A.; Rodgers, K.J. Production of beta-methylamino-L-alanine (BMAA) and Its Isomers by Freshwater Diatoms. Toxins 2019, 11, 512. [Google Scholar] [CrossRef]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial Blooms and the Occurrence of the neurotoxin beta-N-methylamino-L-alanine (BMAA) in South Florida Aquatic Food Webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef]
- Reveillon, D.; Abadie, E.; Sechet, V.; Brient, L.; Savar, V.; Bardouil, M.; Hess, P.; Amzil, Z. Beta-N-methylamino-L-alanine: LC-MS/MS optimization, screening of cyanobacterial strains and occurrence in shellfish from Thau, a French Mediterranean lagoon. Mar. Drugs 2014, 12, 5441–5467. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kiselova, N.; Rosen, J.; Ilag, L.L. Quantification of neurotoxin BMAA (beta-N-methylamino-L-alanine) in seafood from Swedish markets. Sci. Rep. 2014, 4, 6931. [Google Scholar] [CrossRef] [PubMed]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef]
- Chan, S.W.; Dunlop, R.A.; Rowe, A.; Double, K.L.; Rodgers, K.J. L-DOPA is incorporated into brain proteins of patients treated for Parkinson’s disease, inducing toxicity in human neuroblastoma cells in vitro. Exp. Neurol. 2012, 238, 29–37. [Google Scholar] [CrossRef]
- Main, B.J.; Dunlop, R.A.; Rodgers, K.J. The use of L-serine to prevent β-methylamino-L-alanine (BMAA)-induced proteotoxic stress in vitro. Toxicon 2016, 109, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Rodgers, K.J. Assessing the Combined Toxicity of BMAA and Its Isomers 2,4-DAB and AEG In Vitro Using Human Neuroblastoma Cells. Neurotox. Res. 2018, 33, 33–42. [Google Scholar] [CrossRef]
- Potjewyd, G.; Day, P.J.; Shangula, S.; Margison, G.P.; Povey, A.C. L-beta-N-methylamino-l-alanine (BMAA) nitrosation generates a cytotoxic DNA damaging alkylating agent: An unexplored mechanism for neurodegenerative disease. Neurotoxicology 2017, 59, 105–109. [Google Scholar] [CrossRef][Green Version]
- van Onselen, R.; Downing, T.G. BMAA-protein interactions: A possible new mechanism of toxicity. Toxicon 2018, 143, 74–80. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Main, B.J.; Samardzic, K. Cyanobacterial Neurotoxins: Their Occurrence and Mechanisms of Toxicity. Neurotox. Res. 2018, 33, 168–177. [Google Scholar] [CrossRef]
- Al-Tebrineh, J.; Merrick, C.; Ryan, D.; Humpage, A.; Bowling, L.; Neilan, B.A. Community composition, toxigenicity, and environmental conditions during a cyanobacterial bloom occurring along 1,100 kilometers of the Murray River. Appl. Environ. Microbiol. 2012, 78, 263–272. [Google Scholar] [CrossRef]
- Bowling, L.; Baker, P. Major cyanobacterial bloom in the Barwon-Darling River, Australia, in 1991, and underlying limnological conditions. Mar. Freshw. Res. 1996, 47, 643–657. [Google Scholar] [CrossRef]
- Bowling, L.C.; Merrick, C.; Swann, J.; Green, D.; Smith, G.; Neilan, B.A. Effects of hydrology and river management on the distribution, abundance and persistence of cyanobacterial blooms in the Murray River, Australia. Harmful Algae 2013, 30, 27–36. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Milton Park, UK, 2021. [Google Scholar]
- Schneider, T.; Simpson, C.; Desai, P.; Tucker, M.; Lobner, D. Neurotoxicity of isomers of the environmental toxin L-BMAA. Toxicon 2020, 184, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Bowling, L.C.; Padula, M.P.; Bishop, D.P.; Mitrovic, S.M.; Guillemin, G.J.; Rodgers, K.J. Detection of the suspected neurotoxin beta-methylamino-l-alanine (BMAA) in cyanobacterial blooms from multiple water bodies in Eastern Australia. Harmful Algae 2018, 74, 10–18. [Google Scholar] [CrossRef]
- Violi, J.P.; Mitrovic, S.M.; Colville, A.; Main, B.J.; Rodgers, K.J. Prevalence of beta-methylamino-L-alanine (BMAA) and its isomers in freshwater cyanobacteria isolated from eastern Australia. Ecotoxicol. Environ. Saf. 2019, 172, 72–81. [Google Scholar] [CrossRef]
- John, N.; Baker, L.; Ansell, B.R.E.; Newham, S.; Crosbie, N.D.; Jex, A.R. First report of anatoxin-a producing cyanobacteria in Australia illustrates need to regularly up-date monitoring strategies in a shifting global distribution. Sci. Rep. 2019, 9, 10894. [Google Scholar] [CrossRef]
- Blaszczyk, A.; Siedlecka-Kroplewska, K.; Wozniak, M.; Mazur-Marzec, H. Presence of ss-N-methylamino-L-alanine in cyanobacteria and aquatic organisms from waters of Northern Poland; BMAA toxicity studies. Toxicon 2021, 194, 90–97. [Google Scholar] [CrossRef]
- Esterhuizen, M.; Downing, T.G. Beta-N-methylamino-L-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol. Environ. Saf. 2008, 71, 309–313. [Google Scholar] [CrossRef]
- Faassen, E.J.; Gillissen, F.; Lürling, M. A comparative study on three analytical methods for the determination of the neurotoxin BMAA in cyanobacteria. PLoS ONE 2012, 7, e36667. [Google Scholar] [CrossRef]
- Yan, B.; Liu, Z.; Huang, R.; Xu, Y.; Liu, D.; Wang, W.; Zhao, Z.; Cui, F.; Shi, W. Impact factors on the production of β-methylamino-L-alanine (BMAA) by cyanobacteria. Chemosphere 2020, 243, 125355. [Google Scholar] [CrossRef]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spáčil, Z.; Ilag, L.L.; Ronnevi, L.-O.; Rasmussen, U.; Bergman, B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Mun, H.; Shin, H.; Park, S.; Yu, C.; Lee, J.; Yoon, Y.; Chung, H.; Yun, H.; Lee, K. Nitrogen stimulates Microcystis-dominated blooms more than phosphorus in river conditions that favor non-nitrogen-fixing genera. Environ. Sci. Technol. 2020, 54, 7185–7193. [Google Scholar] [CrossRef] [PubMed]
- Barnard, M.A.; Chaffin, J.D.; Plaas, H.E.; Boyer, G.L.; Wei, B.; Wilhelm, S.W.; Rossignol, K.L.; Braddy, J.S.; Bullerjahn, G.S.; Bridgeman, T.B.; et al. Roles of Nutrient Limitation on Western Lake Erie CyanoHAB Toxin Production. Toxins 2021, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Whalen, J.K. Production of the neurotoxin beta-N-methylamino-L-alanine may be triggered by agricultural nutrients: An emerging public health issue. Water Res. 2020, 170, 115335. [Google Scholar] [CrossRef]
- Downing, S.; Banack, S.A.; Metcalf, J.S.; Cox, P.A.; Downing, T.G. Nitrogen starvation of cyanobacteria results in the production of beta-N-methylamino-L-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Programmed Cell Death-Like and Accompanying Release of Microcystin in Freshwater Bloom-Forming Cyanobacterium Microcystis: From Identification to Ecological Relevance. Toxins 2019, 11, 706. [Google Scholar] [CrossRef]
- Ross, C.; Santiago-Vazquez, L.; Paul, V. Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2006, 78, 66–73. [Google Scholar] [CrossRef]
- Jones, G. Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 1994, 28, 871–876. [Google Scholar] [CrossRef]
- Yan, B.; Liu, Z.; Huang, R.; Xu, Y.; Liu, D.; Lin, T.F.; Cui, F. Optimization of the Determination Method for Dissolved Cyanobacterial Toxin BMAA in Natural Water. Anal. Chem. 2017, 89, 10991–10998. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Downing, T. Solid phase extraction of β-N-methylamino-L-alanine (BMAA) from South African water supplies. Water Sa 2011, 37, 523–528. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, S.; Gong, T.; Xian, Q.; Xu, B. Decomposition of β-N-methylamino-L-alanine (BMAA) and 2, 4-diaminobutyric acid (DAB) during chlorination and consequent disinfection byproducts formation. Water Res. 2019, 159, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Vo Duy, S.; Munoz, G.; Dinh, Q.T.; Tien Do, D.; Simon, D.F.; Sauvé, S. Analysis of the neurotoxin β-N-methylamino-L-alanine (BMAA) and isomers in surface water by FMOC derivatization liquid chromatography high resolution mass spectrometry. PLoS ONE 2019, 14, e0220698. [Google Scholar] [CrossRef] [PubMed]
- Stommel, E.W.; Field, N.C.; Caller, T.A. Aerosolization of cyanobacteria as a risk factor for amyotrophic lateral sclerosis. Med. Hypotheses 2013, 80, 142–145. [Google Scholar] [CrossRef]
- Samardzic, K.; Steele, J.R.; Violi, J.P.; Colville, A.; Mitrovic, S.M.; Rodgers, K.J. Toxicity and bioaccumulation of two non-protein amino acids synthesised by cyanobacteria, beta-N-Methylamino-L-alanine (BMAA) and 2,4-diaminobutyric acid (DAB), on a crop plant. Ecotoxicol. Environ. Saf. 2021, 208, 111515. [Google Scholar] [CrossRef]
- Groth, I.; Schumann, P.; Weiss, N.; Martin, K.; Rainey, F.A. Agrococcus jenensis gen. nov., sp. nov., a new genus of actinomycetes with diaminobutyric acid in the cell wall. Int. J. Syst. Bacteriol. 1996, 46, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Reveillon, D.; Sechet, V.; Hess, P.; Amzil, Z. Production of BMAA and DAB by diatoms (Phaeodactylum tricornutum, Chaetoceros sp., Chaetoceros calcitrans and, Thalassiosira pseudonana) and bacteria isolated from a diatom culture. Harmful Algae 2016, 58, 45–50. [Google Scholar] [CrossRef]
- Lage, S.; Burian, A.; Rasmussen, U.; Costa, P.R.; Annadotter, H.; Godhe, A.; Rydberg, S. BMAA extraction of cyanobacteria samples: Which method to choose? Environ. Sci. Pollut. Res. 2016, 23, 338–350. [Google Scholar] [CrossRef]
- Greenstein, K.E.; Zamyadi, A.; Wert, E.C. Comparative Assessment of Physical and Chemical Cyanobacteria Cell Lysis Methods for Total Microcystin-LR Analysis. Toxins 2021, 13, 596. [Google Scholar] [CrossRef]
- Blainey, P.; Krzywinski, M.; Altman, N. Replication. Nat. Methods 2014, 11, 879–880. [Google Scholar] [CrossRef]
- Van der Westhuizen, A.; Eloff, J. Effect of temperature and light on the toxicity and growth of the blue-green alga Microcystis aeruginosa (UV-006). Planta 1985, 163, 55–59. [Google Scholar] [CrossRef]
- Watanabe, M.F.; Oishi, S. Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl. Environ. Microbiol. 1985, 49, 1342–1344. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Lorenzi, A.S.; Chia, M.A.; Mota, E.C.; do Carmo Bittencourt-Oliveira, M. Insights into the impact of increasing temperature, light intensity, and UV-B exposure on the circadian rhythm of microcystin production and release, and the expression of mcy genes in the cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 2022, 34, 231–242. [Google Scholar] [CrossRef]
- Lee, S.; Jang, M.H.; Kim, H.S.; Yoon, B.D.; Oh, H.M. Variation of microcystin content of Microcystis aeruginosa relative to medium N: P ratio and growth stage. J. Appl. Microbiol. 2000, 89, 323–329. [Google Scholar] [CrossRef] [PubMed]
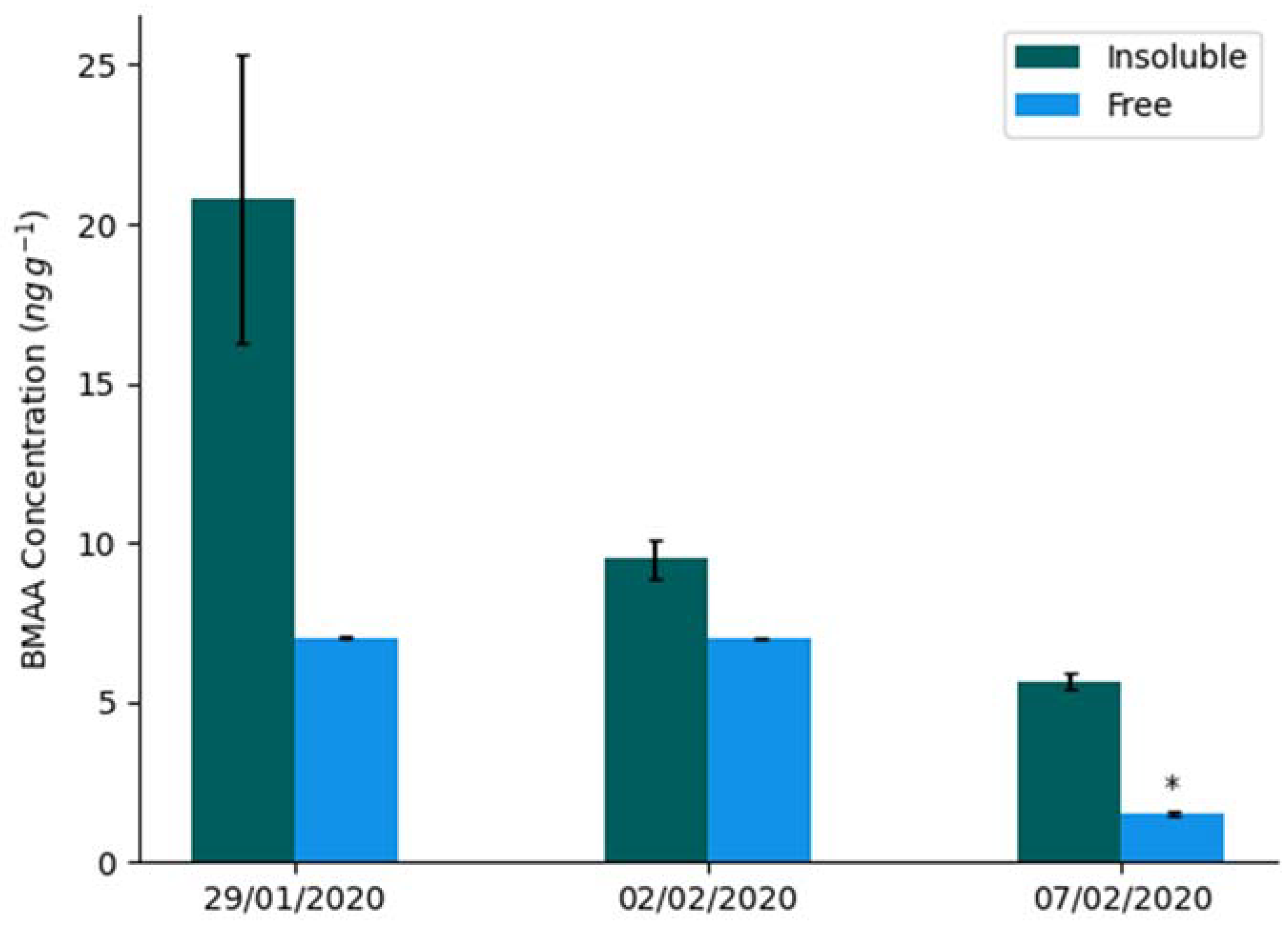

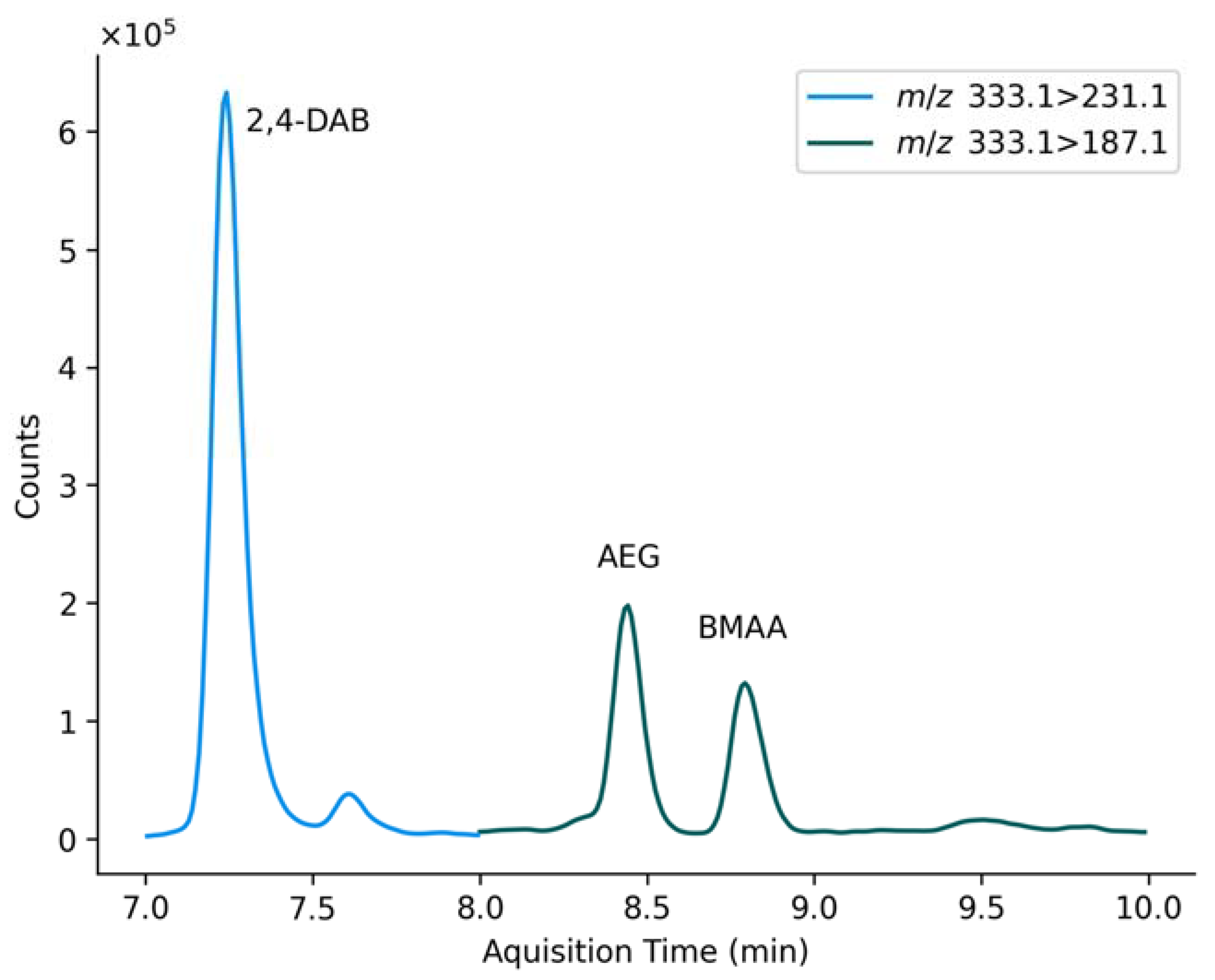
| BMAA (ng g−1) | 2,4-DAB (ng g−1) | AEG (ng g−1) | ||||
|---|---|---|---|---|---|---|
| Date | Free | Insoluble | Free | Insoluble | Free | Insoluble |
| 29 January 2020 | 7.01 ± 0.03 | 20.8 ± 5.5 | <LOQ | 524 ± 105 | 5.16 ± 1.36 | 31.8 ± 2.7 |
| 2 February 2020 | 6.96 ± 0.01 | 9.49 ± 0.76 | <LOQ | 533 ± 137 | 4.65 ± 0.93 | 13.2 ± 5.6 |
| 7 February 2020 Intact | <LOQ | 5.65 ± 0.39 | <LOQ | 221 ± 50 | 4.87 ± 0.48 | 134 ± 47 |
| 7 February 2020 Decayed | <LOQ | 5.65 ± 0.22 | <LOQ | 340 ± 18 | 3.91 ± 0.98 | 86.0 ± 22.2 |
| Compound | Retention Time (min) | Transitions (m/z) | Collision Energy (eV) | |
|---|---|---|---|---|
| 2,4-DAB | 7.3 | 333.1→ | 273.1 231.1 * 142.1 | −20 −12 −28 |
| d5-DAB | 7.3 | 336.1→ | 276.1 * 190.1 102.1 | −10 −17 −28 |
| AEG | 8.3 | 333.1→ | 187.1 * 99.1 88.1 | −16 −27 −30 |
| BMAA | 8.9 | 333.1→ | 187.1 * 159.1 73.1 | −16 −20 −30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, S.J.; Rodgers, K.J.; Mitrovic, S.M.; Bishop, D.P. The Changes in Cyanobacterial Concentration of β-Methylamino-L-Alanine during a Bloom Event. Molecules 2022, 27, 7382. https://doi.org/10.3390/molecules27217382
Peters SJ, Rodgers KJ, Mitrovic SM, Bishop DP. The Changes in Cyanobacterial Concentration of β-Methylamino-L-Alanine during a Bloom Event. Molecules. 2022; 27(21):7382. https://doi.org/10.3390/molecules27217382
Chicago/Turabian StylePeters, Siobhan J., Kenneth J. Rodgers, Simon M. Mitrovic, and David P. Bishop. 2022. "The Changes in Cyanobacterial Concentration of β-Methylamino-L-Alanine during a Bloom Event" Molecules 27, no. 21: 7382. https://doi.org/10.3390/molecules27217382
APA StylePeters, S. J., Rodgers, K. J., Mitrovic, S. M., & Bishop, D. P. (2022). The Changes in Cyanobacterial Concentration of β-Methylamino-L-Alanine during a Bloom Event. Molecules, 27(21), 7382. https://doi.org/10.3390/molecules27217382





