Variations in the Chemical Composition of Essential Oils in Native Populations of Korean Thyme, Thymus quinquecostatus Celak.
Abstract
1. Introduction
2. Results
2.1. The Yield and Color of T. quinquecostatus Essential Oils
2.2. Essential Oil Compositions of T. quinquecostatus Populations
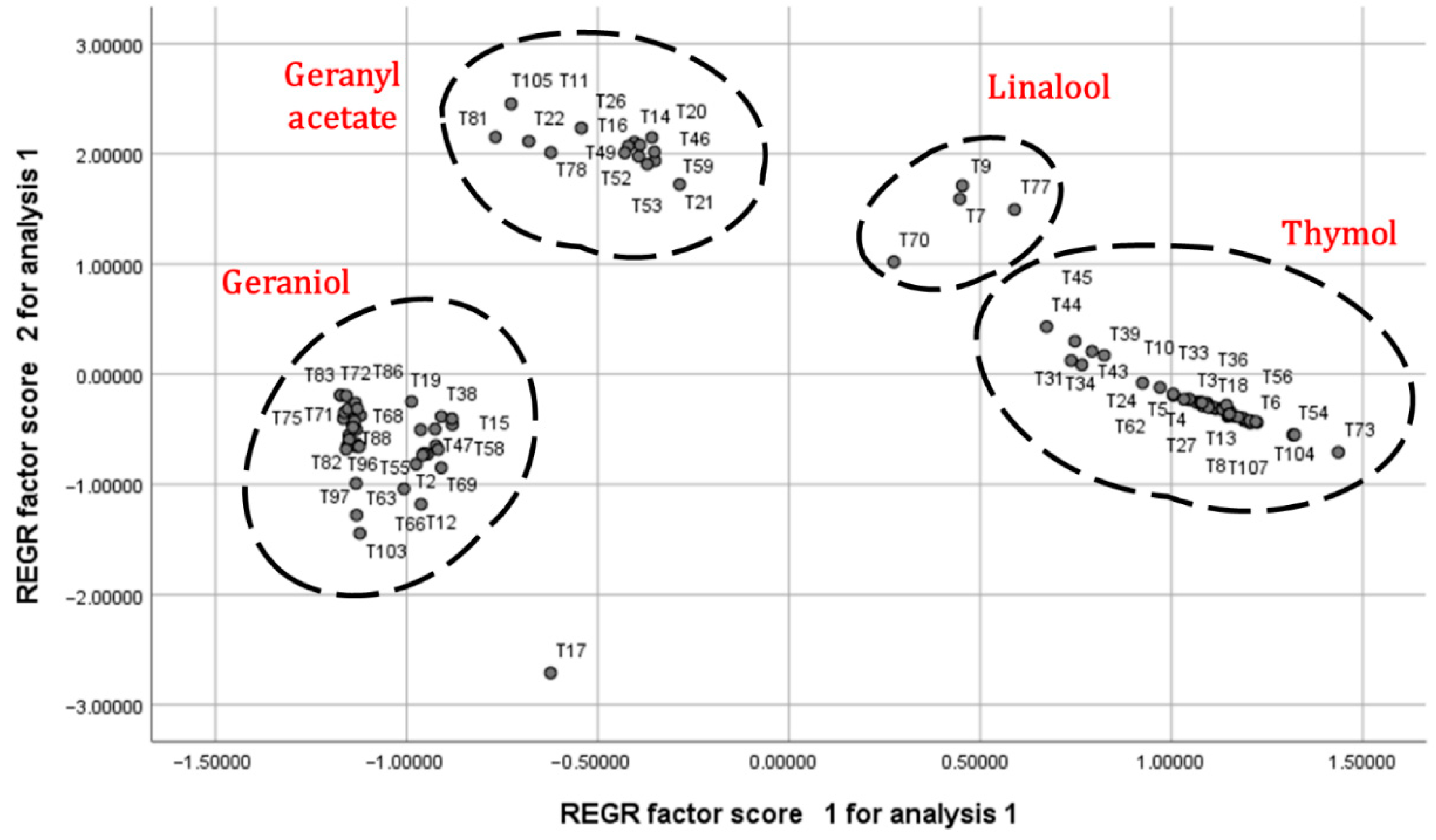
2.3. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Collection and Cultivation of T. quinquecostatus Populations
4.2. Essential Oil Extraction
4.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Kim, S. The Chemical Composition and Biological Activities of Essential Oil from Korean Native Thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak.). Molecules 2022, 27, 4251. [Google Scholar] [CrossRef] [PubMed]
- El Yaagoubi, M.; Mechqoq, H.; El Hamdaoui, A.; Jrv Mukku, V.; El Mousadik, A.; Msanda, F.; El Aouad, N. A review on Moroccan Thymus species: Traditional uses, essential oils chemical composition and biological effects. J. Ethnopharmacol. 2021, 278, 114205. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Moon, J.C.; Kim, S.; Sowndhararajan, K. Morphological, chemical, and genetic characteristics of Korean native thyme Bak-ri-hyang (Thymus quinquecostatus celak.). Antibiotics 2020, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential oil composition of five Thymus species from Bulgaria. Chem. Biodivers. 2021, 18, e2100498. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, S.J.; Hwang, J.W.; Kim, E.K.; Kim, S.E.; Kim, E.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. In vitro protective effects of Thymus quinquecostatus Celak. extracts on t-BHP-induced cell damage through antioxidant activity. Food Chem. Toxicol. 2012, 50, 4191–4198. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Shen, M.; Ren, X.-Y.; He, T.; Wang, L.; Fan, S.-S.; Wang, X.-H.; Li, X.; Wang, X.-P.; Chen, X.-Y.; et al. Multi-Response Extraction Optimization Based on Anti-Oxidative Activity and Quality Evaluation by Main Indicator Ingredients Coupled with Chemometric Analysis on Thymus quinquecostatus Celak. Molecules 2018, 23, 957. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.H. Antifungal activities of essential oils from Thymus quinquecostatus and T. magnus. Planta Med. 2004, 70, 1090–1092. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.-C.; Kim, J.-S. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crop. Prod. 2014, 52, 611–616. [Google Scholar] [CrossRef]
- Jung, H.; Jeong, H.J.; Shin, K.; Kim, Y.S.; Moon, J.H.; Lee, T.H. Protective effect of Thymus quinquecostatus extracts UVB-induced matrix metalloproteinase-1 via suppressing MAPKs phosphorylation in human keratinocyte. J. Appl. Biol. Chem. 2018, 61, 417–421. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.; Ren, X.Y.; Liu, X.Y.; Ma, J.M.; Song, R.L.; Wang, X.H.; Dong, Y.; Yu, A.X.; Fan, Q.Q.; et al. Gut dysbiosis correction contributes to the hepatoprotective effects of Thymus quinquecostatus Celak extract against alcohol through the gut-liver axis. Food Funct. 2021, 12, 10281–10290. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.X.; Zhang, Y.H.; Cheng, S.; Ma, Q.W.; Guo, S.L.; Zhang, J.B. Anti-tumor effect of ethanol extracts from Thymus quinquecostatus Celak on human leukemia cell line. Chin. J. Integr. Med. 2005, 3, 382–385. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, H.; Jeon, W.J.; Baek, S.; Ha, I.H. Antioxidative Effects of Thymus quinquecostatus CELAK through Mitochondrial Biogenesis Improvement in RAW 264.7 Macrophages. Antioxidants 2020, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Liu, X.; Wang, Y.; Ren, X.; Liu, Y.; Dong, Y.; Fan, Q.; Wei, J.; Ma, J.; Yu, A.; et al. Thymus quinquecostatus Celak. ameliorates cerebral ischemia-reperfusion injury via dual antioxidant actions: Activating Keap1/Nrf2/HO-1 signaling pathway and directly scavenging ROS. Phytomedicine 2021, 91, 153673. [Google Scholar] [CrossRef]
- Cebi, N.; Arici, M.; Sagdic, O. The famous Turkish rose essential oil: Characterization and authenticity monitoring by FTIR, Raman and GC–MS techniques combined with chemometrics. Food Chem. 2021, 354, 129495. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Martínez, J.R.; Ruíz, C.A.; Arias, G.; Durán, C.; Salgar, W.; Cala, M. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J. Sep. Sci. 2010, 33, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Torras, J.; Grau, M.D.; López, J.F.; de las Heras, F.X.C. Analysis of essential oils from chemotypes of Thymus vulgaris in Catalonia. J. Sci. Food Agric. 2007, 87, 2327–2333. [Google Scholar] [CrossRef]
- Chen, G.; Tang, Y.; Qu, C.T.; Zhang, Q.Z.; Mu, S.Z. Study on the chemical components of essential oil of Thymus quinquecostatus Celak. from Shandong Yimeng. Jingxi Huagong Zhongjianti 2009, 39, 70–72. [Google Scholar]
- Oh, T.H.; Kim, S.S.; Yoon, W.J.; Kim, J.Y.; Yang, E.J.; Lee, N.H.; Hyun, C.G. Chemical composition and biological activities of Jeju Thymus quinquecostatus essential oils against Propionibacterium species inducing acne. J. Gen. Appl. Microbiol. 2009, 55, 63–68. [Google Scholar]
- He, T.; Li, X.; Wang, X.; Xu, X.; Yan, X.; Li, X.; Sun, S.; Dong, Y.; Ren, X.; Liu, X.; et al. Chemical composition and anti-oxidant potential on essential oils of Thymus quinquecostatus Celak. from Loess Plateau in China, regulating Nrf2/Keap1 signaling pathway in zebrafish. Sci. Rep. 2020, 10, 11280. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.; Choi, Y. Essential oils of Thymus quinquecostatus celakov. and Thymus magnus Nakai. Korean J. Med. Crop Sci. 1994, 2, 234–240. [Google Scholar]
- Jia, P.; Liu, H.; Gao, T.; Xin, H. Glandular trichomes and essential oil of Thymus quinquecostatus. Sci. World J. 2013, 2013, 387952. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, D.; Li, N.; Hou, X.; Wang, D.; Li, D.; Liu, J. Influence of environmental factors on the active substance production and antioxidant activity in Potentilla fruticosa L. and its quality assessment. Sci. Rep. 2016, 6, 28591. [Google Scholar] [CrossRef]
- Judzentiene, A.; Budiene, J. Chemical polymorphism of essential oils of Artemisia vulgaris growing wild in Lithuania. Chem. Biodivers. 2018, 15, e1700257. [Google Scholar] [CrossRef]
- Zielińska, S.; Dąbrowska, M.; Kozłowska, W.; Kalemba, D.; Abel, R.; Dryś, A.; Szumny, A.; Matkowski, A. Ontogenetic and trans-generational variation of essential oil composition in Agastache rugosa. Ind. Crop. Prod. 2017, 97, 612–619. [Google Scholar] [CrossRef]
- Chiang, M.H.; Lee, K.W.; Baik, J. Volatile aroma compounds of several domestic Thymus quinquecostatus by thermal desorption gas chromatograph mass spectrometer. J. Bio-Environ. Con. 2011, 20, 14–20. [Google Scholar]
- Usami, A.; Ishikawa, M.; Hori, K. Heterologous expression of geraniol dehydrogenase for identifying the metabolic pathways involved in the biotransformation of citral by Acinetobacter sp. Tol 5. Biosci. Biotechnol. Biochem. 2018, 82, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.P.; Peng, F.; He, S.L.; Zheng, Y.H.; Jiang, Y.M.; Li, N.W.; Zhang, M.X.; Xia, B. Patch diversity and spatial structure of wild Thymus quinquecostatus. Ying Yong Sheng Tai Xue Bao 2009, 20, 20–26. [Google Scholar]
- Rustaiee, A.R.; Yavari, A.; Nazeri, V.; Shokrpour, M.; Sefidkon, F.; Rasouli, M. Genetic diversity and chemical polymorphism of some Thymus species. Chem. Biodivers. 2013, 10, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.; El-Shazly, H.H.; Sakr, M.; Farid, M.M.; Hamouda, M.; Elkhateeb, E.; Ahmad, H.S. Genetic diversity and volatile oil components variation in Achillea fragrantissima wild accessions and their regenerated genotypes. J. Genet. Eng. Biotechnol. 2021, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Mishra, R.; Singh, A.K.; Srivastava, A.; Lal, R.K. Genetic variability and correlations of essential oil yield with agro-economic traits in Mentha species and identification of promising cultivars. Ind. Crop. Prod. 2017, 95, 726–733. [Google Scholar] [CrossRef]
- Leontaritou, P.; Lamari, F.N.; Papasotiropoulos, V.; Iatrou, G. Exploration of genetic, morphological and essential oil variation reveals tools for the authentication and breeding of Salvia pomifera subsp calycina (Sm.) Hayek. Phytochemistry 2021, 191, 112900. [Google Scholar] [CrossRef]
- Choi, I.Y.; Song, Y.J.; Choi, D.C.; Lee, W.H. A comparative study for obtaining maximum essential oil from six herbs on the basis of harvesting time, cultivation regions & type, and drying methods. Korean J. Hortic. Sci. Technol. 2010, 28, 492–496. [Google Scholar]
- Ghasemi Pirbalouti, A.; Barani, M.; Hamedi, B.; Ataei, K.M.; Karimi, A. Environment effect on diversity in quality and quantity of essential oil of different wild populations of Kerman thyme. Genetika 2013, 45, 441–450. [Google Scholar] [CrossRef]
- Sarfaraz, D.; Rahimmalek, M.; Saeidi, G.; Sabzalian, M.R. Genetic relations among and within wild and cultivated Thymus species based on morphological and molecular markers. 3 Biotech 2020, 10, 289. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Moisa, C.; Lupitu, A.; Pop, G.; Chambre, D.R.; Copolovici, L.; Cioca, G.; Bungau, S.; Copolovici, D.M. Variation of the Chemical Composition of Thymus vulgaris Essential Oils by Phenological Stages. Rev. Chim. 2019, 70, 633–637. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]

| Code No. | Sample Collection Site | Yield (% v/w) | Color of Essential Oil |
|---|---|---|---|
| T1 | 298, Maecheon-ro, Gwangui-myeon, Gurye-gun, Jeollanam-do | 0.2 | Orange |
| T2 | 310-11, Seonhwang-ro, Miam-myeon, Yeongam-gun, Jeollanam-do | 0.5 | Dark yellow |
| T3 | 1679, Gyeonggang-ro, Yongpyeong-myeon, Pyeongchang-gun, Gangwon-do | 0.4 | Orange |
| T4 | 330, Gimhwa-ro, Gimhwa-eup, Cheorwon-gun, Gangwon-do | 0.5 | Orange |
| T5 | 114-5, Heungjeonggyegok-gil, Bongpyeong-myeon, Pyeongchang-gun, Gangwon-do | 0.5 | Orange |
| T6 | 105-19, Ssukgogae-ro, Namwon-si, Jeollabuk-do | 0.6 | Dark yellow |
| T7 | 105-19, Ssukgogae-ro, Namwon-si, Jeollabuk-do | 0.1 | Lemon |
| T8 | 105-19, Ssukgogae-ro, Namwon-si, Jeollabuk-do | 0.5 | Red |
| T9 | 105-19, Ssukgogae-ro, Namwon-si, Jeollabuk-do | 0.1 | Lemon |
| T10 | 105-19, Ssukgogae-ro, Namwon-si, Jeollabuk-do | 0.3 | Yellow |
| T11 | 92, Yangjae-daero, Gwacheon-si, Gyeonggi-do | 0.2 | Yellow |
| T12 | 87, Iryeong-ro 502beon-gil, Jangheung-myeon, Yangju-si, Gyeonggi-do | 0.4 | Lemon |
| T13 | 109-3, Ijin-ri, Bukpyeong-myeon, Haenam-gun, Jeollanam-do | 0.2 | Dark yellow |
| T14 | 256-74, Hosu-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do | 0.3 | Dark yellow |
| T15 | 256-113, Hosu-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do | 0.5 | Lemon |
| T16 | 92, Yangjae-daero, Gwacheon-si, Gyeonggi-do | 0.2 | Yellow |
| T17 | 6, Andong-gil, Gurim-myeon, Sunchang-gun, Jeollabuk-do | 0.3 | Lemon |
| T18 | 1201, Bukbu-ro, Bongyang-eup, Jecheon-si, Chungcheongbuk-do | 0.7 | Dark yellow |
| T19 | 15, Choil-ro 174beon-gil, Hanam-si, Gyeonggi-do | 0.2 | Dark yellow |
| T20 | 80, Seonghyeon-ro, Gwanak-gu, Seoul | 0.1 | Lemon |
| T21 | Iryeong-ro, Jangheung-myeon, Yangju-si, Gyeonggi-do | 0.3 | Dark yellow |
| T22 | Wondang-ro, Deogyang-gu, Goyang-si, Gyeonggi-do | 0.3 | Yellow |
| T23 | 3914, Cheongsong-ro, Bunam-myeon, Cheongsong-gun, Gyeongsangbuk-do | 0.2 | Dark yellow |
| T24 | 563, Yeonju-ro, Jumunjin-eup, Gangneung-si, Gangwon-do | 0.4 | Orange |
| T25 | 52, Choil-ro 105beon-gil, Hanam-si, Gyeonggi-do | 0.4 | Dark yellow |
| T26 | Juam-dong, Gwacheon-si, Gyeonggi-do | 0.4 | Orange |
| T27 | Anseong-si, Gyeonggi-do | 0.5 | Red |
| T28 | 83-18, Gallyeong-gil, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do | 0.4 | Orange |
| T29 | 83-18, Gallyeong-gil, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do | 0.5 | Orange |
| T30 | Cheonbu 3-gil, Buk-myeon, Ulleung-gun, Gyeongsangbuk-do | 0.3 | Orange |
| T31 | Cheonbu 3-gil, Buk-myeon, Ulleung-gun, Gyeongsangbuk-do | 0.4 | Orange |
| T32 | Dodong-ri, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do | 0.5 | Red |
| T33 | Dodong 1-gil, Ulleung-eup, Ulleung-gun, Gyeongsangbuk-do | 0.4 | Red |
| T34 | 128-15, Chusan-gil, Buk-myeon, Ulleung-gun, Gyeongsangbuk-do 40207 | 0.4 | Red |
| T35 | 90, Taeha-ri, Seo-myeon, Ulleung-gun, Gyeongsangbuk-do | 0.7 | Dark yellow |
| T36 | Ulleungsunhwan-ro, Buk-myeon, Ulleung-gun, Gyeongsangbuk-do | 0.3 | Orange |
| T37 | Na-ri, Buk-myeon, Ulleung-gun, Gyeongsangbuk-do | 0.3 | Orange |
| T38 | 256-74, Hosu-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do | 0.4 | Lemon |
| T39 | 415, Gwangneungsumogwon-ro, Soheul-eup, Pocheon-si, Gyeonggi-do | 0.5 | Yellow |
| T40 | 72, Sumogwon-gil, Jeju-si, Jeju-do | 0.6 | Yellow |
| T41 | 72, Sumogwon-gil, Jeju-si, Jeju-do | 0.5 | Yellow |
| T42 | 72, Sumogwon-gil, Jeju-si, Jeju-do | 0.4 | Orange |
| T43 | 72, Sumogwon-gil, Jeju-si, Jeju-do | 0.3 | Orange |
| T44 | 72, Sumogwon-gil, Jeju-si, Jeju-do | 0.3 | Orange |
| T45 | Gyorae-ri, Jocheon-eup, Jeju-si, Jeju-do | 0.2 | Orange |
| T46 | 300, Hallim-ro, Hallim-eup, Jeju-si, Jeju-do | 0.3 | Dark yellow |
| T47 | Misan-dong, Siheung-si, Gyeonggi-do | 0.5 | Lemon |
| T48 | 24, Hongeunjungang-ro 3-gil, Seodaemun-gu, Seoul | 0.5 | Lemon |
| T49 | 72, Magokjungang 1-ro, Gangseo-gu, Seoul | 0.3 | Orange |
| T50 | 11-1, Cheondeoksan-ro 409beon-gil, Namsa-myeon, Cheoin-gu, Yongin-si, Gyeonggi-do | 0.2 | Yellow |
| T51 | 277-1, Maeho-gil, Hyeonnam-myeon, Yangyang-gun, Gangwon-do | 0.6 | Dark yellow |
| T52 | 21-1, Seoin-ro 1222beon-gil, Biin-myeon, Seocheon-gun, Chungcheongnam-do | 0.3 | Dark yellow |
| T53 | 172-31, Jinsan 2-gil, Nam-myeon, Taean-gun, Chungcheongnam-do | 0.4 | Dark yellow |
| T54 | 399-6, Geumgyedong-ro, Gonggeun-myeon, Hoengseong-gun, Gangwon-do | 0.3 | Dark yellow |
| T55 | 248, Howon-ro, Naeseo-eup, Masanhoewon-gu, Changwon-si, Gyeongsangnam-do | 0.4 | Lemon |
| T56 | 47-1, Baegam-ri, Yeomchi-eup, Asan-si, Chungcheongnam-do | 0.2 | Orange |
| T57 | 77, Hyoseongmunhak-gil, Bongpyeong-myeon, Pyeongchang-gun, Gangwon-do | 0.5 | Yellow |
| T58 | 122-1, Yonggang-ri, Dong-eup, Uichang-gu, Changwon-si, Gyeongsangnam-do | 0.3 | Lemon |
| T59 | 616, Hagui-ro, Uiwang-si, Gyeonggi-do | 0.3 | Yellow |
| T60 | 1192, Anyangpangyo-ro, Bundang-gu, Seongnam-si, Gyeonggi-do | Froze to death | |
| T61 | 256-116, Hosu-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do | 0.3 | Lemon |
| T62 | 6, Dongtanjangjicheon 1-gil, Hwaseong-si, Gyeonggi-do | 0.3 | Dark Yellow |
| T63 | 1622, Hoguk-ro, Deogyang-gu, Goyang-si, Gyeonggi-do | 0.5 | Lemon |
| T64 | 91, Yangjae-daero, Gwacheon-si, Gyeonggi-do | 0.8 | Yellow |
| T65 | 256-85, Hosu-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do | 0.4 | Transparent yellow |
| T66 | 49-20, Heonilleung 1-gil, Seocho-gu, Seoul | 0.4 | Lemon |
| T67 | 115, Beolmal-ro, Bucheon-si, Gyeonggi-do | 0.2 | Orange |
| T68 | 175, Angol-gil, Jewon-myeon, Geumsan-gun, Chungcheongnam-do | 0.4 | Lemon |
| T69 | 279-39, Eumnae-ro, Geoje-myeon, Geoje-si, Gyeongsangnam-do | 0.6 | Lemon |
| T70 | 44, Dangseong-ro 364 beon-gil, Songsan-myeon, Hwaseong-si, Gyeonggi-do | 0.3 | Orange |
| T71 | 93-1, Juam-dong, Gwacheon-si, Gyeonggi-do | 0.5 | Pale yellow |
| T72 | 133, Wangsimni-ro, Seongdong-gu, Seoul | 0.5 | Pale yellow |
| T73 | 51, Chusa-ro, Gwacheon-si, Gyeonggi-do | 0.7 | Yellow |
| T74 | 256-98, Hosu-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do | 0.3 | Pale yellow |
| T75 | 217, Beolmal-ro, Bucheon-si, Gyeonggi-do | 0.5 | Transparent yellow |
| T76 | 13, Jeongung-ro, Namsa-myeon, Cheoin-gu, Yongin-si, Gyeonggi-do | Froze to death | |
| T77 | 513, Yongcheon-ro, Geumseong-myeon, Geumsan-gun, Chungcheongnam-do | 0.2 | Lemon |
| T78 | 557, Jungang-ro, Seocho-gu, Seoul | 0.1 | Yellow |
| T79 | 48-44, Hwadong-ro 587beon-gil, Ildong-myeon, Pocheon-si, Gyeonggi-do | 0.3 | Orange |
| T80 | 21-1, Seoin-ro 1222beon-gil, Biin-myeon, Seocheon-gun, Chungcheongnam-do | 0.5 | Orange |
| T81 | 5, Jinjinae 3-gil, Jeungpyeong-eup, Jeungpyeong-gun, Chungcheongbuk-do | 0.2 | Lemon |
| T82 | 22, Mareukbyeokjin-gil, Seo-gu, Gwangju | 0.4 | Pale yellow |
| T83 | 214-1, Samsang-ri, Jangheung-myeon, Yangju-si, Gyeonggi-do | 0.4 | Transparent yellow |
| T84 | 1660, Hoguk-ro, Deogyang-gu, Goyang-si, Gyeonggi-do | 0.5 | Lemon |
| T85 | 75-15, Songtangoga-gil, Jinwi-myeon, Pyeongtaek-si, Gyeonggi-do | 0.5 | Transparent yellow |
| T86 | 1006, Sansu-ro, Toechon-myeon, Gwangju-si, Gyeonggi-do | 0.5 | Transparent yellow |
| T87 | 24-53, Yeyang-gil, Yeondong-myeon, Sejong-si | 0.5 | Pale yellow |
| T88 | 1622, Hoguk-ro, Deogyang-gu, Goyang-si, Gyeonggi-do | 0.7 | Pale yellow |
| T89 | 329, Jeokcheon-ro, Muju-eup, Muju-gun, Jeollabuk-do | 0.6 | Pale yellow |
| T90 | 11, Daewangpangyo-ro 1000beon-gil, Sujeong-gu, Seongnam-si, Gyeonggi-do | 0.6 | Pale yellow |
| T91 | 39, Garim-ro, Gwangmyeong-si, Gyeonggi-do | 0.5 | Pale yellow |
| T92 | 256-74, Hosu-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do | Froze to death | |
| T93 | 44, Dangseong-ro 364beon-gil, Songsan-myeon, Hwaseong-si, Gyeonggi-do | Froze to death | |
| T94 | 2-10, Sinin-gil, Asan-si, Chungcheongnam-do | 0.6 | Pale yellow |
| T95 | 212-114, Hwangmu-ro 330beon-gil, Sindun-myeon, Icheon-si, Gyeonggi-do | 0.6 | Transparent yellow |
| T96 | 852, Gyeryong-ro, Jung-gu, Daejeon | 0.5 | Pale yellow |
| T97 | 256-116, Hosu-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do | 0.3 | Pale yellow |
| T98 | 496, Daehong-ro, Namil-myeon, Geumsan-gun, Chungcheongnam-do | 0.5 | Pale yellow |
| T99 | 53, Gyeongsu-daero, Uiwang-si, Gyeonggi-do | 0.5 | Pale yellow |
| T100 | 1192, Anyangpangyo-ro, Bundang-gu, Seongnam-si, Gyeonggi-do | 0.6 | Pale yellow |
| T101 | 460, Sugaesinam-gil, Namji-eup, Changnyeong-gun, Gyeongsangnam-do | 0.5 | Pale yellow |
| T102 | 115, Beolmal-ro, Bucheon-si, Gyeonggi-do | 0.5 | Pale yellow |
| T103 | 34, Doil-gil, Dong-myeon, Chuncheon-si, Gangwon-do | 0.3 | Pale yellow |
| T104 | 34, Doil-gil, Dong-myeon, Chuncheon-si, Gangwon-do | 0.2 | Yellow |
| T105 | 34, Doil-gil, Dong-myeon, Chuncheon-si, Gangwon-do | 0.3 | Dark Yellow |
| T106 | 114-5, Heungjeonggyegok-gil, Bongpyeong-myeon, Pyeongchang-gun, Gangwon-do | 0.5 | Red |
| T107 | 90-3, Onui-dong, Chuncheon-si, Gangwon-do | 0.4 | Dark Yellow |
| Code | Compound Name | CAS No. | Formula | RI | Count |
|---|---|---|---|---|---|
| C1 | 1-Octen-3-ol | 3391-86-4 | C8H16O | 942 | 89 |
| C2 | L-β-Pinene | 18172-67-3 | C10H16 | 990 | 78 |
| C3 | o-Cymene | 527-84-4 | C10H14 | 1027 | 47 |
| C4 | Eucalyptol | 470-82-6 | C10H18O | 1034 | 58 |
| C5 | 3-Thujene | 353313 | C10H16 | 1057 | 43 |
| C6 | D-α-Pinene | 7785-70-8 | C10H16 | 1059 | 75 |
| C7 | γ-Terpinene | 99-85-4 | C10H16 | 1060 | 85 |
| C8 | Camphene | 79-92-5 | C10H16 | 1063 | 62 |
| C9 | 1-Nonen-3-ol | 21964-44-3 | C9H18O | 1075 | 43 |
| C10 | Terpinolene | 586-62-9 | C10H16 | 1080 | 54 |
| C11 | cis-Thujane-4-ol | 15537-55-0 | C10H18O | 1093 | 74 |
| C12 | Linalool | 78-70-6 | C10H18O | 1100 | 84 |
| C13 | Isoborneol | 10385-78-1 | C10H18O | 1170 | 98 |
| C14 | Terpinen-4-ol | 562-74-3 | C10H18O | 1178 | 82 |
| C15 | α-Terpineol | 98-55-5 | C10H18O | 1190 | 64 |
| C16 | β-Citral | 106-26-3 | C10H16O | 1228 | 40 |
| C17 | Nerol | 106-25-2 | C10H18O | 1238 | 54 |
| C18 | Geraniol | 106-24-1 | C10H18O | 1239 | 68 |
| C19 | Citral | 5392-40-5 | C10H16O | 1254 | 68 |
| C20 | Thymol | 89-83-8 | C10H14O | 1272 | 99 |
| C21 | Carvacrol | 499-75-2 | C10H14O | 1279 | 44 |
| C22 | Geranyl acetate | 105-87-3 | C12H20O2 | 1375 | 52 |
| C23 | Caryophyllene | 87-44-5 | C15H24 | 1419 | 102 |
| C24 | Humulene | 6753-98-6 | C15H24 | 1458 | 100 |
| C25 | β-Cubebene | 13744-15-5 | C15H24 | 1485 | 86 |
| C26 | Elixene | 490377 | C15H24 | 1500 | 43 |
| C27 | Butylated hydroxytoluene | 128-37-0 | C15H24O | 1505 | 74 |
| C28 | β-Bisabolene | 495-61-4 | C15H24 | 1510 | 93 |
| C29 | β-Sesquiphellandrene | 20307-83-9 | C15H24 | 1527 | 56 |
| C30 | Caryophyllene oxide | 1139-30-6 | C15H24O | 1589 | 90 |
| Major Compounds | Code No. of Samples | No. of Samples |
|---|---|---|
| Carvacrol | T39, T43, T44, and T45 | 4 |
| Geraniol | T11, T14, T16, T19, T20, T21, T22, T26, T46, T49, T52, T53, T59, T70, T71, T72, T75, T78, T81, T83, T85, T86, T87, T88, T89, T90, T94, T101, T102, and T105 | 30 |
| Geranyl acetate | T2, T12, T15, T17, T38, T47, T48, T55, T58, T61, T63, T65, T66, T68, T69, T74, T82, T84, T91, T95, T96, T97, T98, T99, T100, and T103 | 26 |
| Linalool | T7, T9, and T77 | 3 |
| o-Cymene | T33 | 1 |
| Thymol | T1, T3, T4, T5, T6, T8, T10, T13, T18, T23, T24, T25, T27, T28, T29, T30, T31, T32, T34, T35, T36, T37, T40, T41, T42, T50, T51, T54, T56, T57, T62, T64, T67, T73, T79, T80, T104, T106, and T107 | 39 |
| No. | Code | Compound Name | Principal Components | |||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |||
| 1 | C1 | 1-Octen-3-ol | 0.597 | −0.033 | −0.038 | 0.275 |
| 2 | C2 | L-β-Pinene | 0.705 | −0.191 | −0.108 | 0.214 |
| 3 | C3 | o-Cymene | 0.617 | −0.140 | −0.113 | 0.330 |
| 4 | C4 | Eucalyptol | −0.209 | 0.269 | −0.166 | −0.111 |
| 5 | C5 | 3-Thujene | 0.693 | −0.133 | −0.097 | 0.296 |
| 6 | C6 | D-α-Pinene | 0.682 | −0.178 | −0.116 | 0.224 |
| 7 | C7 | γ-Terpinene | 0.727 | −0.137 | −0.116 | 0.165 |
| 8 | C8 | Camphene | 0.454 | −0.169 | −0.085 | 0.117 |
| 9 | C9 | 1-Nonen-3-ol | −0.182 | −0.270 | 0.105 | −0.013 |
| 10 | C10 | Terpinolene | 0.640 | −0.142 | −0.102 | 0.182 |
| 11 | C11 | cis-Thujane-4-ol | 0.586 | −0.164 | −0.109 | 0.129 |
| 12 | C12 | Linalool | 0.099 | 0.280 | 0.934 | −0.169 |
| 13 | C13 | Isoborneol | 0.233 | −0.098 | −0.143 | 0.099 |
| 14 | C14 | Terpinen-4-ol | 0.627 | −0.159 | −0.089 | 0.258 |
| 15 | C15 | α-Terpineol | 0.633 | −0.090 | −0.107 | 0.312 |
| 16 | C16 | β-Citral | −0.169 | 0.754 | −0.263 | −0.058 |
| 17 | C17 | Nerol | −0.268 | 0.757 | −0.263 | −0.059 |
| 18 | C18 | Geraniol | −0.922 | 0.285 | −0.220 | −0.103 |
| 19 | C19 | Citral | −0.177 | 0.766 | −0.268 | −0.054 |
| 20 | C20 | Thymol | 0.922 | −0.224 | −0.196 | −0.230 |
| 21 | C21 | Carvacrol | 0.239 | 0.122 | 0.015 | 0.775 |
| 22 | C22 | Geranyl acetate | −0.842 | −0.531 | 0.087 | −0.016 |
| 23 | C23 | Caryophyllene | 0.170 | 0.382 | 0.586 | 0.180 |
| 24 | C24 | Humulene | 0.264 | −0.088 | −0.054 | −0.199 |
| 25 | C25 | β-Cubebene | 0.228 | 0.443 | 0.554 | −0.064 |
| 26 | C26 | Elixene | 0.248 | −0.140 | 0.087 | 0.317 |
| 27 | C27 | Butylated hydroxytoluene | 0.100 | 0.221 | 0.212 | −0.217 |
| 28 | C28 | β-Bisabolene | −0.283 | 0.050 | −0.018 | −0.028 |
| 29 | C29 | β-Sesquiphellandrene | 0.133 | 0.085 | 0.489 | −0.249 |
| 30 | C30 | Caryophyllene oxide | 0.248 | 0.444 | −0.190 | 0.083 |
| % Variance | 65.916 | 13.482 | 10.616 | 3.917 | ||
| Cumulative variance | 65.916 | 79.397 | 90.014 | 93.931 | ||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) | (15) | (16) | (17) | (18) | (19) | (20) | (21) | (22) | (23) | (24) | (25) | (26) | (27) | (28) | (29) | (30) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1)C1 | 1 | 0.560 ** | 0.401 ** | −0.119 | 0.476 ** | 0.349 ** | 0.476 ** | 0.199 * | −0.184 | 0.418 ** | 0.471 ** | −0.042 | 0.051 | 0.308 ** | 0.385 ** | −0.139 | −0.187 | −0.584 ** | −0.161 | 0.501 ** | 0.387 ** | −0.495 ** | 0.187 | 0.342 ** | 0.180 | 0.515 ** | −0.003 | −0.245 * | 0.140 | 0.109 |
| (2)C2 | 1 | 0.544 ** | −0.281 ** | 0.650 ** | 0.616 ** | 0.616 ** | 0.335 ** | 0.037 | 0.587 ** | 0.550 ** | −0.126 | 0.170 | 0.594 ** | 0.584 ** | −0.272 ** | −0.339 ** | −0.703 ** | −0.289 ** | 0.656 ** | 0.273 ** | −0.509 ** | 0.067 | 0.214 * | −0.019 | 0.343 ** | −0.030 | −0.197 * | 0.000 | 0.115 | |
| (3)C3 | 1 | −0.206 * | 0.598 ** | 0.672 ** | 0.343 ** | 0.457 ** | −0.029 | 0.597 ** | 0.273 ** | −0.094 | 0.180 | 0.647 ** | 0.625 ** | −0.202 * | −0.255 ** | −0.586 ** | −0.215 * | 0.544 ** | 0.126 | −0.458 ** | 0.135 | −0.021 | −0.075 | 0.195 * | −0.187 | −0.186 | −0.166 | 0.328 ** | ||
| (4)C4 | 1 | −0.244 * | −0.167 | −0.285 ** | −0.088 | 0.104 | −0.254 ** | −0.221 * | −0.095 | 0.049 | −0.262 ** | −0.145 | 0.392 ** | 0.454** | 0.301 ** | 0.367 ** | −0.196 * | −0.101 | 0.024 | 0.028 | −0.070 | 0.075 | −0.147 | −0.060 | 0.284 ** | 0.145 | 0.108 | |||
| (5)C5 | 1 | 0.721 ** | 0.708 ** | 0.589 ** | −0.210* | 0.702 ** | 0.578 ** | −0.113 | 0.142 | 0.645 ** | 0.652 ** | −0.236 * | −0.304 ** | −0.685 ** | −0.255 ** | 0.599 ** | 0.256 ** | −0.531 ** | 0.098 | −0.098 | −0.140 | 0.366 ** | −0.094 | −0.342 ** | −0.186 | 0.207 * | ||||
| (6)C6 | 1 | 0.607 ** | 0.672 ** | −0.121 | 0.672 ** | 0.425 ** | −0.135 | 0.340 ** | 0.760 ** | 0.715 ** | −0.241 * | −0.296 ** | −0.681 ** | −0.262 ** | 0.608 ** | 0.101 | −0.500 ** | 0.091 | −0.010 | −0.169 | 0.253 ** | −0.140 | −0.211 * | −0.129 | 0.274 ** | |||||
| (7)C7 | 1 | 0.292 ** | −0.239 * | 0.576 ** | 0.769 ** | −0.110 | 0.031 | 0.474 ** | 0.443 ** | −0.238 * | −0.307 ** | −0.700 ** | −0.252 * | 0.672 ** | 0.305 ** | −0.554 ** | 0.074 | 0.003 | −0.065 | 0.424 ** | 0.012 | −0.411 ** | −0.161 | −0.016 | ||||||
| (8)C8 | 1 | −0.129 | 0.487 ** | 0.211 * | −0.109 | 0.603 ** | 0.521 ** | 0.599 ** | −0.202 * | −0.220 * | −0.466** | −0.209 * | 0.428 ** | 0.044 | −0.308 ** | 0.060 | −0.070 | −0.148 | 0.148 | −0.122 | −0.143 | −0.144 | 0.283 ** | |||||||
| (9)C9 | 1 | −0.269 ** | −0.141 | 0.007 | 0.018 | −0.037 | −0.016 | −0.218 * | −0.126 | 0.070 | −0.219 * | −0.122 | −0.100 | 0.304 ** | 0.008 | −0.014 | −0.068 | −0.024 | −0.043 | 0.297 ** | 0.072 | −0.067 | ||||||||
| (10)C10 | 1 | 0.532 ** | −0.095 | 0.133 | 0.720 ** | 0.550 ** | −0.221 * | −0.282 ** | −0.619 ** | −0.234 * | 0.587 ** | 0.155 | −0.477 ** | 0.112 | −0.017 | −0.142 | 0.331 ** | −0.023 | −0.317 ** | −0.129 | 0.084 | |||||||||
| (11)C11 | 1 | −0.111 | −0.027 | 0.394** | 0.432 ** | −0.229 * | −0.272 ** | −0.571 ** | −0.241 * | 0.570 ** | 0.294 ** | −0.417 ** | 0.042 | 0.008 | −0.009 | 0.599 ** | 0.051 | −0.358 ** | −0.128 | −0.104 | ||||||||||
| (12)C12 | 1 | −0.172 | −0.113 | −0.119 | −0.066 | −0.069 | −0.188 | −0.069 | −0.108 | −0.051 | −0.147 | 0.642 ** | −0.025 | 0.671 ** | 0.016 | 0.295 ** | −0.057 | 0.517 ** | −0.064 | |||||||||||
| (13)C13 | 1 | 0.312 ** | 0.324 ** | −0.065 | −0.034 | −0.239 * | −0.046 | 0.237 * | 0.121 | −0.161 | −0.089 | 0.165 | −0.022 | −0.021 | −0.084 | 0.248 * | 0.078 | 0.174 | ||||||||||||
| (14)C14 | 1 | 0.765 ** | −0.275 ** | −0.310 ** | −0.628 ** | −0.288 ** | 0.550 ** | 0.166 | −0.464 ** | 0.055 | −0.002 | −0.113 | 0.186 | −0.002 | −0.098 | −0.057 | 0.222 * | |||||||||||||
| (15)C15 | 1 | −0.147 | −0.194 * | −0.622 ** | −0.178 | 0.540 ** | 0.271 ** | −0.503 ** | 0.036 | −0.013 | −0.033 | 0.264 ** | −0.095 | −0.163 | −0.106 | 0.329 ** | ||||||||||||||
| (16)C16 | 1 | 0.929 ** | 0.394 ** | 0.957 ** | −0.281 ** | −0.095 | −0.266 ** | 0.036 | −0.104 | 0.125 | −0.195 * | −0.055 | 0.062 | −0.088 | 0.338 ** | |||||||||||||||
| (17)C17 | 1 | 0.497 ** | 0.868 ** | −0.369 ** | −0.107 | −0.191 | 0.045 | −0.131 | 0.102 | −0.191 | −0.064 | 0.134 | −0.086 | 0.332** | ||||||||||||||||
| (18)C18 | 1 | 0.405 ** | −0.839 ** | −0.255 ** | .607 ** | −0.191 | −0.243 * | −0.200 * | −0.315 ** | −0.057 | 0.258 ** | −0.193 | −0.090 | |||||||||||||||||
| (19)C19 | 1 | −0.291 ** | −0.086 | −0.266 ** | 0.089 | −0.103 | 0.156 | −0.203 * | 0.121 | 0.110 | −0.099 | .417 ** | ||||||||||||||||||
| (20)C20 | 1 | 0.055 | −0.670 ** | −0.096 | 0.340 ** | 0.032 | 0.171 | 0.054 | −0.281 ** | 0.068 | 0.112 | |||||||||||||||||||
| (21)C21 | 1 | −0.275 ** | 0.131 | 0.080 | 0.177 | 0.31 5** | −0.035 | −0.151 | −0.059 | −0.071 | ||||||||||||||||||||
| (22)C22 | 1 | −0.301 ** | −0.178 | −0.377 ** | −0.131 | −0.183 | 0.202 * | −0.114 | −0.469 ** | |||||||||||||||||||||
| (23)C23 | 1 | −0.161 | 0.507 ** | 0.219 * | 0.341 ** | 0.014 | 0.294 ** | 0.320 ** | ||||||||||||||||||||||
| (24)C24 | 1 | 0.178 | −0.080 | 0.230 * | 0.165 | 0.611 ** | −0.049 | |||||||||||||||||||||||
| (25)C25 | 1 | 0.147 | 0.349 ** | 0.030 | 0.547 ** | 0.127 | ||||||||||||||||||||||||
| (26)C26 | 1 | −0.061 | −0.298 ** | −0.092 | −0.149 | |||||||||||||||||||||||||
| (27)C27 | 1 | 0.081 | 0.306 ** | 0.218 * | ||||||||||||||||||||||||||
| (28)C28 | 1 | 0.427 ** | 0.141 | |||||||||||||||||||||||||||
| (29)C29 | 1 | −0.030 | ||||||||||||||||||||||||||||
| (30)C30 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Sowndhararajan, K.; Deepa, P.; Kim, S. Variations in the Chemical Composition of Essential Oils in Native Populations of Korean Thyme, Thymus quinquecostatus Celak. Molecules 2022, 27, 7203. https://doi.org/10.3390/molecules27217203
Kim M, Sowndhararajan K, Deepa P, Kim S. Variations in the Chemical Composition of Essential Oils in Native Populations of Korean Thyme, Thymus quinquecostatus Celak. Molecules. 2022; 27(21):7203. https://doi.org/10.3390/molecules27217203
Chicago/Turabian StyleKim, Minju, Kandhasamy Sowndhararajan, Ponnuvel Deepa, and Songmun Kim. 2022. "Variations in the Chemical Composition of Essential Oils in Native Populations of Korean Thyme, Thymus quinquecostatus Celak." Molecules 27, no. 21: 7203. https://doi.org/10.3390/molecules27217203
APA StyleKim, M., Sowndhararajan, K., Deepa, P., & Kim, S. (2022). Variations in the Chemical Composition of Essential Oils in Native Populations of Korean Thyme, Thymus quinquecostatus Celak. Molecules, 27(21), 7203. https://doi.org/10.3390/molecules27217203





