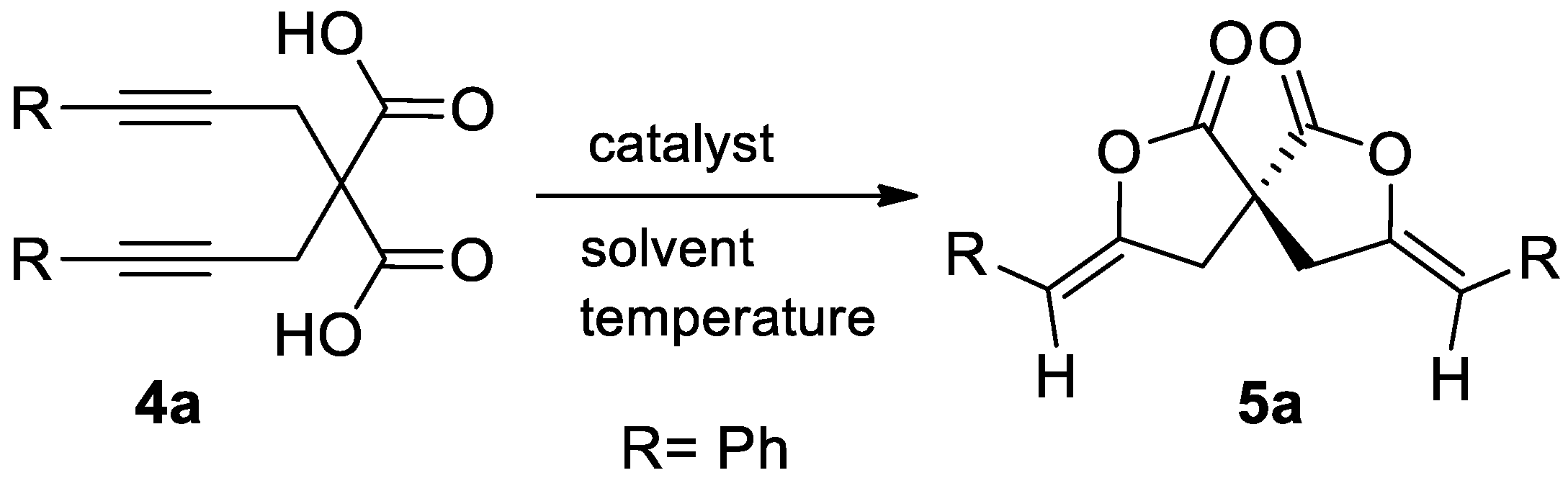
Abstract
The gold-catalyzed cyclization of 2,2-bis(3-arylprop-2-yn1-yl)malonic acid has been proposed as an efficient approach to substituted 3,8-dibenzyl-2,7-dioxaspiro[4.4]nonane-1,6-diones. The reaction proceeds smoothly in mild reaction conditions to give the desired products in quantitative yields in the presence of variously substituted starting materials. In addition, the synthesis of γ-arylidene spirobislactone bearing different substituents on the two aromatic rings has been achieved. This kind of compound could be of great interest in pharmaceutical science given the widespread presence of this scaffold in bioactive natural and synthetic products.
1. Introduction
3,8-dibenzyl-2,7-dioxaspiro[4.4]nonane-1,6-diones are molecules endowed with a complex γ-benzyl-spirobis-γ-lactone scaffold, which gained a great interest from researchers due to their biological activity and appealing conformational features, which include the presence of an element of chirality, namely a stereocenter or a chirality axis. A great number of unique bioactive natural products are endowed with a spirolactone moiety [1]. Among these, some compounds contain a spirobislactone unit or a very similar moiety, as α- and β-levantenolides, biyouyanagin A and B, hyperolactones A and C, clionamide D, longianone (Figure 1) [2]. Moreover, some natural products isolated from Carpesium abrotanoides bearing a similar γ-dilactones scaffold, namely dicarabrol A, dicarabrone C, and dipulchellin A (Figure 1), showed promising biological activities, including anti-inflammatory, antitumor, antiplasmodial, and bactericidal effects [3]. In 2007 a patent reported, among the others, a compound bearing a spirodilactone moiety (Figure 1) with antiviral activity against Flaviviridae family of viruses, in particular HCV [4]. More recently, a granted patent describes the preparation and use of spirobislactones as useful crosslink reagents for the synthesis of polymeric materials to be employed in coating processes for automotive applications [5]. Considering the preparation of spirobislactones, the first synthetic routes have been established by simple methods, using multistep synthesis that includes the cyclization of the two spirolactone rings in separate reaction steps, with low overall yields [6,7,8,9].
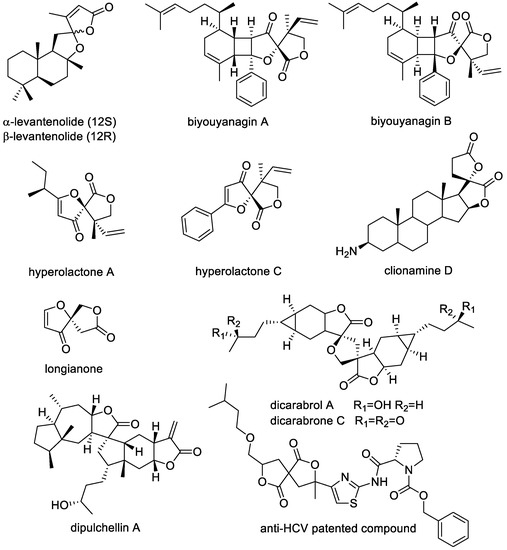
Figure 1.
Bioactive natural and synthetic products containing spirobislactone moiety or similar scaffold.
Roland et al. reported in 1963 the preparation of spirolactones by the reaction of dibromomalononitrile with two equivalents of ethylene, followed by acid hydrolysis [10]. Kotha et al. afforded spirobislactone scaffold by treating diethyl diallylmalonate with sulfuric acid [11]. The diseleno-derivative of spirobislactone was obtained by a Japanese group as a mixture of diastereoisomers by treating diethyl diallylmalonate with phenylselenyl chloride [12,13]. Maldemy et al. reported the cyclization of malonate derivatives with iodine(III) reagents to afford the spirobislactone [14]. The use of acid, base, and metal catalysis played a key role in the synthetic access to spirobislactones. Bronsted acids as triflic acid or concentrated sulfuric acid have been used in the dilactonization of tert-butyl diallylmalonate [15], and diethyl diallylmalonate, respectively [11]. Fairlamb et al. discovered that the treatment of dimethyl diallylmalonate with the Lewis acid SbF5 generated from HSbF6 during a Pd catalyzed cycloisomerization of 1,6-dienes, afforded instead of the desired monocyclized product a mixture of stereoisomer of the dimethyl spirobislactone [16]. Only one example in literature reported the use of polymer-supported bases like PS-DMAP or PS-BEMP to catalyze the spirodilactonization of phenyl glycidyl ether with dimethyl malonate, in a solvent-free reaction [17]. The use of metal catalysts is without any doubt highly efficient for the spirodilactonization process, starting from acyclic precursors. In 1980s two different groups reported an oxidative spirodilactonization reaction of malonic acid in presence of an alkene catalyzed by Mn(III) acetate [18,19,20]. Copper (II) or silver (I) triflates were used by Adrio et al. [21] and Yang et al. [22], respectively, to afford dilactonization of diallymalonic acid to the spirobislactone scaffold, by intramolecular addition of carboxylic acid to the olefin moiety, which selectively led to a spiro-γ-bislactone.
Recently, the use of gold (I) catalysts for the activation of C-C triple bond opened new horizons for the construction of complex molecular scaffolds [23], including for the synthetic access to spirobislactones starting from dipropargylmalonate derivatives. As shown by the wide range of substrate and product scope reported by a recent review [23], gold(I)-catalyzed activation of alkynes is a consolidated method for the construction of molecular complexity. The first examples of gold(I) activation of alkynes were reported by Teles [24] and Tanaka [25], demonstrating the potential of gold(I) in organic synthesis. Currently, gold(I) complexes are the most efficient catalysts for the electrophilic activation of alkynes, and the formation of new carbon−carbon or carbon−heteroatom bonds can be achieved by using different nucleophiles [26,27,28]. Au(I) complexes usually are linear and two-coordinate, as highlighted by a significant number of alkyne-gold complexes that have been characterized and studied in solution or theoretically. Generally, the most effective catalysts for the activation of alkynes are complexes [LAuL′]X or [LAuX], which contain weakly coordinating neutral (L′) or anionic ligand (X−). These complexes can enter catalytic cycles by ligand (often a nitrile) exchange with the unsaturated substrate. Gold(I) complexes selectively activate π-bonds of alkynes in complex way, which has been explained by relativistic effects. Generally, the nucleophilic Markovnikov attack to η2-[AuL]+-activated alkynes 1 forms trans-alkenyl-gold complexes 2 as intermediates (Scheme 1) [23].
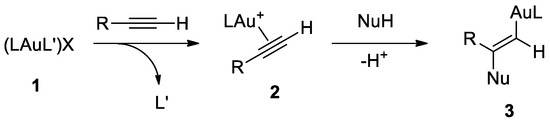
Scheme 1.
Activation of alkynes by electrophilic Au(I) complexes and nucleophilic addition to the C-C triple bond. A labile ligand (L′) is exchanged with the alkyne, forming a π-complex (2), followed by the anti-addition of a nucleophile to the η2-[AuL]+-activated alkyne (2).
In this field, we recently investigated the gold-catalyzed intramolecular hydroarylation reaction of N-ethoxycarbonyl-N-propargylanilines as effective tool for the preparation of 4-substituted-1,2-dihydroquinolines [29], and the synthesis of polycyclic chromene cores through gold (I)-catalyzed intramolecular hydroarylation reaction [30]. Based on these studies, we evaluated the possibility to obtain a gold (I) synthetic approach to spirobislactones. This class of compounds exhibit remarkable features which justify a continue efforts in the development of synthetic procedures to access them. Additionally, at the best of our knowledge, only few examples of gold(I) catalysis successfully employed for the preparation of spirobislactones have been reported in literature. In 2013, Chiarucci et al. reported the synthesis of γ-vinylbutyrolactones by intramolecular oxaallylic alkylation with alcohols. Starting from dimethyl 2,2-bis((E)-4-hydroxybut-2-en-1-yl)malonate they afforded the corresponding γ-methylenespirobislactone by using IMesAuCl/AgOTf as catalyst [31]. A similar γ,γ′-dibenzylspirobislactone was obtained by Zhu et al. by gold-catalyzed homogeneous oxidative carboheterofunctionalization of diallylmalonic acid, in the presence of phenylboronic acid, SIMesAuCl as catalytst, and selectfluor as an oxidant [32]. Gold nanoparticles stabilized by PEG-tagged imidazolium salts have also been used as recyclable catalysts for the cycloisomerization of γ-alkynoic acids into enol-lactones, among the other products, a γ,γ-divinylspirobislactone was obtained starting from dipropargylmalonic acid [33].
Finally, spirobislactones could be prepared as single enantiomers by using chiral Au(I) complexes, as recently reported by Lin et al. [34]. The application of phosphine-nitrogen chiral ligands and related gold (I) complexes used as catalytic systems for the asymmetric reactions have been patented in Chine from the same group [35].
In this paper are reported the results of our studies that led to the development of a method for the synthesis of spirolactones 5 starting from substituted dialkynylmalonic acids 4 (Scheme 2).
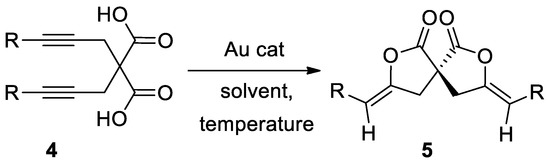
Scheme 2.
Work hypothesis.
2. Results and Discussion
To start our work, we decided to investigated the Au-catalyzed cyclization of 2,2-bis(arylprop-2-ynyl)malonic acids 4 affording 3,8-diarylidene-2,7-dioxaspiro[4.4]nonane-1,6-diones 5 (Table 1). Our preliminary experiments to establish the best reaction conditions for the conversion of 2,2-bis(3-phenylprop-2-ynyl)malonic acid 4a into the corresponding spirolactone 5a are reported in the following Table 1. No cyclization was observed in presence of palladium catalyst or in presence of Au(III) (Table 1, entry 1,2). In contrast, the use of an electrophilic complex of Au(I) with the Buchwald’s sterically bulky biaryl phosphine ligand JohnPhos and acetonitrile as catalyst (Figure 2), led the formation of the desired product in quantitative yields, and in very mild conditions (Table 1, entry 5). Indeed, the best results were obtained at room temperature, very likely due to the decrease of decomposition reactions.

Table 1.
Screening for the best catalyst and reaction conditions for the spirodilactonization to γ-arylmethylene-spirobislactones 5: cyclization of 2,2-bis(3-phenylprop-2-yn-1-yl)malonic acid 4a to 3,8-benzylidene-2,7-dioxaspiro[4.4]nonane-1,6-diones 5a a,b.
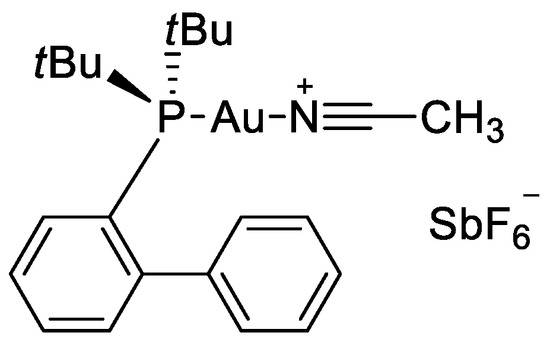
Figure 2.
Chemical structure of JohnPhosAu(MeCN)SbF6 catalyst.
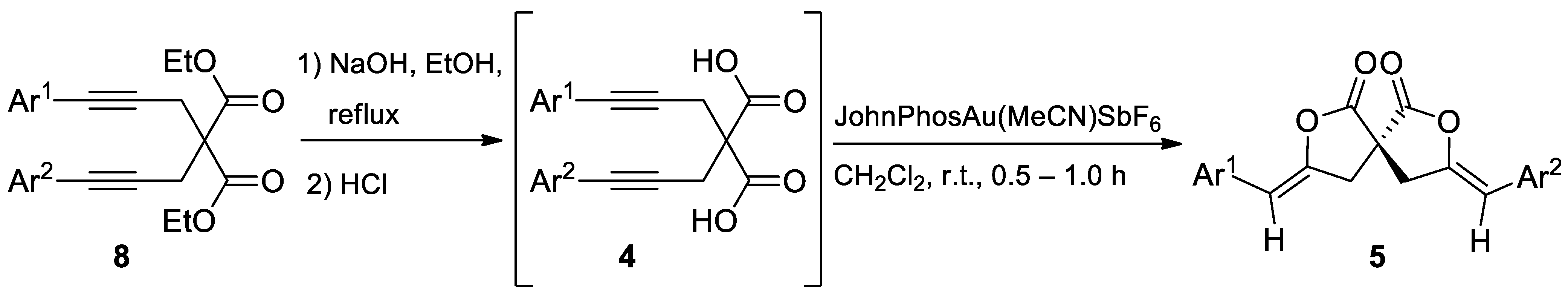
The reaction scope was investigated by using other 2,2-bis(3-arylprop-2-ynyl)malonic acids (4) (Table 2) in the same conditions optimized for substrate 4a. 2,2-bis(3-arylprop-2-ynyl)malonic acids 4 used for these studies were prepared by alkylation of diethyl malonate 6 with propargyl bromide, followed by two Sonogashira cross-coupling with two different aryl halides. Then, the hydrolysis of functionalized diethyl malonate completes the sequences for the preparation of symmetrical or unsymmetrical substrates 4 to be investigated in the gold-catalyzed spirodilactonization reaction (Scheme 3). Since the substituted malonic acid 4a was obtained in a quantitative yield from the last step, the hydrolysis reaction of the other esters 8 was then performed in sequence with the Au (I)-promoted cyclization (Table 2), without isolating the acids 4.

Table 2.
Substrate scope for the gold(I)-catalyzed spirodilactonization to γ-arylidenespirobislactones 5 a,b.
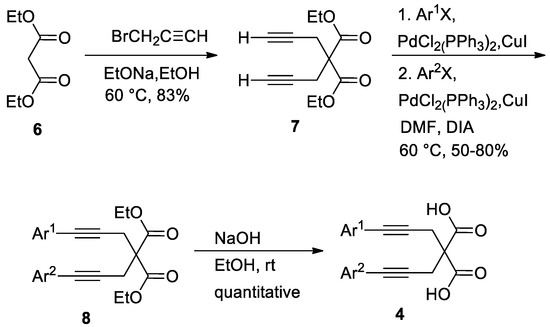
Scheme 3.
Synthesis of symmetrical or unsymmetrical diethyl diarylalkynylmalonates 8 and diarylalkynylmalonic acids 4.
Under the optimized reaction conditions several derivatives bearing a variety of useful functional groups have been prepared and obtained in a quantitative yield. The synthesized compounds are summarized in Table 2.
3. Materials and Methods
3.1. General Experimental Procedures
Melting points were recorded with a Büchi melting point B-545 and are not corrected. 1H and 13C NMR spectra have been acquired with a Bruker Avance 400 spectrometer operating at 400.13 and 100.6 MHz, respectively, at 300 K in DMSO-d6, using 5 mm diameter glass tubes. Chemical shifts were expressed in ppm and coupling constants (J) in hertz (Hz), approximated to 0.1 Hz. The residual solvent peak was used as an internal reference for 1H and 13C NMR spectra. Data for 1H NMR are reported as follows: chemical shift, multiplicity (br = broad, ovrlp = overlapped, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = double doublet), coupling constant, integral. Spectra were processed with the program MestReNova version 6.0.2–5475, FT and zero filling at 64 K. High-resolution (HR) mass spectra were obtained using a Thermo Fischer Exactive mass spectrometer equipped with an ESI source and an Orbitrap analyzer: capillary temperature 275 °C, spray voltage 3.5 kV, sheath gas (N2) 10 arbitrary units, capillary voltage 65 V, tube lens 125 V. Analytical TLC was performed using 0.25 mm Fluka F254 silica gel. The compounds on TLC were revealed by quenching fluorescence (at 254 or 365 nm) using a 4W UV lamp. Otherwise, TLC plates were stained with p-anisaldehyde acidic solution in EtOH or a 10% solution of phosphomolybdic acid in EtOH and heated (T = 120 °C). The product mixture purifications were carried out with silica column chromatography using Fluka 60 Å silica gel (0063–0200 mm, 70–230 mesh). Flash chromatography was performed using 200−400 mesh silica gel. Commercially available reagents and solvents were supplied by Sigma-Aldrich (St. Louis, MI, USA) and used without further purification.
3.2. Procedure for the Screening of Optimal Catalyst, Temperature and Additive for the Gold (I)-Catalyzed Synthesis of 3,8-Dimethylene-2,7-dioxaspiro[4.4]nonane-1,6-diones 5
In a 5 mL Carousel Tube Reactor (Radley Discovery Technology) containing a magnetic stirring bar Au(I) catalyst (0.02 mmol) was dissolved in 1.0 mL of CH2Cl2. Then, 2,2-di(prop-2-ynyl)malonic acid (0.5 mmol) was added and the reaction mixture was stirred for a time ranging from 0.5 to 24 h at a temperature ranging from room temperature to 100 °C. After this time, n-hexane was added to the reaction mixture and then it was centrifuged. The precipitate was separated from the liquid layer to obtain 3,8-dimethylene-2,7-dioxaspiro[4.4]nonane-1,6-dione.
3.3. Procedures for the Synthesis of Stating Materials
Diethyl 2,2-di(prop-2-yl)malonate (7) has been synthetized according to known procedures and their characterization data match our own in all respects [36,37].
diethyl 2,2-di(prop-2-yn-1-yl)malonate (7): colorless oil, 83%. 1H NMR (400 MHz, CDCl3) δ 4.21 (q, J = 7.1 Hz, 4H), 2.96 (d, J = 2.6 Hz, 4H), 2.01 (m, 2H), 1.24 (t, J = 7.1 Hz, 6H). 13C NMR (CDCl3): δ 168.7, 78.5, 71.8, 62.2, 56.4, 22.6, 14.1.
3.4. Typical Procedure for the Preparation of 2,2-Bis(3-phenylprop-2-yn-1-yl)malonate (8a)
Compounds 8a, b, c, f, g, h, i, j, k, l, n have been prepared according to the typical procedure described below, by using suitable aryl iodides.
A flask equipped with a magnetic stirring bar was charged with PdCl2(PPh3)2 (42 mg, 0.06 mmol, 0.02 equiv.) and CuI (22.8 mg, 0.12 mmol, 0.04 equiv.) dissolved in diisopropylamine (12 mL) and N,N-dimethylformamide (6 mL). The resultant solution was stirred under nitrogen at room temperature for 10 min before adding iodobenzene (1346 mg, 736 μL, 6.6 mmol, 2.2 equiv.) and diethyl 2,2-di(prop-2-yn-1-yl)malonate (7) (709 mg, 3.0 mmol, 1.0 equiv.) and stirred for 4 h at room temperature. After this time, the reaction mixture was diluted with Et2O and washed with a saturated NH4Cl solution, HCl 2 N, and with brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by chromatography on SiO2 (25–40 μm), eluting with an 80/20 (v/v) n-hexane/AcOEt mixture to obtain 1106 mg (90% yield) of diethyl 2,2-bis(3-phenylprop-2-yn-1-yl)malonate 8a.
3.5. Typical Procedure for the Preparation of Diethyl 2-(3-(3-methoxyphenyl)prop-2-yn-1-yl)-2-(3-phenylprop-2-yn-1-yl)malonate (8d)
Compounds 8d, e, m were prepared according to the typical procedure described below, by using suitable aryl iodides.
A flask equipped with a magnetic stirring bar was charged with a solution of PdCl2(PPh3)2 (42 mg, 0.06 mmol, 0.02 equiv.) and CuI (22.8 mg, 0.12 mmol, 0.04 equiv.) in diisopropylamine (12 mL) and N,N-dimethylformamide (6 mL), iodobenzene (673 mg, 368 μL, 3.3 mmol, 1.1 equiv.) and diethyl 2,2-di(prop-2-yn-1-yl)malonate (7) (709 mg, 3.0 mmol, 1.0 equiv.). The reaction mixture was stirred at room temperature under argon for 4 h before adding 1-iodo-3-methoxybenzene (772 mg, 3.3 mmol, 1.1 equiv.). After 6 h, the mixture was diluted with Et2O and washed with a saturated NH4Cl solution, HCl 2 N, and with brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by chromatography on SiO2 (25–40 μm), eluting with an 80/20 (v/v) n-hexane/AcOEt mixture to obtain 628 mg (50% yield) of 2-(3-(3-methoxyphenyl)prop-2-yn-1-yl)-2-(3-phenylprop-2-yn-1-yl)malonate (8d).
diethyl 2,2-bis(3-phenylprop-2-yn-1-yl)malonate (8a): yellow pale oil. 1H NMR (400 MHz, CDCl3) δ 7.44–7.37 (m, 4H), 7.30 (m, 6H), 4.30 (q, J = 7.1 Hz, 4H), 3.30 (s, 4H), 1.31 (t, J = 7.1 Hz, 8H). 13C NMR (101 MHz, CDCl3) δ 169.0, 131.8, 128.3, 128.1, 123.2, 84.2, 83.8, 62.1, 57.2, 23.8, 14.2.
diethyl 2,2-bis(3-(4-methoxyphenyl)prop-2-yn-1-yl)malonate (8c): yellow pale oil. 1H NMR (CDCl3): δ 7.33 (d, J = 8.3 Hz, 4H), 6.82 (d, J = 8.3 Hz, 4H), 4.28 (q, J = 7.0 Hz, 4H), 3.81 (s, 6H), 3.25 (s, 4H), 1.30 (t, J = 7.0 Hz, 6H). 13C NMR (CDCl3): δ 169.2, 159.4, 133.1, 115.4, 113.9, 83.5, 82.7, 62.0, 57.3, 55.4, 23.8, 14.2.
diethyl 2-(3-(4-methoxyphenyl)prop-2-yn-1-yl)-2-(3-phenylprop-2-yn-1-yl)malonate (8d): yellow pale oil. 1H NMR (CDCl3): δ 7.45–7.36 (m, 2H), 7.31 (m, 1H), 7.21 (t, J = 7.9 Hz, 1H), 7.01 (d, J = 7.4 Hz, 1H), 6.94 (br s, 1H), 6.87 (m, 1H), 4.30 (q, J = 7.1 Hz, 4H), 3.81 (s, 3H), 3.29 (s, 4H), 1.31 (t, J = 7.2 Hz, 6H). 13C NMR (CDCl3): δ 169.0, 159.4, 131.8, 129.4, 128.3, 128.1, 124.4, 124.3, 123.2, 116.7, 114.6, 84.2, 84.1, 83.8, 83.7, 62.1, 57.2, 55.3, 29.8, 23.8, 14.2.
diethyl 2,2-bis(3-(4-methylphenyl)prop-2-yn-1-yl)malonate (8f): yellow pale oil. 1H NMR (400 MHz, CDCl3): δ 7.30 (d, J = 8.0 Hz, 4H), 7.10 (d, J = 7.8 Hz, 4H), 4.29 (q, J = 7.1 Hz, 4H), 3.28 (s, 4H), 2.35 (s, 6H), 1.30 (t, J = 7.1 Hz, 6H). 13C NMR (CDCl3): δ 169.0, 138.1, 131.6, 129.0, 120.2, 83.8, 83.5, 62.0, 60.4, 57.3, 23.8, 21.5, 14.2.
diethyl 2,2-bis(3-(naphthalen-1-yl)prop-2-yn-1-yl)malonate (8g): yellow pale oil. 1H NMR (CDCl3): δ 8.34 (d, J = 8.2 Hz, 2H), 7.82 (dd, J = 12.2, 8.2 Hz, 6H), 7.65 (d, J = 7.0 Hz, 2H), 7.60–7.46 (m, 6H), 7.40 (t, J = 7.7 Hz, 2H), 4.33 (q, J = 7.1 Hz, 4H), 3.54 (s, 4H), 1.31 (t, J = 7.1 Hz, 6H). 13C NMR (CDCl3): δ 169.3, 133.6, 133.3, 130.7, 128.6, 128.4, 126.9, 126.5, 126.4, 125.3, 121.0, 89.2, 82.0, 62.3, 57.3, 24.5, 14.3.
diethyl 2,2-bis(3-(4-bromo-3-fluorophenyl)prop-2-yn-1-yl)malonate (8i): yellow pale oil. 1H NMR (CDCl3): δ 7.47 (t, J = 7.7 Hz, 1H), 7.13 (d, J = 9.1 Hz, 1H), 7.05 (d, J = 8.2 Hz, 1H), 4.29 (q, J = 7.1 Hz, 2H), 3.23 (s, 2H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (CDCl3): δ 168.8, 160.0, 157.5, 133.5, 128.7, 128.7, 124.2, 124.1, 119.7, 119.5, 109.6, 109.4, 86.3, 82.1, 62.3, 56.9, 23.9, 14.3.
diethyl 2,2-bis(3-(4-chlorophenyl)prop-2-yn-1-yl)malonate (8j): yellow pale oil. 1H NMR (CDCl3): δ 7.32 (d, J = 8.6 Hz, 4H), 7.29–7.23 (m, 4H), 4.29 (q, J = 7.1 Hz, 4H), 3.25 (s, 4H), 1.30 (t, J = 7.1 Hz, 6H). 13C NMR (CDCl3): δ 168.9, 134.2, 133.0, 128.7, 121.7, 85.3, 82.8, 62.2, 57.1, 23.9, 14.2.
diethyl 2,2-bis(3-(4-bromophenyl)prop-2-yn-1-yl)malonate (8k): yellow pale oil. 1H NMR (CDCl3): δ 7.43 (d, J = 8.2 Hz, 4H), 7.26 (t, J = 8.8 Hz, 4H), 4.28 (q, J = 7.1 Hz, 4H), 3.25 (s, 4H), 1.29 (t, J = 7.0 Hz, 6H). 13C NMR (CDCl3): δ 168.9, 139.2, 133.6, 133.2, 131.6, 122.4, 122.1, 85.5, 82.9, 62.2, 57.0, 23.9, 14.2.
diethyl 2,2-bis(3-(2-methyl-5-nitrophenyl)prop-2-yn-1-yl)malonate (8l): yellow pale oil. 1H NMR (CDCl3): δ 8.18 (d, J = 1.8 Hz, 2H), 8.02 (dd, J = 8.4, 1.9 Hz, 2H), 7.33 (d, J = 8.4 Hz, 2H), 4.28 (q, J = 7.1 Hz, 4H), 3.34 (s, 4H), 2.49 (s, 6H), 1.29 (t, J = 7.1 Hz, 6H). 13C NMR (CDCl3): δ 168.7, 147.8, 146.1, 130.3, 127.0, 124.4, 122.9, 90.6, 80.8, 62.4, 56.7, 24.1, 21.1, 14.2.
diethyl 2-(3-(4-chlorophenyl)prop-2-yn-1-yl)-2-(3-(4-methoxyphenyl)prop-2-yn-1-yl)malonate (8m): yellow pale oil. 1H NMR (CDCl3): δ 7.30 (d, J = 3.6 Hz, 2H), 7.28 (d, J = 3.6 Hz, 3H), 7.23 (d, J = 8.5 Hz, 3H), 6.78 (d, J = 8.7 Hz, 2H), 4.25 (q, J = 7.1 Hz, 4H), 3.77 (s, 3H), 3.22 (d, J = 9.1 Hz, 4H), 1.32–1.19 (J = 7.1 Hz, 6H). 13C NMR (CDCl3): δ 169.0, 159.5, 134.1, 133.1, 133.0, 128.6, 121.8, 115.3, 113.9, 85.5, 83.7, 82.7, 82.5, 62.1, 57.2, 55.3, 23.9, 23.8, 14.2.
diethyl 2,2-bis(3-(2-methoxyphenyl)prop-2-yn-1-yl)malonate (8n): yellow pale oil. 1H NMR (CDCl3): δ 7.33 (dd, J = 7.5, 1.4 Hz, 2H), 7.25–7.20 (m, 2H), 6.86–6.81 (m, 4H), 4.24 (q, J = 7.1 Hz, 4H), 3.83 (s, 6H), 3.33 (s, 4H), 1.26 (t, J = 7.1 Hz, 6H). 13C NMR (CDCl3): δ 169.1, 160.2, 133.8, 129.4, 120.4, 112.7, 110.8, 88.7, 79.9, 62.0, 57.5, 55.8, 24.1, 14.2.
3.6. General Procedure for the Synthesis of 3,8-Dimethylenespiro[4.4]nonane-1,6-diones (5)
In a 10 mL round bottom flask, 2,2 disubstituted diethyl malonate (8) (0.5 mmol) was dissolved in 2 mL of EtOH and 1 mL of NaOH 2N was added. The reaction mixture was heated at reflux and stirred for one hour. After cooling, the reaction mixture was acidified with conc. HCl, diluted with Et2O, and washed with brine. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was used without further purification in the next step. In a 5 mL Carousel Tube Reactor (Radley Discovery Technology) containing a magnetic stirring bar JohnPhos Au(MeCN)SbF6 catalyst (0.02 mmol) was dissolved at room temperature in 1.0 mL of CH2Cl2. Then, 2,2-disubstituted malonic acid (0.5 mmol) was added and the reaction mixture was stirred for 1 h at room temperature. After this time, n-hexane was added to the reaction mixture and then it was centrifuged. The precipitate was separated from the liquid layer to obtain 3,8-dimethylene-2,7-dioxaspiro[4.4]nonane-1,6-dione or the corresponding substituted derivative in 96–100% yield.
3,8-dibenzylidene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5a):1H NMR (DMSO): δ 7.52 (s, 2H), 7.38 (d, J = 6.2 Hz, 2H), 7.25 (s, 1H), 5.87 (s, 1H), 3.63 (d, J = 16.3 Hz, 1H), 3.37 (d, J = 22.0 Hz, 2H). 13C NMR (DMSO): δ 171.8, 146.0, 133.6, 128.6, 128.1, 126.9, 104.5, 36.4. HRMS (ESI Orbitrap) m/z 333.11245 [M + H]+ (calcd for C21H17O4+, 333.11214), 355.09397 [M + Na]+ (calcd for C21H16O4Na+, 355.09408).
3,8-dimethylene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5b): white solid; mp: 60–61 °C. 1H NMR (DMSO): δ 4.82 (m, 2H), 4.54 (br s, 2H), 3.41 (d, J = 17.3 Hz, 2H), 3.14 (d, J = 17.3 Hz, 2H); 13C NMR (DMSO): δ 168.6, 131.7, 128.2, 62.1, 56.8, 36.1. HRMS (ESI Orbitrap) m/z 181.04937 [M + H]+ (calcd for C9H9O4+, 181.04954), 203.03167 [M + Na]+ (calcd for C9H8O4Na+, 203.03148).
3,8-bis(4-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5c): white solid; mp: 50–51 °C. 1H NMR (DMSO): δ 7.26 (t, J = 8.0 Hz, 2H), 7.10–7.05 (m, 4H), 6.80 (dd, J1,2 = 6.0 Hz, J2,3 = 2.0 Hz, 2H), 5.81 (s, 2H), 5.73 (s, 1H), 3.73 (s, 6H), 3.58 (d, J = 17.6 Hz, 2H), 3.32 (d, J = 17.6 Hz, 2H); 13C NMR (DMSO): δ 172.2, 159.8, 146.6, 135.3, 130.1, 121.1, 114.2, 112.7, 104.9, 55.5, 50.6, 36.9. HRMS (ESI Orbitrap) m/z 393.13553 [M + H]+ (calcd for C23H21O6+, 393.13326), 415.11464 [M + Na]+ (calcd for C23H20NaO6+, 415.11521).
3-benzylidene-8-(3-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5d): white solid; mp: 40–41 °C. 1H NMR (DMSO): δ 7.53 (d, J = 7.2 Hz, 2H), 7.39–7.25 (m, 4H), 7.13–7.08 (m, 2H), 6.84 (d, J = 7.2 Hz, 1H), 5.87–5.85 (m, 2H), 3.76 (s, 3H), 3.62 (d, J = 17.2 Hz, 2H), 3.37 (d, J = 17.2 Hz, 2H); 13C NMR (DMSO): δ 172.2, 159.8, 146.6, 146.4, 135.3, 134.0, 130.1, 129.1, 128.6, 127.3, 121.1, 114.3, 112.7, 105.0, 104.9, 55.5, 50.6, 36.9. HRMS (ESI Orbitrap) m/z 363.12143 [M + H]+ (calcd for C22H19O5+, 363.12270), 385.10529 [M + Na]+ (calcd for C22H18NaO5+, 385.10464).
3-(4-acetylbenzylidene)-8-benzylidene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5e): white solid; mp: 45–46 °C. 1H NMR (DMSO): δ 7.97 (d, J = 8.0 Hz, 2H), 7.65 (d, J = 8.0 Hz, 2H), 7.52 (d, J = 7.2 Hz, 2H), 7.38 (t, J = 7.6 Hz, 2H), 7.24 (t, J = 7.6 Hz, 1H), 5.98 (s, 1H), 5.88 (s, 1H), 3.69–3.61 (m, 2H), 3.43–3.35 (m, 4H); 13C NMR (DMSO): δ 197.7, 172.2, 172.1, 148.9, 146.4, 138.8, 135.3, 134.0, 129.1, 129.1, 128.6, 127.3, 105.0, 104.2, 50.6, 37.1, 36.9, 27.1. HRMS (ESI Orbitrap) m/z 375.12211 [M + H]+ (calcd for C23H19O5+, 375.12270), 397.10425 [M + Na]+ (calcd for C23H18NaO5+, 397.10464).
3,8-bis(4-methylbenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5f): white solid; mp: 55–56 °C. 1H NMR (DMSO): δ 7.42 (d, J = 7.8 Hz, 4H), 7.19 (d, J = 7.2 Hz, 4H), 5.81 (s, 2H), 3.60 (d, J = 16.8 Hz, 2H), 3.36 (d, J = 16.8 Hz, 2H), 2.30 (s, 6H); 13C NMR (DMSO): δ 172.3, 145.6, 136.6, 131.2, 129.6, 128.5, 104.9, 50.7, 36.9, 21.2. HRMS (ESI Orbitrap) m/z 361.14517 [M + H]+ (calcd for C23H21O4+, 361.14344), 383.12479 [M + Na]+ (calcd for C23H20NaO4+, 383.12538).
3,8-bis(naphthalen-1-ylmethylene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5g): white solid; mp: 45–46 °C. 1H NMR (DMSO): δ 8.20 (d, J = 7.6 Hz, 2H), 7.96 (d, J = 7.4 Hz, 1H), 7.87 (d, J = 8.2 Hz, 1H), 7.79 (d, J = 7.1 Hz, 1H), 7.57 (m, 6H), 6.61 (s, 1H), 3.80 (d, J = 17.2 Hz, 1H), 3.55 (d, J = 17.2 Hz, 1H); 13C NMR (DMSO): δ 172.4, 147.9, 133.8, 131.1, 130.4, 128.9 127.8, 127.1, 126.7, 126.4, 126.0, 124.6, 101.7, 51.3, 36.9. HRMS (ESI Orbitrap) m/z 433.14383 [M + H]+ (calcd for C29H21O4+, 433.14344), 455.12643 [M + Na]+ (calcd for C29H20NaO4+, 455.12538).
3,8-bis(3-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5h): white solid; mp: 65–66 °C. 1H NMR (DMSO): δ 7.30 (t, J = 8.0 Hz, 2H), 7.13 (d, J = 7.7 Hz, 2H), 7.09 (s, 2H), 6.88–6.82 (m, 2H), 5.85 (s, 2H), 3.77 (s, 3H) 3.62 (d, J = 17.4 Hz, 2H), 3.37 (d, J = 17.4 Hz, 2H); 13C NMR (DMSO): δ 172.2, 159.8, 146.6, 135.3, 130.1, 121.1, 114.3, 112.8, 104.9, 55.5, 50.6, 36.9. HRMS (ESI Orbitrap) m/z 393,13415 [M + H]+ (calcd for C23H21O6+, 393,13326), 415.11583 [M + Na]+ (calcd for C23H20NaO6+, 415.11521).
3,8-bis(4-bromo-3-fluorobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5i): white solid; mp: 65–66 °C. 1H NMR (DMSO): δ 7.72 (t, J = 8.0 Hz, 2H), 7.46 (d, J = 10.4 Hz, 2H), 7.33 (d, J = 8.4 Hz, 2H), 5.92 (s, 2H), 3.64 (d, J = 17.6 Hz, 2H), 3.38 (d, J = 17.6 Hz, 2H); 13C NMR (DMSO): δ 171.8, 159.9, 157.5, 148.4, 136.0, 135.9, 134.1, 126.2, 116.2, 116.0, 106.4, 106.2, 103.1, 50.5, 36.9. HRMS (ESI Orbitrap) m/z 524.91356 [M + H]+ (calcd for C21H13Br2F2O4+, 524.91432), 546.89517 [M + Na]+ (calcd for C21H12Br2F2NaO4+, 546.89626).
3,8-bis(4-chlorobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5j): white solid; mp: 65–66 °C. 1H NMR (DMSO): δ 7.51 (d, J = 7.6 Hz, 4H), 7.41 (d, J = 7.6 Hz, 4H), 5.86 (m, 2H), 3.59 (d, J = 17.2 Hz, 2H), 3.34 (d, J = 17.2 Hz, 2H); 13C NMR (DMSO): δ 172.0, 147.2, 133.0, 131.7, 130.2, 129.1, 103.8, 50.6, 36.9. HRMS (ESI Orbitrap) m/z 401.03474 [M + H]+ (calcd for C21H15Cl2O4+, 401.03419), 423.01581 [M + Na]+ (calcd for C21H14Cl2NaO4+, 423.01614).
3,8-bis(4-bromobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5k): white solid; mp: 67–68 °C. 1H NMR (DMSO): δ 7.58 (d, J = 8.4 Hz, 4H), 7.48 (d, J = 8.4 Hz, 4H), 5.88 (s, 2H), 3.62 (d, J = 18.0 Hz, 2H), 3.35 (d, J = 18.0 Hz, 2H); 13C NMR (DMSO): δ 172.0, 147.3, 133.3, 132.0, 130.5, 120.2, 103.9, 50.6, 36.9. HRMS (ESI Orbitrap) m/z 488.93451 [M + H]+ (calcd for C21H15Br2O4+, 488.93316), 510.91493 [M + Na]+ (calcd for C21H14Br2NaO4+, 510.91511).
3,8-bis(2-methyl-5-nitrobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5l): white solid; mp: 57–58 °C. 1H NMR (DMSO): δ 8.46 (d, J = 2.4 Hz, 2H), 8.03 (dd, J1,2 = 6.0 Hz, J2,3 = 2.4 Hz, 2H), 7.53 (d, J = 8.8 Hz, 2H), 6.09 (s,2H), 3.75 (d, J = 18.0 Hz, 2H), 3.46 (d, J = 18.0 Hz, 2H), 2.45 (s, 6H); 13C NMR (DMSO): δ 171.9, 149.0, 146.4, 144.2, 134.1, 131.8, 123.2, 122.0, 100.9, 50.9, 37.0, 20.4. HRMS (ESI Orbitrap) m/z 451.11426 [M + H]+ (calcd for C23H19N2O8+, 451.11359), 473.09627 [M + Na]+ (calcd for C23H18N2NaO8+, 473.09554).
3-(4-chlorobenzylidene)-8-(4-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5m): white solid; mp: 55–56 °C. 1H NMR (DMSO): δ 7.54 (d, J = 8.4 Hz, 2H), 7.46 (t, J = 7.2 Hz, 4H), 6.95 (d, J = 8.8 Hz, 2H), 5.89 (s,1H), 5.80 (s, 1H), 3.76 (s, 3H), 3.60 (d, J = 18.0 Hz, 2H), 3.46 (d, J = 18.0 Hz, 2H); 13C NMR (DMSO): δ 171.7, 171.6, 158.1, 146.7, 144.0, 132.5, 131.1, 129.7, 129.4, 128.6, 126.1, 125.7, 114.1, 114.0, 104.2, 103.4, 103.3, 97.3, 55.1, 50.2, 36.5, 36.3. HRMS (ESI Orbitrap) m/z 397.08365 [M + H]+ (calcd C22H18ClO5+, 397.08373), 419.06529 [M + Na]+ (calcd for C22H17ClNaO5+, 419.06567).
3,8-bis(2-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5n): white solid; mp: 61–62 °C. 1H NMR (DMSO): δ 7.69 (dd, J = 7.8, 1.4 Hz, 2H), 7.29–7.22 (m, 2H), 7.03 (d, J = 8.3 Hz, 2H), 6.99 (t, J = 7.8 Hz, 2H), 6.04 (s, 2H), 3.82 (s, 6H), 3.67 (dd, J = 17.3, 1.6 Hz, 2H), 3.38 (dd, J = 17.3, 1.6 Hz, 2H). 13C NMR (DMSO): δ 172.3, 156.0, 146.3, 129.4, 128.8, 122.2, 120.9, 111.5, 98.9, 56.0, 50.8, 37.1. HRMS (ESI Orbitrap) m/z 393.13428 [M + H]+ (calcd for C23H21O6+, 393.13326), 415.11581 [M + Na]+ (calcd for C23H20NaO6+, 415.11521).
4. Conclusions
The activation of alkynes by gold(I) complexes is a versatile tool for the selective functionalization of C-C triple bond with several nucleophiles, including heteroatoms. The intramolecular version of this reaction leads to cyclic or heterocyclic intermediates, that are useful for the synthesis of high value pharmaceutically relevant compounds. We demonstrated that functionalized symmetric or unsymmetric biarylacetylenic malonic acids may be efficiently cyclized under extremely mild conditions at room temperature in presence of JohnPhosAu(MeCN)SbF6 catalyst, and without any additives. The corresponding γ-arylmethylene-spirobislactones were isolated in excellent yields (96–100%). This process constitutes an easy and efficient access to highly valuable building blocks of natural products or biologically active compounds. The high reactivity of gold(I) catalyst towards the activation of C-C triple bond, associated with very mild reactions conditions would allow further synthesis of lactones.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28010300/s1, Figure S1: 1H NMR of 3,8-dibenzylidene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5a); Figure S2: 13C NMR of 3,8-dibenzylidene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5a); Figure S3: 1H NMR of 3,8-dimethylene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5b); Figure S4: 13C NMR of 3,8-dimethylene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5b); Figure S5: 1H NMR of 3,8-bis(4-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5c); Figure S6: 13C NMR of 3,8-bis(4-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5c); Figure S7: 1H NMR of 3-benzylidene-8-(3-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5d); Figure S8: 13C NMR of 3-benzylidene-8-(3-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5d); Figure S9: 1H NMR of 3-(4-acetylbenzylidene)-8-benzylidene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5e); Figure S10: 13C NMR of 3-(4-acetylbenzylidene)-8-benzylidene-2,7-dioxaspiro[4.4]nonane-1,6-dione (5e); Figure S11: 1H NMR of 3,8-bis(4-methylbenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5f); Figure S12: 13C NMR of 3,8-bis(4-methylbenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5f); Figure S13: 1H NMR of 3,8-bis(naphthalen-1-ylmethylene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5g); Figure S14: 1H NMR of 3,8-bis(naphthalen-1-ylmethylene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5g); Figure S15: 1H NMR of 3,8-bis(3-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5h); Figure S16: 13C NMR of 3,8-bis(3-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5h); Figure S17: 1H NMR of 3,8-bis(4-bromo-3-fluorobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5i); Figure S18: 13C NMR of 3,8-bis(4-bromo-3-fluorobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5i); Figure S19: 1H NMR of 3,8-bis(4-chlorobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5j); Figure S20: 13C NMR of 3,8-bis(4-chlorobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5j); Figure S21: 1H NMR of 3,8-bis(4-bromobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5k); Figure S22: 13C NMR of 3,8-bis(4-bromobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5k); Figure S23: 1H NMR of 3,8-bis(2-methyl-5-nitrobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5l); Figure S24. 13C NMR of 3,8-bis(2-methyl-5-nitrobenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5l); Figure S25: 1H NMR of 3-(4-chlorobenzylidene)-8-(4-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5m); Figure S26: 13C NMR of 3-(4-chlorobenzylidene)-8-(4-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5m); Figure S27: 1H NMR of 3,8-bis(2-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5n); Figure S28: 1H NMR of 3,8-bis(2-methoxybenzylidene)-2,7-dioxaspiro[4.4]nonane-1,6-dione (5n).
Author Contributions
A.I.: writing, editing and revision. D.A.: synthesis of compounds. A.C.: conceptualization, methodology, writing and editing the draft. G.F. and A.G.: conceptualization, methodology, revision, and advice. G.M. and V.V.: chromatography and compound purification. A.I., A.S. and R.V.: synthesis of compounds and NMR. G.F. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the MIUR (Ministry of Education University and Research) under Grant number 2017SXBSX4 (PRIN project 2017, “Targeting Hedgehog pathway: virtual screening identification and sustainable synthesis of novel Smo and Gli inhibitors and their pharmacological drug delivery strategies for improved therapeutic effects in tumors”); MIUR under the Excellence Departments Grant to the Department of Chemistry and Technology of Drugs, and Sapienza University of Rome under Grant “Progetti Ateneo 2019”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Krasavin, M. Spirocyclic Motifs in Natural Products. Molecules 2019, 24, 4165. [Google Scholar] [CrossRef] [PubMed]
- Quintavalla, A. Spirolactones: Recent Advances in Natural Products, Bioactive Compounds and Synthetic Strategies. Curr. Med. Chem. 2018, 25, 917–962. [Google Scholar] [CrossRef]
- Wu, J.-W.; Tang, C.; Ke, C.-Q.; Yao, S.; Liu, H.-C.; Lin, L.-G.; Ye, Y. Dicarabrol A, dicarabrone C and dipulchellin A, unique sesquiterpene lactone dimers from Carpesium abrotanoides. RSC Adv. 2017, 7, 4639–4644. [Google Scholar] [CrossRef]
- Schmitz, F.U.; Roberts, C.D.; Abadi, A.D.M.; Griffith, R.C.; Laivers, M.R.; Slobodov, I.; Rai, R. N-(5-Membered Aromatic Ring)-Amido Anti-Viral Compounds. U.S. Patent US2007265265A1, 21 June 2007. [Google Scholar]
- Andersonc, A.G. Preparation and Use of Gamma-Butyrolactones as Cross-Linking Agents. U.S. Patent 6423850B1, 23 July 2002. [Google Scholar]
- Tiecco, M.; Tingoli, M.; Testaferri, L.; Bartoli, D. Simple Synthesis of γ-Lactones from Olefinic Nitriles. Synth. Commun. 1989, 19, 2817–2824. [Google Scholar] [CrossRef]
- Kochikyan, T.V.; Samvelyan, M.A.; Arutyunyan, V.S.; Avetisyan, A.A. Synthesis and Some Transformations of Ethyl 5-Alkoxymethyl-3-(2-methyl-3-oxobutyl)-2-oxotetrahydrofuran-3-carboxylates. Russ. J. Org. Chem. 2003, 39, 1329–1333. [Google Scholar] [CrossRef]
- Ghochikyan, T.V.; Muzalevskiy, V.M.; Samvelyan, M.A.; Galstyan, A.S.; Nenajdenko, V.G. Efficient synthesis of triazole-containing spiro dilactones. Mendeleev Commun. 2016, 26, 11–13. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Ghochikyan, T.V.; Spinelli, D.; Galstyan, A.S.; Geronikaki, A.; Samvelyan, M.A.; Hakobyan, E.K.; Hovakimyan, A.A. Synthesis of novel 1,2,3-triazole-based hybrids via click reactions. Arkivoc 2021, 2022, 7–21. [Google Scholar] [CrossRef]
- Roland, J.R.; Little, E.L.; Winberg, H.E. Bis(2-bromoalkyl)malononitriles by Addition of Dibromomalononitrile to Alkenes. J. Org. Chem. 1963, 28, 2809–2811. [Google Scholar] [CrossRef]
- Kotha, S.; Dipak, M.K.; Mobin, S.M. Serendipitous and acid catalyzed synthesis of spirolactones. Tetrahedron 2011, 67, 4616–4619. [Google Scholar] [CrossRef]
- Toshimitsu, A.; Aoai, T.; Owada, H.; Uemura, S.; Okano, M. Phenylselenenyl chloride in acetonitrile-water: A highly convenient reagent for hydroxyselenation of olefins and preparation of cyclic ethers from dienes. Tetrahedron 1985, 41, 5301–5306. [Google Scholar] [CrossRef]
- Uemura, S.; Toshimitsu, A.; Aoai, T.; Okano, M. Phenylselenenyl chloride as a reagent for the facile preparation of cyclic ethers from diolefins. Tetrahedron Lett. 1980, 21, 1533–1536. [Google Scholar] [CrossRef]
- Malmedy, F.; Wirth, T. Cyclization of Malonate Derivatives with Iodine(III) Reagents. Eur. J. Org. Chem. 2017, 2017, 786–789. [Google Scholar] [CrossRef]
- Muñoz, M.P.; Lloyd-Jones, G.C. Triflic Acid Mediated Dealkylative Lactonisation via NMR-Observable Alkyloxonium Intermediates. Eur. J. Org. Chem. 2009, 2009, 516–524. [Google Scholar] [CrossRef]
- Fairlamb, I.J.S.; Grant, S.; Tommasi, S.; Lynam, J.M.; Bandini, M.; Dong, H.; Lin, Z.; Whitwood, A.C. Phosphinite Ligand Effects in Palladium(II)-Catalysed Cycloisomerisation of 1,6-Dienes: Bicyclo[3.2.0]heptanyl Diphosphinite (B[3.2.0]DPO) Ligands Exhibit Flexible Bite Angles, an Effect Derived from Conformational Changes (exo-or endo-Envelope) in the Bicyclic Ligand Scaffold. Adv. Synth. Catal. 2006, 348, 2515–2530. [Google Scholar]
- Angelini, T.; Fringuelli, F.; Lanari, D.; Pizzo, F.; Vaccaro, L. A catalytic approach to the base-promoted reaction of epoxides with activated methylenes. Tetrahedron Lett. 2010, 51, 1566–1569. [Google Scholar] [CrossRef]
- Ito, N.; Nishino, H.; Kurosawa, K. The Reaction of Olefins with Malonic Acid in the Presence of Manganese(III) Acetate. Bull. Chem. Soc. Jpn. 1983, 56, 3527–3528. [Google Scholar] [CrossRef]
- Fristad, W.E.; Hershberger, S.S. Manganese(III)-mediated spirodilactonization. J. Org. Chem. 1985, 50, 1026–1031. [Google Scholar] [CrossRef]
- Fristad, W.E.; Peterson, J.R.; Ernst, A.B. Manganese(III). gamma.-lactone annulation with substituted acids. J. Org. Chem. 1985, 50, 3143–3148. [Google Scholar] [CrossRef]
- Adrio, L.A.; Quek, L.S.; Taylor, J.G.; Kuok Hii, K. Copper-catalysed intramolecular O–H addition to unactivated alkenes. Tetrahedron 2009, 65, 10334–10338. [Google Scholar] [CrossRef]
- Yang, C.-G.; Reich, N.W.; Shi, Z.; He, C. Intramolecular Additions of Alcohols and Carboxylic Acids to Inert Olefins Catalyzed by Silver(I) Triflate. Org. Lett. 2005, 7, 4553–4556. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef] [PubMed]
- Teles, J.H.; Brode, S.; Chabanas, M. Cationic Gold(I) Complexes: Highly Efficient Catalysts for the Addition of Alcohols to Alkynes. Angew. Chem. Int. Ed. Engl. 1998, 37, 1415–1418. [Google Scholar] [CrossRef]
- Mizushima, E.; Sato, K.; Hayashi, T.; Tanaka, M. Highly Efficient AuI-Catalyzed Hydration of Alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 4563–4565. [Google Scholar] [CrossRef]
- Arcadi, A.; Ciogli, A.; Fabrizi, G.; Fochetti, A.; Franzini, R.; Ghirga, F.; Goggiamani, A.; Iazzetti, A. Synthesis of pyrano[2,3-f]chromen-2-ones vs. pyrano[3,2-g]chromen-2-ones through site controlled gold-catalyzed annulations. Org. Biomol. Chem. 2019, 17, 10065–10072. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A. Construction of the 1,5-Benzodiazepine Skeleton from o-Phenylendiamine and Propargylic Alcohols via a Domino Gold-Catalyzed Hydroamination/Cyclization Process. Org. Lett. 2016, 18, 3511–3513. [Google Scholar] [CrossRef]
- Cera, G.; Piscitelli, S.; Chiarucci, M.; Fabrizi, G.; Goggiamani, A.; Ramón, R.S.; Nolan, S.P.; Bandini, M. One-Pot Gold-Catalyzed Synthesis of Azepino[1,2-a]indoles. Angew. Chem. Int. Ed. 2012, 51, 9891–9895. [Google Scholar] [CrossRef]
- Arcadi, A.; Calcaterra, A.; Fabrizi, G.; Fochetti, A.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Marsicano, V.; Mazzoccanti, G.; Serraiocco, A. Synthesis of 4-Substituted-1,2-Dihydroquinolines by Means of Gold-Catalyzed Intramolecular Hydroarylation Reaction of N-Ethoxycarbonyl-N-Propargylanilines. Molecules 2021, 26, 3366. [Google Scholar] [CrossRef]
- Arcadi, A.; Fabrizi, G.; Fochetti, A.; Franzini, R.; Ghirga, F.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Serraiocco, A. Synthesis of Polycyclic Chromene Cores through Gold (I)-Catalyzed Intramolecular Hydroarylation Reaction (IMHA). Eur. J. Org. Chem. 2021, 2021, 1676–1687. [Google Scholar] [CrossRef]
- Chiarucci, M.; Locritani, M.; Cera, G.; Bandini, M. Gold(I)-catalyzed synthesis of γ-vinylbutyrolactones by intramolecular oxaallylic alkylation with alcohols. Beilstein J. Org. Chem. 2011, 7, 1198–1204. [Google Scholar] [CrossRef]
- Zhu, S.; Ye, L.; Wu, W.; Jiang, H. N-Heterocyclic carbene–gold(I)-catalyzed carboheterofunctionalization of alkenes with arylboronic acids. Tetrahedron 2013, 69, 10375–10383. [Google Scholar] [CrossRef]
- Fernández, G.; Bernardo, L.; Villanueva, A.; Pleixats, R. Gold nanoparticles stabilized by PEG-tagged imidazolium salts as recyclable catalysts for the synthesis of propargylamines and the cycloisomerization of γ-alkynoic acids. New J. Chem. 2020, 44, 6130–6141. [Google Scholar] [CrossRef]
- Lin, B.; Yang, T.; Zhang, D.; Zhou, Y.; Wu, L.; Qiu, J.; Chen, G.-Q.; Che, C.-M.; Zhang, X. Gold-Catalyzed Desymmetric Lactonization of Alkynylmalonic Acids Enabled by Chiral Bifunctional P,N ligands. Angew. Chem. Int. Ed. 2022, 61, e202201739. [Google Scholar]
- Lin, B.; Chen, G.; Zhi, Z.; Zhang, X. Application of Phosphine-Nitrogen Ligands and Complexes Thereof in Catalysis of Asymmetric Reactions. CN Patent CN113527360A, 19 September 2021. [Google Scholar]
- König, N.F.; Al Ouahabi, A.; Poyer, S.; Charles, L.; Lutz, J.-F. A Simple Post-Polymerization Modification Method for Controlling Side-Chain Information in Digital Polymers. Angew. Chem. Int. Ed. Engl. 2017, 56, 7297–7301. [Google Scholar] [CrossRef]
- Al Ouahabi, A.; Charles, L.; Lutz, J.-F. Synthesis of Non-Natural Sequence-Encoded Polymers Using Phosphoramidite Chemistry. J. Am. Chem. Soc. 2015, 137, 5629–5635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).