Recent Research Progress on Natural Stilbenes in Dendrobium Species
Abstract
1. Introduction
2. Phenanthrenes
2.1. Simple Phenanthrene
2.2. Dihydrophenanthrene
2.3. Other Phenanthrenes
3. Bibenzyls
3.1. Simple Bibenzyl
3.2. Bridged Carbon Bibenzyl
3.3. Bibenzyl Derivatives
4. Pharmacological Activity of Stilbenes in Dendrobium Species
4.1. Anti-Oxidant Activity
| No. | Compound | Dendrobium Species | DPPH Radical Scavenging | ABTS Radical Scavenging | Ref. |
|---|---|---|---|---|---|
| 1 | 1 | D. loddigesii | 62.2 µM | - | [52] |
| 2 | 2 | D. nobile | IC50: 35.71 ± 0.19 µM | - | [61] |
| 3 | 7 | D. nobile | IC50: 29.67 ± 1.11 µM | - | [61] |
| 4 | 14 | D. nobile | IC50: 12.90 ± 0.35 µM | - | [61] |
| 5 | 26 | D. nobile | IC50: 34.78 ± 0.04 µM | - | [61] |
| 6 | 27 | D. loddigesii | IC50: 26.1 µM | - | [52] |
| 7 | 31 | D. nobile | IC50: 144.5 ± 2.65 µM | - | [61] |
| 8 | 43 | D. loddigesii | IC50: 14.1 µM | - | [51] |
| 9 | 46 | D. draconis | IC50: 11.7 µM | - | [36] |
| 10 | 53 | D. draconis | IC50: 283.3 ± 13.7 µM | - | [36] |
| 11 | 73 | D. draconis | IC50: 22.3 ± 1.0 µM | - | [36] |
| 12 | 79 | D. aphyllum | 5.25% | - | [9] |
| 13 | 99 | D. loddigesii | IC50: 23.2 µM | - | [51] |
| 14 | 126 | D. signatum | IC50: 9.91 ± 0.3 µM | IC50: 12.0 ± 0.3 µM | [78] |
| 15 | 130 | D. chrysanthum | IC50: 14.70 µM | - | [24] |
| 16 | 132 | D. williamsonii | IC50: 19.5 µM | - | [132] |
| 17 | 133 | D. loddigesii | IC50: 85.8 µM | - | [51] |
| 18 | 135 | D. heterocarpum | 0.68 ± 1.45% | 31.13 ± 3.19% | [43] |
| 19 | 140 | D. heterocarpum | 33.13 ± 2.20% | 28.32 ± 2.94% | [43] |
| 20 | 145 | D. candidum | IC50: 15.6 µM | - | [92] |
| 21 | 147 | D. nobile | IC50: 40.3 ± 0.1µM | - | [115] |
| 22 | 148 | D. nobile | IC50: 14.5 ± 0.3 µM | - | [115] |
| 23 | 149 | D. nobile | IC50: 14.0 ± 0.1 µM | - | [115] |
| 24 | 150 | D. heterocarpum | 5.36 ± 1.09% | 40.97 ± 6.55% | [43] |
| 25 | 151 | D. heterocarpum | 1.93 ± 1.24% | 34.88 ± 4.11% | [43] |
| 26 | 157 | D. signatum | IC50: 14.0 ± 0.1 µM | IC50: 18.5 ± 0.2 µM | [78] |
| 27 | 161 | D. secundum | IC50: 15.87 ± 1.48 µM | - | [132] |
| 28 | 166 | D. loddigesii | 89.411% | - | [50] |
| 29 | 167 | D. catenatum | IC50: 34.45 ± 1.07 µM | IC50: 9.01 ± 1.39 µM | [97] |
| 30 | 185 | D. nobile | IC50: 21.8 ± 0.4 µM | - | [115] |
| 31 | 191 | D. loddigesii | 29.292% | - | [50] |
| 32 | 192 | D. loddigesii | 12.041% | - | [50] |
| 33 | 196 | D. heterocarpum | 21.79 ± 1.01% | 16.58 ± 2.75% | [43] |
| 34 | 197 | D. nobile | IC50: 19.9 ± 0.8 µM | - | [115] |
| 35 | 198 | D. candidum | IC50: 34.2 µM | - | [92] |
| 36 | 199 | D. candidum | IC50: 34.5 µM | - | [92] |
| 37 | 208 | D. loddigesii | 35.276% | - | [50] |
| 38 | 215 | D. aphyllum | 87.97% | - | [9] |
| 39 | 216 | D. loddigesii | IC50: 60.1 µM | - | [51] |
| 40 | 218 | D. heterocarpum | 21.08 ± 1.19% | 29.89 ± 5.38% | [43] |
| 41 | 219 | D. candidum | IC50: 32.4 µM | - | [93] |
| 42 | 220 | D. candidum | IC50: 36.8 µM | - | [94] |
| 43 | 221 | D. candidum | IC50: 70.2 µM | - | [94] |
| 44 | 223 | D. nobile | IC50: 21.0 ± 0.4 µM | - | [115] |
| 45 | 224 | D. signatum | IC50: 8.9 ± 0.1 µM | IC50: 18.0 ± 0.1 µM | [78] |
| 46 | 225 | D. signatum | IC50: 15.6 ± 0.2 µM | IC50: 8.1 ± 0.0 µM | [78] |
| 47 | 226 | D. candidum | IC50: 19.8 µM | - | [93] |
| 48 | 227 | D. candidum | IC50: 45.0 µM | - | [94] |
| 49 | 229 | D. candidum | IC50: 87.6 µM | - | [94] |
| 50 | 230 | D. candidum | IC50: 50.4 µM | - | [94] |
| 51 | 231 | D. catenatum | - | IC50: 10.03 ± 0.88 µM | [97] |
| 52 | 232 | D. candidum | IC50: 40.5 µM | - | [94] |
| 53 | 233 | D. candidum | IC50: 22.3 µM | - | [94] |
| 54 | 234 | D. candidum | IC50: 30.3 µM | - | [94] |
| 55 | 237 | D. findlayanum | 37.563 ± 0.099% | - | [106] |
| 56 | 255 | D. findlayanum | 18.115 ± 0.478% | - | [106] |
| 57 | 256 | D. findlayanum | 27.632 ± 0.347% | - | [106] |
| 58 | 257 | D. signatum | IC50: 10.2 ± 0.1 µM | IC50: 24.0 ± 0.2 µM | [78] |
4.2. Anti-Infammatory Activity
4.3. α-. Glucosidase and Pancreatic Lipase Inhibitory Activities
4.4. Antitumor Activity
4.5. Antimicrobial Activity
4.6. Neuroprotective Activity
4.7. Anti-Platelet Aggregation Activity
4.8. Others
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cakova, V.; Bonte, F.; Lobstein, A. Dendrobium: Sources of Active Ingredients to Treat Age-Related Pathologies. Aging Dis. 2017, 8, 827–849. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Su, Q.; Bai, L.; Li, M.; Liu, J.; Liu, X.; Zhang, C.; Jiang, Z.; He, J.; Shi, J.; et al. Recent research progress on natural small molecule bibenzyls and its derivatives in Dendrobium species. Eur. J. Med. Chem. 2020, 204, 112530. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.F.; Wu, C.T.; Huang, W.Y.; Lin, W.S.; Wu, H.W.; Huang, T.K.; Chang, M.Y.; Lin, Y.S. Antioxidation and Melanogenesis Inhibition of Various Dendrobium tosaense Extracts. Molecules 2018, 23, 1810. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ma, J.; Li, G.; Pan, H.; Zhu, Y.; Jin, Q.; Cai, Y.; Han, B. Natural Composition and Biosynthetic Pathways of Alkaloids in Medicinal Dendrobium Species. Front. Plant Sci. 2022, 13, 850949. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Ng, T.B. The medicinal and pharmaceutical importance of Dendrobium species. Appl. Microbiol. Biotechnol. 2017, 101, 2227–2239. [Google Scholar] [CrossRef]
- Majumder, P.L.; Guha, S.; Sen, S. Bibenzyl derivatives from the orchid Dendrobium amoenum. Phytochemistry 1999, 52, 1365–1369. [Google Scholar] [CrossRef]
- Yang, D.; Cheng, Z.Q.; Ding, Z.T.; Zhou, J.; Hu, J.M. Phenolic chemical constituents of Dendrobium aphyllum. Chin. Tradit. Herb. Drugs 2017, 48, 2839–2842. [Google Scholar]
- Zhang, C.F.; Shao, L.; Wang, W.H.; Wang, L.; Wang, Z.T.; Xu, L.S. Phenolic components from herbs of Dendrobium aphyllum. China J. Chin. Mater. Med. 2008, 33, 2922–2925. [Google Scholar]
- Yang, D.; Liu, L.Y.; Cheng, Z.Q.; Xu, F.Q.; Fan, W.W.; Zi, C.T.; Dong, F.W.; Zhou, J.; Ding, Z.T.; Hu, J.M. Five new phenolic compounds from Dendrobium aphyllum. Fitoterapia 2015, 100, 11–18. [Google Scholar] [CrossRef]
- Shang, Z.M.; Cheng, L.; Liu, G.Y.; Zhang, M.S.; Li, X.F.; Shao, S.J. Chemical constituents of Dendrobium bellatulum. Chin. Tradit. Herb. Drugs 2019, 50, 2036–2040. [Google Scholar]
- Klongkumnuankarn, P.; Busaranon, K.; Chanvorachote, P.; Sritularak, B.; Jongbunprasert, V.; Likhitwitayawuid, K. Cytotoxic and Antimigratory Activities of Phenolic Compounds from Dendrobium brymerianum. Evid. Based Complement. Altern. Med. 2015, 2015, 350410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Yu, H.; Liu, Y. Chemical constituents from Dendrobium brymerianum Rchb. f. Biochem. Syst. Ecol. 2014, 57, 175–177. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.L.; Wang, F.F.; Dong, H.L.; Guo, S.X.; Yang, J.S.; Shao, P.G. Chemical constituents of Dendrobium candidum. China J. Chin. Mater. Med. 2010, 35, 1715–1719. [Google Scholar]
- Li, R.S.; Yang, X.; He, p.; Gan, N. Studies on Phenanthrene Constituents from Stems of Dendrobium candidum. J. Chin. Med. Mater. 2009, 32, 4. [Google Scholar] [CrossRef] [PubMed]
- San, H.T.; Boonsnongcheep, P.; Putalun, W.; Mekboonsonglarp, W.; Sritularak, B.; Likhitwitayawuid, K. α-Glucosidase Inhibitory and Glucose Uptake Stimulatory Effects of Phenolic Compounds from Dendrobium christyanum. Nat. Prod. Commun. 2020, 15, 1934578x20913453. [Google Scholar]
- Hu, J.M.; Fan, W.W.; Dong, F.W.; Miao, Z.H.; Zhou, J. Chemical Components of Dendrobium chrysotoxum. Chin. J. Chem. 2012, 30, 1327–1330. [Google Scholar] [CrossRef]
- Li, Y.-P.; Qing, C.; Fang, T.-T.; Liu, Y.; Chen, Y.-G. Chemical constituents of Dendrobium chrysotoxum. Chem. Nat. Compd. 2009, 45, 414–416. [Google Scholar] [CrossRef]
- Yang, H.; Chou, G.X.; Wang, Z.T.; Hu, Z.B.; Xu, L.S. Two new fluorenones from Dendrobium chrysotoxum. J. Asian Nat. Prod. Res. 2004, 6, 35–38. [Google Scholar] [CrossRef]
- Li, S.; He, S.; Zhong, S.; Duan, X.; Ye, H.; Shi, J.; Peng, A.; Chen, L. Elution-extrusion counter-current chromatography separation of five bioactive compounds from Dendrobium chrysototxum Lindl. J. Chromatogr. A 2011, 1218, 3124–3128. [Google Scholar] [CrossRef]
- Yang, H.; Gong, Y.Q.; Wang, Z.T.; Xu, L.S.; Hu, Z.B.; Xu, G.J. Studies on chemical constituents of Dendrobium chrysotoxum. Chin. Tradit. Herb. Drugs 2001, 32, 973–974. [Google Scholar]
- Yang, H.; Wang, Z.T.; Xu, L.S.; Hu, Z.B. Chemical constituents of Dendrobium chrysotoxum. J. China Pharm. Univ. 2002, 33, 367–369. [Google Scholar]
- Yang, L.; Qin, L.H.; Bligh, S.W.; Bashall, A.; Zhang, C.F.; Zhang, M.; Wang, Z.T.; Xu, L.S. A new phenanthrene with a spirolactone from Dendrobium chrysanthum and its anti-inflammatory activities. Bioorg. Med. Chem. 2006, 14, 3496–3501. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.H.; Zhao, W.M.; Qin, G.W. New fluorenone and phenanthrene derivatives from Dendrobium chrysanthum. Nat. Prod. Res. 2003, 17, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Wang, Y.J.; Chen, L.L. Antioxidant Bibenzyls, Phenanthrenes, and Fluorenones from Dendrobium chrysanthum. Chem. Nat. Compd. 2016, 52, 90–92. [Google Scholar] [CrossRef]
- Cai, J.Y.; Ni, J.; Chen, T.H.; Zhang, T. A new phenanthrene from Dendrobium chrysanthum. Chin. Tradit. Herb. Drugs 2017, 48, 3. [Google Scholar]
- Wen, R.X.; LI, Y.P.; Chen, L.J.; Huang, R.; Tao, R.S.; Wang, J.; LI, S.; Wen, X.L.; Zhang, G.H. Chemical Constituents from the Herbs of Dendrobium Chrysanthum. J. Kunming Med. Univ. 2017, 38, 6–9. [Google Scholar]
- Li, C.-B.; Wang, C.; Fan, W.-W.; Dong, F.-W.; Xu, F.-Q.; Wan, Q.-L.; Luo, H.-R.; Liu, Y.-Q.; Hu, J.-M.; Zhou, J. Chemical components of Dendrobium crepidatum and their neurite outgrowth enhancing activities. Nat. Prod. Bioprospect. 2013, 3, 70–73. [Google Scholar] [CrossRef]
- Sun, J.W.; Liu, J.M.; Chen, R.D.; Liu, Y.Y.; Li, Y.; Cen, S.; Chen, S.M.; Guo, S.X.; Dai, J.G. Study on chemical bibenzyls in Dendrobium gratiosissimum. China J. Chin. Mater. Med. 2020, 45, 4929–4937. [Google Scholar]
- Lin, Y.; Wang, F.; Yang, L.J.; Chun, Z.; Bao, J.K.; Zhang, G.L. Anti-inflammatory phenanthrene derivatives from stems of Dendrobium denneanum. Phytochemistry 2013, 95, 242–251. [Google Scholar] [CrossRef]
- Li, F.; Pan, H.M.; Liu, X.; Chen, B.; Tang, Y.X.; Xi, X.J.; Wang, M.K. New phenanthrene glycosides from Dendrobium denneanum and their cytotoxic activity. Phytochem. Lett. 2013, 6, 640–644. [Google Scholar] [CrossRef]
- Ying, L.; Jin-He, J.; Yan, Z.; Ye-Gao, C. Chemical constituents of Dendrobium aurantiacum var. denneanum. Chem. Nat. Compd. 2009, 45, 525–527. [Google Scholar] [CrossRef]
- Su, L.; Liu, X.W.; Wang, J.R.; Ye, Q.; Guo, L.; Zhang, T.M. Chemical constituents of Dendrobium aurantiacum Rchb.f.var. denneanum. Chin. Tradit. Pat. Med. 2015, 37, 3. [Google Scholar]
- Fan, C.; Wang, W.; Wang, Y.; Qin, G.; Zhao, W. Chemical constituents from Dendrobium densiflorum. Phytochemistry 2001, 57, 1255–1258. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Y.; Ding, Y.; Zhao, J.; Xu, H.; Chou, G. Four new compounds from Dendrobium devonianum. Nat. Prod. Res. 2019, 33, 2160–2168. [Google Scholar] [CrossRef]
- Meng, Z.X.; Dong, H.L.; Wang, C.L.; Guo, S.X. Chemical Constituents of Dendrobium devonianum. Chin. Pharm. J. 2013, 48, 855–859. [Google Scholar]
- Sritularak, B.; Anuwat, M.; Likhitwitayawuid, K. A new phenanthrenequinone from Dendrobium draconis. J. Asian Nat. Prod. Res. 2011, 13, 251–255. [Google Scholar] [CrossRef]
- Tanagornmeatar, K.; Chaotham, C.; Sritularak, B.; Likhitwitayawuid, K.; Chanvorachote, P. Cytotoxic and anti-metastatic activities of phenolic compounds from Dendrobium ellipsophyllum. Anticancer Res. 2014, 34, 6573–6579. [Google Scholar]
- Xu, F.Q.; Xu, F.C.; Hou, B.; Fan, W.W.; Zi, C.T.; Li, Y.; Dong, F.W.; Liu, Y.Q.; Sheng, J.; Zuo, Z.L.; et al. Cytotoxic bibenzyl dimers from the stems of Dendrobium fimbriatum Hook. Bioorg. Med. Chem. Lett. 2014, 24, 5268–5273. [Google Scholar] [CrossRef]
- Xu, F.Q.; Fan, W.W.; Zi, C.T.; Dong, F.W.; Yang, D.; Zhou, J.; Hu, J.M. Four new glycosides from the stems of Dendrobium fimbriatum Hook. Nat. Prod. Res. 2017, 31, 797–801. [Google Scholar] [CrossRef]
- Bi, Z.M.; Wang, Z.T.; Xu, L.S.; Xu, G.J. Studies on the chemical constituents of Dendrobium fimbriatum. Acta Pharm. Sin. 2003, 38, 526–529. [Google Scholar]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Rev. Bras. De Farmacogn. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Shang, Z.M.; Xia, D.; Chen, L.; Liu, G.Y.; Zhang, M.S.; Zhang, J.Y.; LI, X.F.; Xiao, S.J. Chemical constituents from Dendrobium hancockii. Chin. Tradit. Herb. Drugs 2019, 50, 3760–3763. [Google Scholar]
- Yang, X.L.; Yan, S.; Hu, J.M.; Zhou, J. Chemical constituents from Dendrobium heterocarpum Lindl. Nat. Prod. Res. Dev. 2019, 31, 1745–1752. [Google Scholar]
- Chen, Y.G.; Yu, H.; Lian, X. Isolation of Stilbenoids and Lignans from Dendrobium hongdie. Trop. J. Pharm. Res. 2015, 14, 2055. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.Y.; Song, X.Q.; Mei, W.L.; Zuo, W.J.; Cai, C.H.; Chen, J.; Dai, H.F. Chemical Constituents from Dendrobium hainanense (Orchidaceae) in Hainan. J. Trop. Subtrop. Bot. 2015, 23, 317–322. [Google Scholar]
- Zhang, Y.Y.; Wang, P.; Song, X.Q.; Zuo, W.J.; Wang, H.; Chen, L.L.; Mei, W.L.; Dai, H.F. Chemical constituents from Dendrobium hainanense. J. Asian Nat. Prod. Res. 2019, 21, 873–880. [Google Scholar] [CrossRef]
- Na Ranong, S.; Likhitwitayawuid, K.; Mekboonsonglarp, W.; Sritularak, B. New dihydrophenanthrenes from Dendrobium infundibulum. Nat. Prod. Res. 2019, 33, 420–426. [Google Scholar] [CrossRef]
- Qin, M.L.; Chen, Y.P.; Wu, H.; Yu, J.D.; Zheng, J.R.; Li, Y.P.; Li, S.H. Phytochemical and chemotaxonomic study on Dendrobium lindleyi Stendel. Biochem. Syst. Ecol. 2022, 102, 104412. [Google Scholar] [CrossRef]
- Khoonrit, P.; Mirdogan, A.; Dehlinger, A.; Mekboonsonglarp, W.; Likhitwitayawuid, K.; Priller, J.; Bottcher, C.; Sritularak, B. Immune modulatory effect of a novel 4,5-dihydroxy-3,3′,4 -trimethoxybibenzyl from Dendrobium lindleyi. PLoS ONE 2020, 15, e0238509. [Google Scholar] [CrossRef]
- Ma, R.J.; Yang, L.; Bai, X.; Li, J.Y.; Yuan, M.Y.; Wang, Y.Q.; Xie, Y.; Hu, J.M.; Zhou, J. Phenolic Constituents with Antioxidative, Tyrosinase Inhibitory and Anti-aging Activities from Dendrobium loddigesii Rolfe. Nat. Prod. Bioprospect. 2019, 9, 329–336. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, H.P.; He, W.B.; Yang, W.L.; Ni, F.Y.; Huang, Z.W.; Hu, H.Y.; Wang, J. Polyphenols from Dendrobium loddigesii and their biological activities. Acta Sci. Nat. Univ. Sunyatseni 2019, 58, 96–102. [Google Scholar]
- Ito, M.; Matsuzaki, K.; Wang, J.; Daikonya, A.; Wang, N.; Yao, X.; Kitanaka, S. New phenanthrenes and stilbenes from Dendrobium loddigesii. Chem. Pharm. Bull. 2010, 58, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kuang, M.; Hu, G.; Wu, R.; Wang, J.; Liu, L.; Lin, Y. Loddigesiinols G-J: α-glucosidase inhibitors from Dendrobium loddigesii. Molecules 2014, 19, 8544–8555. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Chen, J.J.; Yu, H.; Zhao, Y.X.; Zhou, J. Five new compounds from Dendrobium longicornu. Planta Med. 2008, 74, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Li, J.T.; Yin, B.L.; Liu, Y. Bibenzyls, 9,10-dihydrophenanthrenes, and phenanthraquinone from Dendrobium longicornu. Chem. Nat. Compd. 2010, 46, 790–791. [Google Scholar] [CrossRef]
- Zhou, X.M.; Zheng, C.J.; Gan, L.S.; Chen, G.Y.; Zhang, X.P.; Song, X.P.; Li, G.N.; Sun, C.G. Bioactive Phenanthrene and Bibenzyl Derivatives from the Stems of Dendrobium nobile. J. Nat. Prod. 2016, 79, 1791–1797. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, W. New alloaromadendrane, cadinene and cyclocopacamphane type sesquiterpene derivatives and bibenzyls from Dendrobium nobile. Planta Med. 2002, 68, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, J.D.; Baek, N.I.; Kim, S.I.; Ahn, B.Z. In vitro and in vivo antitumoral phenanthrenes from the aerial parts of Dendrobium nobile. Planta Med. 1995, 61, 178–180. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, S.A.; Hong, S.S.; Han, X.H.; Lee, C.; Kang, S.J.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; et al. Phenanthrenes from Dendrobium nobile and their inhibition of the LPS-induced production of nitric oxide in macrophage RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2010, 20, 3785–3787. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, D.L.; Zhang, M.S.; Linghu, L.; Fu, S.B.; Deng, Y.; He, Y.Q.; Xiao, S.J. Dihydrophenanthrofurans and bisbibenzyl derivatives from the stems of Dendrobium nobile. Fitoterapia 2020, 143, 104586. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.K.; Wang, N.L.; Kurihara, H.; Yao, X.S. Antioxidant phenanthrenes and lignans from Dendrobium nobile. J. Chin. Pharm. Sci. 2008, 17, 314–318. [Google Scholar]
- Zhou, W.; Zeng, Q.F.; Xia, J.; Wang, L.; Tao, L.; Shen, X.C. Antitumor Phenanthrene Constituents of Dendrobium nobile. Chin. Pharm. J. 2018, 53, 1722–1725. [Google Scholar]
- Kim, J.; Oh, S.; Han, S.; Uddin, G.; Kim, C.; Lee, J. Anti-inflammatory effects of Dendrobium nobile derived phenanthrenes in LPS-stimulated murine macrophages. Arch. Pharmacal Res. 2015, 38, 1117–1126. [Google Scholar] [CrossRef]
- Lin, T.H.; Chang, S.J.; Chen, C.C.; Wang, J.P.; Tsao, L.T. Two phenanthraquinones from Dendrobium moniliforme. J. Nat. Prod. 2001, 64, 1084–1086. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.J.; Yin, G.; Wang, S.H.; Lu, Y.; LI, X.F. Study on the active components of antioxidant in Dendrobium moniliforme. Sw. Chin. J. Mod. Drug Appl. 2012, 6, 62–63. [Google Scholar]
- Zhao, C.S.; Zhao, W.M. A New Bibenzyl Glycoside from Dendrobium moniliforme. Chin. Chem. Lett. 2003, 14, 276–277. [Google Scholar]
- Ren, G.; Deng, W.Z.; Xie, Y.F.; Wu, C.H.; Li, W.Y.; Xiao, C.Y.; Chen, Y.L. Bibenzyl Derivatives From Leaves of Dendrobium officinale. Nat. Prod. Commun. 2020, 15, 1934578×20908678. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Wang, J.H.; Xu, H.; Chou, G.X.; Wang, Z.T. Bibenzyls from Dendrobium officinale. China J. Chin. Mater. Med. 2021, 46, 3853–3858. [Google Scholar]
- Cui, Y.D.; Lu, Y.L.; Zhao, Y.M.; Liu, M.X.; Zhang, G.G. Isolation and identification of chemical constituents from Dendrobium officinale Kimura et Migo. J. Shenyang Pharm. Univ. 2019, 36, 7–11. [Google Scholar]
- Kyokong, N.; Muangnoi, C.; Thaweesest, W.; Kongkatitham, V.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. A new phenanthrene dimer from Dendrobium palpebrae. J. Asian Nat. Prod. Res. 2019, 21, 391–397. [Google Scholar] [CrossRef]
- Kongkatitham, V.; Muangnoi, C.; Kyokong, N.; Thaweesest, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. Anti-oxidant and anti-inflammatory effects of new bibenzyl derivatives from Dendrobium parishii in hydrogen peroxide and lipopolysaccharide treated RAW264.7 cells. Phytochem. Lett. 2018, 24, 31–38. [Google Scholar] [CrossRef]
- Yamaki, M.; Honda, C. The stilbenoids from Dendrobium plicatile. Phytochemistry 1996, 43, 207–208. [Google Scholar] [CrossRef]
- Chen, D.N.; Wang, Y.Y.; Liu, W.J.; Chen, Y.J.; Wu, Y.P.; Wang, J.X.; He, F.; Jiang, L. Stilbenoids from aerial parts of Dendrobium plicatile. Nat. Prod. Res. 2020, 34, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Honda, C.; Yamaki, M. Phenanthrenes from Dendrobium plicatile. Phytochemistry 2000, 53, 987–990. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhao, Y.X.; Miao, Z.H.; Zhou, J. Chemical Components of Dendrobium polyanthum. Bull. Korean Chem. Soc. 2009, 30, 2098–2100. [Google Scholar]
- Chanvorachote, P.; Kowitdamrong, A.; Ruanghirun, T.; Sritularak, B.; Mungmee, C.; Likhitwitayawuid, K. Anti-Metastatic Activities of Bibenzyls from Dendrobium Pulchellum. Nat. Prod. Commun. 2013, 8, 115–118. [Google Scholar] [CrossRef]
- Khumploy, P.; Raksat, A.; Choodej, S.; Aree, T.; Ngamrojanavanich, N.; Pudhom, K. Picrotoxane sesquiterpene and alpha-pyrone derivative from Dendrobium signatum and their free radical scavenging potency. J. Nat. Med. 2021, 75, 967–974. [Google Scholar] [CrossRef]
- Mittraphab, A.; Muangnoi, C.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. A New Bibenzyl-phenanthrene Derivative from Dendrobium signatum and its Cytotoxic Activity. Nat. Prod. Commun. 2016, 11, 657–659. [Google Scholar] [CrossRef]
- Sarakulwattana, C.; Mekboonsonglarp, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. New bisbibenzyl and phenanthrene derivatives from Dendrobium scabrilingue and their alpha-glucosidase inhibitory activity. Nat. Prod. Res. 2020, 34, 1694–1701. [Google Scholar] [CrossRef]
- Sritularak, B.; Duangrak, N.; Likhitwitayawuid, K. A new bibenzyl from Dendrobium secundum. Z Nat. C J. Biosci. 2011, 66, 205–208. [Google Scholar]
- Chen, X.J.; Mei, W.L.; Zuo, W.J.; Zeng, Y.B.; Guo, Z.K.; Song, X.Q.; Dai, H.F. A new antibacterial phenanthrenequinone from Dendrobium sinense. J. Asian Nat. Prod. Res. 2013, 15, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Mei, W.L.; Zhao, Y.X.; Huang, S.Z.; Kong, F.D.; Yang, N.N.; Song, X.Q.; Dai, H.F. Chemical Constituents from Dendrobium sinense. J. Trop. Subtrop. Bot. 2017, 25, 189–194. [Google Scholar]
- Qin, Z.M.; Zhu, X.Y.; Fu, H.; Hu, J.; Yang, M.H. Study on Chemical Constituents of Dendrobium Stuposum. Chin. J. Ethnomed. Ethnopharm. 2019, 28, 19–22. [Google Scholar]
- Zhang, G.N.; Zhang, Z.F.; Wang, Z.T.; Xu, L.S. Studies on Chemical Constituents of Dendrobium thyrsiflorum Rchb. f (Ⅰ). Chin. J. Nat. Med. 2004, 2, 17–21. [Google Scholar]
- Liu, Y.Y.; Yu, H.; Chen, Y.G. Chemical constituents of Dendrobium thyrsiflorum. Chem. Nat. Compd. 2011, 47, 275–276. [Google Scholar] [CrossRef]
- Zhang, G.; Zhong, L.; Bligh, S.; Guo, Y.; Zhang, C.; Zhang, M.; Wang, Z.; Xu, L. Bi-bicyclic and bi-tricyclic compounds from Dendrobium thyrsiflorum. Phytochemistry 2005, 66, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Chen, J.J.; Yu, H.; Zhao, Y.X.; Zhou, J. Two novel bibenzyls from Dendrobium trigonopus. J. Asian Nat. Prod. Res. 2008, 10, 653–657. [Google Scholar] [CrossRef]
- Sukphan, P.; Sritularak, B.; Mekboonsonglarp, W.; Lipipun, V.; Likhitwitayawuid, K. Chemical constituents of Dendrobium venustum and their antimalarial and anti-herpetic properties. Nat. Prod. Commun. 2014, 9, 825–827. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.J.; Yang, L.; Yuan, M.Y.; Li, J.Y.; Hou, B.; Li, H.M.; Yang, X.Z.; Ding, C.C.; Hu, J.M. Sesquiterpene amino ether and cytotoxic phenols from Dendrobium wardianum Warner. Fitoterapia 2017, 122, 76–79. [Google Scholar] [CrossRef]
- Li, J.J.; Ren, F.C.; Hu, J.M.; Zhou, J. Chemical constituents and cytotoxic activities of Dendrobium wardianum. Chin. Tradit. Herb. Drugs 2020, 51, 1819–1824. [Google Scholar]
- Li, Y.; Wang, C.L.; Guo, S.X.; Yang, J.S.; Xiao, P.G. Two new compounds from Dendrobium candidum. Chem. Pharm. Bull. 2008, 56, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.L.; Wang, Y.J.; Guo, S.X.; Yang, J.S.; Chen, X.M.; Xiao, P.G. Three new bibenzyl derivatives from Dendrobium candidum. Chem. Pharm. Bull. 2009, 57, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.L.; Wang, Y.J.; Wang, F.F.; Guo, S.X.; Yang, J.S.; Xiao, P.G. Four new bibenzyl derivatives from Dendrobium candidum. Chem. Pharm. Bull. 2009, 57, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.L.; Zhao, H.J.; Guo, S.X. Eight new bibenzyl derivatives from Dendrobium candidum. J. Asian Nat. Prod. Res. 2014, 16, 1035–1043. [Google Scholar] [CrossRef]
- LV, Y.J.; Chen, Q. Chemical Constituents of Dendrobium Candidum and Their Effects on Metabolism of Cholesterol in HepG2 Cell Line. Chin. Arch. Tradit. Chin. Med. 2016, 34, 4. [Google Scholar]
- Phechrmeekha, T.; Sritularak, B.; Likhitwitayawuid, K. New phenolic compounds from Dendrobium capillipes and Dendrobium secundum. J. Asian Nat. Prod. Res. 2012, 14, 748–754. [Google Scholar] [CrossRef]
- Zhu, L.J.; Wang, M.Q.; Qin, Y.; Wang, M.N.; Zhang, G.Q.; Niu, L.T.; Chen, J.B.; Zhang, X.; Yao, X.S. Two new dibenzyl derivatives from the stems of Dendrobium catenatum. J. Asian Nat. Prod. Res. 2021, 23, 955–960. [Google Scholar] [CrossRef]
- Dong, F.W.; Luo, H.R.; Wan, Q.L.; Xu, F.Q.; Fan, W.W.; Wang, K.J.; Li, N.; Hu, J.M. Two New Bibenzyl Glucosides from Dendrobium chrysotoxum. Bull. Korean Chem. Soc. 2012, 33, 2247–2250. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Zhang, C.F.; Zhang, M.; Wang, Z.T.; Xu, L.S. Study on Chemical Constituents from Stems of Dendrobium crepidatum. Pharm. Clin. Res. 2011, 19, 136–138. [Google Scholar]
- Ding, X.; Zou, Y.; Liu, J.; Wang, X.; Hu, Y.; Liu, X.; Zhang, C. Dendrocrepidamine, a novel octahydroindolizine alkaloid from the roots of Dendrobium crepidatum. J. Asian Nat. Prod. Res. 2021, 23, 1085–1092. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.F.; Wang, Z.T.; Zhang, M.; Xu, L.S. Five new compounds from Dendrobium crystallinum. J. Asian Nat. Prod. Res. 2009, 11, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.F.; Wang, Z.T.; Zhang, M.; Xu, L.S. Chemical constituents of Dendrobium crystallinum. Chin. Tradit. Herb. Drugs 2011, 42, 31–33. [Google Scholar]
- Yang, D.; Cheng, Z.Q.; Ding, Z.T.; Zhou, J.; Hu, J.M. Chemical constituents of Dendrobium crystallinum. Guihaia 2017, 37, 5. [Google Scholar]
- Wang, L.; Zhang, C.F.; Wang, Z.T.; Zhang, M.; Shao, L.; Xu, L.S. Studies on chemical constituents of Dendrobium crystallinum. China J. Chin. Mater. Med. 2008, 33, 2. [Google Scholar]
- Liu, G.Y.; Tan, L.; Cheng, L.; Ding, L.S.; Zhou, Y.; Deng, Y.; He, Y.Q.; Guo, D.L.; Xiao, S.J. Dendrobine-type alkaloids and bibenzyl derivatives from Dendrobium findlayanum. Fitoterapia 2020, 142, 104497. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Cheng, Z.Q.; Yang, L.; Hou, B.; Yang, J.; Li, X.N.; Zi, C.T.; Dong, F.W.; Liu, Z.H.; Zhou, J.; et al. Seco-Dendrobine-Type Alkaloids and Bioactive Phenolics from Dendrobium findlayanum. J. Nat. Prod. 2018, 81, 227–235. [Google Scholar] [CrossRef]
- Zhang, C.F.; Wang, M.; Wang, L.; Iinuma, M.; Zhang, M.; Xu, L.S.; Wang, Z.T. Chemical constituents of Dendrobium gratiosissimum and their cytotoxic activities. Indian J. Chem. B 2008, 47, 952–956. [Google Scholar]
- Li, Q.M.; Jiang, H.; Zha, X.Q.; Wu, D.L.; Pan, L.H.; Duan, J.; Liu, J.; Luo, J.P. Anti-inflammatory bibenzyls from the stems of Dendrobium huoshanense via bioassay guided isolation. Nat. Prod. Res. 2020, 34, 563–566. [Google Scholar] [CrossRef]
- Su, S.Q.; Jiang, H.; Li, Q.M.; Cha, X.Q.; Luo, J.P. Study on chemical constituents of Dendrobium huoshanense stems and their anti-inflammatory activity. China J. Chin. Mater. Med. 2020, 45, 3452–3458. [Google Scholar]
- Zhao, H.S.; Xu, F.Q.; Chen, X.X.; Hu, J.M. Chemical constituents of Dendrobium huoshanense C. Z. Tang et S. J. Chen. Nat. Prod. Res. Dev. 2021, 33, 1491–1498. [Google Scholar]
- Maitreesophone, P.; Khine, H.E.E.; Nealiga, J.Q.L.; Kongkatitham, V.; Panuthai, P.; Chaotham, C.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory effects and anti-adipogenic activity of dendrofalconerol B, a bisbibenzyl from Dendrobium harveyanum. South Afr. J. Bot. 2022, 146, 187–195. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Z.Y.; Shao, Z.M.; Zhang, M.S.; LI, X.F.; Zhang, Y.J.; Xiao, S.J. Chemical constituents of Dendrobium hercoglossum. Chin. Tradit. Herb. Drugs 2020, 51, 3126–3130. [Google Scholar]
- LI, C.Y.; Lu, Y.; Chen, Y.; Zheng, J.W.; Wang, J. Chemical Components of Dendrobium loddigesii. Acta Sci. Nat. Univ. Sunyatseni 2013, 52, 73–76. [Google Scholar]
- Hu, J.M.; Fan, W.W.; Zhou, L.; Zhao, Y.X.; Zhou, J. A New Phenolic Compound from Dendrobium longicornu. Bull. Korean Chem. Soc. 2010, 31, 3025–3026. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Xu, J.K.; Wang, J.; Wang, N.L.; Kurihara, H.; Kitanaka, S.; Yao, X.S. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J. Nat. Prod. 2007, 70, 24–28. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, H.; Wang, N.L.; Yao, X.S. Three new bibenzyl derivatives from Dendrobium nobile. J. Asian Nat. Prod. Res. 2006, 8, 113–118. [Google Scholar] [CrossRef]
- Liu, Q.F.; Chen, W.L.; Tang, J.; Zhao, W.M. Novel Bis(bibenzyl) and (Propylphenyl)bibenzyl Derivatives from Dendrobium nobile. Helv. Chim. Acta 2007, 90, 1745–1750. [Google Scholar] [CrossRef]
- Zhang, M.S.; Linghu, L.; Zhang, J.Y.; Nie, X.Q.; Li, X.F.; Guo, D.L.; Xiao, S.J. Bibenzyl Derivatives from Dendrobium nobile. Chin. J. Org. Chem. 2019, 39, 3289–3293. [Google Scholar] [CrossRef]
- Xiao, S.J.; Liu, Z.; Zhang, M.S.; Chen, Y.Z.; Nie, X.Q.; Zhang, J.Y.; He, Y.Q.; Shi, J.S. A new bibenzyl compound from Dendrobium nobile. Acta Pharm. Sin. 2016, 51, 1117–1120. [Google Scholar]
- Zhao, N.; Yang, G.; Zhang, Y.; Chen, L.; Chen, Y. A new 9,10-dihydrophenanthrene from Dendrobium moniliforme. Nat. Prod. Res. 2016, 30, 174–179. [Google Scholar] [CrossRef]
- Chu, C.; Li, T.; Pedersen, H.A.; Kongstad, K.T.; Yan, J.Z.; Staerk, D. Antidiabetic constituents of Dendrobium officinale as determined by high-resolution profiling of radical scavenging and α-glucosidase and α-amylase inhibition combined with HPLC-PDA-HRMS-SPE-NMR analysis. Phytochem. Lett. 2019, 31, 47–52. [Google Scholar] [CrossRef]
- Liu, M.; Cui, Y.D.; Deng, B.W.; Shi, S.; Zhang, C.Y.; Zhang, G.G. Isolation and identification of chemical constituents from dendrobium officinale Kimura et Migo. J. Shenyang Pharm. Univ. 2018, 35, 739–743+749. [Google Scholar]
- Zhao, G.Y.; Deng, B.W.; Zhang, C.Y.; Cui, Y.D.; Bi, J.Y.; Zhang, G.G. New phenanthrene and 9, 10-dihydrophenanthrene derivatives from the stems of Dendrobium officinale with their cytotoxic activities. J. Nat. Med. 2018, 72, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Warinhomhoun, S.; Muangnoi, C.; Buranasudja, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Antioxidant Activities and Protective Effects of Dendropachol, a New Bisbibenzyl Compound from Dendrobium pachyglossum, on Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Keratinocytes. Antioxidants 2021, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Pann Phyu, M.; Kongkatitham, V.; Mekboonsonglarp, W.; Likhitwitayawuid, K.; Sritularak, B. Phenanthrenes from Dendrobium senile and their pancreatic lipase inhibitory activity. J. Asian Nat. Prod. Res. 2022, 24, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Mei, W.L.; Cai, C.H.; Guo, Z.K.; Song, X.Q.; Dai, H.F. Four new bibenzyl derivatives from Dendrobium sinense. Phytochem. Lett. 2014, 9, 107–112. [Google Scholar] [CrossRef]
- Weng, R.; Yupeng, L.I.; Shuang, L.I.; Zhang, G.; Wang, Y.; Wen, X.; Zhang, J.; Huang, R. Extraction and Structure Elucidation of Phenols from Dendrobium thyrsiflorum. Agric. Basic Sci. Technol. 2015, 16, 2144–2145. [Google Scholar]
- Zhang, T.; Zhang, C.F.; Wang, Z.T.; Xu, L.S. Studies on Chemical Constituents of Dendrobium trigonopus Rchb. f. Chin. J. Nat. Med. 2005, 3, 32–34+3. [Google Scholar]
- Yang, M.; Zhang, Y.; Chen, L.; Chen, Y. A new (propylphenyl)bibenzyl derivative from Dendrobium williamsonii. Nat. Prod. Res. 2018, 32, 1699–1705. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Raju, M.S.; Subbaraju, G.V. Synthesis and biological activity of isoamoenylin, a metabolite of Dendrobium amoenum. Biosci. Biotechnol. Biochem. 2002, 66, 2236–2238. [Google Scholar] [CrossRef][Green Version]
- Thant, S.; Morales, N.; Buranasudja, V.; Sritularak, B.; Luechapudiporn, R. Protective Effect of Lusianthridin on Hemin-Induced Low-Density Lipoprotein Oxidation. Pharmaceuticals 2021, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Rungwichaniwat, P.; Sritularak, B.; Likhitwitayawuid, K. Chemical Constituents of Dendrobium williamsonii. Pharmacogn. J. 2014, 6, 36–41. [Google Scholar] [CrossRef]
- Xue, Y.; Deng, Q.; Zhang, Q.; Ma, Z.; Chen, B.; Yu, X.; Peng, H.; Yao, S.; Liu, J.; Ye, Y.; et al. Gigantol ameliorates CCl-induced liver injury via preventing activation of JNK/cPLA2/12-LOX inflammatory pathway. Sci. Rep. 2020, 10, 22265. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Pan, S.; Guh, J.; Liao, C.; Huang, D.; Chen, C.; Teng, C. Moscatilin induces apoptosis in human colorectal cancer cells: A crucial role of c-Jun NH2-terminal protein kinase activation caused by tubulin depolymerization and DNA damage. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 4250–4258. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Xu, X.; Jin, D. Moscatilin induces apoptosis of pancreatic cancer cells via reactive oxygen species and the JNK/SAPK pathway. Mol. Med. Rep. 2017, 15, 1195–1203. [Google Scholar] [CrossRef]
- Chen, C.; Chen, C.; Shen, C.; Chang, H.; Chen, Y. Moscatilin induces apoptosis and mitotic catastrophe in human esophageal cancer cells. J. Med. Food 2013, 16, 869–877. [Google Scholar] [CrossRef]
- Yang, H.; Lee, P.; Jeong, E.; Kim, H.; Kim, Y. Selective apoptosis in hepatic stellate cells mediates the antifibrotic effect of phenanthrenes from Dendrobium nobile. Phytother. Res. PTR 2012, 26, 974–980. [Google Scholar] [CrossRef]
- Yu, C.; Weng, M.; Chen, W.; Chien, K.; Chi, C.; Chung, C.; Huang, C.; Wang, P.; Chen, C.; Tsai, A.; et al. Moscatilin Inhibits Metastatic Behavior of Human Hepatocellular Carcinoma Cells: A Crucial Role of uPA Suppression via Akt/NF-κB-Dependent Pathway. Int. J. Mol. Sci. 2021, 22, 2930. [Google Scholar] [CrossRef]
- Pai, H.; Chang, L.; Peng, C.; Chang, Y.; Chen, C.; Shen, C.; Teng, C.; Pan, S. Moscatilin inhibits migration and metastasis of human breast cancer MDA-MB-231 cells through inhibition of Akt and Twist signaling pathway. J. Mol. Med. 2013, 91, 347–356. [Google Scholar] [CrossRef]
- Lee, E.; Han, A.; Nam, B.; Kim, Y.; Jin, C.; Kim, J.; Eun, Y.; Jung, C. Moscatilin Induces Apoptosis in Human Head and Neck Squamous Cell Carcinoma Cells via JNK Signaling Pathway. Molecules 2020, 25, 901. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Xin, W.; Liu, N.; Zhang, H. Nudol, a phenanthrene derivative from Dendrobium nobile, induces cell cycle arrest and apoptosis and inhibits migration in osteosarcoma cells. Drug Des. Dev. Ther. 2019, 13, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Han, M.; Zhang, B.; Sun, L.; Jin, X.; Li, T. Chrysotoxene induces apoptosis of human hepatoblastoma HepG2 cells in vitro and in vitro via activation of the mitochondria-mediated apoptotic signaling pathway. Oncol. Lett. 2018, 15, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Bhummaphan, N.; Petpiroon, N.; Prakhongcheep, O.; Sritularak, B.; Chanvorachote, P. Lusianthridin targeting of lung cancer stem cells via Src-STAT3 suppression. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 62, 152932. [Google Scholar] [CrossRef] [PubMed]
- Wattanathamsan, O.; Treesuwan, S.; Sritularak, B.; Pongrakhananon, V. Cypripedin, a phenanthrenequinone from Dendrobium densiflorum, sensitizes non-small cell lung cancer H460 cells to cisplatin-mediated apoptosis. J. Nat. Med. 2018, 72, 503–513. [Google Scholar] [CrossRef]
- Lu, T.; Han, C.; Chang, Y.; Lu, T.; Huang, H.; Bao, B.; Wu, H.; Huang, C.; Li, C.; Wu, T. Denbinobin, a phenanthrene from Dendrobium nobile, impairs prostate cancer migration by inhibiting Rac1 activity. Am. J. Chin. Med. 2014, 42, 1539–1554. [Google Scholar] [CrossRef]
- Petpiroon, N.; Sritularak, B.; Chanvorachote, P. Phoyunnanin E inhibits migration of non-small cell lung cancer cells via suppression of epithelial-to-mesenchymal transition and integrin αv and integrin β3. BMC Complementary Altern. Med. 2017, 17, 553. [Google Scholar] [CrossRef] [PubMed]
- Phiboonchaiyanan, P.; Petpiroon, N.; Sritularak, B.; Chanvorachote, P. Phoyunnanin E Induces Apoptosis of Non-small Cell Lung Cancer Cells via p53 Activation and Down-regulation of Survivin. Anticancer Res. 2018, 38, 6281–6290. [Google Scholar] [CrossRef]
- Song, J.; Shaw, P.; Sze, C.; Tong, Y.; Yao, X.; Ng, T.; Zhang, Y. Chrysotoxine, a novel bibenzyl compound, inhibits 6-hydroxydopamine induced apoptosis in SH-SY5Y cells via mitochondria protection and NF-κB modulation. Neurochem. Int. 2010, 57, 676–689. [Google Scholar] [CrossRef]
- Bhummaphan, N.; Pongrakhananon, V.; Sritularak, B.; Chanvorachote, P. Cancer Stem Cell-Suppressing Activity of Chrysotoxine, a Bibenzyl from Dendrobium pulchellum. J. Pharmacol. Exp. Ther. 2018, 364, 332–346. [Google Scholar] [CrossRef]
- Pinkhien, T.; Petpiroon, N.; Sritularak, B.; Chanvorachote, P. Batatasin III Inhibits Migration of Human Lung Cancer Cells by Suppressing Epithelial to Mesenchymal Transition and FAK-AKT Signals. Anticancer Res. 2017, 37, 6281–6289. [Google Scholar]
- Petpiroon, N.; Bhummaphan, N.; Tungsukruthai, S.; Pinkhien, T.; Maiuthed, A.; Sritularak, B.; Chanvorachote, P. Chrysotobibenzyl inhibition of lung cancer cell migration through Caveolin-1-dependent mediation of the integrin switch and the sensitization of lung cancer cells to cisplatin-mediated apoptosis. Phytomedicine 2019, 58, 152888. [Google Scholar] [CrossRef] [PubMed]
- Hlosrichok, A.; Sumkhemthong, S.; Sritularak, B.; Chanvorachote, P.; Chaotham, C. A bibenzyl from Dendrobium ellipsophyllum induces apoptosis in human lung cancer cells. J. Nat. Med. 2018, 72, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, P.; Liu, J.; Cao, Y. Erianin inhibits indoleamine 2, 3-dioxygenase -induced tumor angiogenesis. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 88, 521–528. [Google Scholar] [CrossRef] [PubMed]
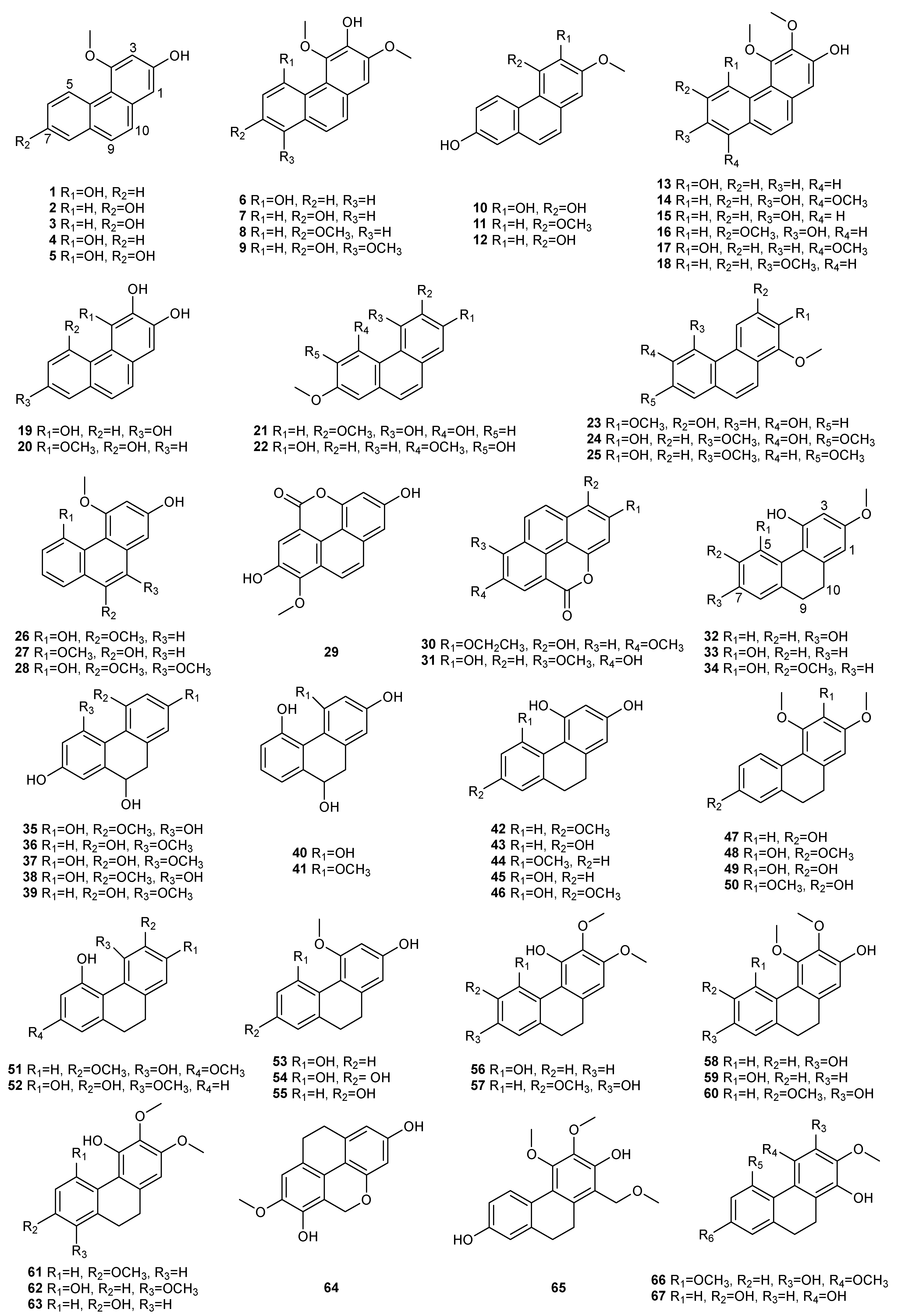
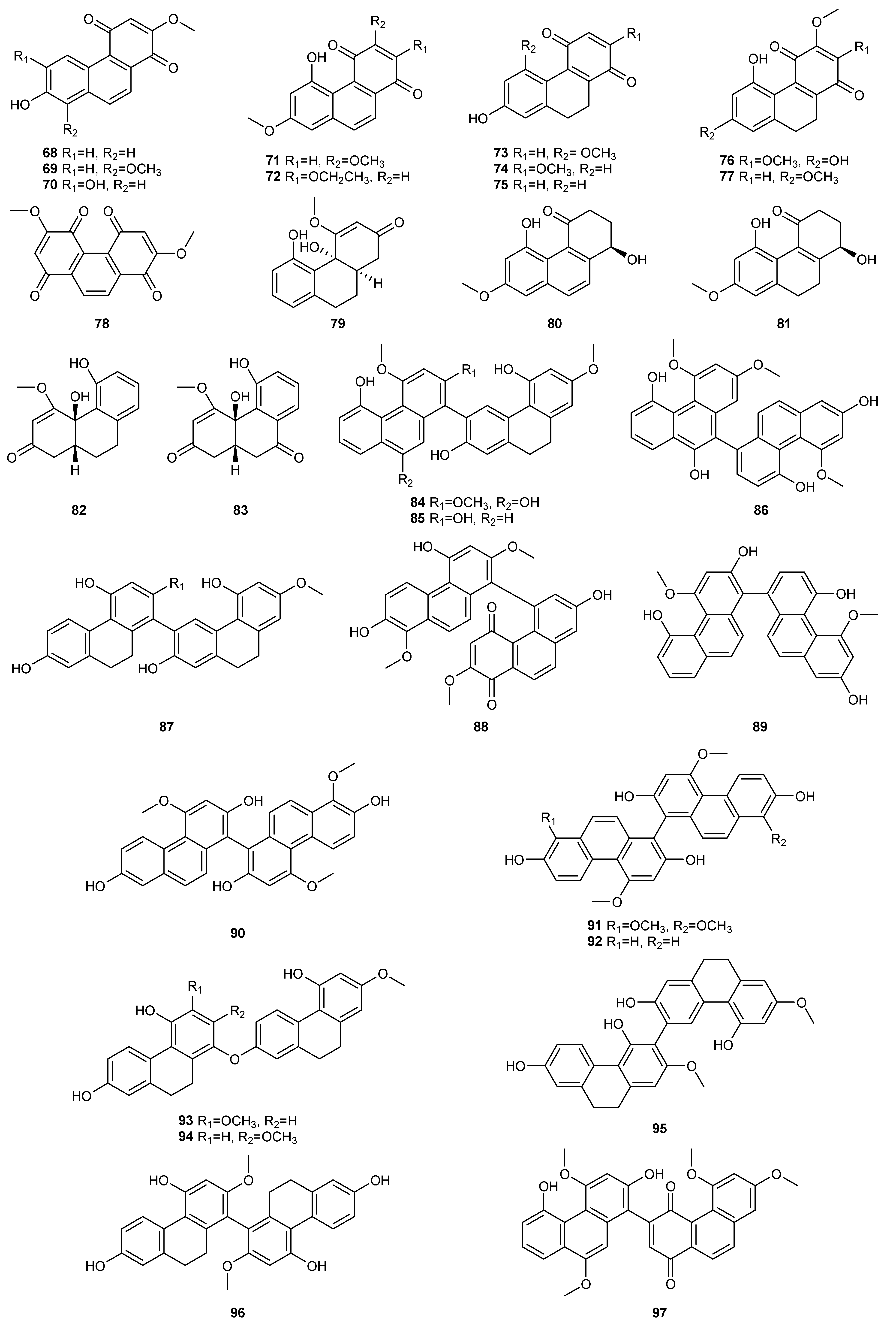
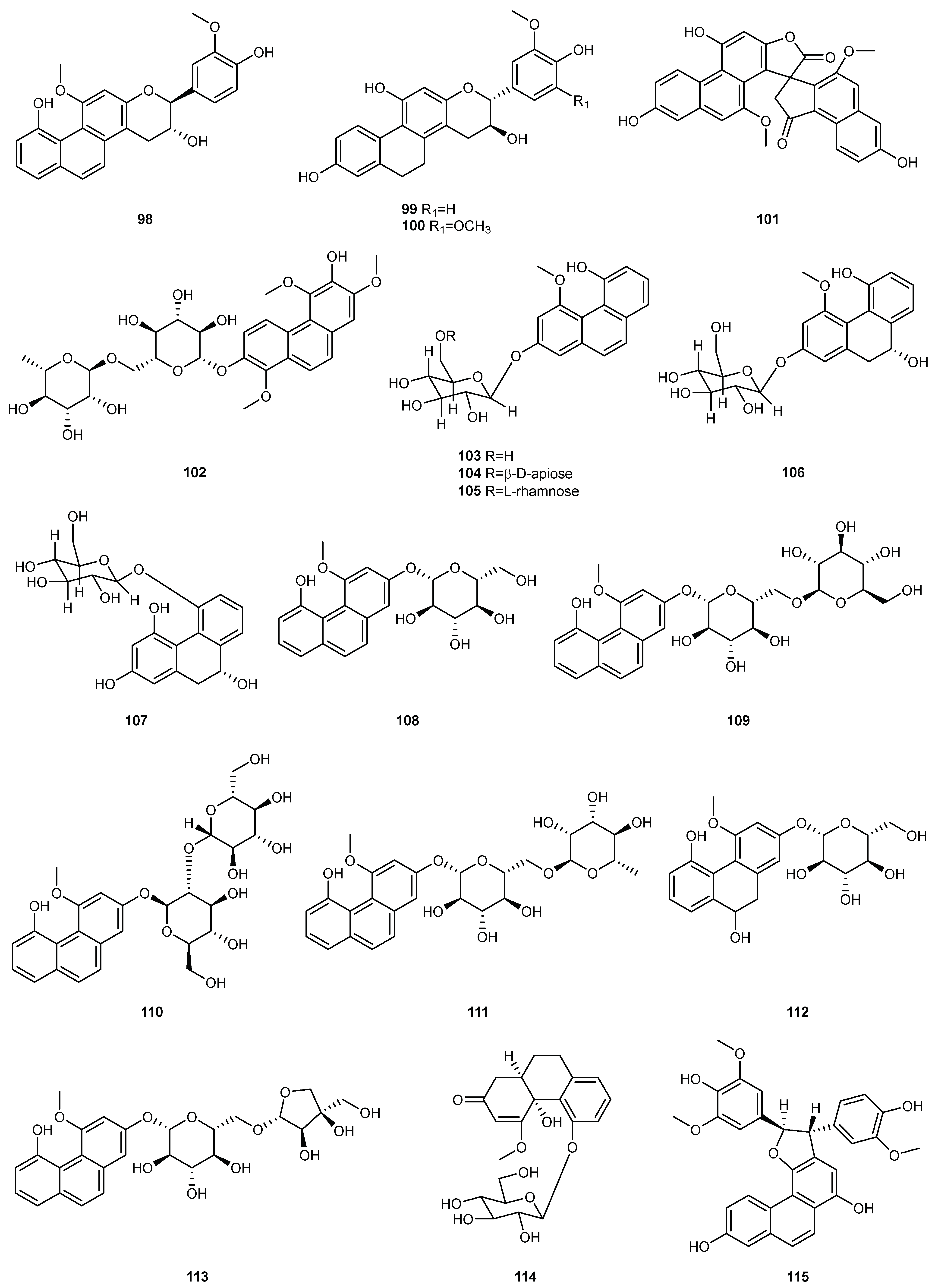
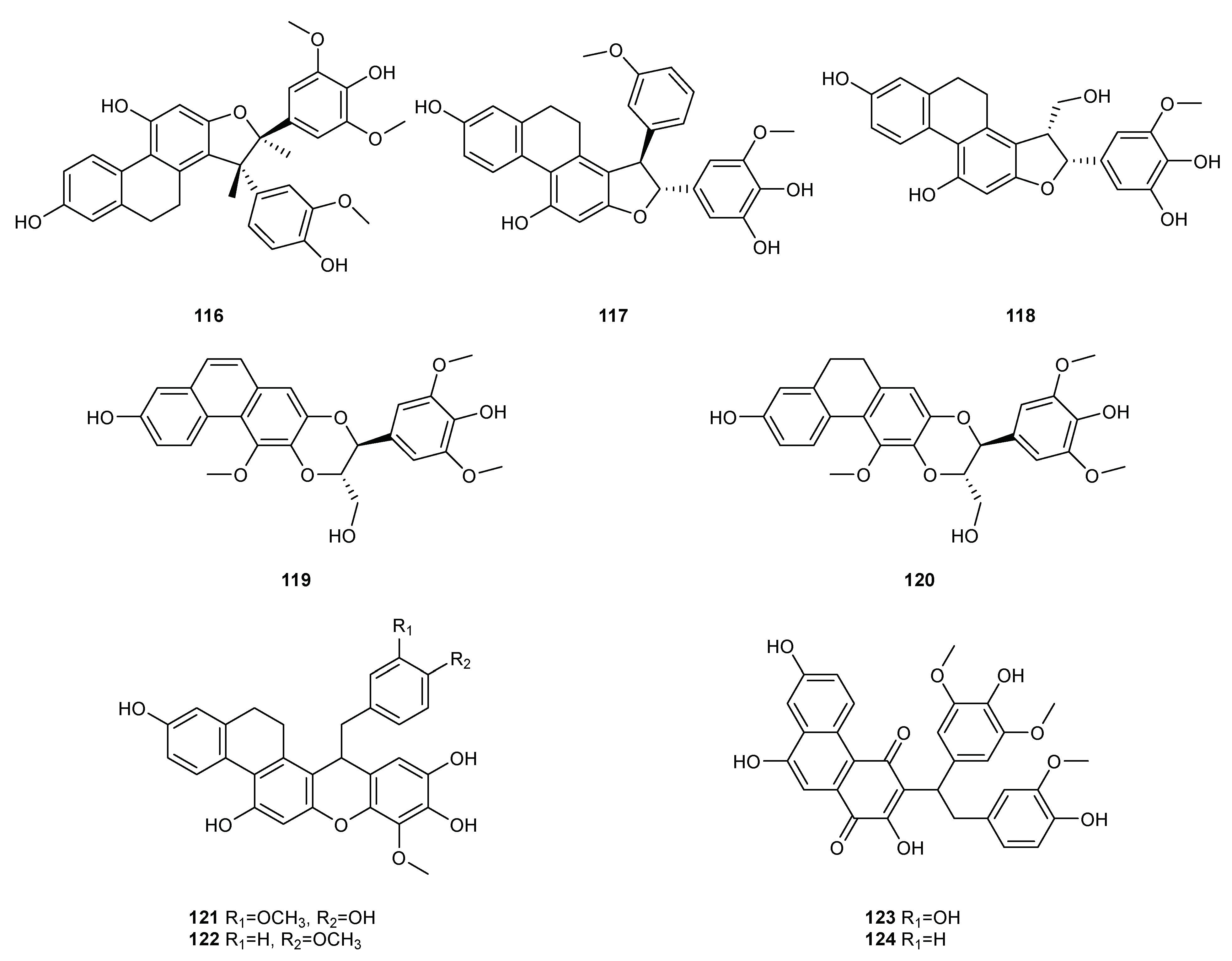
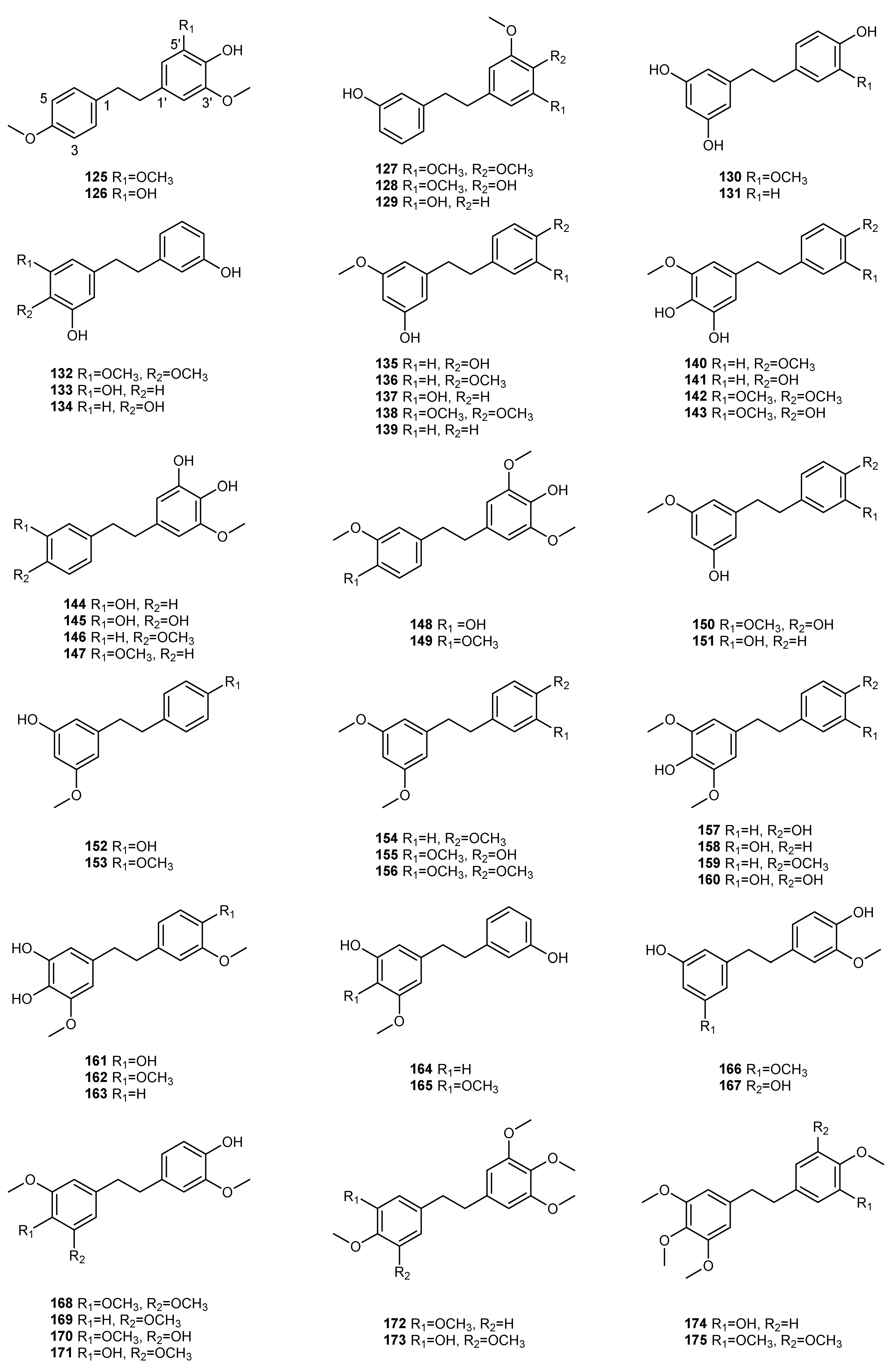
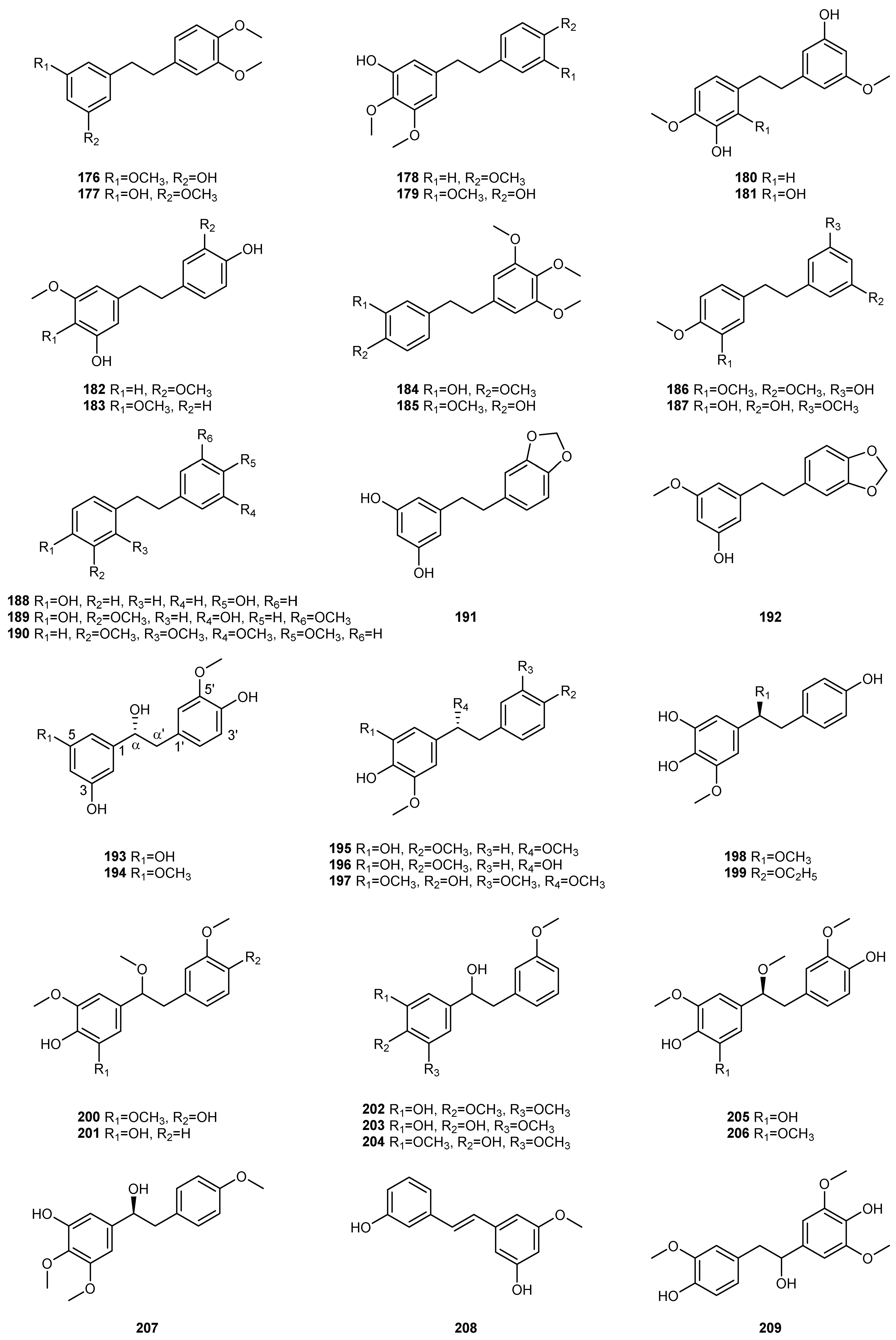
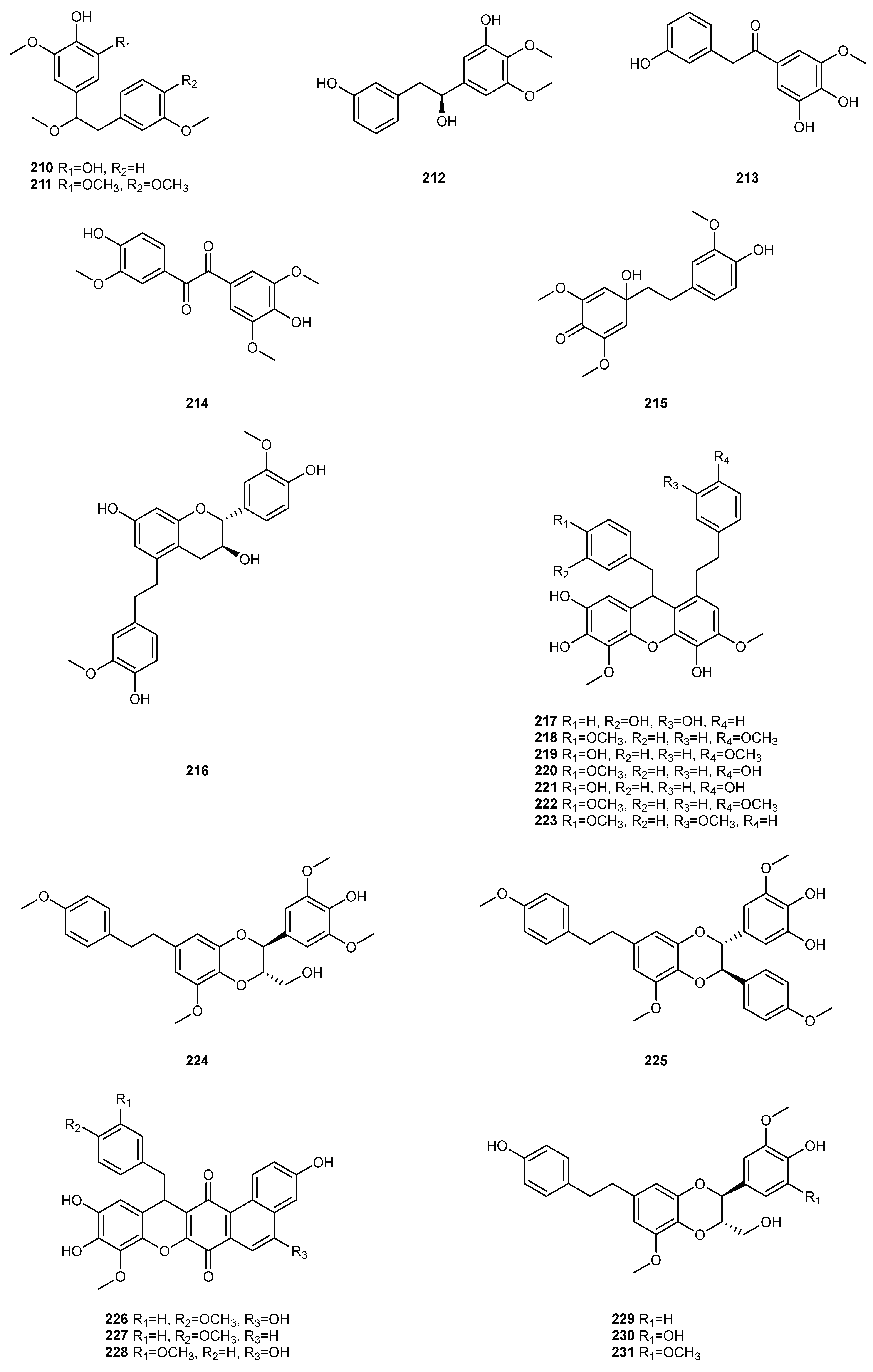
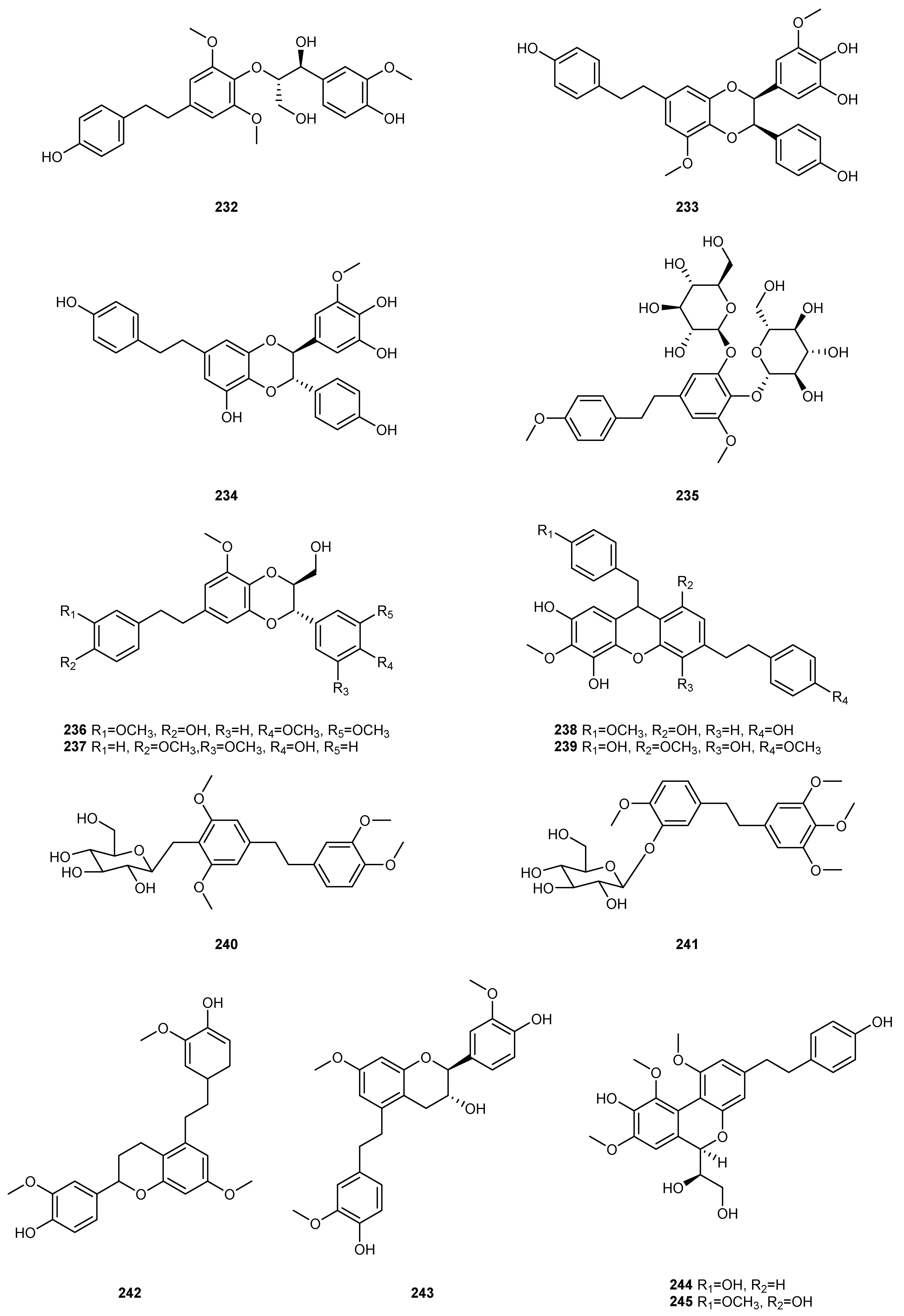
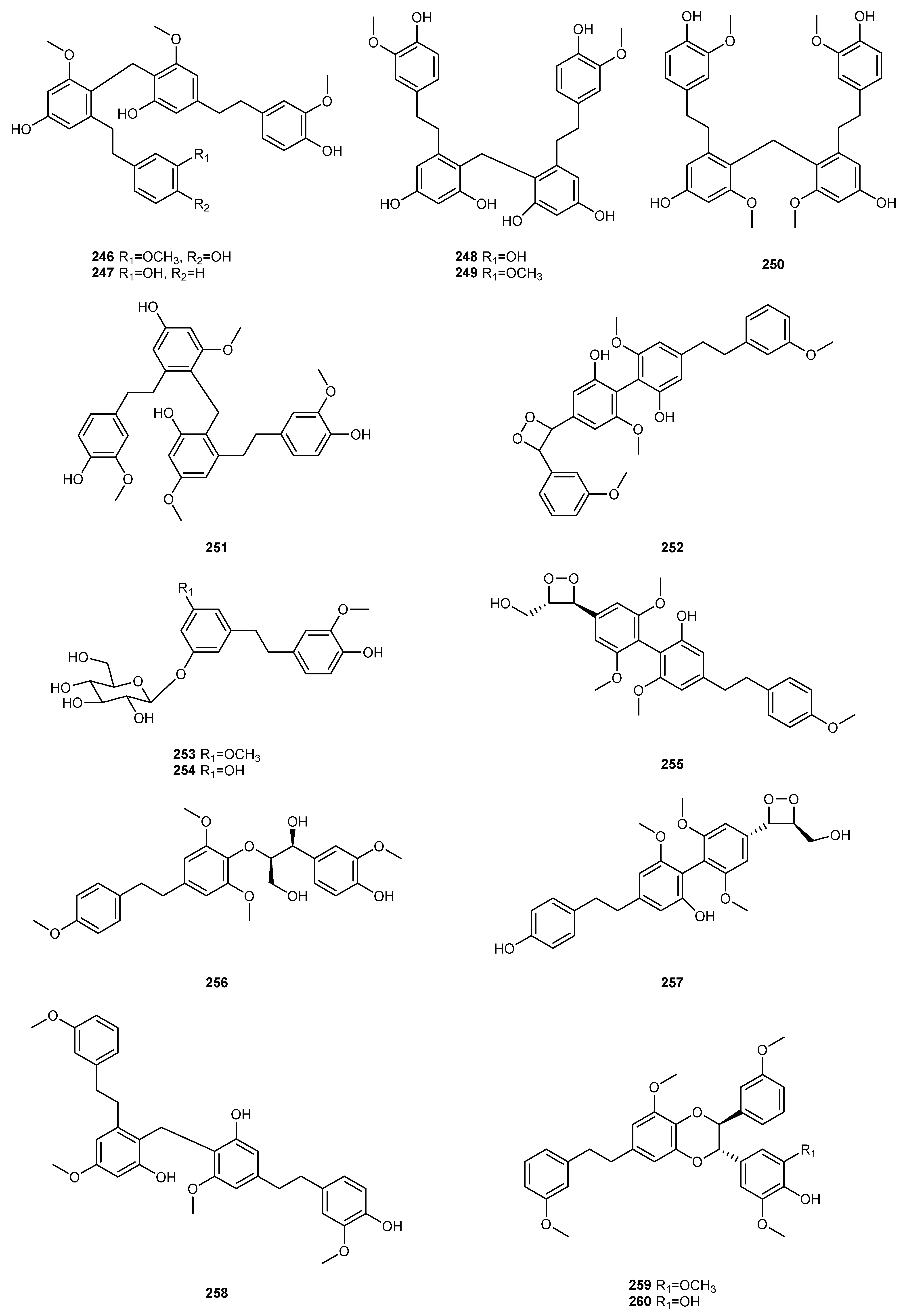
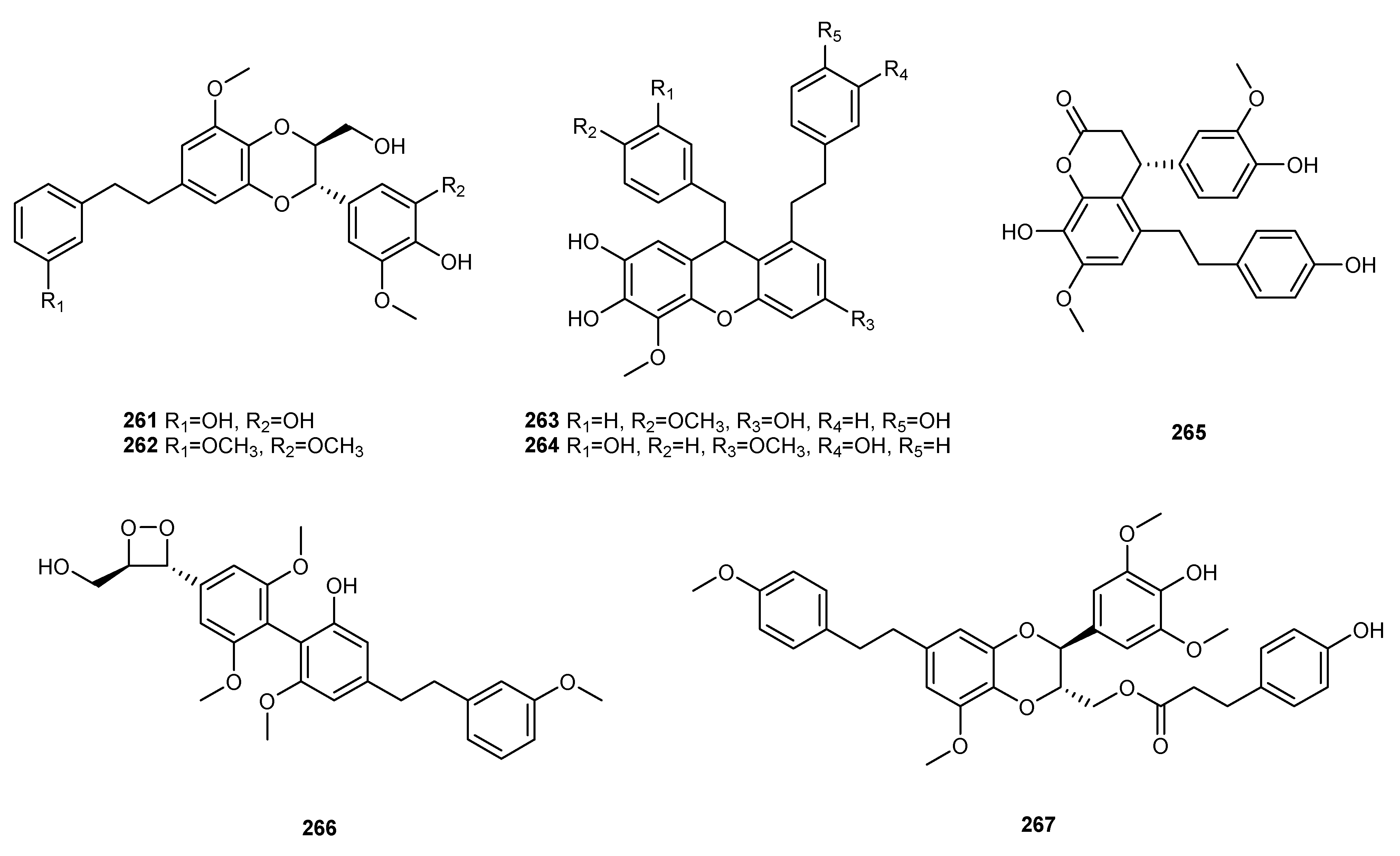

| NO. | Dendrobium Species | Phenanthrenes | Ref. |
|---|---|---|---|
| 1 | D. amoenum | Flaccidin (64) | [6] |
| 2 | D. aphyllum | moscatin (1), lusianthridin (32), 4-methoxy-2,5,7,9-tetrahydroxy-9,10-dihydrophenanthrene (35), 5-methoxy-4,7,9-trihydroxy-9,10-dihydrophenanthrene (36), 2,4-dihydroxy-7-methoxy-9,10-dihydro-phenanthrene (42), 2,4,7-trihydroxy-9,10-dihydrophenanthrene (43), hircinol (53), aphyllone A (79) | [7,8,9] |
| 3 | D. bellatulum | 2,5,7-trihydroxy-4-methoxy-9,10-dihydrophenanthrene (54) | [10] |
| 4 | D. brymerianum | flavanthrinin (2), lusianthridin (32), hircinol (52), densiflorol B (62) | [11,12] |
| 5 | D. candidum | bulbophyllanthrin (6), 2,5-dihydroxy-3,4-dimethoxyphenanthrene (13), confusarin (14), nudol (15), 2,3,4,7-tetramethoxyphenanthrene (19), 2,4,7-trihydroxy-9,10-dihydrophenanthrene (43), denbinobin (71) | [13,14] |
| 6 | D. capillipes | - | - |
| 7 | D. catenatum | - | - |
| 8 | D. christyanum | 4,5-dihydroxy-2-methoxy-9,10-dihydrophenanthrene (33) | [15] |
| 9 | D. chrysotoxum | moscatin (1), 3,7-dihydroxy-2,4-dimethoxyphenanthrene (7), confusarin (14), nudol (15), 2,7-dihydr-oxy-3,4,6-trimethoxyphenanthrene (16), chrysotoxene (23), 2,5-dihydroxy-4,9-dimethoxyphenanthr-ene (26), 2,7-dihydroxy-8-methoxyphenanthro [4,5-bcd] pyran-5(5H)-one (29), 4,5-dihydroxy-2,6-dimethoxy-9,10-dihydrophenanthrene (34), 2,4,7-trihydroxy-9,10-dihydrophenanthrene (43), eriant-hridin (58), densiflorol B (68), chrysotoxol A (99), chrysotoxol B (100) | [16,17,18,19,20,21] |
| 10 | D. chrysanthum | moscatin (1), flavanthrinin (2), 2,5-dihydroxy-4,9-dimethoxyphenanthrene (26), loddigesiinol A (27), 2,4-dihydroxy-5-methoxy-9,10-dihydrophenanthrene (44), 2,4,5-trihydroxy-9,10-dihydrophenanthr-ene (45), hircinol (53), coelonin (55), dendrochrysanene (111), denchryside A (112) | [22,23,24,25,26] |
| 11 | D. crepidatum | confusarin (14), hircinol (53) | [27] |
| 12 | D. crystallinum | cystalltone (30) | [28] |
| 13 | D. denneanum | moscatin (1), 4,5-dihydroxy-2-methoxy-9,10-dihydrophenanthrene (33), 5-methoxy-2,4,7,9S-tetrahy-droxy-9,10-dihydrophenanthrene (37), 4-methoxy-2,5,7,9S-tetrahydroxy-9,10-dihydrophenanthrene (38), 5-methoxy-4,7,9S-trihydroxy-9,10-dihydrophenanthrene (39), 2,5-dihydroxy-4-methoxy-phena-nthrene 2-O-β-D-glucopyranoside (103), 2,5-dihydroxy-4-methoxy-phenanthrene 2-O-β-D-apiofuran-osyl-(1–6)-β-D-glucopyranoside (104), 2,5-dihydroxy-4-methoxy-phenanthrene 2-O-α-L-rhamnopyr-anosyl-(1–6)-β-D-glucopyranoside (105), 9,10-dihydrophenanthrene 2-O-β-D-glucopyranoside (106), 1,2,5,9R-tetrahydroxy-9,10-dihydrophenanthrene 5-O-β-D-glucopyranoside (107), denneanoside A (108), denneanoside B (109), denneanoside C (110), denneanoside D (111), denneanoside F (112), denneanoside E (113) | [29,30,31,32] |
| 14 | D. densiflorum | 2,6-dihydroxy-1,5,7-trimethoxyphenanthrene (24), lusianthridin (32), densiflorl B (68), cypripedin (69) | [33] |
| 15 | D. devonianum | 4-methoxy-2,7-dihydroxyphenanthrene (3), 4-methoxy-2,5-dihydroxyphenanthrene (4), 4,5-dihydro-xy-2-methoxy-9,10-dihydrophenanthrene (33), 4,5-dihydroxy-2,6-dimethoxy-9,10-dihydrophenant-hrene (34), 2,4,7-trihydroxy-9,10-dihyrophenanthrene (43), hircinol (53), dendrodevonin A (80), den-drodevonin B (81) | [34,35] |
| 16 | D. draconis | 7-methoxy-9,10-dihydrophenanthrene-2,4,5-triol (46), hircinol (53), 5-methoxy-7-hydroxy-9,10-dihy-dro-1,4-phenanthrenequinone (73) | [36] |
| 17 | D. ellipsophyllum | 4,5-dihydroxy-2,3-dimethoxy-9,10-dihydrophenanthrene (56) | [37] |
| 18 | D. fimbriatum | moscatin (1), confusarin (14), fimbriatone (31), lusianthridin (32), (S)-2,4,5,9-tetrahydroxy-9,10-dihy-drophenanthrene (40), 2,4,7-trihydroxy-9,10-dihydrophenanthrene (43), 2,4-dimethoxy-9,10-dihy-drophenanthren-7-ol (47), hircinol (53), aphyllone A (79), 9,10-dihydro-aphyllone A-5-O-β-D-glucop-yranoside (114) | [38,39,40] |
| 19 | D. findlayanum | - | - |
| 20 | D. formosum | confusarin (14), nudol (15), lusianthridin (32), hircinol (53), 2,5,7-trihydroxy-4-methoxy-9,10-dihy-drophenanthrene (54), coelonin (55), erianthridin (58), 5-methoxy-7-hydroxy-9,10-dihydro-1,4-phen-anthrenequinone (73) | [41] |
| 21 | D. gratiosissimum | flavanthrinin (2), cannithrene 2 (51) | [28] |
| 22 | D. huoshanense | - | - |
| 23 | D. hancockii | moscatin (1), 2,5-dihydroxy-4,9-dimethoxyphenanthrene (26), ephemeranthoquinone (74) | [42] |
| 24 | D. harveyanum | - | - |
| 25 | D. heterocarpum | coelonin (55) | [43] |
| 26 | D. hercoglossum | - | - |
| 27 | D. hongdie | moscatin (1), nudol (15), ephemeranthoquinone (74) | [44] |
| 28 | D. hainanense | 3,7-dihydroxy-2,4-dimethoxyphenanthrene (7), 3-hydroxy-2,4,7-trimethoxy-phenanthrene (8), 3,4,7-trihydroxy-2-methoxyphenanthrene (10), nudol (15), 3-hydroxy-2,4,7-trimethoxy-9,10-dihydro-phenanthrene (48), flavanthridin (49), 7-hydroxy-2,3,4-trimethoxy-9,10-dihydrophenanthrene (50), erianthridin (58), 3,4-dimethoxy-1-(methoxymethyl)-9,10-dihydrophenanthrene-2,7-diol (65) | [45,46] |
| 29 | D. infundibulum | dendroinfundin A (61), dendroinfundin B (62), ephemeranthol A (63) | [47] |
| 30 | D. lindleyi | flavanthrinin (2), dehydroorchinol (11), lusianthridin (32), coelonin (55), densiflorol B (68), cypriped-in (69) | [48,49] |
| 31 | D. loddigesii | moscatin (1), loddigesiinol A (27), lusianthridin (32), plicatol C (41), 2,4,7-trihydroxy-9,10-dihydro-phenanthrene (43), hircinol (53), chrysotoxol A (99), loddigesiinol B (98), loddigesiinol I (115), loddigesiinol J (116), loddigesiinol G (123), loddigesiinol H (124) | [50,51,52,53] |
| 32 | D. longicornu | 7-methoxy-9,10-dihydrophenanthrene-2,4,5-triol (46), hircinol (53), coelonin (55), 5-methoxy-7-hydr-oxy-9,10-dihydro-1,4-phenanthrenequinone (73), ephemeranthoquinone (74) | [54,55] |
| 33 | D. nobile | moscatin (1), flavanthrinin (2), 3,7-dihydroxy-2,4-dimethoxyphenanthrene (7), denthyrsinin (9), dehydroorchinol (11), lusianthrin (12), confusarin (14), nudol (15), 3,4,8-trimethoxyphenanthrene-2,5-diol (17), fimbriol B (20), 4,5-dihydroxy-3,7-dimethoxyphenanthrene (21), 5,7-dimethoxyphenan-threne-2,6-diol (22), 2,5-dihydroxy-4,9-dimethoxyphenanthrene (26), fimbriaton (31), lusianthridin (32), 4,5-dihydroxy-2-methoxy-9,10-dihydrophenanthrene (33), ephemeranthol C (52), hircinol (53), coelonin (55), erianthridin (58), ephemeranthol A (63), densiflorol B (68), cypripedin (69), 6,7-dihydr-oxy-2-methoxy-1,4-phenanthrenedione (70), denbinobin (71), 7-hydroxy-9,10-dihydro-1,4-phenanth-renedione (75), denobilone B (82), denobilone C (83), denthyrsinol A (84), denthyrsinol B (85), denthy-rsinol C (86), denthyrsinol (89), phochinenin D (93), phochinenin G (95), 4,4′,7,7′-tetrahydroxy-2,2′-dimethoxy-9,9′,10,l0′-tetrahhydro-1,1′-phenanthrene (96), dendronbibisline A (117), dendronbibis-line B (118) | [56,57,58,59,60,61,62,63] |
| 34 | D. moniliforme | 3,7-dihydroxy-2,4-dimethoxyphenanthrene (7), confusarin (14), hircinol (53), 1,5-dihydroxy-3,4,7-trimethoxy-9,10-dihydrophenanthrene (66), denbinobin (71), moniliformin (78) | [64,65,66] |
| 35 | D. officinale | confusarin (14), nudol (15), 2,4,7-trihydroxy-9,10-dihydrophenanthrene (43), 2,4-dimethoxy-9,10-dihydrophenanthren-7-ol (47), erianthridin (58), ephemeeranthol A (63), dendrocandin P1 (119), den-drocandin P2 (120) | [67,68,69] |
| 36 | D. pachyglossum | 4,5-dihydroxy-2,3-dimethoxy-9,10-dihydrophenanthrene (56) | [70] |
| 37 | D. palpebrae | 2,6-dihydroxy-1,5,7-trimethoxyphenanthrene (24), 2,5-dihydroxy-4,9-dimethoxyphenanthrene (26), lusianthridin (32), dendropalpebrone (97) | [71] |
| 38 | D. parishii | flavanthrinin (2), dendroparishiol (121). | [72] |
| 39 | D. plicatile | 3,7-dihydroxy-2,4-dimethoxyphenanthrene (7), denthyrsinin (9), nudol (15), plicatol A (28), lusianth-ridin (32), plicatol C (41), flavanthridin (49), hircinol (53), coelonin (55), erianthridin (58), ephemeran-thol A (63), 1,4,7-trihydroxy-2-methoxy-9,10-dihydrophenanthrene (67), ephemeranthoquinone (74), 2,2′-dimethoxy-9,9′,10,l0′-tetrahhydro-1,1′-phenanthrene (96) | [73,74,75] |
| 40 | D. polyanthum | moscatin (1), hircinol (53), 2,4,7-trihydroxy-9,10-dihydrophenanthrene (43) | [76] |
| 41 | D. pulchellum | fimbriaton (31) | [77] |
| 42 | D. signatum | dendrosignatol (122) | [78] |
| 43 | D. scabrilingue | dendroscabrol A (19), lusianthridin (32), coelonin (55) | [79] |
| 44 | D. secundum | - | - |
| 45 | D. senile | moscatin (2), 2,5,7-trihydroxy-4-methoxyphenanthrene (5), 2,5-dihydroxy-4,9-dimethoxyphenanthr-ene (26), bleformin G (90), 4,4′,8,8′-tetramethoxy [1,10-biphenanthrene]-2,2′,7,7′-tetrol (91), 2,2′,7,7′-tetrahydroxy-4,4′-dimethoxy-1,10-biphenanthrene (92) | [80] |
| 46 | D. sinense | 2,5,7-trihydroxy-4-methoxy-9,10-dihydrophenanthrene (54), 4,5-dihydroxy-2,3-dimethoxy-9,10-dih-ydrophenanthrene (56), 4,7-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene (57), 2,5-dihydr-oxy-3,4-dimethoxy-9,10-dihydrophenanthrene (59), 2,7-dihydroxy-3,4,6-trimethoxy-9,10-dihydrop-henanthrene (60), ephemeranthol A (63), denbinobin B (76) | [81,82] |
| 47 | D. stuposum | 2,5-dihydroxy-4,9-dimethoxyphenanthrene (26), loddigesiinol A (27) | [83] |
| 48 | D. thyrsiflorum | moscatin (1), denthyrsinin (9), 2,6-dihydroxy-1,5,7-trimethoxyphenanthrene (24), hircinol (53), densi-florol B (68), denthyrsinol (89), denthyrsinone (88) | [84,85,86] |
| 49 | D. trigonopus | moscatin (1), hircinol (53) | [87] |
| 50 | D. venustum | flavanthrinin (2), lusianthridin (32), densiflorol B (68), phoyunnanin C (87), phoyunnanin E (94) | [88] |
| 51 | D. wardianum | moscatin (1), loddigesiinol A (27), coelonin (55), denbinobin (71), 3-ethoxy-5-hydroxy-7-methoxy-1,4-phenanthrenequinone (72), 9,10-dihydrodenbinobin (77) | [89,90] |
| 52 | D. williamsonii | - | - |
| NO. | Dendrobium Species | Bibenzyls | Ref. |
|---|---|---|---|
| 1 | D. amoenum | amoenylin (125), isoamoenylin (127), 3,3′,4-trihydroxybibenzyl (134), moscatilin (148) | [6] |
| 2 | D. aphyllum | tristin (130), dihydroresveratrol (131), moscatilin(148), gigantol (150), batatasin III (151), 4,4′-dihy-droxy-3,5-dimethoxybibenzyl (157), aphyllal C (191), aphyllal D (193), aphyllal E (194), aphyllone B (214), trigonopol B (216) | [8,9] |
| 3 | D. bellatulum | aloifol I (128), 3,3′-dihydroxy-4,5-dimethoxybiphezyl (132), dendrosinen B (144), batatasin III (151), 4′,5-dihydroxy-3,3′-dimethoxybiphezyl (166), dendrosinen D (217) | [10] |
| 4 | D. brymerianum | tristin (130), moscatilin (148), gigantol (150) | [11] |
| 5 | D. candidum | 3,4’-dihydroxy-5-methoxybibenzyl (135), 3,4-dihydroxy-4′,5-dimethoybibenzyl (140), dendrocandin E (145), moscatilin (148), chrysotoxine (149), gigantol (150), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), chrysotobibenzyl (172), 3-O-methylgigantol (176), erianin(184), dendrocanol (188), dendro-bibenzy (190), dendrocandin A (195), dendrocandin C (198), dendrocandin D (199), dendrocandin F (218), dendrocandin G (219), dendrocandin J (220), dendrocandin K (221), dendrocandin B (224), dendrocandin I (225), dendrocandin H (226), dendrocandin L (227), dendrocandin N (229), dend-rocandin O (230), dendrocandin M (232), dendrocandin P (233), dendrocandin Q (234), dendromoni-liside E (235) | [13,91,92,93,94,95] |
| 6 | D. capillipes | moscatilin (148), chrysotoxine (149), gigantol (150), chrysotobibenzyl (172), crepidatin (185) | [96] |
| 7 | D. catenatum | dihydroresveratrol (131), 3,4’-dihydroxy-5-methoxybibenzyl (135), 3,4′,5-trihydroxy-3′-methoxy-bibenzyl (167), dendrocandin V (228), dendrocandin N (229), dendrocandin U (231), dendrocandin W (236), dendrofindlaphenol B (237) | [97] |
| 8 | D. christyanum | aloifol I (128), dendrosinen B (144), moscatilin (148), gigantol (150), batatasin III (151) | [15] |
| 9 | D. chrysotoxum | batatasin (129), tristin (130), moscatilin (148), chrysotoxine (149), gigantol (150), batatasin III (151), 4’,5-dihydroxy-3,3’-dimethoxybiphezyl(166), chrysotobibenzyl (172), erianin (184), trigonopol B (216), 3,3′,4′,5-tetramethoxybibenzyl-4-O-β-D-glucopyranoside (240), 3,4,4′,5-tetramethoxybibenzyl-3′-O-β-D-glucopyranoside (230) | [16,17,19,20,21,98] |
| 10 | D. chrysanthum | tristin (130), moscatilin (148), chrysotoxine (149), gigantol (150), batatasin III (151), chrysotobibenzyl (172), crepidatin (185) | [22,24,26] |
| 11 | D. crepidatum | moscatilin (148), 4′-hydroxy-3,4,5,3′-tetramethoxybibenzyl (168), erianin (184), crepidatin (185), crepidatuol A (242), crepidatuol B (243) | [27,99,100] |
| 12 | D. crystallinum | dihydroresveratrol (131), 3,4’-dihydroxy-5-methoxybibenzyl (135), gigantol (145), batatasin III (151), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), 3,4′,5-trihydroxy-3′-methoxybibenzyl (167), 4′-hydroxy-3,3′,5-trimethoxybibenzyl (169), 3,5′-dihydroxy-3′,4-dimethoxybiphezyl (180), 5′-hydroxy-3,3′,4-trimethoxybibenzyl (186), dencryol A (238), dencryol B (239) | [101,102,103,104] |
| 13 | D. denneanum | moscatilin (148), gigantol (150), crepidatin (185) | [31] |
| 14 | D. densiflorum | tristin (130), moscatilin (148), gigantol (150), densiflorol A (192) | [33] |
| 15 | D. devonianum | tristin (130), 3-hydroxy-4′,5-dimethoxybibenzyl (136), 3,4′,5-trihydroxybibenzyl (154) | [35] |
| 16 | D. draconis | gigantol (150), batatasin III (151) | [36] |
| 17 | D. ellipsophyllum | moscatilin (148), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), 4,5,4′-trihydroxy-3,3′-dimethoxybibe-nzyl (161) | [37] |
| 18 | D. fimbriatum | tristin (130), moscatilin (148), gigantol (150), batatasin III (151), 3,4’-dihydroxy-3′,4,5-trimethoxy-bibenzyl (170), crepidatin (185), 4,4′-dihydroxy-3,3′,5,α-tetramethoxybibenzyl (200), fimbriadimerbi-benzyl B (246), fimbriadimerbibenzyl C (247), fimbriadimerbibenzyl D (248), fimbriadimerbibenzyl F (249), fimbriadimerbibenzyl E (250), fimbriadimerbibenzyl G (251), gigantol-5-O-β-D-glucopyrano-side (253), trisin-5-O-β-D-glucopyranoside (254), fimbriadimerbibenzyl A (258) | [38,40] |
| 19 | D. findlayanum | 3,4’-dihydroxy-5-methoxybibenzyl (135), 3,3’-dihydroxy-5-methoxybibenzyl (137), 3,4,4′-trihydroxy-5-methoxybibenzy (141), 3,4-dihydroxy-3’,4’,5-trimethoxybibenzyl (142), 4,4′-dihydroxy-3,5-dimeth-oxybibenzyl (157), 3′,4-dihydroxy-3,5-dimethoxybiphezyl (158), 4,4′-dihydroxy-3,5,3′-trimethoxy-bibenzyl (171), 3,3′-dihydroxy-4,5′-dimethoxybiphezyl (187), (R)-3,α-dihydroxy-4,4ʹ,5-trimethoxy-bibenzyl (207), dendrofindlaphenol B (237), dendrofindlaphenol A (255), dendrofindlaphenol C (256), 6″-de-O-methyldendrofindlaphenol A (257) | [105,106] |
| 20 | D. formosum | moscatilin (148), gigantol (150), batatasin III (151) | [41] |
| 21 | D. gratiosissimum | amoenylin (125), tristin (130), 3,4’-dihydroxy-5-methoxybibenzyl (135), 3-methylgigantol (138), 3,4-dihydroxy-4′,5-dimethoxybibenzyl (140), 3,4-dihydroxy-3’,4’,5-trimethoxybibenzyl (142), 3,4,4′-trihydroxy-5,3′-dimethoxybibenzyl (143), moniliformine (146), moscatilin (148), chrysotoxine (149), gigantol (150), batatasin III (151), 3,4′,5-trihydroxybibenzyl (154), aloifol (155), gigantol tetramethyl ether (156), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), 4-hydroxy-3,5,4′-trimethoxybibenzyl (159), 3,4’-dihydroxy-3′,4,5-trimethoxybibenzyl (170), dendrogratiol A (178), dencryol A (238), dencryol B (239) | [107,28] |
| 22 | D. huoshanense | 3,4’-dihydroxy-5-methoxybibenzyl (135), 3-hydroxy-4′,5-dimethoxybibenzyl (136), 3-hydroxy-5-methoxybibenzyl (139), batatasin III (151), 5,4′-dihydroxy-3-methoxybibenzyl (152), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), 3’,4-dihydroxy-3,5′-dimethoxybibenzyl (189), dendrocandin B (224), dendrocandin U (231) | [108,109,110] |
| 23 | D. hancockii | 3,4′,5-trihydroxy-3′-methoxybibenzyl (167), 4,4′-dihydroxy-3,5,3′-trimethoxybibenzyl (171), 3′,4-dihydroxy-3,5′-dimethoxybibenzyl (189), 3,α-dihydroxy-4,5,3′-trimethoxybibenzyl (202), crepidatuol B (243) | [42] |
| 24 | D. harveyanum | 3,4-dihydroxy-4′,5-dimethoxybibenzyl (140), dendrofalconerol A (222), dendrocandin B (224), dendr-ofalconerol B (263) | [111] |
| 25 | D. heterocarpum | 3,4’-dihydroxy-5-methoxybibenzyl (135), 3-hydroxy-4′,5-dimethoxybibenzyl (136), 3,4-dihydroxy-4′,5-dimethoxybibenzyl (140), moscatilin (148), gigantol (150), batatasin III (151), 3-O-methylgigantol (176), densiflorol A (192), dendrocandin A (195), (S)-3,4,α-trihydroxy-4′,5-dimethoxybibenzyl (196), dendrocandin F (218), dendrocandin I (225) | [43] |
| 26 | D. hercoglossum | 3′,4-dihydroxy-3,5-dimethoxybiphezyl (158), 4,4′-dihydroxy-3,5,3′-trimethoxybibenzyl (171), 4,5-dihydroxy-3,3′,α-trimethoxybibenzyl (201), 3,4,α-trihydroxy-5,3′-dimethoxybibenzyl (203), 4,α-dihydroxy-3,5,3′-trimethoxybibenzyl (207) | [112] |
| 27 | D. hongdie | tristin (130), gigantol (150), batatasin III (151) | [44] |
| 28 | D. hainanense | - | - |
| 29 | D. infundibulum | aloifol I (128), 3,3′-dihydroxy-4,5-dimethoxybiphezyl (132), dendrosinen B (144), moscatilin (148), batatasin III (151), 5,4′-dihydroxy-3,4,3′-trimethoxybibenzyl (179) | [47] |
| 30 | D. lindleyi | tristin (130), 3,3′,5-trihydroxybibenzyl (133), 3-dendrobin A (147), moscatilin (148), chrysotoxine (149), gigantol (150), batatasin III (151), 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl (162), thunalbene (206) | [48,49] |
| 31 | D. loddigesii | tristin (130), 3,3′,5-trihydroxybibenzyl (133), moscatilin (148), gigantol (150), batatasin III (151), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), 4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl (161), 4′,5-dihy-droxy-3,3′-dimethoxybiphezyl (166), crepidatin (185), aphyllal C (191), densiflorol A (192), loddigesii-nol C (205), (R)-4,5,4ʹ-trihydroxy-3,3ʹ,α-trimethoxybibenzyl (208), loddigesiinol D (215), trigonopol B (216), crepidatuol B (243) | [50,51,53,54,113] |
| 32 | D. longicornu | aloifol I (128), batatasin (129), tristin (130), 3,3′-dihydroxy-4,5-dimethoxybiphezyl (132), 3,3′,4-trihy-droxybibenzyl (134), moscatilin (148), gigantol (150), batatasin III (151), 3,4’-dihydroxy-3′,4,5-trimeth-oxybibenzyl (170), cannabistilbene II (181), longicornuol A (244), trigonopol A (245) | [54,55,114] |
| 33 | D. nobile | tristin (130), 3,3′,5-trihydroxybibenzyl (133), 3-hydroxy-5-methoxybibenzyl (139), dendrobin A (147), moscatilin (148), chrysotoxine (149), gigantol (150), batatasin III (151), 3′,4-dihydroxy-3,5-dimethoxy-biphezyl (158), 4,5-dihydroxy-3,3′-dimethoxybibenzy (163), 5,3’-dihydroxy-3-methoxybibenzyl (164), 4′,5-dihydroxy-3,3′-dimethoxybiphezyl (166), 4′-hydroxy-3,3′,5-trimethoxybibenzyl (169), 4,4′-dihy-droxy-3,5,3′-trimethoxybibenzyl (171), chrysotobibenzyl (172), 3-O-methylgigantol (176), 3,4′-dihy-droxy-5,5′-dimethoxydihydrostilbene (182), crepidatin (185), nobilin D (197), 3′,4-dihydroxy-3,5′-dimethoxybibenzyl (189), 4,5-dihydroxy-3,3′,α-trimethoxybibenzyl (201), 4,α-dihydroxy-3,5,3′-trim-ethoxybibenzyl (204), nobilin B (209), nobilin A (212), nobilin C (213), nobilin E (223), dendronophen-ol A (252),dendronbibisline D (259), didendronbiline A (260), dendronbiline B (261), dendronbibisline C (262), dendronophenol B (266) | [56,57,59,60,115,116,117,118,119] |
| 34 | D. moniliforme | aloifol I (128), 3,3′-dihydroxy-4,5-dimethoxybiphezyl (132), moscatilin (148), gigantol (150), 3,4’-dihydroxy-3′,4,5-trimethoxybibenzyl (170), dendromoniliside E (235), longicornuol A (244) | [120,66] |
| 35 | D. officinale | amoenylin (125), tristin (130), 3,3′-dihydroxy-4,5-dimethoxybiphezyl (132), 3,4’-dihydroxy-5-methox-ybibenzyl (135), 3-hydroxy-4′,5-dimethoxybibenzyl (136), 3,4-dihydroxy-4′,5-dimethoxybibenzyl (140), 3,4,4′-trihydroxy-5-methoxybibenzy (141), dendrosinen B (144), moscatilin (148), gigantol (150), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), 3,4′,5-trihydroxy-3′-methoxybibenzyl (167), 3-O-methylgigantol (176), 3,4′-dihydroxy-4,5-dimethoxybibenzyl (183), erianin(184), densiflorol A (192), (S)-3,4,α-trihydroxy-4′,5-dimethoxybibenzyl (196), trigonopol B (216), dendrocandin B (224), dendro-candin N (229), dendrocandin U (231), 6″-de-O-methyldendrofindlaphenol A (257), dendrocandin X (265), denofficin (267) | [67,68,121,122,123] |
| 36 | D. pachyglossum | Moscatilin (148), gigantol (150), 4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl (161) | [124] |
| 37 | D. palpebrae | moscatilin (148), gigantol (150), 4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl (161) | [70] |
| 38 | D. parishii | dendrocandin E (145), 4,3′,4′-trihydroxy-3,5-dimethoxybibenzyl (160), 4,5,4′-trihydroxy-3,3′-dimeth-oxybibenzyl (161) | [71] |
| 39 | D. plicatile | moscatilin (148), batatasin III (151), 5,3’-dihydroxy-3-methoxybibenzyl (164), 3’-hydroxy-3,4,4’,5-tetramethoxybibenzyl (174), 3-O-methylgigantol (176), 3,3’,4’-trimethoxy-5-hydroxybibenzyl (177) | [72,73] |
| 40 | D. polyanthum | moscatilin (148), gigantol (150), batatasin III (151) | [75] |
| 41 | D. pulchellum | moscatilin (138), chrysotoxine (139), chrysotobibenzyl (162), crepidatin (175) | [76] |
| 42 | D. signatum | 4′,5′-dihydroxy-3′,4-dimethoxybiphezyl (126), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), dendro-falconerol A (222), dendrocandin B (224), dendrocandin I (225), 6″-de-O-methyldendrofindlaphenol A (257) | [77,78] |
| 43 | D. scabrilingue | aloifol I (128), gigantol (150), batatasin III (151), dendroscabrol B (264) | [79] |
| 44 | D. secundum | moscatilin (148), 4,5,4′-trihydroxy-3,3′-dimethoxybibenzyl (161), 5-hydroxy-3,4,3′,4′,5′-pentamethox-ybibenzyl (173), brittonin A (175) | [96,125] |
| 45 | D. senile | aloifol I (128), moscatilin (148) | [80] |
| 46 | D. sinense | aloifol I (128), dendrosinen B (144), chrysotoxine (149), 5,3′-dihydroxy-3,4-dimethoxybibenzyl (165), 5,4′-dihydroxy-3,4,3′-trimethoxybibenzyl (179), dendrosinen A (210), dendrosinen C (211), dendrosi-nen D (217), longicornuol A (244), trigonopol A (245) | [82,126] |
| 47 | D. stuposum | 4,4′-dihydroxy-3,5,3′-trimethoxybibenzyl (171) | [83] |
| 48 | D. thyrsiflorum | tristin (130), moscatilin (148), gigantol (150), batatasin III (151), 4′,5-dihydroxy-3,3′-dimethoxy-biphezyl (166), 3,4′,5-trihydroxy-3′-methoxybibenzyl (167), 4,4′-dihydroxy-3,5,3′-trimethoxybibenzyl (171), erianin (184) | [84,86,127] |
| 49 | D. trigonopus | tristin (130), moscatilin (148), gigantol (150), trigonopol B (216), trigonopol A (245) | [87,128] |
| 50 | D. venustum | gigantol (150), batatasin III (151) | [88] |
| 51 | D. wardianum | 3,3′-dihydroxy-4,5-dimethoxybiphezyl (132), 3,4-dihydroxy-4′,5-dimethoxybibenzyl (140), moscatili-n (148), gigantol (150), 5-hydroxy-3,4′-dimethoxybibenzyl (153), 4-hydroxy-3,5,4′-trimethoxybibenzyl (159), 4′-hydroxy-3,3′,5-trimethoxybibenzyl (169), dendrocandin A (195), dendrocandin V (228), dendrocandin U (231) | [89,90] |
| 52 | D. williamsonii | amoenylin (125), aloifol I (128), 3,3′-dihydroxy-4,5-dimethoxybiphezyl (132), moniliformine (146), moscatilin (148), 4,4′-dihydroxy-3,5-dimethoxybibenzyl (157), dendrofindlaphenol A (256) | [129] |
| NO. | Compound | Dendrobium Species | NO Inhibition (%) | IC50 (µM) | Ref. |
|---|---|---|---|---|---|
| 1 | 1 | D. aphyllum | 32.48 (25 μM) | - | [9] |
| 2 | 17 | D. nobile | - | 20.4 ± 0.8 | [59] |
| 3 | 20 | D. nobile | - | 28.9 ± 0.6 | [59] |
| 4 | 22 | D. nobile | - | 35.7 ± 0.6 | [59] |
| 5 | 27 | D. loddigesii | - | 2.6 | [52] |
| 6 | 32 | D. nobile | - | 9.6 ± 0.3 | [59] |
| 7 | 37 | D. denneanum | 90 ± 7 (50 μM) | 3.1 | [29] |
| 8 | 38 | D. denneanum | 86 ± 2 (50 μM) | 4.2 | [29] |
| 9 | 39 | D. denneanum | 58 ± 8 (50 μM) | - | [29] |
| 10 | 41 | D. loddigesii | - | 29.1 | [52] |
| 11 | 43 | D. loddigesii | - | 8.6 | [51] |
| 12 | 49 | D. nobile | - | 34.1 ± 0.9 | [59] |
| 13 | 52 | D. nobile | - | 17.6 ± 0.4 | [59] |
| 14 | 53 | D. nobile | - | 26.4 ± 0.2 | [59] |
| 15 | 55 | D. nobile | - | 10.2 ± 0.2 | [59] |
| 16 | 57 | D. nobile | - | 19.5 ± 0.4 | [59] |
| 17 | 63 | D. nobile | - | 12.0 ± 0.3 | [59] |
| 18 | 79 | D. aphyllum | 12.21 (25 μM) | - | [9] |
| 19 | 98 | D. loddigesii | - | 9.9 | [51] |
| 20 | 99 | D. loddigesii | - | 10.9 | [51] |
| 21 | 103 | D. denneanum | 92 ± 2 (50 μM) | 4.6 | [29] |
| 22 | 104 | D. denneanum | 76 ± 4 (50 μM) | 16.9 | [29] |
| 23 | 105 | D. denneanum | 62 ± 1 (50 μM) | 41.5 | [29] |
| 24 | 106 | D. denneanum | 68 ± 2 (50 μM) | - | [29] |
| 25 | 107 | D. denneanum | 92 ± 5 (50 μM) | 0.7 | [29] |
| 26 | 115 | D. loddigesii | - | 7.5 | [51] |
| 27 | 116 | D. loddigesii | - | 14.6 | [51] |
| 28 | 130 | D. aphyllum | 27.23 (25 μM) | - | [9] |
| 29 | 131 | D. aphyllum | 25.82 (25 μM) | - | [9] |
| 30 | 133 | D. loddigesii | - | 13.1 | [51] |
| 31 | 148 | D. nobile | - | 27.6 ± 0.5 | [59] |
| 32 | 150 | D. nobile | - | 32.9 | [115] |
| 33 | 151 | D. loddigesii | - | 21.9 | [51] |
| 34 | 157 | D. loddigesii | - | 49.3 | [51] |
| 35 | 172 | D. nobile | - | 48.2 | [115] |
| 36 | 185 | D. crepidatum | - | 3.04 ± 1.15 | [100] |
| 37 | 191 | D. aphyllum | 22.07 (25 μM) | - | [9] |
| 38 | 193 | D. aphyllum | 14.96 (25 μM) | - | [9] |
| 39 | 197 | D. nobile | - | 15.3 | [115] |
| 40 | 209 | D. crepidatum | - | 26.64 ± 0.51 | [100] |
| 41 | 214 | D. loddigesii | - | 69.7 | [52] |
| 42 | 215 | D. aphyllum | 19.72 (25 μM) | - | [9] |
| 43 | 216 | D. loddigesii | - | 26.3 | [51] |
| 44 | 223 | D. nobile | - | 19.2 | [115] |
| 45 | 257 | D. findlayanum | - | 21.4 ± 1.0 | [106] |
| No. | Compound | Dendrobium Species | α-Glucosidase (µM) | Pancreatic Lipase (µM) | Ref. |
|---|---|---|---|---|---|
| 1 | 1 | D. senile | - | 57.60 ± 3.30 | [80] |
| 2 | 14 | D. formosum | 189.78 ± 1.11 | 154.61 ± 8.58 | [41] |
| 3 | 18 | D. scabrilingue | 96.2 ± 12.0 | - | [79] |
| 4 | 26 | D. senile | - | 58.60 ± 3.40 | [80] |
| 5 | 32 | D. scabrilingue | 112.9 ± 5.3 | - | [79] |
| 6 | 33 | D. christyanum, | 133.11 ± 10.82 | - | [15] |
| 7 | 43 | D. loddigesii | 119.2 | - | [51] |
| 8 | 55 | D. scabrilingue | 131.4 ± 6.6 | - | [79] |
| 9 | 73 | D. formosum | 126.88 ± 0.66 | 69.45 ± 10.14 | [41] |
| 10 | 98 | D. loddigesii | 52.8 | - | [51] |
| 11 | 99 | D. loddigesii | 39.6 | - | [51] |
| 12 | 115 | D. loddigesii | 5.5 | - | [51] |
| 13 | 116 | D. loddigesii | 5.8 | - | [51] |
| 14 | 123 | D. loddigesii | 16.7 | - | [53] |
| 15 | 124 | D. loddigesii | 10.9 | - | [53] |
| 16 | 131 | D. catenatum | 36.05 ± 0.67 | - | [97] |
| 17 | 133 | D. loddigesii | 31.8 | - | [51] |
| 18 | 135 | D. catenatum | 159.59 ± 0.86 | - | [97] |
| 19 | 140 | D. harveyanum | 32.0 ± 1.5 | NA | [111] |
| 20 | 141 | D. officinale | 403.4 | - | [121] |
| 21 | 144 | D. infundibulum | 213.9 ± 2.4 | 295.01 ± 37.90 | [47] |
| 22 | 150 | D. christyanum, | 79.87 ± 14.20 | - | [15] |
| 23 | 151 | D. infundibulum | 148.8 ± 8.4 | NA | [47] |
| 24 | 157 | D. loddigesii | 94.2 | - | [51] |
| 25 | 222 | D. harveyanum | 71.6 ± 5.5 | 6.60 ± 0.10 | [111] |
| 26 | 231 | D. officinale | 9.46 | - | [121] |
| 27 | 235 | D. harveyanum | 51.2 ± 3.4 | 4.90 ± 0.09 | [111] |
| 28 | 243 | D. loddigesii | 18.9 | - | [53] |
| 29 | 253 | D. scabrilingue | 9.4 ± 0.7 | - | [75] |
| Compound | MCF-7 | HL-60 | A549 | SMMC-7721 | SW480 | Ref. |
|---|---|---|---|---|---|---|
| 1 | 23.75 ± 0.82 | NA | 16.29 ± 0.25 | NA | 18.97 ± 1.04 | [89] |
| 71 | 13.13 ± 0.47 | 3.08 ± 0.12 | 19.68 ± 1.12 | NA | 16.81 ± 0.13 | [89] |
| 77 | 3.63 ± 0.03 | 2.33 ± 0.12 | 14.79 ± 0.64 | 14.84 ± 0.41 | 6.66 ± 0.71 | [89] |
| 148 | 6.20 | 2.18 | 5.73 | 4.07 | 6.88 | [38] |
| 170 | 37.12 | 24.32 | >40 | >40 | >40 | [38] |
| 228 | 38.48 ± 1.16 | NA | NA | NA | NA | [89] |
| 246 | 12.06 | 15.79 | 15.55 | 16.63 | 18.19 | [38] |
| 247 | 24.84 | 22.33 | >40 | >40 | >40 | [38] |
| 249 | 13.67 | 19.08 | 16.64 | 17.47 | 18.83 | [38] |
| 250 | 5.85 | 16.91 | 16.49 | 18.89 | 21.23 | [38] |
| 251 | 7.75 | 16.91 | 16.49 | 18.89 | 21.23 | [38] |
| 255 | 4.7 ± 0.7 | 2.3 ± 0.2 | 4.4 ± 0.5 | 5.1 ± 0.4 | 5.3 ± 0.8 | [106] |
| 257 | 19.4 ± 2.7 | 23.5 ± 2.0 | 20.8 ± 1.6 | 34.4 ± 2.3 | >40 | [106] |
| 258 | 12.67 | 16.13 | 16.34 | 18.27 | 17.15 | [106] |
| NO. | Compound | Dendrobium Species | Cancer cell | IC50 (µM) | Ref. |
|---|---|---|---|---|---|
| 1 | 3 | D. devonianum | HT-29 | 30.30 | [34] |
| 2 | 7 | D. plicatile | HepG2 | 6.12 | [73] |
| MDA-MB231 | 12.46~19.61 | ||||
| A549 | 11.68~43.88 | ||||
| 3 | 9 | D. thyrsiflorum | Hela | 2.7 | [86] |
| K-562 | 2.3 | ||||
| MCF-7 | 4.8 | ||||
| 4 | 14 | D. officinale | HL-60 | 18.95 ± 0.70 | [123] |
| THP-1 | 11.51 ± 0.12 | ||||
| 5 | 15 | D. plicatile | HepG2 | 4.20 | [73] |
| A549 | 9.12 | ||||
| 6 | 26 | D. chrysotoxum | K562 | 45.64 | [17] |
| HL-60 | 1~10 | ||||
| A549 | 8.65 | ||||
| BEL-7402 | 1.79 | ||||
| SGC-7901 | 2.89 | ||||
| D. plicatile | HepG2 | 11.27~38.68 | [73] | ||
| 7 | 32 | D. brymerianum | H460 | 65.0 | [11] |
| D. nobile | A549 | 9.8 | [58] | ||
| HL-6 | 7.7 | ||||
| SK-OV-3 | 9.4 | ||||
| D. plicatile | HepG2 | 8.70 | [73] | ||
| MDA-MB231 | 8.04 | ||||
| 8 | 43 | D. officinale | HL-60 | 29.53 ± 0.22 | [123] |
| THP-1 | 26.53 ± 0.58 | ||||
| 9 | 47 | D. officinale | HL-60 | 11.96 ± 0.58 | [123] |
| THP-1 | 8.92 ± 0.67 | ||||
| 10 | 53 | D. plicatile | HepG2 | 11.27~38.68 | [73] |
| A549 | 11.27~38.68 | ||||
| D. thyrsiflorum | K-562 | 6.3 | [86] | ||
| 11 | 55 | D. plicatile | HepG2 | 9.35 | [73] |
| MDA-MB231 | 5.36 | ||||
| A549 | 5.79 | ||||
| 12 | 58 | D. plicatile | HepG2 | 8.40 | [73] |
| MDA-MB231 | 12.46~19.61 | ||||
| A549 | 11.68~43.88 | ||||
| 13 | 63 | D. officinale | HL-60 | 39.35 ± 1.58 | [123] |
| THP-1 | 36.34 ± 2.21 | ||||
| D. plicatile | HepG2 | 11.27~38.68 | [73] | ||
| MDA-MB231 | 12.46~19.61 | ||||
| A549 | 11.68~43.88 | ||||
| 14 | 64 | D. plicatile | HepG2 | 11.27~38.68 | [73] |
| 15 | 67 | D. plicatile | HepG2 | 8.84 | [73] |
| MDA-MB231 | 7.69 | ||||
| A549 | 8.92 | ||||
| 16 | 71 | D. nobile | SK-OV-3 | 3.5 | [58] |
| 17 | 88 | D. thyrsiflorum | Hela | 9.9 | [86] |
| K-562 | 6.5 | ||||
| MCF-7 | 3.0 | ||||
| 18 | 89 | D. thyrsiflorum | Hela | 9.3 | [86] |
| K-562 | 1.6 | ||||
| 19 | 108 | D.denneanum | SNU387 | 4.38 | [30] |
| 20 | 117 | D.denneanum | SNU387 | 8.40 | [30] |
| D. nobile | HepG2 | 4.81 ± 0.04 | [60] | ||
| 21 | 118 | D.denneanum | SNU387 | 11.21 | [30] |
| D. nobile | HepG2 | 19.47 ± 1.11 | [60] | ||
| 22 | 119 | D. officinale | HL-60 | 35.32 ± 1.76 | [123] |
| THP-1 | 20.78 ± 1.80 | ||||
| 23 | 120 | D. officinale | THP-1 | 45.32 ± 2.39 | [123] |
| 24 | 125 | D. gratiosissimum | HepG2 | 10.15 | [28] |
| BGC823 | 23.12 | ||||
| 25 | 128 | D. sinense | SGC-7901 | 12.8 ± 0.6 | [126] |
| D. williamsonii | HL-60 | 5.10 | [129] | ||
| 26 | 130 | D. officinale | HL-60 | 29.06 ± 1.13 | [123] |
| THP-1 | 13.53 ± 1.10 | ||||
| 27 | 132 | D. williamsonii | KB | 195.0 | [132] |
| MCF-7 | 187.7 | ||||
| 28 | 135 | D. gratiosissimum | HL-60 | 89.8 | [107] |
| 29 | 137 | D. findlayanum | SHSY5Y | 19.40 ± 1.95 | [105] |
| Hela | 26.71 ± 2.26 | ||||
| 30 | 139 | D. gratiosissimum | HL-60 | 45.6 | [107] |
| 31 | 140 | D. gratiosissimum | HL-60 | 25.6 | [107] |
| U87-MG | 24.24 | [28] | |||
| HepG2 | 35.19 | ||||
| 32 | 142 | D. findlayanum | A172 | 12.26 ±1.48 | [105] |
| 33 | 143 | D. gratiosissimum | HepG2 | 31.40 | [28] |
| 34 | 146 | D. williamsonii | HL-60 | 10.69 | [129] |
| 35 | 148 | D. brymerianum | H460 | 196.7 | [11] |
| D. capillipes | KB | 2.2 | [96] | ||
| NCI-H187 | 10.5 | ||||
| D. ellipsophyllum | H292 | 226.09 ± 5.67 | [37] | ||
| D. officinale | Hela | 16.8 ± 2.6 | [67] | ||
| D. plicatile | HepG2 | 11.27~38.68 | [73] | ||
| D. pulchellum | H23 | 33.41 ± 5.36 | [76] | ||
| D. thyrsiflorum | K-562 | 7.1 | [86] | ||
| 36 | 149 | D. capillipes | KB | 60.5 | [96] |
| NCI-H187 | 65.6 | ||||
| D. pulchellum | H23 | 198.38 ± 9.28 | [76] | ||
| 37 | 150 | D. brymerianum | H460 | 23.4 | [11] |
| D. capillipes | KB | 61.9 | [96] | ||
| NCI-H187 | 71.6 | ||||
| MCF-7 | 67.8 | ||||
| D. gratiosissimum | HL-60 | 10.6 | [107] | ||
| D. officinale | Hela | 92.4 ± 6.4 | [67] | ||
| THP-1 | 23.34 ± 0.83 | [123] | |||
| 38 | 151 | D. plicatile | HepG2 | 11.27~38.68 | [73] |
| MDA-MB231 | 12.46~19.61 | ||||
| A549 | 11.68~43.88 | ||||
| 39 | 157 | D. ellipsophyllum | H292 | 197.74 ± 0.78 | [37] |
| D. findlayanum | A172 | 13.14 ± 2.31 | [105] | ||
| SHSY5Y | 4.05 ± 0.76 | ||||
| Hela | 5.99 ± 1.15 | ||||
| D. gratiosissimum | HCT116 | 33.66 | [28] | ||
| HepG2 | 15.25 | ||||
| BGC823 | 22.07 | ||||
| PC9 | 45.36 | ||||
| 40 | 158 | D. findlayanum | SHSY5Y | 13.37 ± 1.12 | [105] |
| 41 | 159 | D. gratiosissimum | HepG2 | 47.44 | [28] |
| PC9 | 30.63 | ||||
| 42 | 161 | D. ellipsophyllum | H292 | 96.56 ± 0.22 | [37] |
| D. capillipes | KB | 48.3 | [96] | ||
| NCI-H187 | 63.8 | ||||
| MCF-7 | 62.6 | ||||
| 43 | 165 | D. sinense | SGC-7901 | 7.8 ± 0.05 | [126] |
| BEL-7402 | 11.7 ± 0.5 | ||||
| K562 | 15.7 ± 0.2 | ||||
| 44 | 171 | D. findlayanum | A172 | 3.77 ± 0.60 | [105] |
| SHSY5Y | 1.65 ± 0.16 | ||||
| Hela | 2.22 ± 0.25 | ||||
| 45 | 172 | D. capillipes | KB | 132.4 | [96] |
| NCI-H187 | 123.7 | ||||
| D. pulchellum | H23 | 252.14 ± 12.21 | [76] | ||
| 46 | 173 | D. capillipes | NCI-H187 | 87.8 | [96] |
| 47 | 176 | D. plicatile | HepG2 | 11.27~38.68 | [73] |
| MDA-MB231 | 12.46~19.61 | ||||
| A549 | 11.68~43.88 | ||||
| D. sinense | SGC-7901 | 16.7 ± 0.4 | [126] | ||
| 48 | 184 | D. thyrsiflorum | K-562 | 0.0014 | [86] |
| 49 | 185 | D. capillipes | KB | 14.4 | [96] |
| NCI-H187 | 13.7 | ||||
| D. pulchellum | H23 | 157.77 ± 11.21 | [76] | ||
| 50 | 207 | D. findlayanum | A172 | 34.69 ± 2.95 | [105] |
| SHSY5Y | 10.63 ± 1.05 | ||||
| Hela | 15.74 ± 1.87 | ||||
| 51 | 224 | D. officinale | Hela | 91.1 ± 11.2 | [67] |
| 52 | 231 | D. officinale | Hela | 41.5 ± 2.4 | [67] |
| 53 | 232 | D. sinense | BEL-7402 | 10.0 ± 0.4 | [126] |
| K562 | 10.3 ± 0.1 | ||||
| 54 | 238 | D. gratiosissimum | HL-60 | 2.1 | [107] |
| 55 | 239 | D. gratiosissimum | HL-60 | 6.4 | [107] |
| 56 | 259 | D. nobile | HepG2 | 1.25 ± 0.06 | [60] |
| 57 | 262 | D. nobile | HepG2 | 11.99 ± 1.02 | [60] |
| 58 | 267 | D. officinale | Hela | 20.2 ± 1.3 | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, D.; Lv, X.; Chen, J.; Peng, M.; Cai, J. Recent Research Progress on Natural Stilbenes in Dendrobium Species. Molecules 2022, 27, 7233. https://doi.org/10.3390/molecules27217233
Zhai D, Lv X, Chen J, Peng M, Cai J. Recent Research Progress on Natural Stilbenes in Dendrobium Species. Molecules. 2022; 27(21):7233. https://doi.org/10.3390/molecules27217233
Chicago/Turabian StyleZhai, Denghui, Xiaofa Lv, Jingmei Chen, Minwen Peng, and Jinyan Cai. 2022. "Recent Research Progress on Natural Stilbenes in Dendrobium Species" Molecules 27, no. 21: 7233. https://doi.org/10.3390/molecules27217233
APA StyleZhai, D., Lv, X., Chen, J., Peng, M., & Cai, J. (2022). Recent Research Progress on Natural Stilbenes in Dendrobium Species. Molecules, 27(21), 7233. https://doi.org/10.3390/molecules27217233





