Efficient Synthesis of Key Chiral Intermediate in Painkillers (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanamine by Bienzyme Cascade System with R-ω-Transaminase and Alcohol Dehydrogenase Functions
Abstract
1. Introduction
2. Results and Discussion
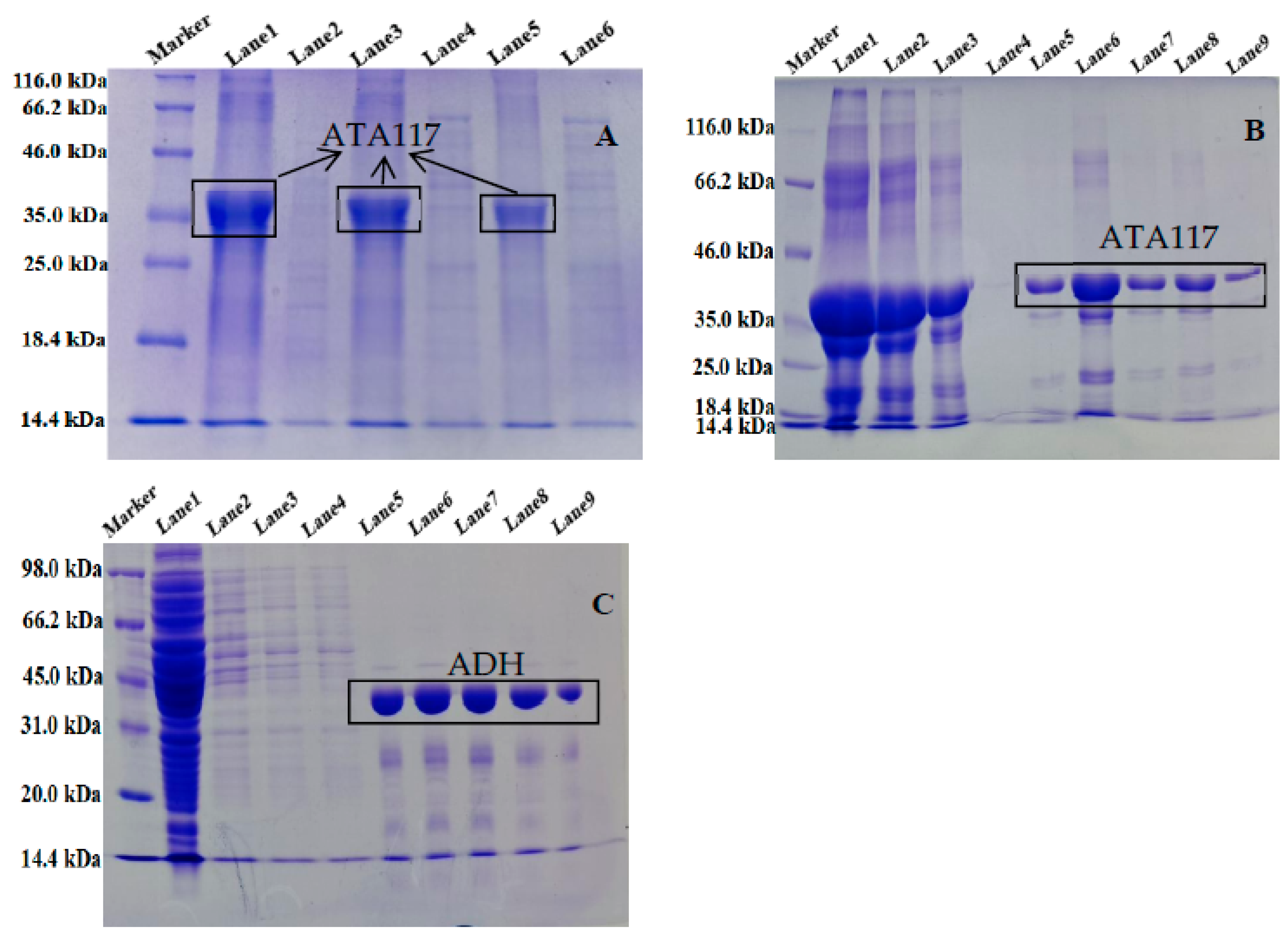
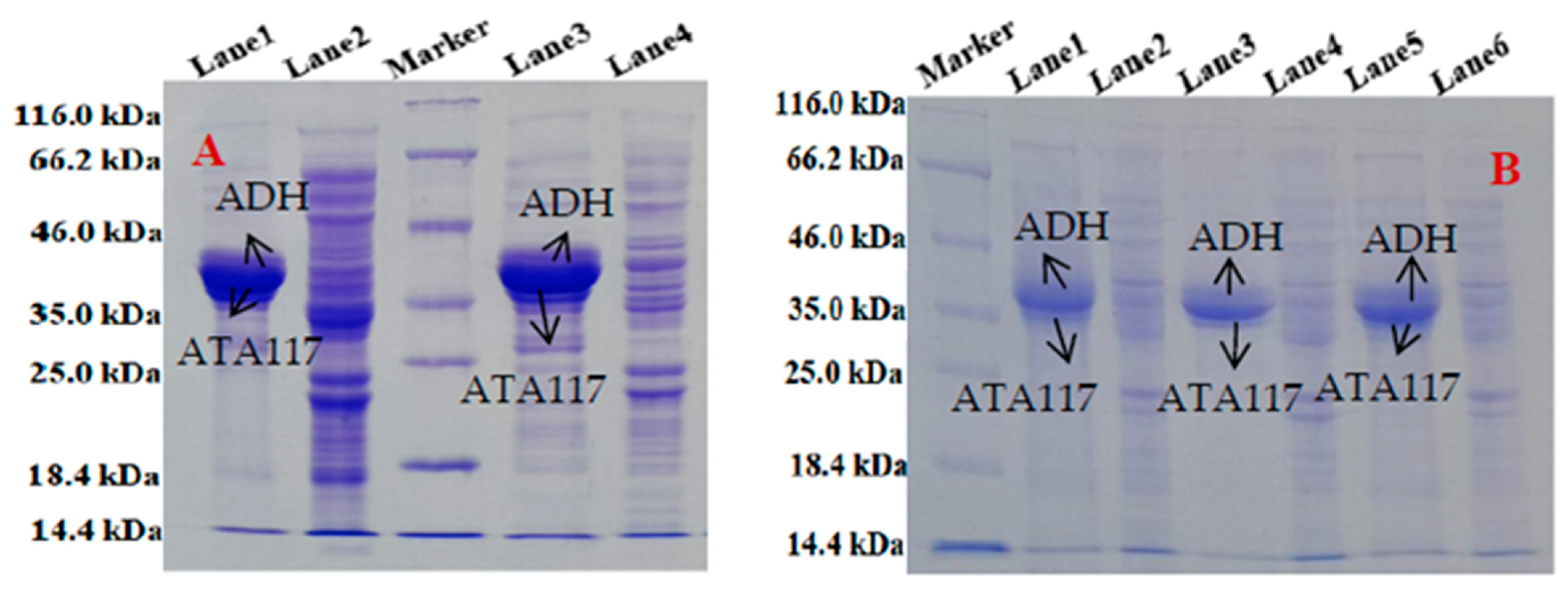
2.1. Expression and Purification of ATA117 and ADH from Single Expression System
2.2. Catalytic Properties of Purified ATA117 Expressed by the E. coli BL21(DE3)/pETDuet-ATA117 Recombinant Engineering Bacteria
2.3. Comparison of ATA117 Activity from E. coli BL21(DE3)/pETDuet-ATA117 Whole Cells and Crude Enzyme Solution
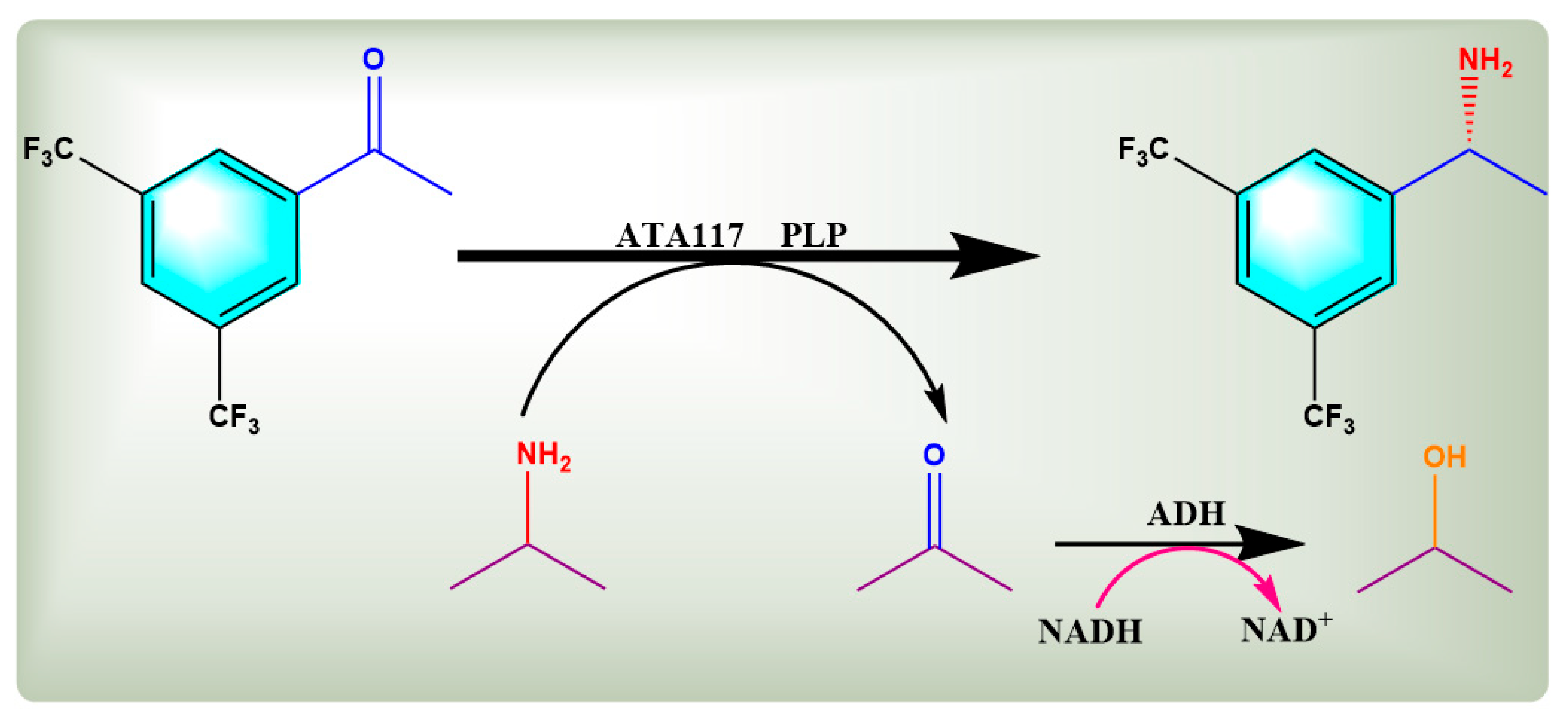
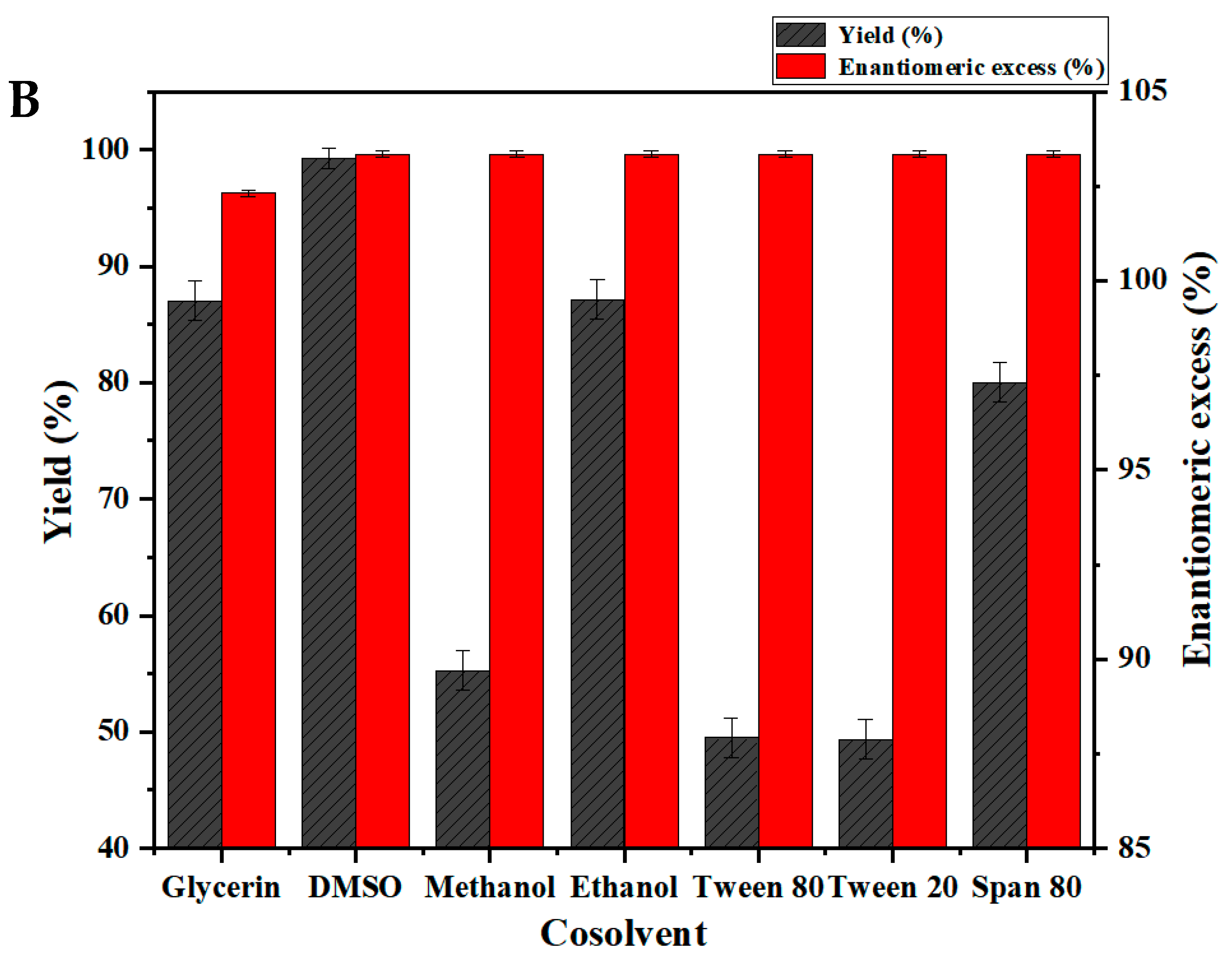
2.4. Asymmetric Synthesis of R-BPA from BPO by Free Bienzyme and Co-Expression Plasmid Engineered Bacteria
2.4.1. Preparation of R-BPA by Bienzyme Cascade Reaction with Free Enzyme Mixture Containing ATA117 and ADH
2.4.2. Preparation of R-BPA by Bienzyme Cascade System via Co-Expression Engineering Bacteria
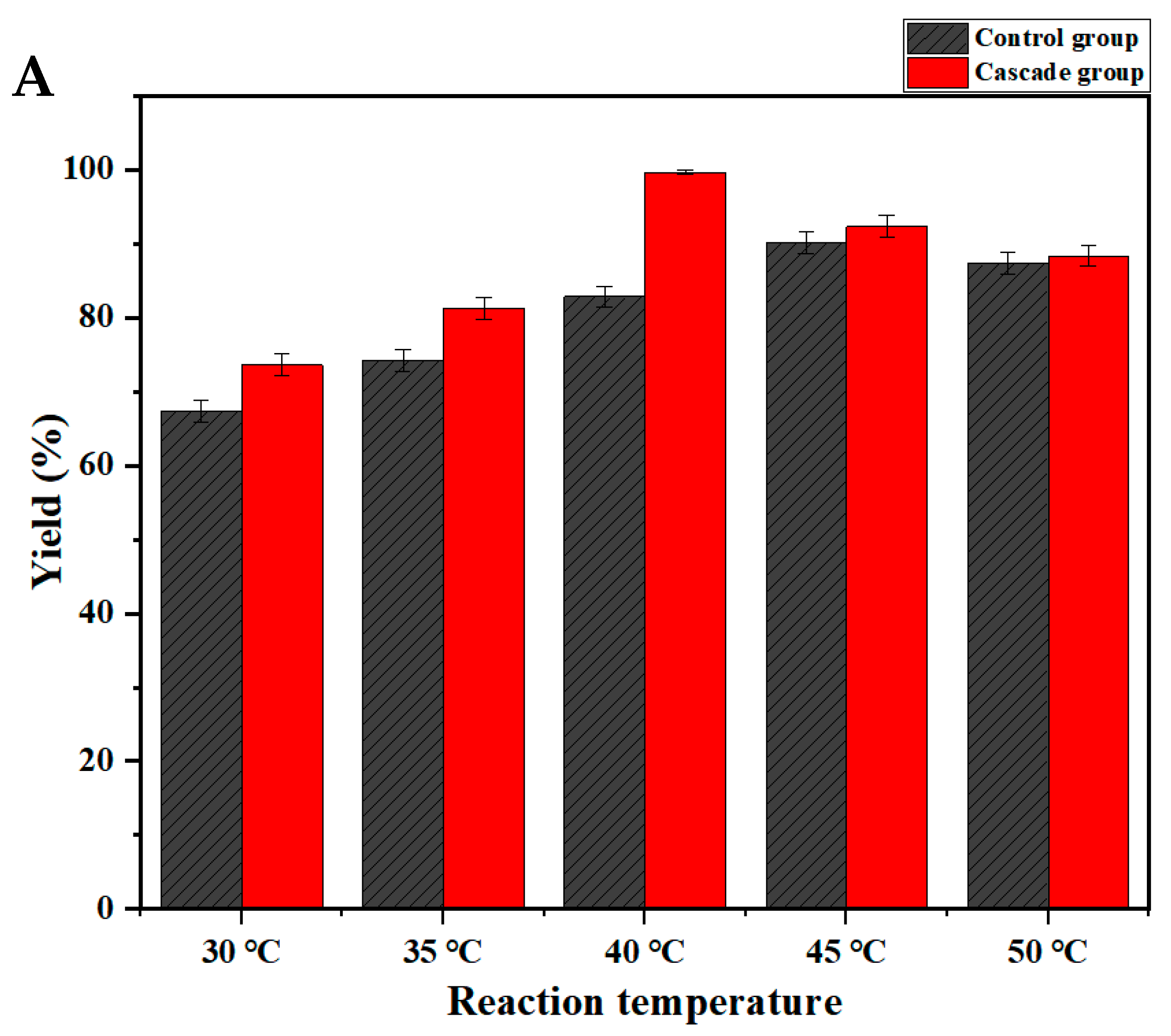
2.4.3. Comparison of Asymmetric Synthesis of R-BPA Catalyzed by Free Bienzyme and Co-Expression Engineered Bacteria
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Expression and Purification of ATA117 and ADH from Single Expression System
3.4. Enzyme Activity Assays
3.4.1. Activity of ATA117
3.4.2. Activity of ADH
3.5. Catalytic Properties of Purified ATA117 Expressed by the E. coli BL21(DE3)/pETDuet-ATA117 Recombinant Engineering Bacteria
3.5.1. Substrate Specificity and Enantioselectivity
3.5.2. Effects of pH and Temperature on Enzyme Activity
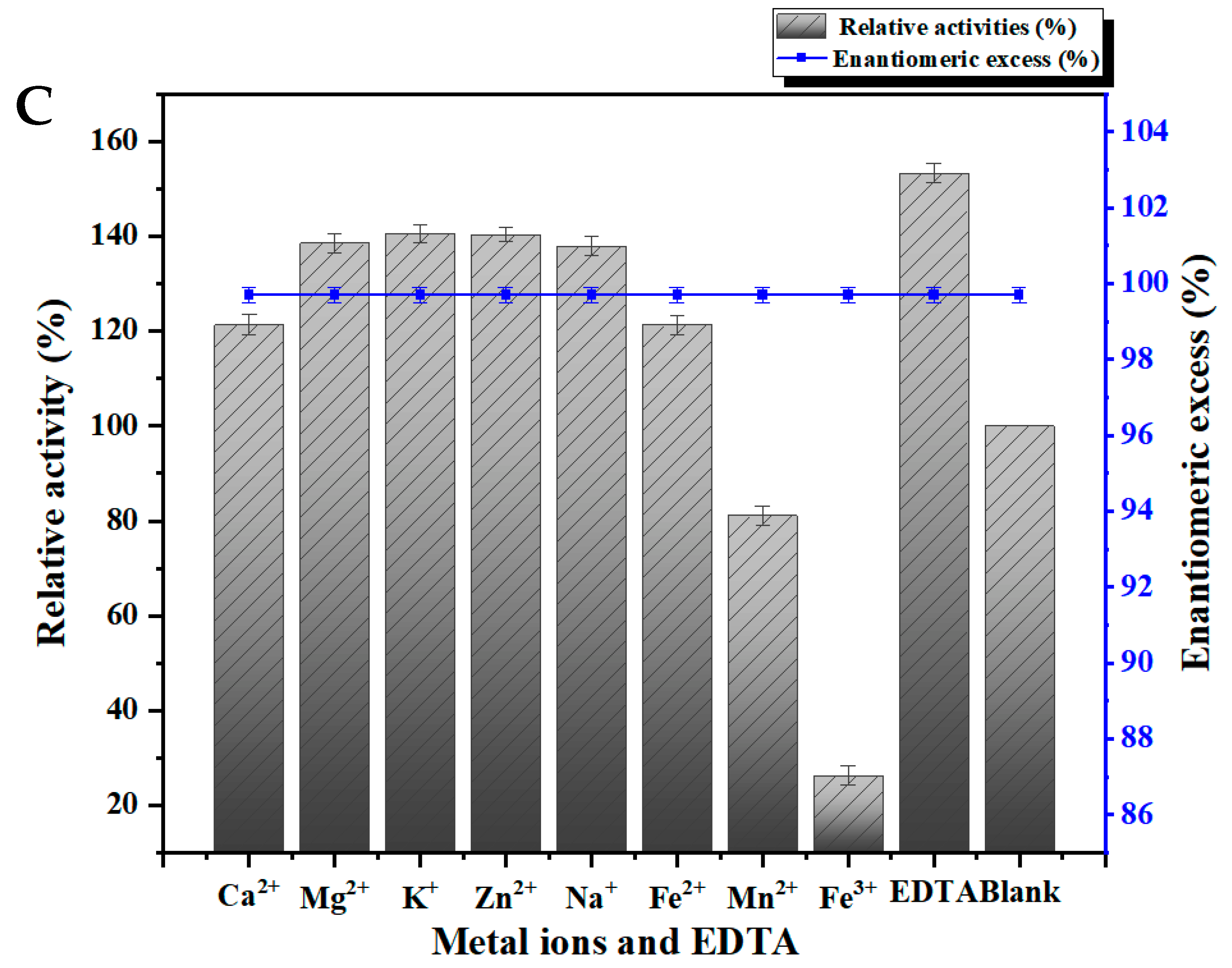
3.5.3. Effects of Metal Ions and Reagents on Enzyme Activity
3.5.4. Comparison of ATA117 Activity from E. coli BL21(DE3)/pETDuet-ATA117 Whole Cells and Crude Enzyme Solution
3.6. Preparation of Bienzyme Cascade System by Two Different Ways: Free Single Enzyme and Co-Expression Engineering Bacteria
3.6.1. Preparation of R-BPA by Bienzyme Cascade Reaction with Free Enzyme Mixture Containing ATA117 and ADH
3.6.2. Preparation of R-BPA by Bienzyme Cascade System via Co-Expression Engineering Bacteria Constructed by Two Double-Gene Tandem Co-Expression Systems
3.6.3. Preparation of R-BPA by Bienzyme Cascade System via Co-Expression Engineering Bacteria Constructed by Two-Plasmid Co-Expression System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.W.; Li, Y.; Nie, W.; Chang, Z.; Yu, Z.-A.; Zhao, Y.-F.; Lu, X.; Fu, Y. Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines. Nat. Commun. 2021, 12, 1313. [Google Scholar] [CrossRef] [PubMed]
- He, Y.L.; Song, H.Y.; Chen, J.; Zhu, S. NiH-catalyzed asymmetric hydroarylation of N-acyl enamines to chiral benzylamines. Nat. Commun. 2021, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, E.E.; Monti, D. Amine transaminases in chiral amines synthesis: Recent advances and challenges. World J. Microbiol. Biotechnol. 2018, 34, 13. [Google Scholar] [CrossRef] [PubMed]
- Boyko, K.M.; Nikolaeva, A.Y.; Timofeev, V.I.; Popov, V.O.; Bezsudnova, E.Y. Three-dimensional structure of branched-chain amino acid transaminase from thermoproteus uzoniensisin complex with L-norvaline. Crystallogr. Rep. 2020, 65, 740–743. [Google Scholar] [CrossRef]
- Dong, L.H.; Meng, Q.L.; Ramirez-Palacios, C.; Wijma, H.J.; Marrink, S.J.; Janssen, D.B. Asymmetric Synthesis of Optically Pure Aliphatic Amines with an Engineered Robust ω-Transaminase. Catalysts 2020, 10, 1310. [Google Scholar] [CrossRef]
- Kim, G.H.; Jeon, H.; Khobragade, T.P.; Patil, M.D.; Sung, S.; Yoon, S.; Won, Y.; Choi, I.S.; Yun, H. Enzymatic synthesis of sitagliptin intermediate using a novel ω-transaminase. Enzym. Microb. Technol. 2018, 120, 52–60. [Google Scholar] [CrossRef]
- Heckmann, C.M.; Gourlay, L.J.; Dominguez, B.; Paradisi, F. An (R)-selective transaminase from thermomyces stellatus: Stabilizing the tetrameric form. Front. Bioeng. Biotechnol. 2020, 8, 707. [Google Scholar] [CrossRef]
- Bezsudnova, E.Y.; Popov, V.O.; Boyko, K.M. Structural insight into the substrate specificity of PLP fold type IV transaminases. Appl. Microbiol. Biotechnol. 2020, 104, 2343–2357. [Google Scholar] [CrossRef]
- Taday, F.; Ryan, J.; Argent, S.P.; Caprio, V.; Maciá, B.; O’Reilly, E. Asymmetric construction of alkaloids by employing a key omega-transaminase cascade. Chem. Eur. J. 2020, 26, 3729–3732. [Google Scholar] [CrossRef]
- Kelly, S.A.; Pohle, S.; Wharry, S.; Mix, S.; Allen, C.C.; Moody, T.S.; Gilmore, B.F. Application of ω-transaminases in the pharmaceutical industry. Chem. Rev. 2018, 118, 349–367. [Google Scholar] [CrossRef]
- Gourbeyre, L.; Heuson, E.; Charmantray, F.; Hélaine, V.; Debard, A.; Petit, J.-L.; de Berardinis, V.; Gefflaut, T. Biocatalysed synthesis of chiral amines: Continuous colorimetric assays for mining amine-transaminases. Catal. Sci. Technol. 2021, 11, 904–911. [Google Scholar] [CrossRef]
- Buss, O.; Voss, M.; Delavault, A.; Gorenflo, P.; Syldatk, C.; Bornscheuer, U.; Rudat, J. β-phenylalanine ester synthesis from stable β-keto ester substrate using engineered ω-transaminases. Molecules 2018, 23, 1211. [Google Scholar] [CrossRef] [PubMed]
- Gomm, A.; O’Reilly, E. Transaminases for chiral amine synthesis. Curr. Opin. Chem. Biol. 2018, 43, 106–112. [Google Scholar] [CrossRef]
- Luo, W.; Hu, J.G.; Lu, J.P.; Zhang, H.; Wang, X.; Liu, Y.; Dong, L.; Yu, X. One pot cascade synthesis of L-2-aminobutyric acid employing omega-transaminase from Paracoccus pantotrophus. Mol. Catal. 2021, 515, 111890. [Google Scholar] [CrossRef]
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Recent advances in ω-transaminase-mediated biocatalysis for the enantioselective synthesis of chiral amines. Catalysts 2018, 8, 254. [Google Scholar] [CrossRef]
- Xie, D.F.; Fang, H.; Mei, J.Q.; Gong, J.-Y.; Wang, H.-P.; Shen, X.-Y.; Huang, J.; Mei, L.-H. Improving thermostability of (R)-selective amine transaminase from Aspergillus terreus through introduction of disulfide bonds. Appl. Biochem. 2018, 65, 255–262. [Google Scholar] [CrossRef]
- Gao, X.X.; Zhang, X.; Zhu, N.Q.; Mou, Y.; Zhang, H.; Liu, X.; Wei, P. Reshaping the substrate binding region of (R)-selective ω-transaminase for asymmetric synthesis of (R)-3-amino-1-butanol. Appl. Microbiol. Biotechnol. 2020, 104, 3959–3969. [Google Scholar] [CrossRef]
- Zeifman, Y.S.; Boyko, K.M.; Nikolaeva, A.Y.; Timofeev, V.I.; Rakitina, T.V.; Popov, V.; Bezsudnova, E.Y. Functional characterization of PLP fold type IV transaminase with a mixed type of activity from Haliangium ochraceum. Biochim. Biophys. Acta. Proteins Proteom. 2019, 1867, 575–585. [Google Scholar] [CrossRef]
- Kim, E.M.; Park, J.H.; Kim, B.G.; Seo, J.H. Identification of (R)-selective ω-aminotransferases by exploring evolutionary sequence space. Enzyme Microb. Technol. 2018, 110, 46–52. [Google Scholar] [CrossRef]
- Iglesias, C.; Panizza, P.; Giordano, S.R. Identification, expression and characterization of an R-ω-transaminase from Capronia semiimmersa. Appl. Microbiol. Biotechnol. 2017, 101, 5677–5687. [Google Scholar] [CrossRef]
- Corti, M.; Rinaldi, F.; Monti, D.; Ferrandi, E.; Marrubini, G.; Temporini, C.; Tripodo, G.; Kupfer, T.; Conti, P.; Terreni, M.; et al. Development of an integrated chromatographic system for ω-transaminase-IMER characterization useful for flow-chemistry applications. J. Pharm. Biomed. Anal. 2019, 169, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.Q.; Wang, J.Y.; Hu, H.; Liu, S.; Zhang, C.; Zhu, Y.; Bocola, M.; Sun, L.; Ji, Y.; Zhou, A.; et al. Transaminase engineering and process development for a whole-cell neat organic process to produce (R)-alpha-phenylethylamine. Org. Process Res. Dev. 2021, 12, 31. [Google Scholar] [CrossRef]
- Marx, L.; Rios-Lombardia, N.; Farnberger, J.F.; Kroutil, W.; Benítez-Mateos, A.I.; López-Gallego, F.; Morís, F.; González-Sabín, J.; Berglund, P. Chemoenzymatic approaches to the synthesis of the calcimimetic agent cinacalcet employing transaminases and ketoreductases. Adv. Synth. Catal. 2018, 360, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Chen, X.L.; Li, M.Y.; Zhang, X.-J.; Jia, D.-X.; Wang, Y.-J.; Liu, Z.-Q.; Zheng, Y.-G. Creation of a robust and R-selective ω-amine transaminase for the asymmetric synthesis of sitagliptin intermediate on a kilogram scale. Enzym. Microb. Technol. 2020, 141, 109655. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Mix, S.; Moody, T.S.; Gilmore, B.F. Transaminases for industrial biocatalysis: Novel enzyme discovery. Appl. Microbiol. Biotechnol. 2020, 104, 4781–4794. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, X.L.; Xiang, C.; Liu, Z.-Q.; Wang, Y.-J.; Zheng, Y.-G. Fluorescence-based high-throughput screening system for R-ω-transaminase engineering and its substrate scope extension. Appl. Microbiol. 2020, 104, 2999–3009. [Google Scholar] [CrossRef]
- Jia, D.X.; Peng, C.; Li, J.L.; Wang, F.; Liu, Z.-Q.; Zheng, Y.-G. Redesign of (R)-omega-transaminase and its application for synthesizing amino acids with bulky side chain. Appl. Biochem. Biotechnol. 2021, 193, 3624–3640. [Google Scholar] [CrossRef]
- Molnar, Z.; Farkas, E.; Lako, A.; Erdélyi, B.; Kroutil, W.; Vértessy, B.G.; Paizs, C.; Poppe, L. Immobilized whole-cell transaminase biocatalysts for continuous-flow kinetic resolution of amines. Catalysts 2019, 9, 438. [Google Scholar] [CrossRef]
- Garcia-Ramos, M.; Lavandera, I. Transaminases as suitable catalysts for the synthesis of enantiopure beta, beta-difluoroamines. Org. Biomol. Chem. 2022, 20, 984–988. [Google Scholar] [CrossRef]
- Cutlan, R.; De Rose, S.; Isupov, M.N.; Littlechild, J.A.; Harmer, N.J. Using enzyme cascades in biocatalysis: Highlight on transaminases and carboxylic acid reductases. Biochim. Biophys. Acta. Proteins Proteom. 2020, 1868, 140322. [Google Scholar] [CrossRef]
- Chen, F.F.; Zhang, Y.H.; Zhang, Z.J.; Liu, L.; Wu, J.-P.; Xu, J.-H.; Zheng, G.-W. An ammonium-formate-driven trienzymatic cascade for ω-transaminase-catalysed (R)-selective amination. J. Org. Chem. 2019, 84, 14987–14993. [Google Scholar] [CrossRef] [PubMed]
- Telzerow, A.; Hobisch, M.; Muller, M.; Schürmann, M.; Schwab, H.; Steiner, K. A co-expression system to shift the equilibrium of transamination reactions toward the synthesis of enantiomerically pure amines. Mol. Catal. 2019, 471, 38–43. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Fernie, A.R. The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation. Nat. Commun. 2018, 9, 2136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Hess, H. Toward rational design of high-efficiency enzyme cascades. ACS Catal. 2017, 7, 6018–6027. [Google Scholar] [CrossRef]
- Schmid-Dannert, C.; Lopez-Gallego, F. Advances and opportunities for the design of self-sufficient and spatially organized cell-free biocatalytic systems. Curr. Opin. Chem. Biol. 2019, 49, 97–104. [Google Scholar] [CrossRef]
- Leskovac, V.; Trivi, S.; Periin, D.; Trivic, S.; Pericin, D. The three zinc-containing alcohol dehydrogenases from baker’s yeast, saccharomyces cerevisiae. FEMS Yeast Res. 2002, 2, 481–494. [Google Scholar] [CrossRef]
- Tavanti, M.; Parmeggiani, F.; Castellanos, J.R.G.; Mattevi, A.; Turner, N.J. One-pot biocatalytic double oxidation of α-isophorone for the synthesis of ketoisophorone. ChemCatChem 2017, 9, 3338–3348. [Google Scholar] [CrossRef]
- Simon, R.C.; Richter, N.; Busto, E.; Kroutil, W. Recent developments of cascade reactions involving ω-transaminases. ACS Catalysis 2014, 4, 129–143. [Google Scholar] [CrossRef]
- Vachal, P.; Duffy, J.L.; Campeau, L.-C.; Amin, R.P.; Mitra, K.; Murphy, B.A.; Shao, P.P.; Sinclair, P.J.; Ye, F.; Katipally, R.; et al. Invention of MK-8262, a Cholesteryl Ester Transfer Protein (CETP) Inhibitor Backup to Anacetrapib with Best-in-Class Properties. J. Med. Chem. 2021, 64, 13215–13258. [Google Scholar] [CrossRef]
- Ninh, P.H.; Honda, K.; Sakai, T.; Okano, K.; Ohtake, H. Assembly and multiple gene expression of thermophilic enzymes in Escherichia coli for in vitro metabolic engineering. Biotechnol. Bioeng. 2015, 112, 189–196. [Google Scholar] [CrossRef]
- Chen, H.; Huang, R.; Zhang, Y.H.P. Systematic comparison of co-expression of multiple recombinant thermophilic enzymes in Escherichia coli BL21(DE3). Appl. Microbiol. Biotechnol. 2017, 101, 4481–4493. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, H.E.; Lee, K.H.; Han, W.; Yi, M.-J.; Jeong, J.; Oh, B.-H. Two-promoter vector is highly efficient for overproduction of protein complexes. Protein Sci. 2004, 13, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Lu, M.M.; Zhang, X.H.; Cheng, F.; Xu, J.-M.; Xue, Y.-P.; Jin, L.-Q.; Wang, Y.-S.; Zheng, Y.-G. Significant improvement of the nitrilase activity by semi-rational protein engineering and its application in the production of iminodiacetic acid. Int. J. Biol. Macromol. 2018, 116, 563–571. [Google Scholar] [CrossRef] [PubMed]

| Vectors | Target Gene | PCR Template | Primer | Sequence 5′ → 3′ | Restriction | |
|---|---|---|---|---|---|---|
| single gene part | pETDuet-1 | ATA117 | PUC57-ATA117 | pETDuet-ATA-F | CGGGATCCGATGGCGTTCTCAGCGGACACCC | BamHI |
| pETDuet-ATA-R | TTCCGCTCGAGGTACTGTACCGGGGTCAGCC | XhoI | ||||
| pET28a | ATA117 | pETDuet-ATA117 | pET28a-ATA117-F | CGGATCCATGGCGTTCTCAGCGG | BamHI | |
| pET28a-ATA117-R | GCTCGAGGTACTGTACCGGGGTCAG | XhoI | ||||
| pETDuet-1 | ADH | pET28a-ADH | pETDuet-ADH-F | AGCCAGGATCCGATGAGCATTCCGG | BamHI | |
| pETDuet-ADH-R | CAGACTCGAGTTTGCTGGTATCAACAAC | XhoI | ||||
| co-expression part | pETDuet-1 | ATA117 | pETDuet-ATA117 | pETDuet-ATA117-F4 | cacagccaggatccgaattcgATGGCGTTCTCAGCGGACA | EcoRI |
| pETDuet-ATA117-R3 | gcggccgcaagcttgtcgacGTACTGTACCGGGGTCAG | SalI | ||||
| pETDuet-ATA117 | ADH | pET28a-ADH | pETDuet-ATA117-ADH -F3 | agaaggagatatacatatgATGAGCATTCCGGAAACCCAGA | NdeI | |
| pETDuet-ATA117-ADH-R3 | gtttctttaccagactcgagTTTGCTGGTATCAACAACATAACGA | XhoI | ||||
| pACYCDuet | ATA117 | pETDuet-ATA117 | pACYCDuet-ATA117-F2 | cacagccaggatccgaattcGATGGCGTTCTCAGCGGACA | EcoRI | |
| pACYCDuet-ATA117-R2 | gcggccgcaagcttgtcgacGTACTGTACC GGGGTCAG | SalI | ||||
| pACYCDuet-ATA117 | ADH | pET28a-ADH | pACYCDuet-ATA117-ADH -F2 | agaaggagatatacatatgATGAGCATTCCGGAAACCCAGA | NdeI | |
| pACYCDuet-ATA117-ADH-R2 | gtttctttaccagactcgagTTTGCTGGTATCAACAACATAACGA | XhoI |
| Enzyme Expression Form | Original Plasmid | Recombinant Plasmid | Growth Temperature (°C) | Optimum Induction Temperature (°C) | Optimum Inducer Concentration (mmol/L) | Optimal Induction time (h) | Protein Concentration (mg/mL) | Specific Activity of ATA117 (U/mg) | Specific Activity of ADH (U/mg) | |
|---|---|---|---|---|---|---|---|---|---|---|
| single enzyme | ATA117 | pETDuet | pETDuet-ATA117 | 37 | 18 | 1 | 15 | 0.19 | 0.18 | / |
| pACYCDuet | pACYCDuet-ATA117 | 37 | 18 | 1 | 16 | 0.11 | 0.09 | / | ||
| pET28a | pET28a-ATA117 | 37 | 18 | 1 | 12 | 0.15 | 0.14 | / | ||
| ADH | pETDuet | pETDuet-ADH | 37 | 18 | 0.5 | 15 | 0.20 | / | 0.16 | |
| pET28a | pET28a-ADH | 37 | 30 | 1 | 12 | 0.16 | / | 0.15 | ||
| bienzyme | ATA117/ADH co-expression | pETDuet | pETDuet-ATA117-ADH | 37 | 18 | 1 | 8 | 0.37 | 0.18 | 0.15 |
| pACYCDuet | pACYCDuet-ATA117-ADH | 37 | 18 | 1 | 8 | 0.21 | 0.09 | 0.08 | ||
| pETDuet and pET28a | pETDuet-ATA117/pET28a-ADH | 37 | 23 | 1 | 14 | 0.34 | 0.12 | 0.10 | ||
| pACYCDuet and pET28a | pACYCDuet-ATA117/pET28a-ADH | 37 | 23 | 1 | 14 | 0.26 | 0.11 | 0.15 | ||
| pACYCDuet and pETDuet | pACYCDuet-ATA117/pETDuet-ADH | 37 | 18 | 1 | 15 | 0.31 | 0.11 | 0.16 | ||
| Substrate | Yield (%) | e.e. (%) |
|---|---|---|
| Acetophenone | 44.49 | 72.7 |
| Benzyl acetone | 73.56 | 22.4 |
| P-methoxypropiophenone | 55.38 | 32.45 |
| 3,5-bistrifluoromethylacetophenone | 100 | 100 |
| Ethyl levulinate | 0 | 0 |
| Ethyl 4-acetobutyrate | 2.35 | 100 |
| Methyl acetoacetate | 100 | 91 |
| Ethyl acetoacetate | 100 | 73.59 |
| Tert-Butyl acetoacetate | 61 | 77 |
| Sitagliptin precursor ketone | 100 | 100 |
| Ethyl benzoyl formate | 0 | 0 |
| Ethyl benzoylacetate | 0 | 0 |
| Ethyl 2-oxo-4-phenylbutyrate | 0 | 0 |
| Catalyst | Substrate Concentration (mM) | Yield (%) | Enantiomeric Excess (%) | |
|---|---|---|---|---|
| Free enzyme | the mixture of ATA117 and ADH | 166.6 | 99.9 | 99.9 |
| 207.8 | 91.7 | 99.9 | ||
| ATA117 single enzyme | 166.6 | 82.9 | 99.9 | |
| 207.8 | 73.8 | 99.9 | ||
| Whole cell | E. coli BL21(DE3)/pETDuet-ATA117-ADH | 207.8 | 99.9 | 99.9 |
| E. coli BL21(DE3)/pACYCDuet-ATA117-ADH | 207.8 | 56.2 | 99.9 | |
| E.coli BL21(DE3)/pETDuet-ATA117/pET28a-ADH | 207.8 | 91.8 | 99.9 | |
| E. coli BL21(DE3)/pACYCDuet-ATA117/pET28a-ADH | 207.8 | 69.6 | 99.9 | |
| E. coli BL21(DE3)/pACYCDuet-ATA117/pETDuet-ADH | 207.8 | 83.1 | 99.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Wang, J.; Xu, H.; Zhang, C.; Cheng, P.; Du, L.; Tang, L.; Li, J.; Ou, Z. Efficient Synthesis of Key Chiral Intermediate in Painkillers (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanamine by Bienzyme Cascade System with R-ω-Transaminase and Alcohol Dehydrogenase Functions. Molecules 2022, 27, 7331. https://doi.org/10.3390/molecules27217331
Lu Y, Wang J, Xu H, Zhang C, Cheng P, Du L, Tang L, Li J, Ou Z. Efficient Synthesis of Key Chiral Intermediate in Painkillers (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanamine by Bienzyme Cascade System with R-ω-Transaminase and Alcohol Dehydrogenase Functions. Molecules. 2022; 27(21):7331. https://doi.org/10.3390/molecules27217331
Chicago/Turabian StyleLu, Yuan, Jinmei Wang, Haobo Xu, Chuyue Zhang, Pengpeng Cheng, Lihua Du, Lan Tang, Jinghua Li, and Zhimin Ou. 2022. "Efficient Synthesis of Key Chiral Intermediate in Painkillers (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanamine by Bienzyme Cascade System with R-ω-Transaminase and Alcohol Dehydrogenase Functions" Molecules 27, no. 21: 7331. https://doi.org/10.3390/molecules27217331
APA StyleLu, Y., Wang, J., Xu, H., Zhang, C., Cheng, P., Du, L., Tang, L., Li, J., & Ou, Z. (2022). Efficient Synthesis of Key Chiral Intermediate in Painkillers (R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethanamine by Bienzyme Cascade System with R-ω-Transaminase and Alcohol Dehydrogenase Functions. Molecules, 27(21), 7331. https://doi.org/10.3390/molecules27217331







