Synthesis and Characterization of Thermally Stable Lignosulfonamides
Abstract
1. Introduction
2. Results and Discussion
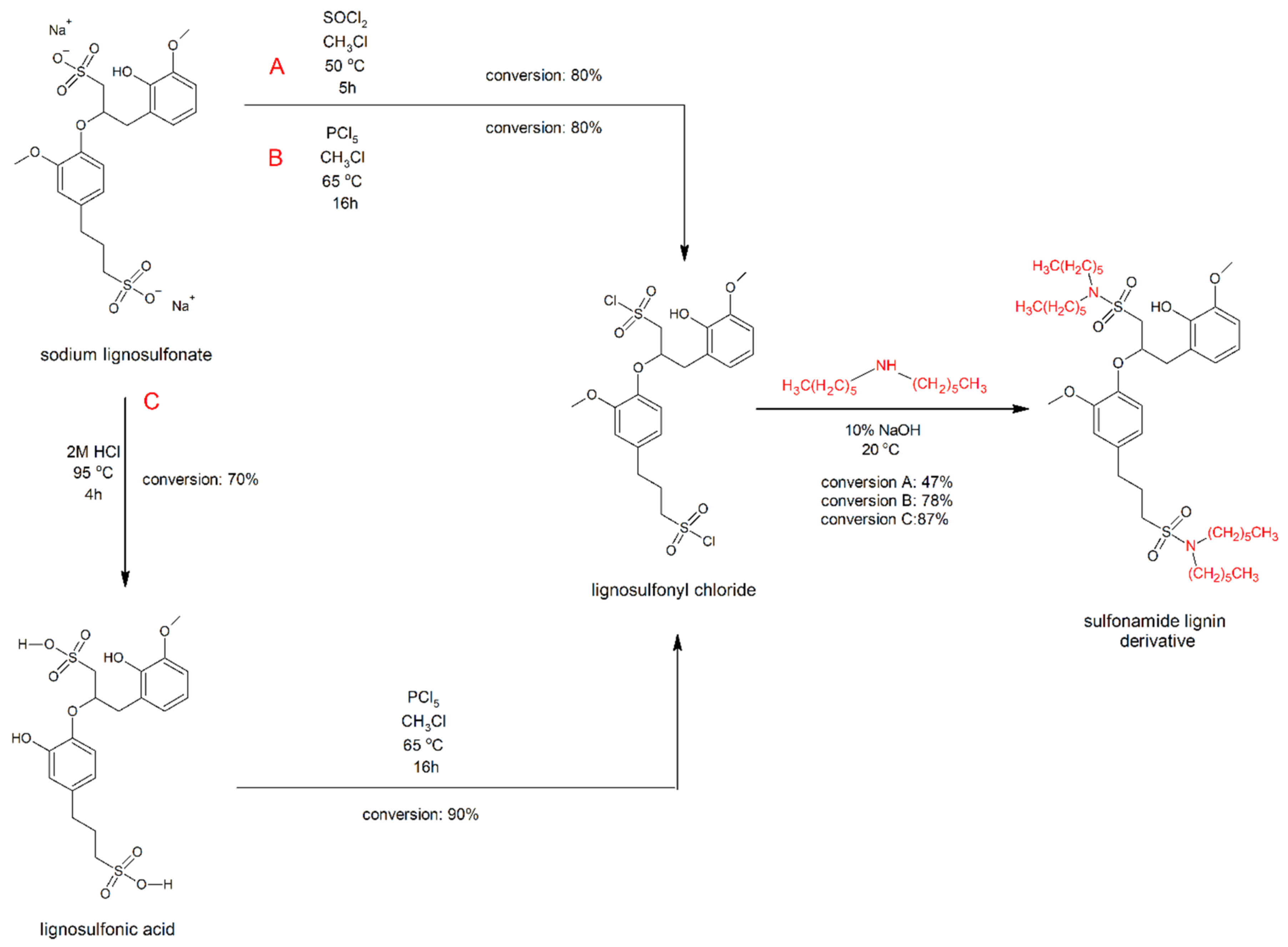
2.1. Routes of Lignosulfonate Chemical Modification
2.2. Fourier Transform Infrared (FTIR) Spectroscopy
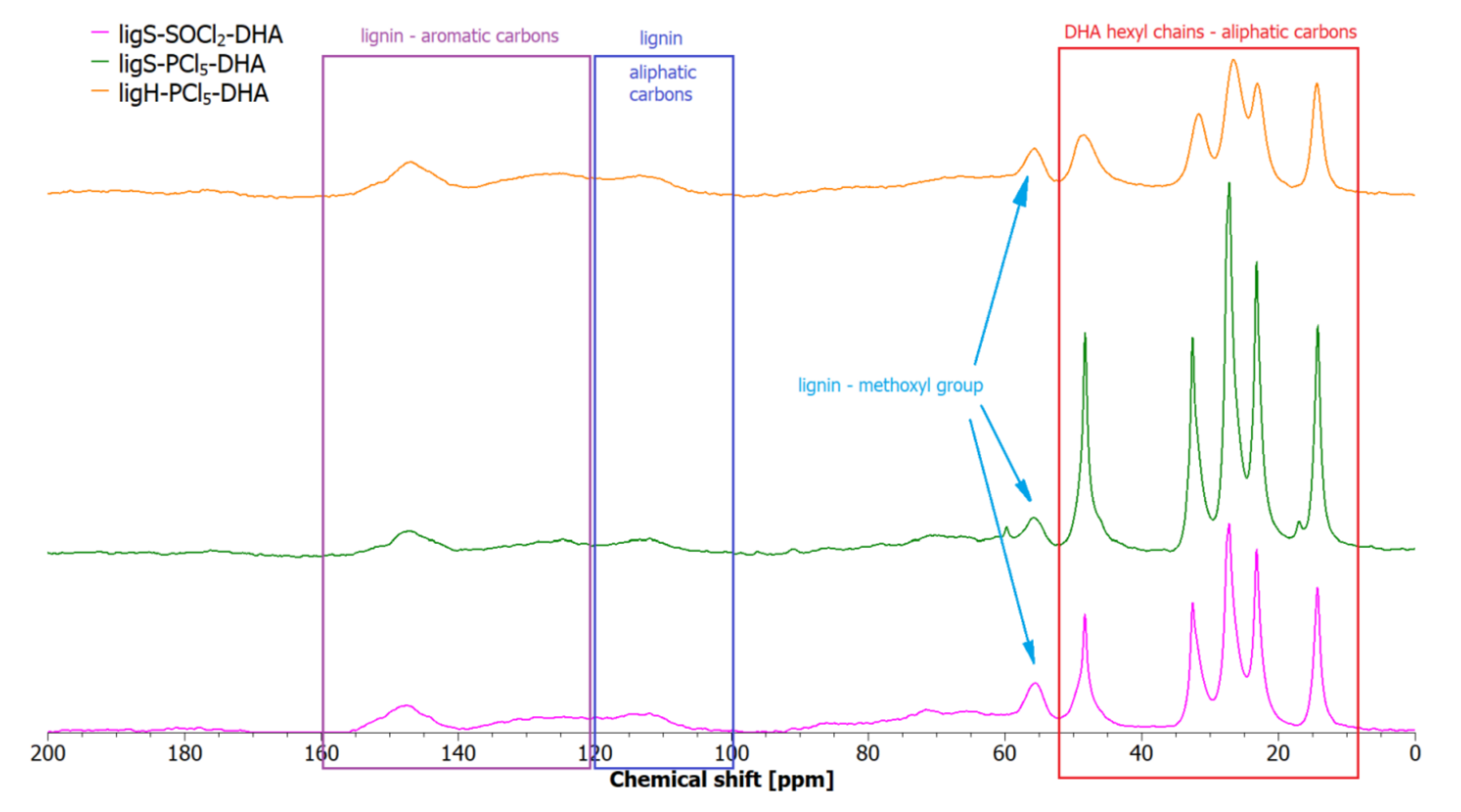
2.3. Solid-State Nuclear Magnetic Resonance (SS-NMR)
2.4. Wide-Angle X-ray Diffraction (WAXD)
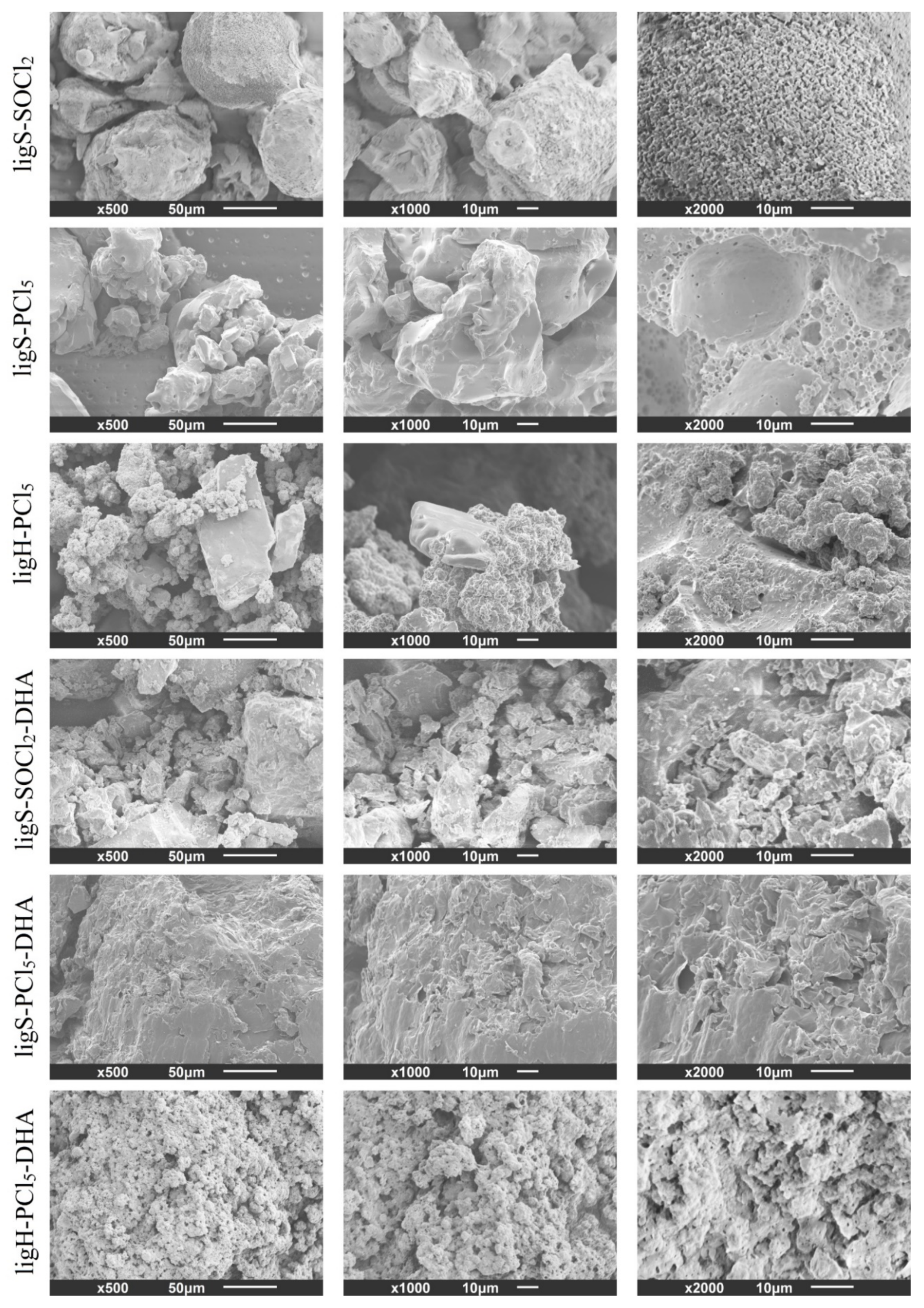
2.5. Scanning Electron Microscopy (SEM)
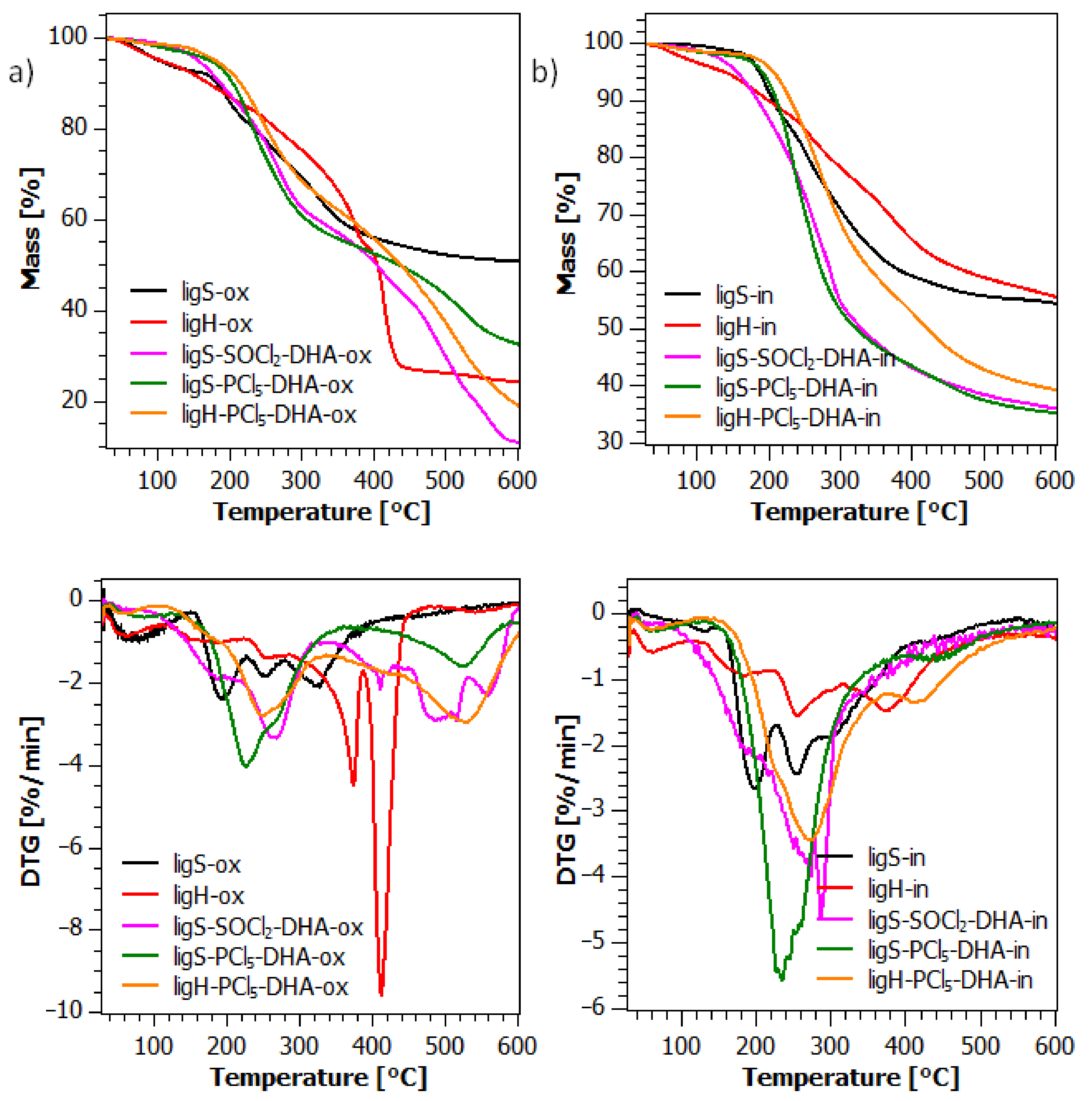
2.6. Thermogravimetric Analysis (TGA)
3. Materials and Methods
3.1. Materials
3.2. Chemical Modification of Sodium Lignosulfonate
3.2.1. Modification with Thionyl Chloride (Synthesis Route A)
3.2.2. Modification with Phosphorus Pentachloride (Synthesis Route B)
3.2.3. Modification with Phosphorus Pentachloride with Additional Acidolysis (Synthesis Route C)
3.2.4. Second Step of Modification
3.3. FTIR Spectroscopy
3.4. Solid-State Nuclear Magnetic Resonance
3.5. WAXD
3.6. SEM
3.7. TGA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerriero, G.; Hausman, J.F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Lignocellulosic biomass: Biosynthesis, degradation, and industrial utilization. Eng. Life Sci. 2016, 16, 1–16. [Google Scholar] [CrossRef]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the potential of lignin-containing cellulose nanofibrils (LCNFs): A review on properties and applications. Cellulose 2020, 27, 1853–1877. [Google Scholar] [CrossRef]
- Ajao, O.; Jeaidi, J.; Benali, M.; Restrepo, A.M.; El Mehdi, N.; Boumghar, Y. Quantification and variability analysis of lignin optical properties for colour-dependent industrial applications. Molecules 2018, 23, 377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Hao, N.; Shinde, S.; Pu, Y.; Kang, X.; Ragauskas, A.J.; Yuan, J.S. Defining lignin nanoparticle properties through tailored lignin reactivity by sequential organosolv fragmentation approach (SOFA). Green Chem. 2019, 21, 245–260. [Google Scholar] [CrossRef]
- Nge, T.T.; Tobimatsu, Y.; Yamamura, M.; Takahashi, S.; Takata, E.; Umezawa, T.; Yamada, T. Effect of heat treatment on the chemical structure and thermal properties of softwood-derived glycol lignin. Molecules 2020, 25, 1167. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef] [PubMed]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Alipoormazandarani, N.; Benselfelt, T.; Wang, L.; Wang, X.; Xu, C.; Wågberg, L.; Willför, S.; Fatehi, P. Functional Lignin Nanoparticles with Tunable Size and Surface Properties: Fabrication, Characterization, and Use in Layer-by-Layer Assembly. ACS Appl. Mater. Interfaces 2021, 13, 26308–26317. [Google Scholar] [CrossRef] [PubMed]
- Gurrala, L.; Kumar, M.M.; Sharma, S.; Paek, C.; Vinu, R. Selective production of C9 monomeric phenols via hydrogenolysis of lignin using Pd-(W/Zr/Mo oxides)-supported on biochar catalyst. Fuel 2022, 308, 121818. [Google Scholar] [CrossRef]
- Han, T.; Ding, S.; Yang, W.; Jönsson, P. Catalytic pyrolysis of lignin using low-cost materials with different acidities and textural properties as catalysts. Chem. Eng. J. 2019, 373, 846–856. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Nord, F.F.; Schubert, W.J. Lignin. Sci. Am. 1958, 199, 104–113. [Google Scholar] [CrossRef]
- Rossberg, C.; Janzon, R.; Saake, B.; Leschinsky, M. Effect of process parameters in pilot scale operation on properties of Organosolv lignin. BioResources 2019, 14, 4543–4559. [Google Scholar]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Yan, D.; Han, Y.; Ma, Z.; Wang, Q.; Wang, X.; Li, Y.; Sun, G. Magnesium lignosulfonate-derived N, S co-doped 3D flower-like hierarchically porous carbon as an advanced metal-free electrocatalyst towards oxygen reduction reaction. Int. J. Biol. Macromol. 2022, 209, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Karimov, O.K.; Teptereva, G.A.; Chetvertneva, I.A.; Movsumzade, E.M.; Karimov, E.K. The structure of lignosulfonates for production of carbon catalyst support. IOP Conf. Ser. Earth Environ. Sci. 2021, 839, 022086. [Google Scholar] [CrossRef]
- Fattahanisa, A.; Setiati, R.; Ristawati, A.; Siregar, S.; Marhaendrajana, T.; Wahyuningrum, D. The effect of middle phase emulsion and interfacial tension of Sodium Lignosulfonate surfactant synthesized from bagasse to Enhanced Oil Recovery. IOP Conf. Ser. Earth Environ. Sci. 2021, 1089, 062021. [Google Scholar] [CrossRef]
- Hu, B.; Lin, L.; Fang, Y.; Zhou, M.; Zhou, X. Application of Chitosan-Lignosulfonate Composite Coating Film in Grape Preservation and Study on the Difference in Metabolites in Fruit Wine. Coatings 2022, 12, 494. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, Y.; Lu, M.; Guo, X.; Yang, D.; Lou, H.; Qiu, X.; Guo, C.F. Direct Construction of Catechol Lignin for Engineering Long-Activity Conductive, Adhesive and UV-Blocking Hydrogel Bioelectronics. Small Methods 2021, 5, 2001311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, M.; Yuan, Q.; Cheng, G. Controlled Preparation of Corncob Lignin Nanoparticles and their Size-Dependent Antioxidant Properties: Toward High Value Utilization of Lignin. ACS Sustain. Chem. Eng. 2019, 7, 17166–17174. [Google Scholar] [CrossRef]
- Hambardzumyan, A.; Foulon, L.; Bercu, N.B.; Pernes, M.; Maigret, J.E.; Molinari, M.; Chabbert, B.; Aguié-Béghin, V. Organosolv lignin as natural grafting additive to improve the water resistance of films using cellulose nanocrystals. Chem. Eng. J. 2015, 264, 780–788. [Google Scholar] [CrossRef]
- Setälä, H.; Alakomi, H.L.; Paananen, A.; Szilvay, G.R.; Kellock, M.; Lievonen, M.; Liljeström, V.; Hult, E.L.; Lintinen, K.; Österberg, M.; et al. Lignin nanoparticles modified with tall oil fatty acid for cellulose functionalization. Cellulose 2020, 27, 273–284. [Google Scholar] [CrossRef]
- Chen, K.; Lei, L.; Lou, H.; Niu, J.; Yang, D.; Qiu, X.; Qian, Y. High internal phase emulsions stabilized with carboxymethylated lignin for encapsulation and protection of environmental sensitive natural extract. Int. J. Biol. 2020, 158, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Triviño, E.; Valencia, C.; Franco, J.M. Thickening Castor Oil with a Lignin-Enriched Fraction from Sugarcane Bagasse Waste via Epoxidation: A Rheological and Hydrodynamic Approach. ACS Sustain. Chem. Eng. 2021, 9, 10503–10512. [Google Scholar] [CrossRef]
- Gouveia, J.R.; Garcia, G.E.S.; Antonino, L.D.; Tavares, L.B.; Dos Santos, D.J. Epoxidation of kraft lignin as a tool for improving the mechanical properties of epoxy adhesive. Molecules 2020, 25, 2513. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ding, H.; Qi, G.; Guo, J.; Xu, F.; Li, C.; Puglia, D.; Kenny, J.; Ma, P. Enhancing the Radical Scavenging Activity and UV Resistance of Lignin Nanoparticles via Surface Mannich Amination toward a Biobased Antioxidant. Biomacromolecules 2021, 22, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.W.; Dietz, C.; Trosien, S.; Mehlhase, S.; Bitsch, M.J.; Nau, M.; Meckel, T.; Geissler, A.; Siegert, G.; Huong, J.; et al. Co-curing of epoxy resins with aminated lignins: Insights into the role of lignin homo crosslinking during lignin amination on the elastic properties. Holzforschung 2021, 75, 390–398. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Wang, B.; Wu, Y.; Ma, M.; Zhang, X. Synthesis of Magnetic Lignin-Based Hollow Microspheres: A Highly Adsorptive and Reusable Adsorbent Derived from Renewable Resources. ACS Sustain. Chem. Eng. 2016, 4, 5523–5532. [Google Scholar] [CrossRef]
- Dai, K.; Zhao, G.; Wang, Z.; Peng, X.; Wu, J.; Yang, P.; Li, M.; Tang, C.; Zhuang, W.; Ying, H. Novel Mesoporous Lignin-Calcium for Efficiently Scavenging Cationic Dyes from Dyestuff Effluent. ACS Omega 2021, 6, 816–826. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Si, C.; Bae, J.H.; Jeong, H.; Kim, Y.S. One-step silanization and amination of lignin and its adsorption of Congo red and Cu(II) ions in aqueous solution. Int. J. Biol. Macromol. 2020, 159, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chang, Q.; Yang, H. Selective absorption of ofloxacin and ciprofloxacin from a binary system using lignin-based absorbents: Quantitative analysis, adsorption mechanisms, and structure-activity relationships. Sci. Total 2021, 765, 144427. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Bartczak, P.; Wysokowski, M.; Jankowska, M.; Kabat, K.; Jesionowski, T. Silica conjugated with kraft lignin and its use as a novel ‘green’ sorbent for hazardous metal ions removal. Chem. Eng. J. 2015, 260, 684–693. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Carbohydrate biopolymers, lignin based adsorbents for removal of heavy metals (Cd2+, Pb2+, Zn2+) from wastewater, regeneration and reuse for spent adsorbents including latent fingerprint detection: A review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef]
- Naseer, A.; Jamshaid, A.; Hamid, A.; Muhammad, N.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Khuram, S.; Shah, N.S. Lignin and lignin based materials for the removal of heavy metals from waste water—An overview. Z. Phys. Chem. 2018, 233, 325–345. [Google Scholar] [CrossRef]
- Gioia, C.; Colonna, M.; Tagami, A.; Medina, L.; Sevastyanova, O.; Berglund, L.A.; Lawoko, M. Lignin-Based Epoxy Resins: Unravelling the Relationship between Structure and Material Properties. Biomacromolecules 2020, 21, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Wu, X.; Wang, D.; Zhou, S.; Han, S.; Wang, H.; Sun, F. Simultaneously reinforcing and toughening of shape-memory epoxy resin with carboxylated lignosulfonate: Facile preparation and effect mechanism. Int. J. Biol. Macromol. 2022, 217, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Fang, X.; Liao, J.; Zhou, X.; Zhou, S.; Bai, F.; Peng, S. High solid content production of environmentally benign ultra-thin lignin-based polyurethane films: Plasticization and degradation. Polymer 2019, 178, 1215872. [Google Scholar] [CrossRef]
- Yuan, Z.; Shang, X.; Fang, J.; Li, H. A simple method for preparation of lignin/TiO2 nanocomposites by sulfonation degree regulation and their application in polyurethane films. Int. J. Biol. Macromol. 2022, 198, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Md Tahir, P.; Halis, R. Lignin-based copolymer adhesives for composite wood panels—A review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Li, R.J.; Gutierrez, J.; Chung, Y.L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Arafat, S.; Kumar, N.; Wasiuddin, N.M.; Owhe, E.O.; Lynam, J.G. Sustainable lignin to enhance asphalt binder oxidative aging properties and mix properties. J. Clean. Prod. 2019, 217, 456–468. [Google Scholar] [CrossRef]
- Borrero-Lopéz, A.M.; Valencia, C.; Ibarra, D.; Ballesteros, I.; Franco, J.M. Lignin-enriched residues from bioethanol production: Chemical characterization, isocyanate functionalization and oil structuring properties. Int. J. Biol. Macromol. 2022, 195, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Alwadani, N.; Fatehi, P. Synthetic and lignin-based surfactants: Challenges and opportunities. Carbon Resour. Convers. 2018, 1, 126–138. [Google Scholar] [CrossRef]
- Qu, W.; Yang, J.; Sun, X.; Bai, X.; Jin, H.; Zhang, M. Towards producing high-quality lignin-based carbon fibers: A review of crucial factors affecting lignin properties and conversion techniques. Int. J. Biol. Macromol. 2021, 189, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Trogen, M.; Le, N.D.; Sawada, D.; Guizani, C.; Lourençon, T.V.; Pitkänen, L.; Sixta, H.; Shah, R.; O’Neill, H.; Balakshin, M.; et al. Cellulose-lignin composite fibres as precursors for carbon fibres. Part 1—Manufacturing and properties of precursor fibres. Carbohydr. Polym. 2021, 252, 117133. [Google Scholar] [CrossRef]
- Wu, Y.; Qian, Y.; Zhang, A.; Lou, H.; Yang, D.; Qiu, X. Light color dihydroxybenzophenone grafted lignin with high UVS/UVB absorbance ratio for efficient and safe natural sunscreen. Ind. Eng. Chem. Res. 2020, 59, 17037–17068. [Google Scholar] [CrossRef]
- Gujjala, L.K.S.; Kim, J.; Won, W. Technical lignin to hydrogels: An Eclectic review on suitability, synthesis, applications, challenges and future prospects. J. Clean. Prod. 2022, 363, 132585. [Google Scholar] [CrossRef]
- Wang, S.H.; Chang, F.C. Cu(II) and Au(III) recovery with electrospun lignosulfonate CO2-activated carbon fiber. Int. J. Biol. Macromol. 2022, 203, 505–514. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, B.; Lv, J.; He, Y.; Zhang, H.; Li, W.; Li, Y.; Wågberg, T.; Hu, G. Facile synthesis of sodium lignosulfonate/polyethyleneimine/sodium alginate beads with ultra-high adsorption capacity for Cr(VI) removal from water. J. Hazard. Mater 2022, 436, 129270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, S.; Xi, C.; Zhang, F. Modification of the crosslinked hyperbranched polyamide-amines by thiourea and its selective adsorption for Cu (II). Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Xia, L.; Feng, H.; Zhang, Q.; Luo, X.; Fei, P.; Li, F. Centrifugal Spinning of Lignin Amine/Cellulose Acetate Nanofiber for Heavy Metal Ion Adsorption. Fibers Polym. 2022, 23, 77–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wu, C. Dual-Modified Lignin-Assembled Multilayer Microsphere with Excellent Pb2+ Capture. Polymers 2022, 14, 2824. [Google Scholar] [CrossRef]
- Heo, J.W.; An, L.; Chen, J.; Bae, J.H.; Kim, Y.S. Preparation of amine-functionalized lignins for the selective adsorption of Methylene blue and Congo red. Chemosphere 2022, 295, 133815. [Google Scholar] [CrossRef]
- Borsalani, H.; Nikzad, M.; Ghoreyshi, A.A. Extraction of Lignosulfonate from Black Liquor into Construction of a Magnetic Lignosulfonate-Based Adsorbent and Its Adsorption Properties for Dyes from Aqueous Solutions. J. Polym. Environ. 2022, 30, 4068–4085. [Google Scholar] [CrossRef]
- Pakzad, K.; Orooji, Y.; Nasrollahzadeh, M.; Tajbakhsh, M. Copper complex stabilized on magnetic lignosulfonate: A magnetically recyclable catalyst for removal of wastewater contaminants. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Ren, R.; Zhong, Y.; Fan, Y. A high-performance electrode based on reduced graphene oxide/lignosulfonate/carbon microspheres film for flexible supercapacitors. BioResources 2022, 17, 1729–1744. [Google Scholar] [CrossRef]
- Mondal, A.K.; Xu, D.; Wu, S.; Zou, Q.; Huang, F.; Ni, Y. Design of Fe3+-Rich, High-Conductivity Lignin Hydrogels for Supercapacitor and Sensor Applications. Biomacromolecules 2022, 23, 766–778. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, X.; Huang, X.; Zhang, Y.; Shi, M.; Zhao, Y. High-efficient lignin-based polymerizable macromolecular photoinitiator with UV-blocking property for visible light polymerization. Int. J. Biol. Macromol. 2022, 204, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, C.; Li, M.; Ding, K.; Huang, X.; Liang, X.; Lei, Y.; Jiang, Q.; Wang, Y. Sodium lignosulfonate cross-linked bioprosthetic heart valve materials for enhanced cytocompatibility, improved hemocompatibility, and reduced calcification. Compos. Part B Eng. 2022, 234, 109669. [Google Scholar] [CrossRef]
- Sgarzi, M.; Gigli, M.; Giuriato, C.; Crestini, C. Simple Strategies to Modulate the pH-Responsiveness of Lignosulfonate-Based Delivery Systems. Materials 2022, 15, 1857. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, N.S.; Galiwango, E.; Al-Marzougi, A.H.; Mahmoud, E. Sodium lignosulfonate: A renewable corrosion inhibitor extracted from lignocellulosic waste. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, Y.F.; Li, Y.; Zhong, R. Effect of sodium lignosulfonate/nano calcium carbonate composite filler on properties of isotactic polypropylene. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Yan, W.-J.; Xu, S.; Tian, X.-Y.; Min, J.-J.; Liu, S.-C.; Ding, C.-J.; Wang, N.-L.; Hu, Y.; Fan, Q.-X.; Li, J.-S.; et al. Novel bio-based lignosulfonate and Ni(OH)2 nanosheets dual modified layered double hydroxide as an eco-friendly flame retardant for polypropylene. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130195. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, M.; Yang, D. Preparation of uniform lignosulfonate-based colloidal spheres for UV-absorbing thermoplastics. Int. J. Biol. Macromol. 2022, 219, 663–671. [Google Scholar] [CrossRef]
- Song, S.; Xu, Z.; Zhen, X.; Wang, Z.; Ge, T. Preparation of Lignosulfonate-Based Phenol Formaldehyde Foam with Excellent Thermal Performance. Macromol. Chem. Phys. 2022, 223, 2200159. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, L.; Qin, W.; Loy, D.A.; Wu, Z.; Chen, H.; Zhang, Q. Preparation, characterization and antioxidant properties of curcumin encapsulated chitosan/lignosulfonate micelles. Carbohydr. Polym. 2022, 281, 119080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wu, X.; Ouyang, X.; Lou, H.; Yang, D.; Qian, Y.; Qiu, X. Preparation of Light-Colored Lignosulfonate Sunscreen Microcapsules with Strengthened UV-Blocking and Adhesion Performance. ACS Sustain. Chem. Eng. 2022, 10, 9381–9388. [Google Scholar] [CrossRef]
- Komisarz, K.; Majka, T.M.; Pielichowski, K. Chemical Transformation of Lignosulfonates to Lignosulfonamides with Improved Thermal Characteristics. Fibers 2022, 10, 20. [Google Scholar] [CrossRef]
- Kim, C.S.Y. Lignosulfonamide and a Process for Its Preparation. U.S. Patent 3,438,960, 15 April 1969. [Google Scholar]
- DeBons, F.E.; Whittington, L.E.; Pedersen, L.D. Method of Enhanced Oil Recovery and Compositions Useful Therein. U.S. Patent 4,548,721, 22 October 1985. [Google Scholar]
- Kim, C.S.Y. Nitrogen and Lignin Containing Products and Process for Obtaining Them. U.S. Patent 3,538,071, 3 November 1970. [Google Scholar]
- Schilling, P. Sulfomethylated Lignin Amines. U.S. Patent 4,786,720, 22 November 1988. [Google Scholar]
- Schilling, P. Sulfomethylated Lignin Amines. U.S. Patent 4,859,362, 22 August 1989. [Google Scholar]
- Zhou, H.; Shi, X.; Wu, W.; An, X.; Tian, Y.; Qiao, Y. Facile preparation of lignosulfonate/N-methylaniline composite and its application in efficient removal of Cr(VI) from aqueous solutions. Int. J. Biol. Macromol. 2020, 154, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, H.; Yao, X.; Yu, P.; Zhou, L.; Jia, D. The aggregation structure regulation of lignin by chemical modification and its effect on the property of lignin/styrene–butadiene rubber composites. J. Appl. Polym. Sci. 2018, 135, 45759. [Google Scholar] [CrossRef]
- Gallus-Olender, J.; Franc, B. The Identification of Oxidation and Decomposition Products of Phosphorus Trichloride by Infrared Spectroscopy. Spectrosc. Lett. 1975, 8, 551–560. [Google Scholar] [CrossRef]
- Gil, A.M.; Neto, C.P. Solid-State Nmr Studies of Wood and Other Lignocellulosic Materials. In Annual Reports on NMR Spectroscopy, 1st ed.; Webb, G.A., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 37, pp. 75–117. [Google Scholar]
- Brudin, S.; Schoenmakers, P. Analytical methodology for sulfonated lignins. J. Sep. Sci. 2010, 33, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Lutnaes, B.F.; Myrvold, B.O.; Lauten, R.A.; Endeshaw, M.M. 1H and 13C NMR data of benzylsulfonic acids—Model compounds for lignosulfonate. Magn. Reson. Chem. 2008, 46, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Addala, S.; Bouhdjer, L.; Chala, A.; Bouhdjar, A.; Halimi, O.; Boudine, B.; Sebais, M. Structural and optical properties of a NaCl single crystal doped with CuO nanocrystals. Chin. Phys. B 2013, 22, 098103. [Google Scholar] [CrossRef]
- Stein, S.E. On the high temperature chemical equilibria of polycyclic aromatic hydrocarbons. J. Phys. Chem. 1978, 82, 566–571. [Google Scholar] [CrossRef]
- Korshak, V.V.; Khomoutov, V.A.; Doroshenko, Y.Y. A study of thermal stability in a number of aromatic and nitrogen containing polycyclic compounds. Polym. Sci. USSR 1976, 18, 597–603. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Kuhnt, T.; Foster, E.J.; Weder, C. Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 2013, 14, 1223–1230. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef] [PubMed]




| Material | Description |
|---|---|
| ligS | Pure sodium lignosulfonate |
| ligH | Lignosulfonic acid, obtained via reaction of ligS with hydrochloric acid (HCl) |
| ligS-SOCl2 | Lignosulfonyl chloride, a product of the reaction of ligS with SOCl2 |
| ligS-PCl5 | Lignosulfonyl chloride, a product of the reaction of ligS with PCl5 |
| ligH-PCl5 | Lignosulfonyl chloride, a product of the reaction of ligH with PCl5 |
| ligS-SOCl2-DHA | Main products, sulfonamide derivatives of lignin, formed in the reaction of the corresponding lignosulfonyl chloride with dihexylamine |
| ligS-PCl5-DHA | |
| ligH-PCl5-DHA |
| Title 1 | Degradation in Synthetic Air | Degradation in Nitrogen | ||||||
|---|---|---|---|---|---|---|---|---|
| T5% [°C] | T20% [°C] | Tmax [°C] | Char at 600 °C [%] | T5% [°C] | T20% [°C] | Tmax [°C] | Char at 600 °C [%] | |
| ligS | 102 | 234 | 327 | 50.9 | 187 | 255 | 199 | 54.5 |
| ligH | 104 | 264 | 412 | 24.4 | 139 | 285 | 374 | 55.7 |
| ligS-SOCl2 | 131 | 268 | 481 | 22.7 | 167 | 273 | 285 | 50.8 |
| ligS-PCl5 | 110 | 205 | 183 | 41.3 | 115 | 204 | 190 | 51.9 |
| ligH-PCl5 | 91 | 220 | 156 | 59.2 | 121 | 230 | 188 | 62.5 |
| ligS-SOCl2-DHA | 155 | 237 | 268 | 11.0 | 156 | 229 | 287 | 36.0 |
| ligS-PCl5-DHA | 172 | 232 | 226 | 32.6 | 191 | 232 | 235 | 35.4 |
| ligH-PCl5-DHA | 179 | 250 | 249 | 19.1 | 207 | 265 | 274 | 39.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komisarz, K.; Majka, T.M.; Kurczab, M.; Pielichowski, K. Synthesis and Characterization of Thermally Stable Lignosulfonamides. Molecules 2022, 27, 7231. https://doi.org/10.3390/molecules27217231
Komisarz K, Majka TM, Kurczab M, Pielichowski K. Synthesis and Characterization of Thermally Stable Lignosulfonamides. Molecules. 2022; 27(21):7231. https://doi.org/10.3390/molecules27217231
Chicago/Turabian StyleKomisarz, Karolina, Tomasz M. Majka, Monika Kurczab, and Krzysztof Pielichowski. 2022. "Synthesis and Characterization of Thermally Stable Lignosulfonamides" Molecules 27, no. 21: 7231. https://doi.org/10.3390/molecules27217231
APA StyleKomisarz, K., Majka, T. M., Kurczab, M., & Pielichowski, K. (2022). Synthesis and Characterization of Thermally Stable Lignosulfonamides. Molecules, 27(21), 7231. https://doi.org/10.3390/molecules27217231








