Microscopical Evaluation of the Effects of High-Pressure Processing on Milk Casein Micelles
Abstract
1. Introduction
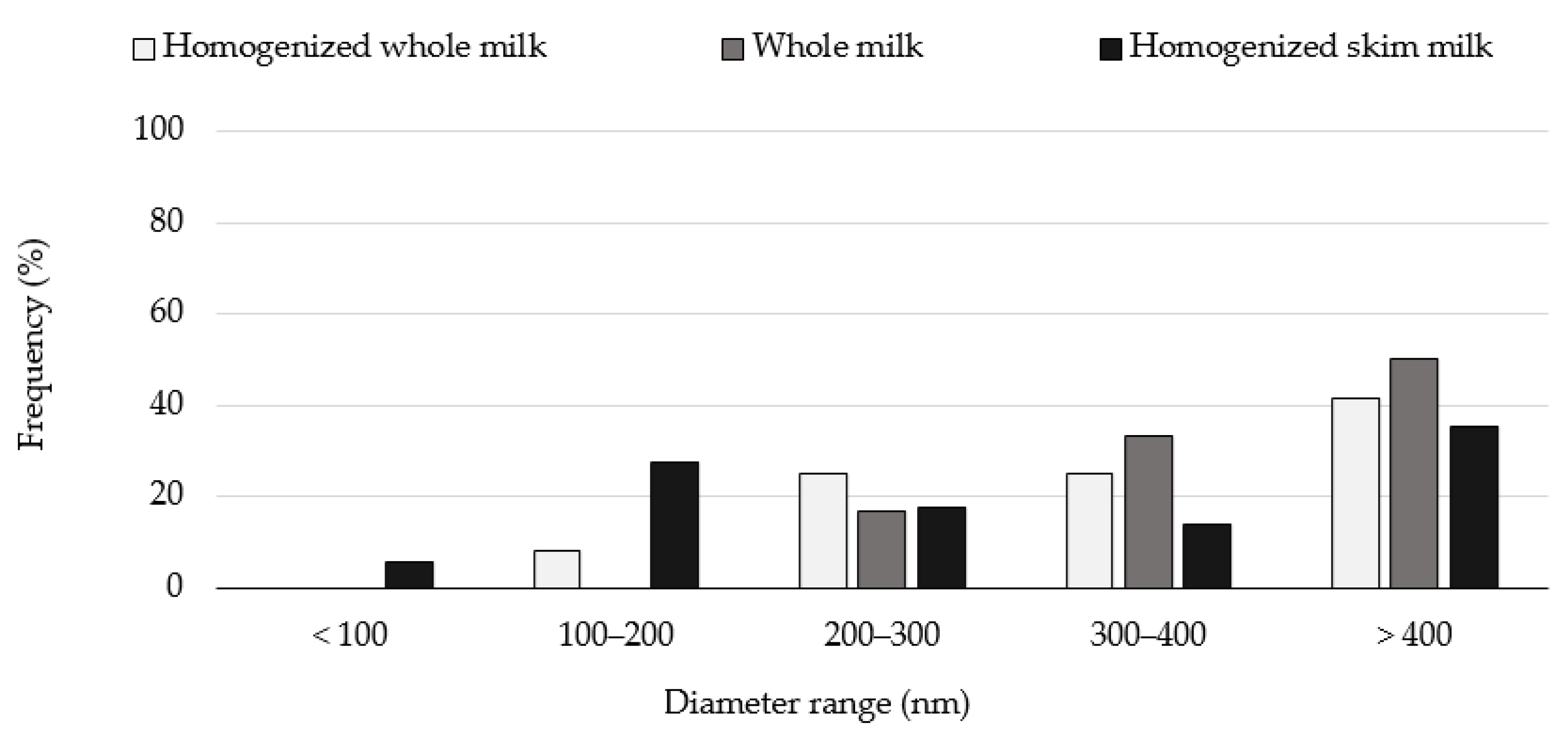
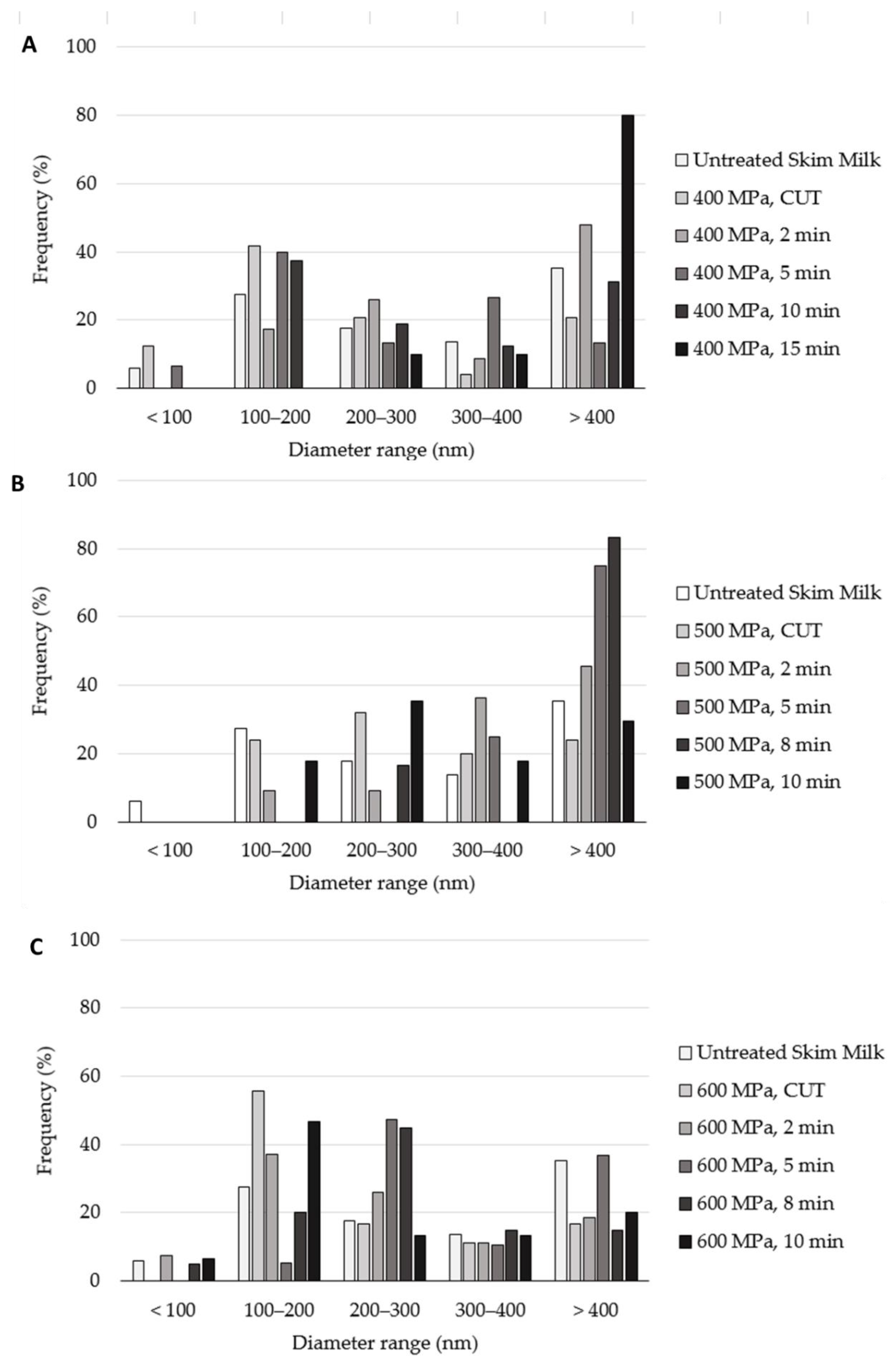
2. Results
Effect of the HPP Treatments on the Diameter of the Casein Micelles
3. Materials and Methods
3.1. Milk Samples
3.2. High-Pressure Processing
3.3. Scanning Electron Microscopy (SEM)
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stratakos, A.C.; Inguglia, E.S.; Linton, M.; Tollerton, J.; Murphy, L.; Corcionivoschi, N.; Koidis, A.; Tiwari, B.K. Effect of high pressure processing on the safety, shelf life and quality of raw milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef]
- Roobab, U.; Shabbir, M.A.; Khan, A.W.; Arshad, R.N.; Bekhit, A.E.; Zeng, X.; Inam-Ur-Raheem, M.; Aadil, R.M. High-pressure treatments for better quality clean-label juices and beverages: Overview and advances. LWT 2021, 149, 111828. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Arshad, R.N.; Radicetti, E.; Tedeschi, P.; Shahbaz, M.U.; Walayat, N.; Nawaz, A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Processing for Sustainable Food Supply. Sustainability 2021, 13, 13908. [Google Scholar] [CrossRef]
- Liu, G.; Carøe, C.; Qin, Z.; Munk, D.M.E.; Crafack, M.; Petersen, M.A.; Ahrné, L. Comparative study on quality of whole milk processed by high hydrostatic pressure or thermal pasteurization treatment. LWT 2020, 127, 109370. [Google Scholar] [CrossRef]
- Serna-Hernandez, S.O.; Escobedo-Avellaneda, Z.; García-García, R.; de Rostro-Alanis, M.J.; Welti-Chanes, J. High hydrostatic pressure induced changes in the physicochemical and functional properties of milk and dairy products: A review. Foods 2021, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Özer, B.; Yaman, H. Milk and Milk Products: Microbiology of Liquid Milk. Encycl. Food Microbiol. Second Ed. 2014, 2, 721–727. [Google Scholar] [CrossRef]
- Chavan, R.S.; Sehrawat, R.; Mishra, V.; Bhatt, S. Milk: Processing of Milk, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780123849533. [Google Scholar]
- Liepa, M.; Zagorska, J.; Galoburda, R. High-pressure processing as novel technology in dairy industry: A review. Res. Rural Dev. 2016, 1, 76–83. [Google Scholar]
- Dalgleish, D.G.; Corredig, M. The Structure of the Casein Micelle of Milk and Its Changes During Processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein Proteins: Structural and Functional Aspects. In Milk Proteins—From Structure to Biological Properties and Health Aspects; InTech: Rijeka, Croatia, 2016; pp. 3–18. [Google Scholar] [CrossRef]
- Cadesky, L.; Walkling-Ribeiro, M.; Kriner, K.T.; Karwe, M.V.; Moraru, C.I. Structural changes induced by high-pressure processing in micellar casein and milk protein concentrates. J. Dairy Sci. 2017, 100, 7055–7070. [Google Scholar] [CrossRef] [PubMed]
- Trejo, R.; Dokland, T.; Jurat-Fuentes, J.; Harte, F. Cryo-transmission electron tomography of native casein micelles from bovine milk. J. Dairy Sci. 2011, 94, 5770–5775. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Okda, T. Nanoscale observation of the natural structure of milk-fat globules and casein micelles in the liquid condition using a scanning electron assisted dielectric microscopy. Biochem. Biophys. Res. Commun. 2017, 491, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Hemar, Y.; Xu, C.; Wu, S.; Ashokkumar, M. Size reduction of “reformed casein micelles” by high-power ultrasound and high hydrostatic pressure. Ultrason. Sonochem. 2020, 63, 104929. [Google Scholar] [CrossRef] [PubMed]
- DOF NORMA Oficial Mexicana NOM-155-SCFI-2012, Leche-Denominaciones, Especificaciones Fisicoquímicas, Información Comercial y Métodos de Prueba. Available online: https://www.dof.gob.mx/normasOficiales/4692/seeco/seeco.htm (accessed on 1 July 2021).
- Yang, S.; Liu, G.; Munk, D.M.E.; Qin, Z.; Petersen, M.A.; Cardoso, D.R.; Otte, J.; Ahrné, L. Cycled high hydrostatic pressure processing of whole and skimmed milk: Effects on physicochemical properties. Innov. Food Sci. Emerg. Technol. 2020, 63, 102378. [Google Scholar] [CrossRef]
- Naik, L.; Sharma, R.; Rajput, Y.; Manju, G. Application of High Pressure Processing Technology for Dairy Food Preservation—Future Perspective: A Review. J. Anim. Prod. Adv. 2013, 3, 232. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Sihag, M.; Kaushik, R. High Pressure Processing and Its Impact on Milk Proteins: A Review. Res. Rev. J. Dairy Sci. Technol. 2013, 2, 12–20. [Google Scholar]
- Huppertz, T.; Fox, P.F.; de Kruif, K.G.; Kelly, A.L. High pressure-induced changes in bovine milk proteins: A review. Biochim. Biophys. Acta Proteins Proteom. 2006, 1764, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.H.; Gamlath, C.J.; Martin, G.J.O.; Ashokkumar, M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems—A review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. High pressure treatment of bovine milk: Effects on casein micelles and whey proteins. J. Dairy Res. 2004, 71, 97–106. [Google Scholar] [CrossRef] [PubMed]
- DOF NORMA Oficial Mexicana NOM-243-SSA1-2010, Productos y Servicios. Leche, Fórmula Láctea, Producto Lácteo Combinado y Derivados Lácteos. Disposiciones y Especificaciones Sanitarias. Métodos de Prueba. Available online: https://dof.gob.mx/normasOficiales/4156/salud2a/salud2a.htm (accessed on 1 July 2021).
- Serna-Hernandez, S.O. Effects of High Hydrostatic Pressure in the Microbiological, Microscopical and Physicochemical Properties of Milk; Instituto Tecnologico y de Estudios Superiores de Monterrey: Monterrey, Mexico, 2021. [Google Scholar]
- Das Murtey, M.; Ramasamy, P. Sample Preparations for Scanning Electron Microscopy—Life Sciences. In Modern Electron Microscopy in Physical and Life Sciences; Janecek, M., Kral, R., Eds.; Chapter 8; BoD—Books on Demand: London, UK, 2016; pp. 161–185. ISBN 978-953-51-2252-4. [Google Scholar]

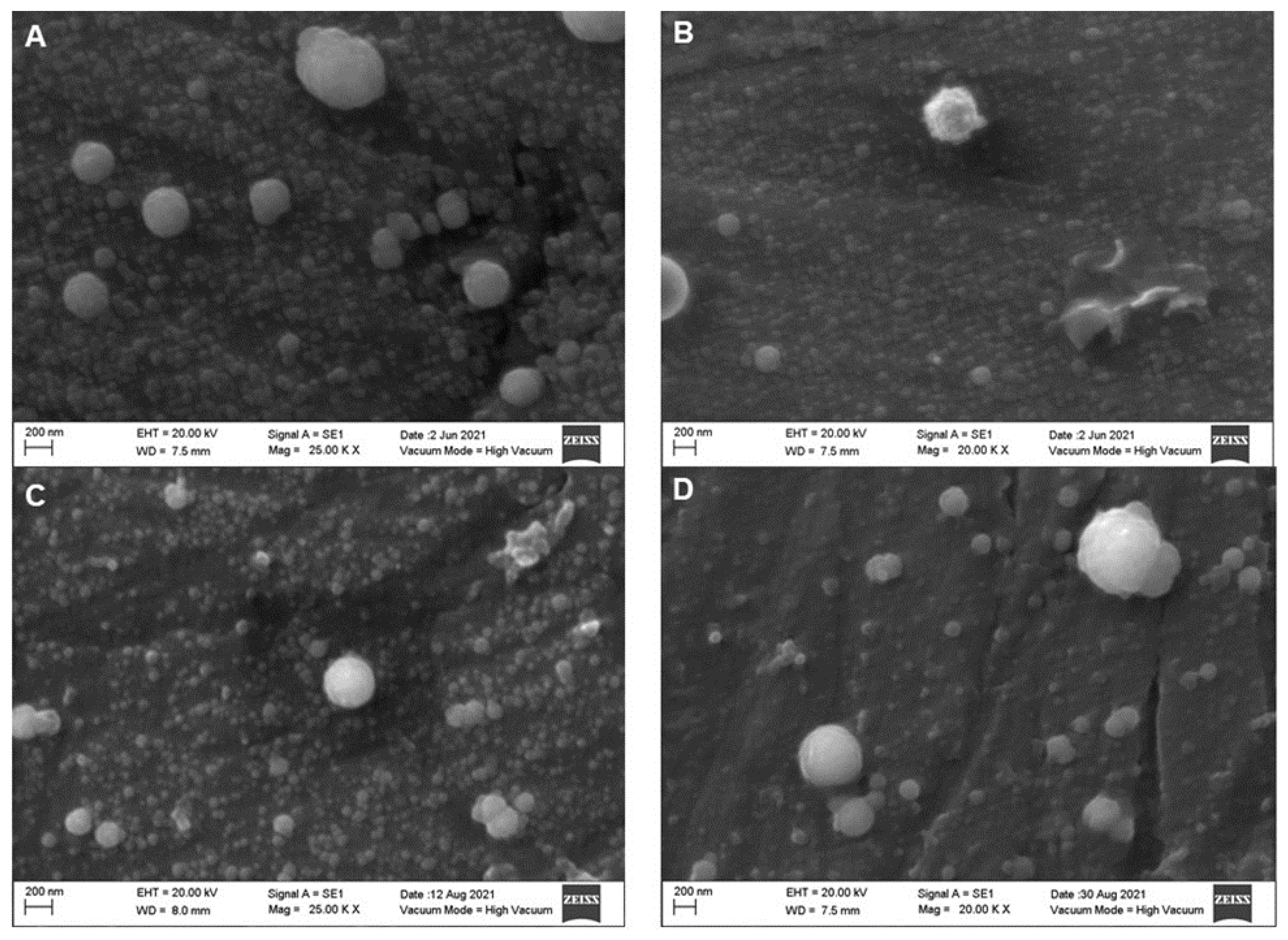
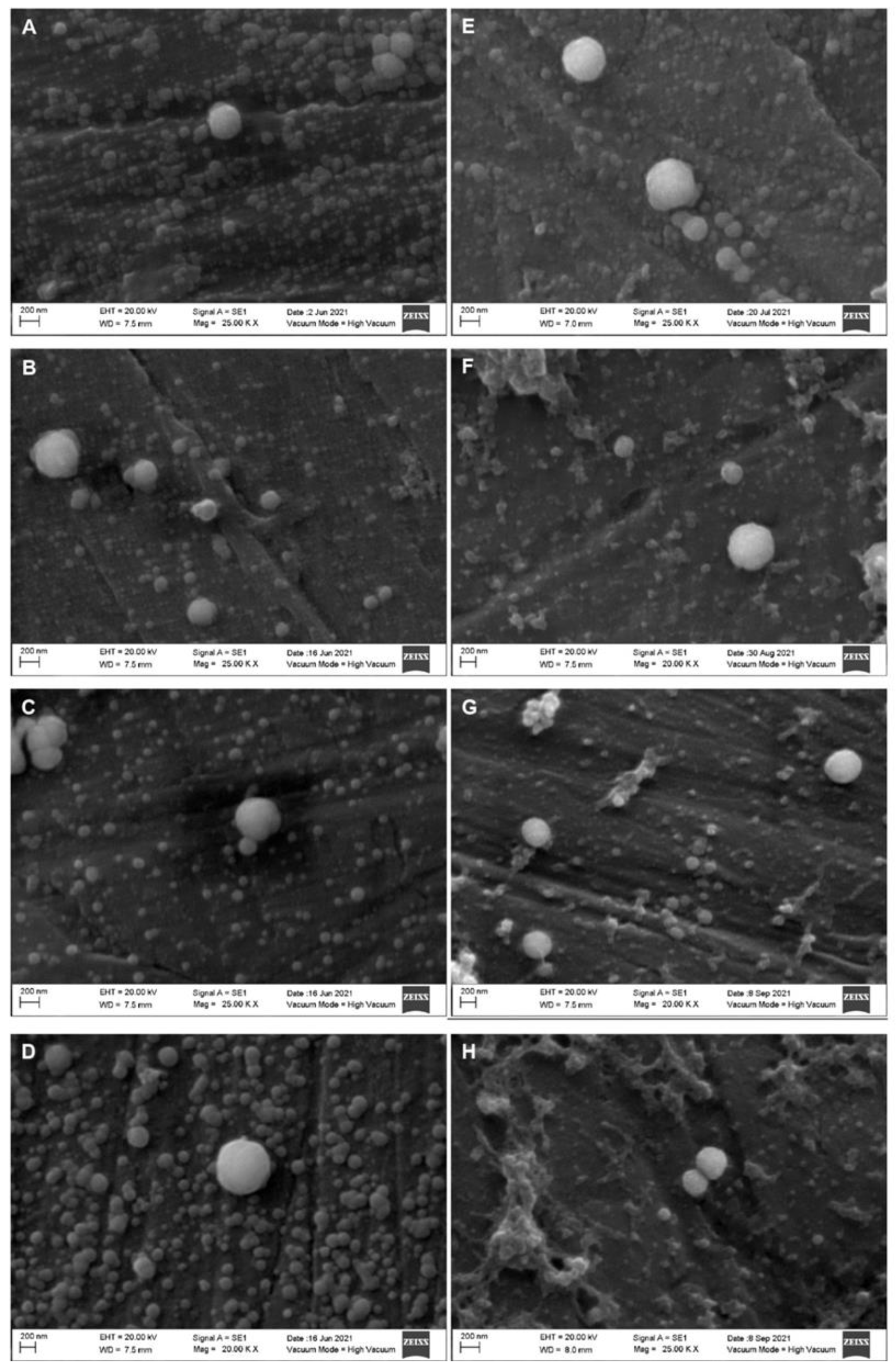
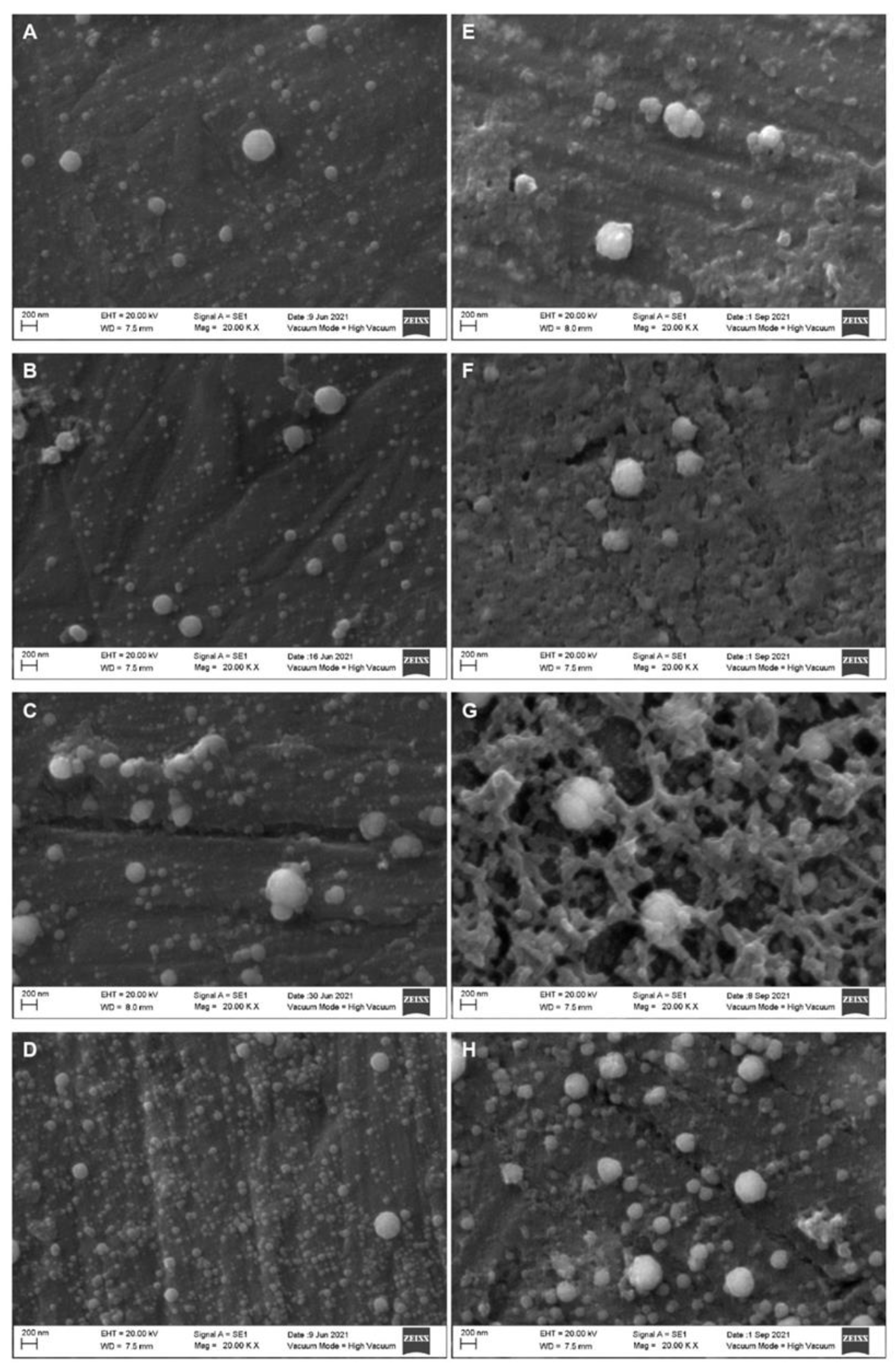
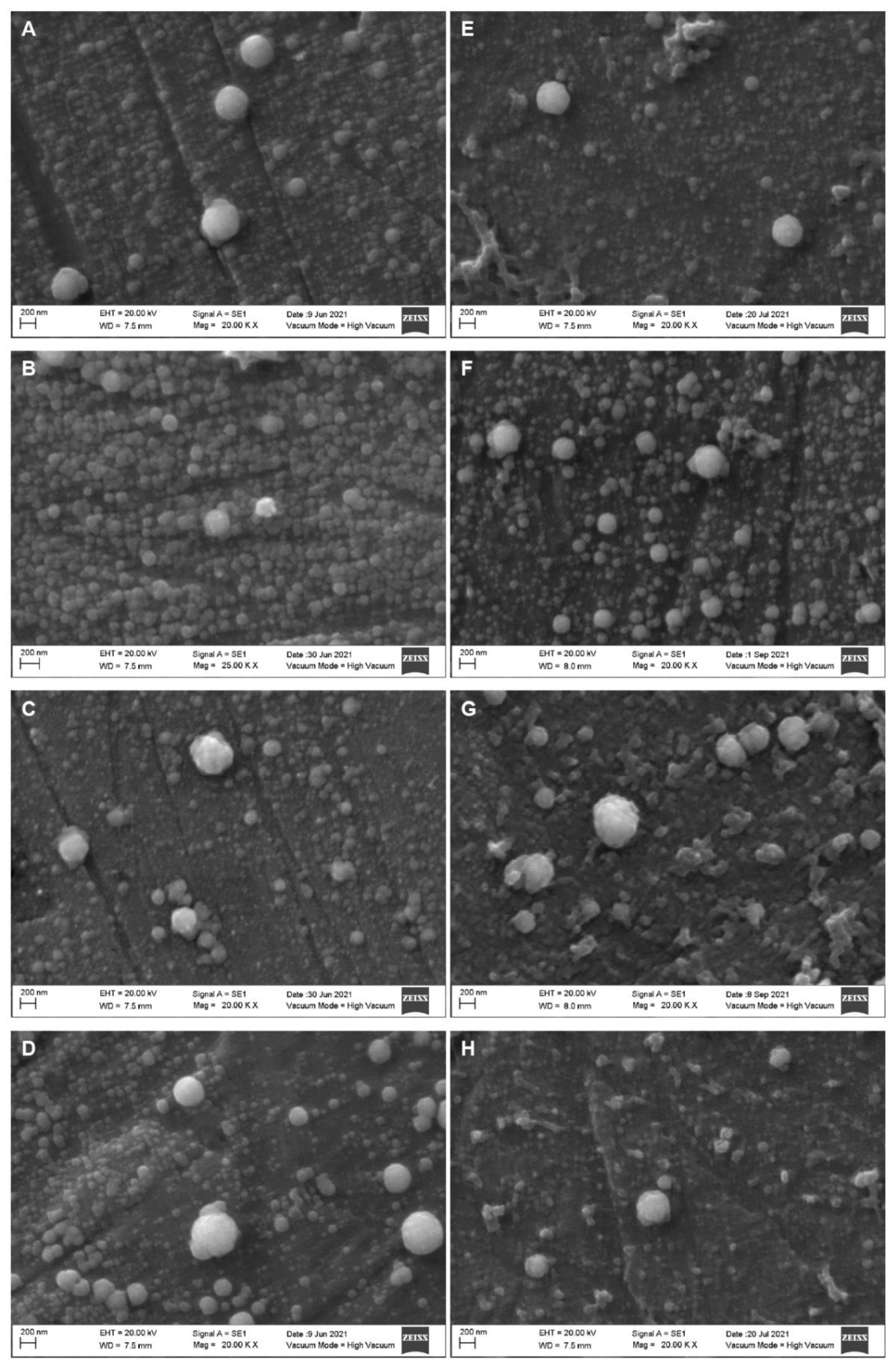

| Parameter | Whole Milk | Skim Milk |
|---|---|---|
| pH | 6.65 ± 0.05 | 6.70 ± 0.02 |
| Acidity (g/L, (lactic acid equivalents) | 1.62 ± 0.00 | 1.64 ± 0.03 |
| Protein (g/L) | 29.30 ± 0.90 | 31.30 ± 1.10 |
| Fat (g/L) | 3.50 ± 0.00 | 1.00 ± 0.00 |
| Total Solids (g/L) | 11.35 ± 0.03 | 11.31 ± 0.11 |
| Treatments | Whole Milk | Skim Milk |
|---|---|---|
| Mean Diameter (nm) | ||
| Homogenized and untreated | 391 ± 112 a | 370 ± 266 bc |
| Untreated | 370 ± 175 ab | ND |
| 400 MPa, CUT | 319 ± 82 abcd | 233 ± 146 c |
| 400 MPa, 2 min | 169 ± 9 cd | 408 ± 204 abc |
| 400 MPa, 5 min | 159 ± 109 cd | 263 ± 129 c |
| 400 MPa, 10 min | 178 ± 142 bcd | 281 ± 129 c |
| 400 MPa, 15 min | 208 ± 114 bcd | 588 ± 254 a |
| 500 MPa, CUT | 273 ± 121 abcd | 322 ± 130 c |
| 500 MPa, 2 min | 190 ± 97 bcd | 448 ± 190 abc |
| 500 MPa, 5 min | 198 ± 159 bcd | 587 ± 146 ab |
| 500 MPa, 8 min | ND | 625 ± 170 ab |
| 500 MPa, 10 min | 284 ± 165 abc | 313 ± 118 c |
| 600 MPa, CUT | 237 ± 130 abcd | 238 ± 117 c |
| 600 MPa, 2 min | 135 ± 72 d | 252 ± 132 c |
| 600 MPa, 5 min | 166 ± 123 cd | 374 ± 182 abc |
| 600 MPa, 8 min | ND | 268 ± 137 c |
| 600 MPa, 10 min | 239 ± 148 abcd | 236 ± 147 c |
| HPP Treatment | T (°C) | |
|---|---|---|
| P (MPa) | t (min) | |
| 400 | CUT | 26.2 ± 3.6 |
| 2 | 34.1 ± 11.1 | |
| 5 | 30.6 ± 4.2 | |
| 10 | 29.3 ± 4.7 | |
| 15 | 28.6 ± 3.8 | |
| 500 | CUT | 23.9 ± 5.5 |
| 2 | 31.7 ± 6.6 | |
| 5 | 31.9 ± 6.6 | |
| 8 * | 30.2 ± 6.4 | |
| 10 | 29.4 ± 5.8 | |
| 600 | CUT | 25.3 ± 10.8 |
| 2 | 33.3 ± 7.5 | |
| 5 | 33.3 ± 7.5 | |
| 8 * | 31.5 ± 7.5 | |
| 10 | 31.1 ± 7.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Hernandez, S.O.; Escobedo-Avellaneda, Z.; García-García, R.; Rostro-Alanis, M.d.J.; Welti-Chanes, J. Microscopical Evaluation of the Effects of High-Pressure Processing on Milk Casein Micelles. Molecules 2022, 27, 7179. https://doi.org/10.3390/molecules27217179
Serna-Hernandez SO, Escobedo-Avellaneda Z, García-García R, Rostro-Alanis MdJ, Welti-Chanes J. Microscopical Evaluation of the Effects of High-Pressure Processing on Milk Casein Micelles. Molecules. 2022; 27(21):7179. https://doi.org/10.3390/molecules27217179
Chicago/Turabian StyleSerna-Hernandez, Sergio O., Zamantha Escobedo-Avellaneda, Rebeca García-García, Magdalena de Jesús Rostro-Alanis, and Jorge Welti-Chanes. 2022. "Microscopical Evaluation of the Effects of High-Pressure Processing on Milk Casein Micelles" Molecules 27, no. 21: 7179. https://doi.org/10.3390/molecules27217179
APA StyleSerna-Hernandez, S. O., Escobedo-Avellaneda, Z., García-García, R., Rostro-Alanis, M. d. J., & Welti-Chanes, J. (2022). Microscopical Evaluation of the Effects of High-Pressure Processing on Milk Casein Micelles. Molecules, 27(21), 7179. https://doi.org/10.3390/molecules27217179







