Bimetallic Au–Ag Nanoparticles: Advanced Nanotechnology for Tackling Antimicrobial Resistance
Abstract
1. Introduction
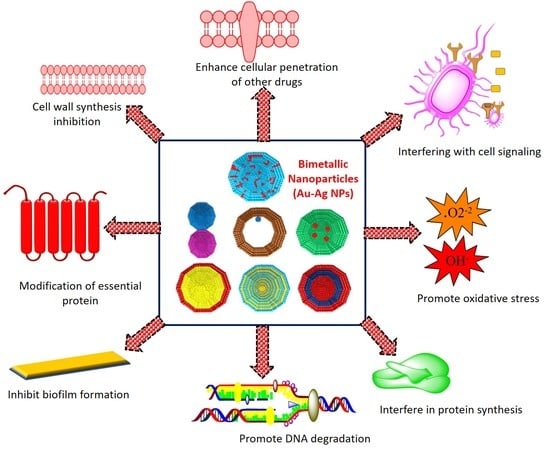
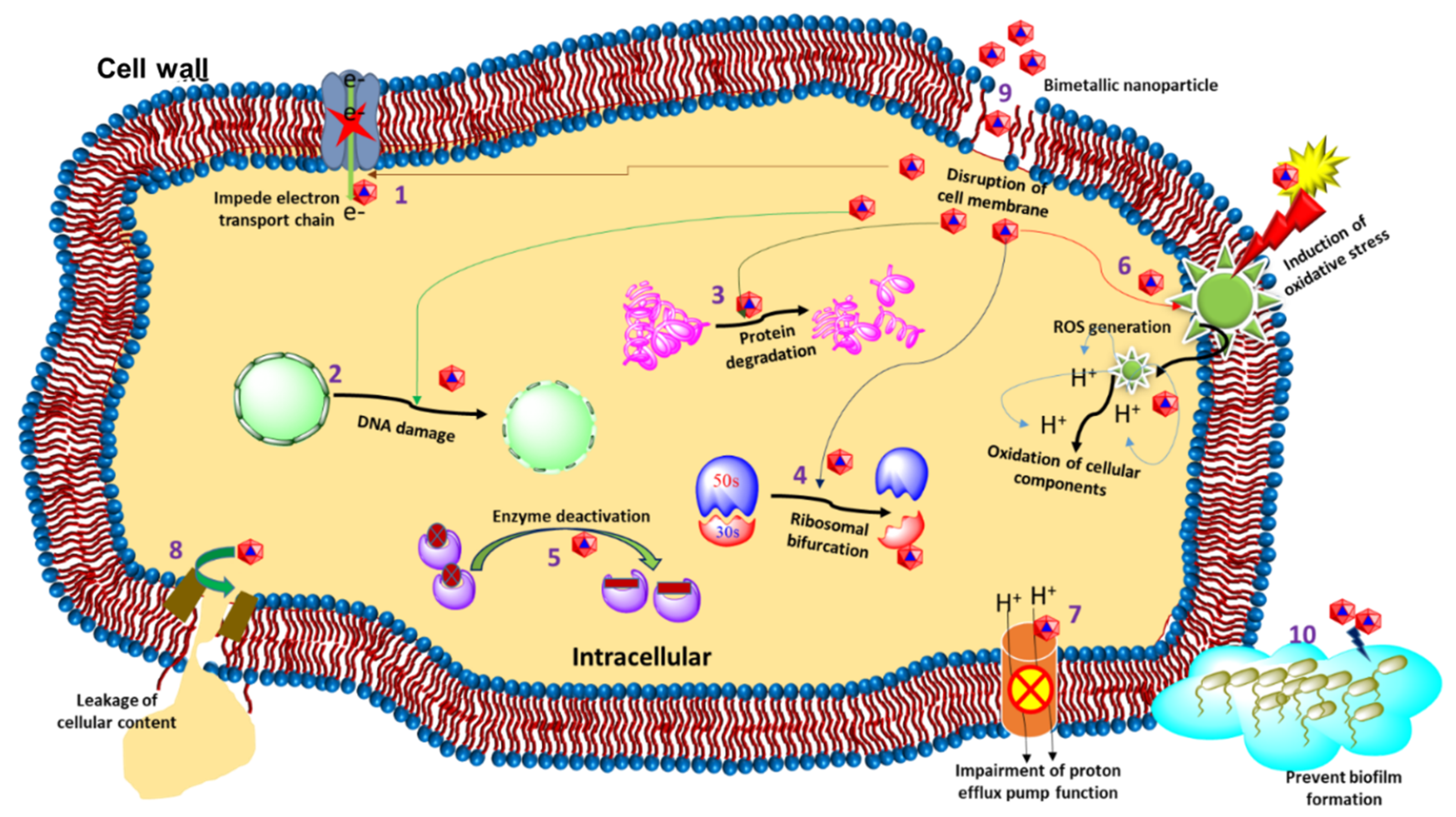
2. The Antibacterial Mode of Action of Au–Ag NPs
2.1. Cell Membrane Degradation
2.2. Disturbance in Homeostasis
2.3. Oxidative Stress and the Production of Reactive Oxygen Species (ROS)
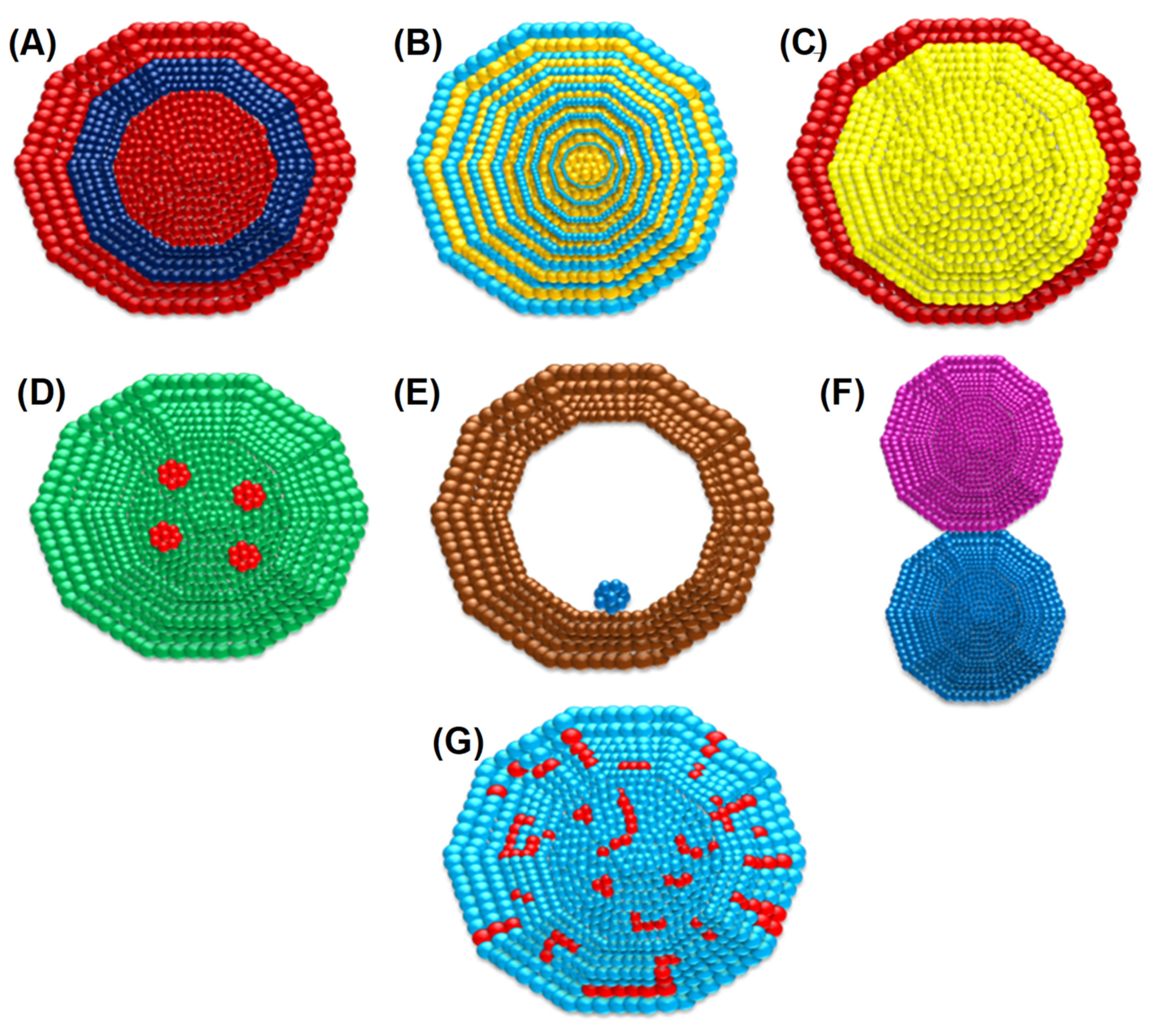
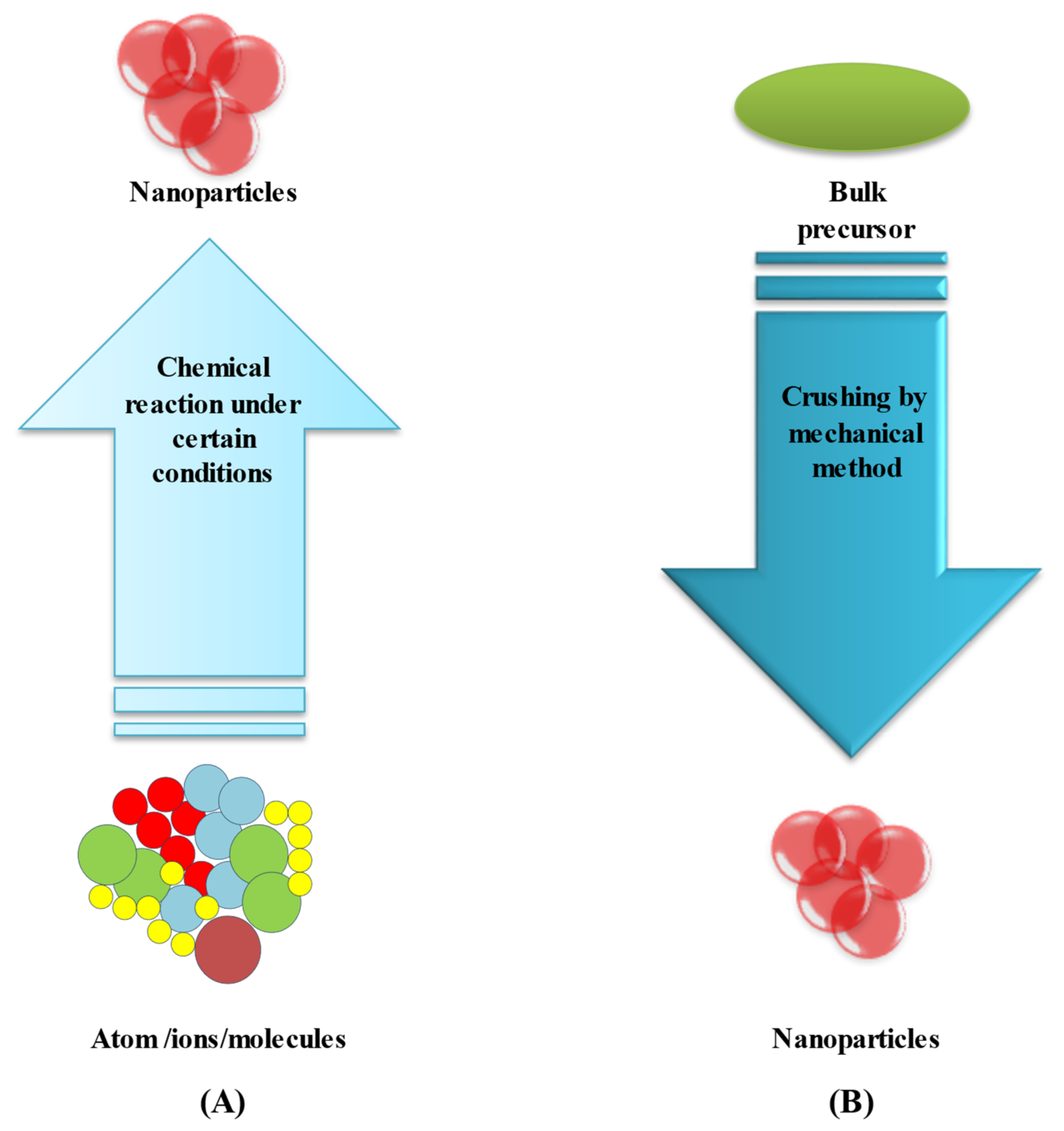
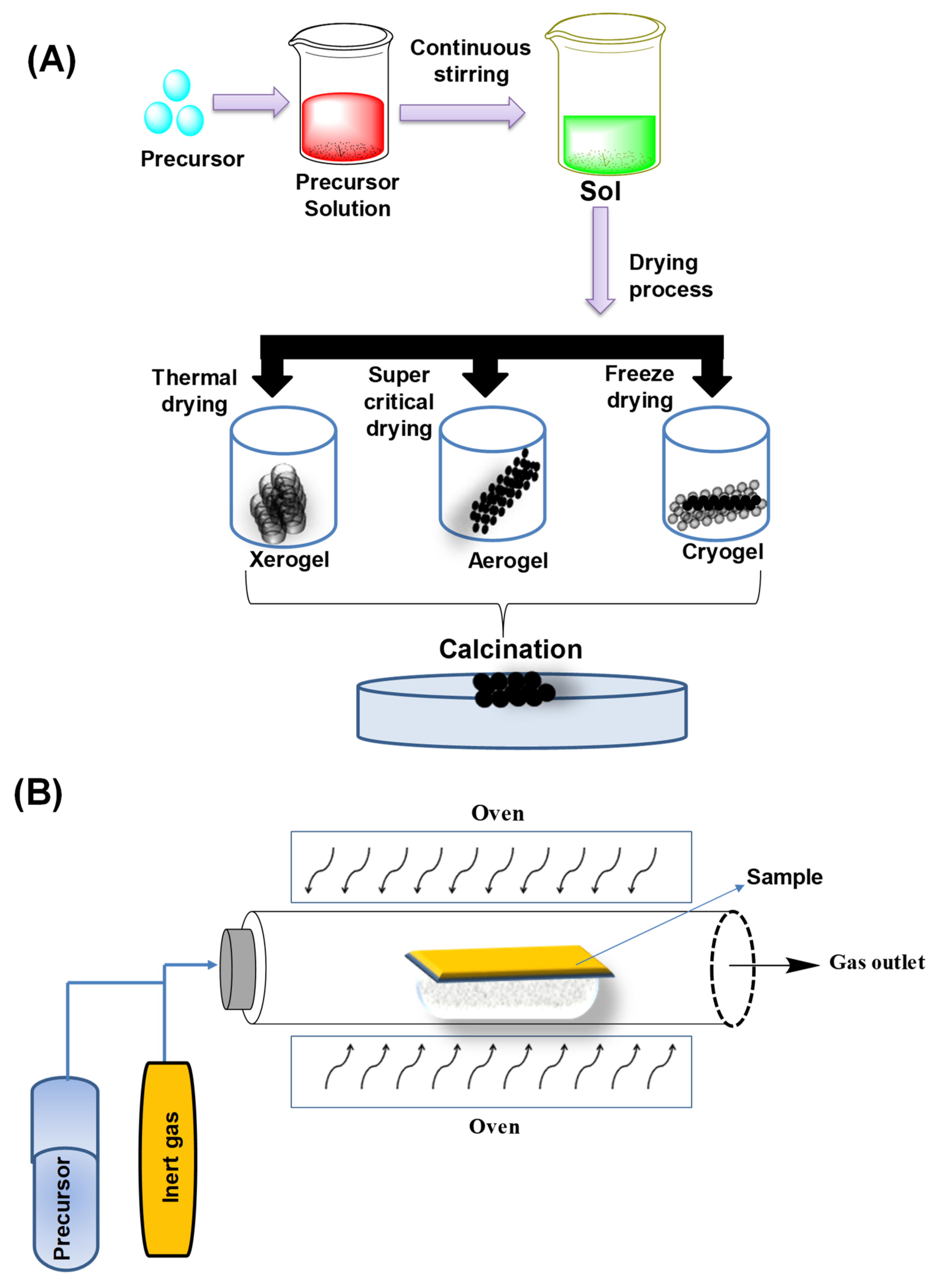
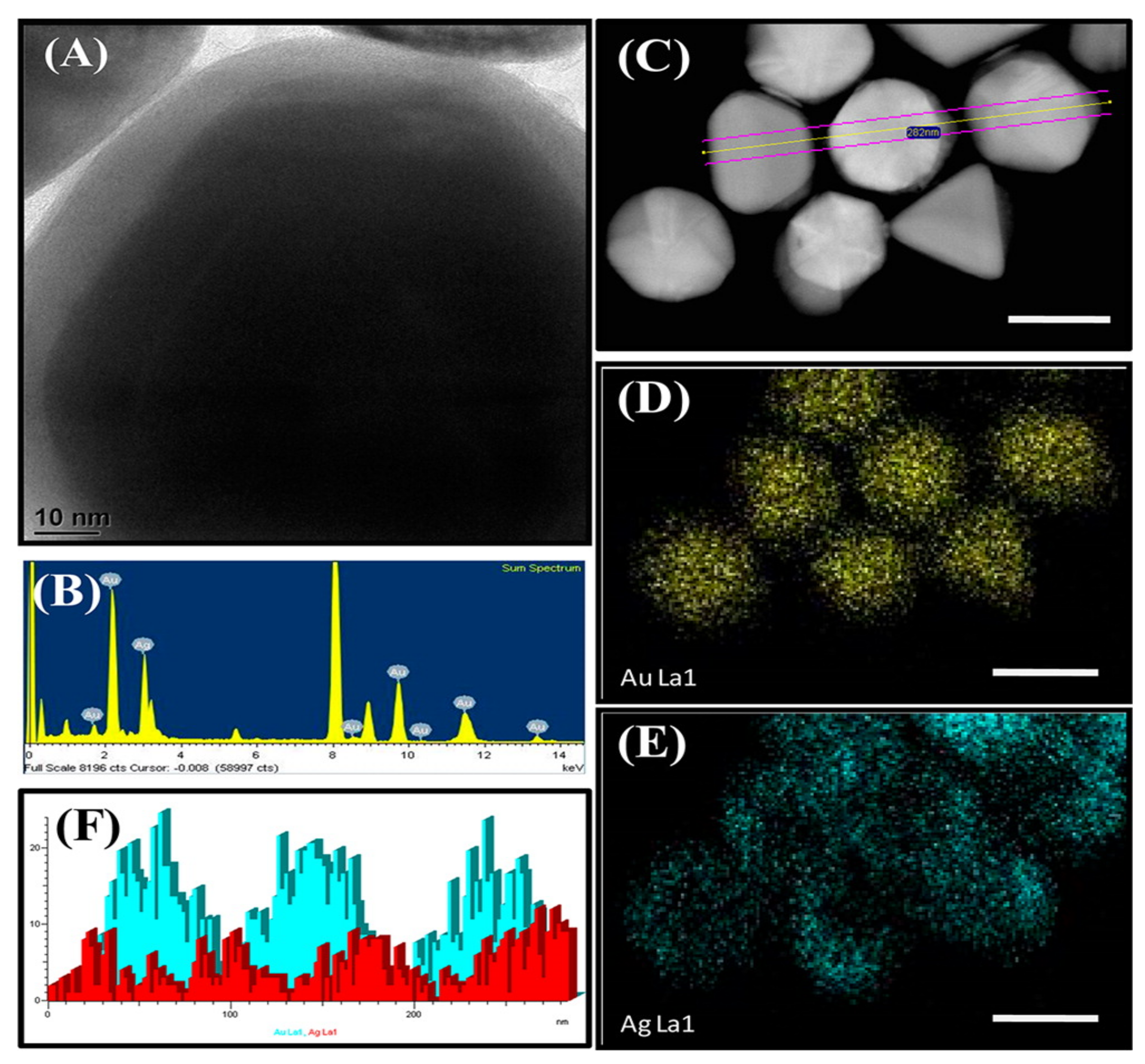
3. Synthesis Routes of BIMETALLIC Au–Ag Nanoparticles
3.1. Bottom-Up Method
3.1.1. Sol–Gel Method
3.1.2. Chemical Vapor Deposition (CVD) Method
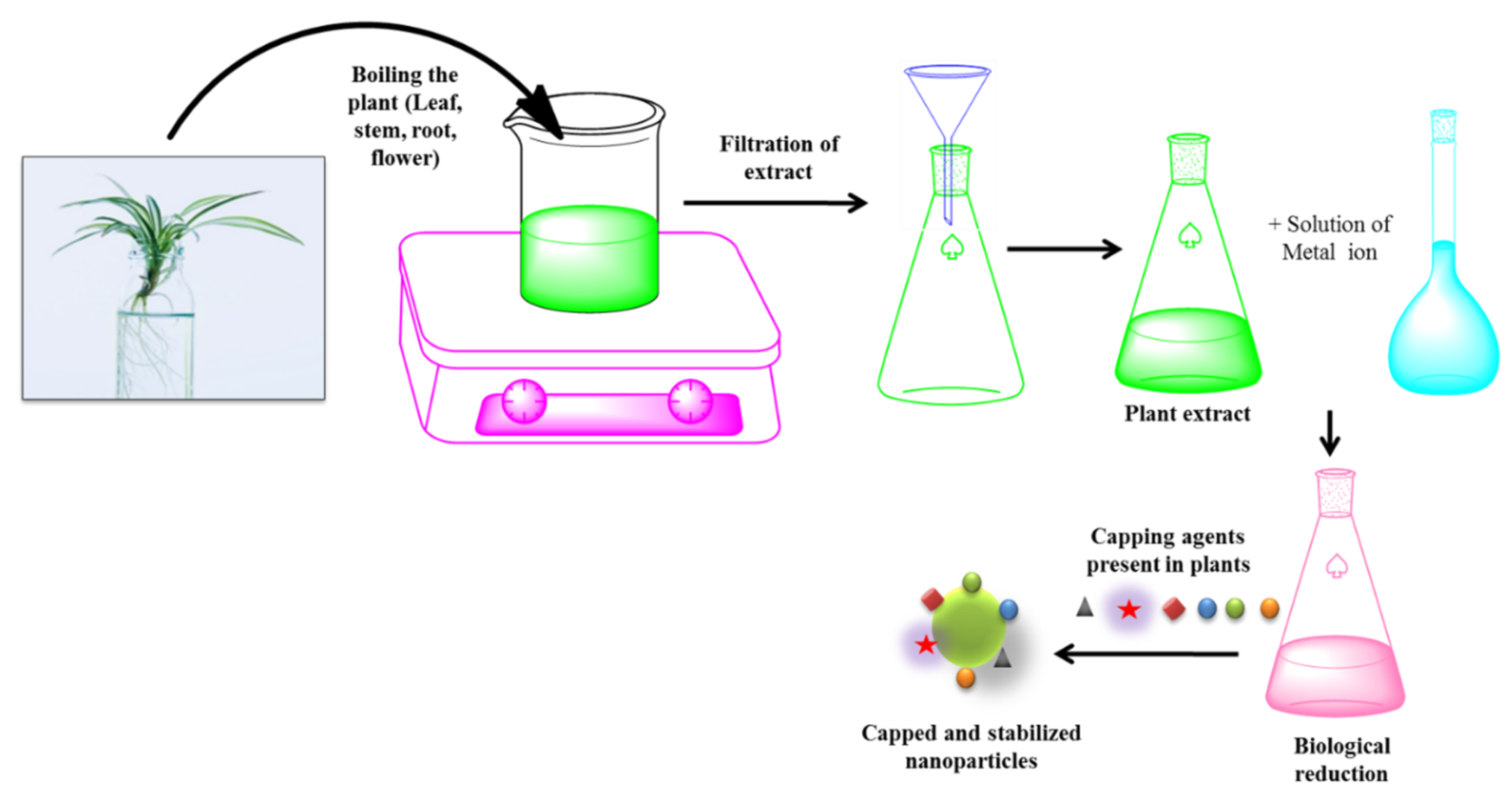
3.1.3. Green Synthesis
3.1.4. Co-Precipitation
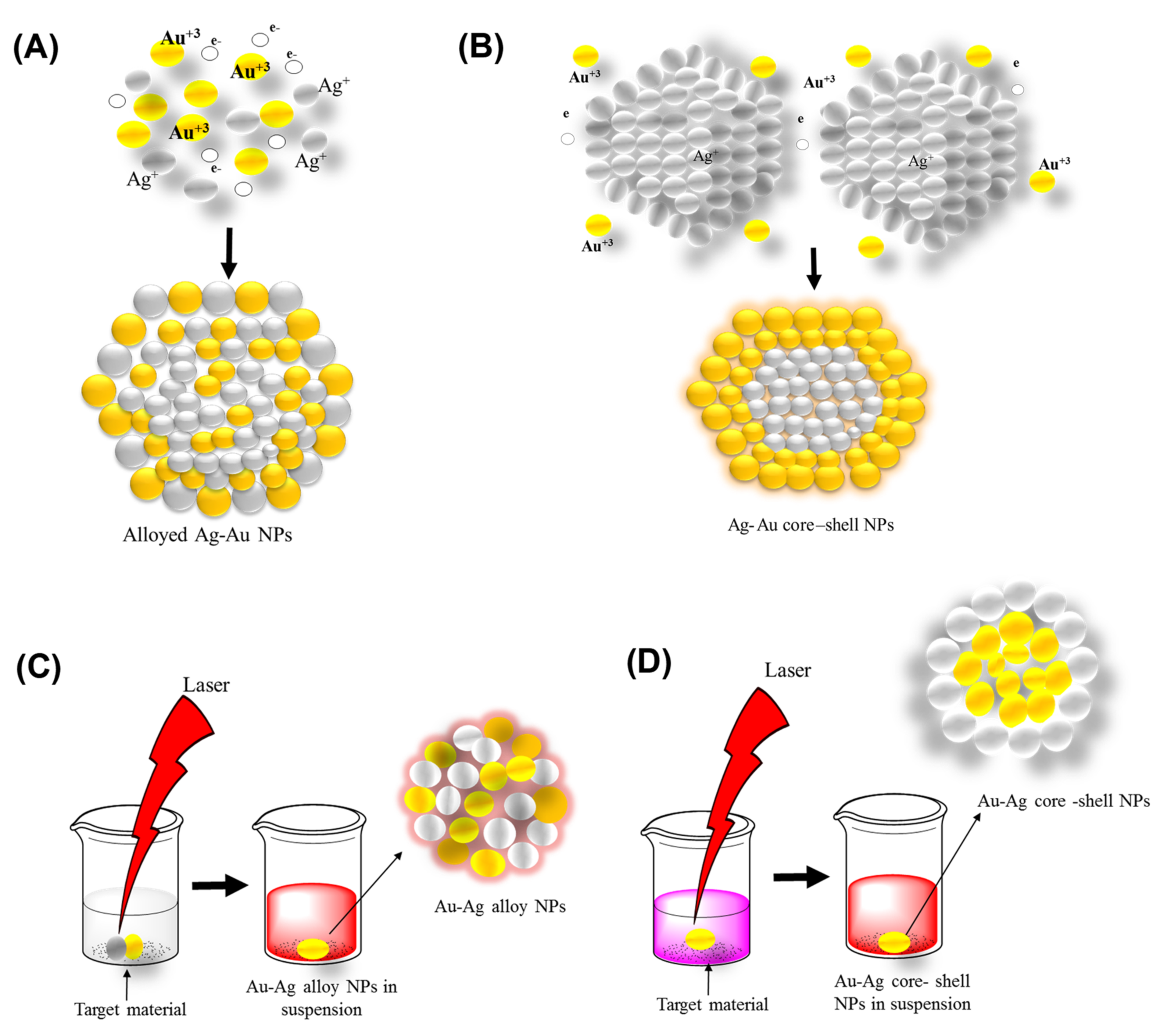
3.2. Top-Down Method
Laser Ablation
4. Antibacterial Properties of Bimetallic Au–Ag NPs
5. Bimetallic Nanoparticles Targeting Multidrug-Resistant Bacteria
6. Gold, Silver, and Gold–Silver Nanomaterials for Wound Healing
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopatkin, A.J.; Bening, S.C.; Manson, A.L.; Stokes, J.M.; Kohanski, M.A.; Badran, A.H.; Earl, A.M.; Cheney, N.J.; Yang, J.H.; Collins, J.J. Clinically relevant mutations in core metabolic genes confer antibiotic resistance. Science 2021, 371, eaba0862. [Google Scholar] [CrossRef] [PubMed]
- Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Mott, D.M.; Anh, D.T.N.; Singh, P.; Shankar, C.; Maenosono, S. Electronic transfer as a route to increase the chemical stability in gold and silver core–shell nanoparticles. Adv. Colloid Interface Sci. 2012, 185, 14–33. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Cao, J.; Gong, C.; Zuo, J.; Zhang, N.; Zhao, Y. Hyaluronic acid and antimicrobial peptide-modified gold/silver hybrid nanocages to combat bacterial multidrug resistance. Int. J. Pharm. 2020, 586, 119505. [Google Scholar] [CrossRef]
- Mahmudin, L.; Suharyadi, E.; Utomo, A.B.S.; Abraha, K. Optical properties of silver nanoparticles for surface plasmon resonance (SPR)-based biosensor applications. J. Mod. Phys. 2015, 6, 1071. [Google Scholar] [CrossRef]
- Rossi, A.; Zannotti, M.; Cuccioloni, M.; Minicucci, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Silver Nanoparticle-Based Sensor for the Selective Detection of Nickel Ions. Nanomaterials 2021, 11, 1733. [Google Scholar] [CrossRef]
- Feng, L.; Gao, G.; Huang, P.; Wang, K.; Wang, X.; Luo, T.; Zhang, C. Optical properties and catalytic activity of bimetallic gold-silver nanoparticles. Nano Biomed. Eng. 2010, 2, 258–267. [Google Scholar] [CrossRef]
- Al-Zaban, M.I.; AlHarbi, M.A.; Mahmoud, M.A.; Bahatheq, A.M. Production of biodiesel from oleaginous fungal lipid using highly catalytic bimetallic gold-silver core-shell nanoparticle. J. Appl. Microbiol. 2021, 132, 381–389. [Google Scholar] [CrossRef]
- Reñones, P.; Collado, L.; Iglesias-Juez, A.; Oropeza, F.E.; Fresno, F.; de la Peña, O.S. Silver-gold bimetal-loaded TiO2 Photocatalysts for CO2 Reduction; U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Aazam, E.S.; Zaheer, Z. Gold@ Silver bimetallic nanoparticles: Fabrication and removal of toxic chromium (VI). J. Mater. Sci. Mater. Electron. 2021, 32, 11043–11058. [Google Scholar] [CrossRef]
- Jia, X.; Yao, Y.; Yu, G.; Qu, L.; Li, T.; Li, Z.; Xu, C. Synthesis of gold-silver nanoalloys under microwave-assisted irradiation by deposition of silver on gold nanoclusters/triple helix glucan and antifungal activity. Carbohydr. Polym. 2020, 238, 116169. [Google Scholar] [CrossRef]
- Fereja, S.L.; Li, P.; Guo, J.; Fang, Z.; Zhang, Z.; Zhuang, Z.; Zhang, X.; Liu, K.; Chen, W. Silver-enhanced fluorescence of bimetallic Au/Ag nanoclusters as ultrasensitive sensing probe for the detection of folic acid. Talanta 2021, 233, 122469. [Google Scholar] [CrossRef]
- Kazancioglu, E.O.; Aydin, M.; Arsu, N. Photochemical synthesis of bimetallic gold/silver nanoparticles in polymer matrix with tunable absorption properties: Superior photocatalytic activity for degradation of methylene blue. Mater. Chem. Phys. 2021, 269, 124734. [Google Scholar] [CrossRef]
- GÜRSOY, N. Fungus-mediated synthesis of silver nanoparticles (agnp) and inhibitory effect on Aspergillus spp. in combination with antifungal agent. Cumhur. Sci. Journa. 2020, 41, 311–318. [Google Scholar] [CrossRef]
- Dat, N.M.; Khang, P.T.; Anh, T.N.M.; Quan, T.H.; Thinh, D.B.; Thien, D.T.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Synthesis, characterization, and antibacterial activity investigation of silver nanoparticle-decorated graphene oxide. Mater. Lett. 2021, 285, 128993. [Google Scholar]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. A sol–gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. Appl. Surf. Sci. 2021, 535, 147675. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Wang, Z.; Zhao, Y. Controlled synthesis of Ag nanoparticles with different morphologies and their antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 397–404. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. A facile method to prepare size-tunable silver nanoparticles and its antibacterial mechanism. Adv. Powder Technol. 2018, 29, 407–415. [Google Scholar] [CrossRef]
- Asharani, P.; Wu, Y.L.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef]
- Shehata, A.M.; Salem, F.M.; El-Saied, E.M.; Abd El-Rahman, S.S.; Mahmoud, M.Y.; Noshy, P.A. Evaluation of the ameliorative effect of zinc nanoparticles against silver nanoparticle–induced toxicity in liver and kidney of rats. Biol. Trace Elem. Research 2021, 200, 1201–1211. [Google Scholar] [CrossRef]
- Soares, T.; Ribeiro, D.; Proença, C.; Chisté, R.C.; Fernandes, E.; Freitas, M. Size-dependent cytotoxicity of silver nanoparticles in human neutrophils assessed by multiple analytical approaches. Life Sci. 2016, 145, 247–254. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A review on the toxicity of silver nanoparticles on human health. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Li, T.; Albee, B.; Alemayehu, M.; Diaz, R.; Ingham, L.; Kamal, S.; Rodriguez, M.; Bishnoi, W.S. Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Anal. Bioanal. Chem. 2010, 398, 689–700. [Google Scholar] [CrossRef]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Mohammed, M.K. Studying the bactericidal ability and biocompatibility of gold and gold oxide nanoparticles decorating on multi-wall carbon nanotubes. Chem. Pap. 2020, 74, 4033–4046. [Google Scholar] [CrossRef]
- Ni, Z.; Gu, X.; He, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Synthesis of silver nanoparticle-decorated hydroxyapatite (ha@ag) poriferous nanocomposites and the study of their antibacterial activities. RSC Adv. 2018, 8, 41722–41730. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Fabrication of silver nanoparticles embedded into polyvinyl alcohol (ag/pva) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced raman scattering (sers) activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 462–469. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical intermediate-modified gold nanoparticles: Against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Gucchait, A.; Paul, S.; Saha, T.; Acharya, S.; Hoque, K.M.; Misra, A.K.; Chatterjee, B.K.; Chatterjee, T.; Chakrabarti, P. Virstatin-conjugated gold nanoparticle with enhanced antimicrobial activity against the vibrio cholerae el tor biotype. ACS Appl. Bio Mater. 2021, 4, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic nanoparticles for antimicrobial applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Majhi, R.K.; Singh, A.; Mishra, M.; Tiwari, A.; Chawla, S.; Guha, P.; Satpati, B.; Mohapatra, H.; Goswami, L.; et al. Carbohydrate-coated gold–silver nanoparticles for efficient elimination of multidrug resistant bacteria and in vivo wound healing. ACS Appl. Mater. Interfaces 2019, 11, 42998–43017. [Google Scholar] [CrossRef]
- Bahrami, K.; Nazari, P.; Nabavi, M.; Golkar, M.; Almasirad, A.; Shahverdi, A.R. Hydroxyl capped silver-gold alloy nanoparticles: Characterization and their combination effect with different antibiotics against Staphylococcus aureus. Nanomed. J. 2014, 1, 155–161. [Google Scholar]
- Medina-Cruz, D.; Saleh, B.; Vernet-Crua, A.; Nieto-Argüello, A.; Lomelí-Marroquín, D.; Vélez-Escamilla, L.Y.; Cholula-Díaz, J.L.; García-Martín, J.M.; Webster, T. Bimetallic nanoparticles for biomedical applications: A review. In Racing for the Surface; Springer: Berlin/Heidelberg, Germany, 2020; pp. 397–434. [Google Scholar]
- Guo, B.; Alivio, T.E.; Fleer, N.A.; Feng, M.; Li, Y.; Banerjee, S.; Sharma, V.K. Elucidating the role of dissolved organic matter and sunlight in mediating the formation of Ag–Au bimetallic alloy nanoparticles in the aquatic environment. Environ. Sci. Technol. 2021, 55, 1710–1720. [Google Scholar] [CrossRef]
- Simon, J.; Nampoori, V.; Kailasnath, M. Concentration dependent thermo-optical properties and nonlinear optical switching behavior of bimetallic Au-Ag nanoparticles synthesized by femtosecond laser ablation. Opt. Laser Technol. 2021, 140, 107022. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Wang, X.; Wang, M.; Su, X. One-step fabrication of wavelength-tunable luminescence of gold-silver bimetallic nanoclusters: Robust performance for α-glucosidase assay. Sens. Actuators B Chem. 2021, 345, 130407. [Google Scholar] [CrossRef]
- Navya, P.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Srinivas, S.P.; Jain, D.; Amin, M.H.; Bhargava, S.K.; Daima, H.K. Single step formation of biocompatible bimetallic alloy nanoparticles of gold and silver using isonicotinylhydrazide. Mater. Sci. Eng. C 2019, 96, 286–294. [Google Scholar] [CrossRef]
- Mohsin, M.; Jawad, M.; Yameen, M.A.; Waseem, A.; Shah, S.H.; Shaikh, A.J.J.P. An insight into the coating behavior of bimetallic silver and gold core-shell nanoparticles. Plasmonics 2020, 15, 1599–1612. [Google Scholar] [CrossRef]
- Sierra, M.A.; Casarrubios, L.; de la Torre, M.C. Bio-organometallic derivatives of antibacterial drugs. Chemistry 2019, 25, 7232–7242. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359. [Google Scholar] [CrossRef]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent developments in the medicinal applications of silver-nhc complexes and imidazolium salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.L.; Gu, Y.; Huang, H.; Zhang, G.W. Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukherjee, P.; Senapati, D.; Srinivasan, R. Bimetallic gold-silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, D.K.; Cha, H.G.; Kim, C.W.; Kang, Y.C.; Kang, Y.S. Preparation and characterization of the antibacterial Cu nanoparticle formed on the surface of SiO2 nanoparticles. J. Phys. Chem. B 2006, 110, 24923–24928. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotech. 2017, 15, 65. [Google Scholar] [CrossRef]
- Wang, Y.; Malkmes, M.J.; Jiang, C.; Wang, P.; Zhu, L.; Zhang, H.; Zhang, Y.; Huang, H.; Jiang, L. Antibacterial mechanism and transcriptome analysis of ultra-small gold nanoclusters as an alternative of harmful antibiotics against Gram-negative bacteria. J. Hazard. Mater. 2021, 416, 126236. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef]
- Keren, I.; Wu, Y.; Inocencio, J.; Mulcahy, L.R.; Lewis, K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 2013, 339, 1213–1216. [Google Scholar] [CrossRef]
- Boonstra, J.; Post, J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 2004, 337, 1–13. [Google Scholar] [CrossRef]
- Długosz, O.; Sochocka, M.; Ochnik, M.; Banach, M. Metal and bimetallic nanoparticles: Flow synthesis, bioactivity and toxicity. J. Colloid. Interface Sci. 2021, 586, 807–818. [Google Scholar] [CrossRef]
- Nasrabadi, H.T.; Abbasi, E.; Davaran, S.; Kouhi, M.; Akbarzadeh, A. Bimetallic nanoparticles: Preparation, properties, and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 376–380. [Google Scholar] [CrossRef]
- Devi, N.; Sahoo, S.; Kumar, R.; Singh, R.K. A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 2021, 13, 11679–11711. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.T.; McMurtrey, M.D. Development of Bismuth and Platinum Bi-Metallic Nanoparticles to Enhance Melt Wire Temperature Resolution, Idaho National Lab. (INL), Idaho Falls, ID (United States). 2021. Available online: https://www.osti.gov/biblio/1813574 (accessed on 16 September 2022).
- Kawai, S.; Mardis, M.; Machmudah, S.; Kanda, H.; Zhao, Y.; Goto, M. Bimetallic nanoparticle generation from Au− TiO2 film by pulsed laser ablation in an aqueous medium. Alex. Eng. J. 2021, 60, 2225–2234. [Google Scholar]
- Zhang, J.; Chen, T.; Jiao, Y.; Cheng, M.; Wang, L.-L.; Wang, J.-L. Improved activity of Ni–Mo/SiO2 bimetallic catalyst synthesized via sol-gel method for methylcyclohexane cracking. Pet. Sci. 2021, 18, 1530–1542. [Google Scholar] [CrossRef]
- Devarajan, S.; Bera, P.; Sampath, S. Bimetallic nanoparticles: A single step synthesis, stabilization, and characterization of Au–Ag, Au–Pd, and Au–Pt in sol–gel derived silicates. J. Colloid Interface Sci. 2005, 290, 117–129. [Google Scholar] [CrossRef]
- Wack, S.; Lunca Popa, P.; Adjeroud, N.; Vergne, C.; Leturcq, R. Two-step approach for conformal chemical vapor-phase deposition of ultra-thin conductive silver films. ACS Appl. Mater. Interfaces 2020, 12, 36329–36338. [Google Scholar] [CrossRef]
- Williams-Godwin, L.; Brown, D.; Livingston, R.; Webb, T.; Karriem, L.; Graugnard, E. Open-source automated chemical vapor deposition system for the production of two- dimensional nanomaterials. PLoS ONE 2019, 14, e0210817. [Google Scholar] [CrossRef]
- Boies, A.M.; Roberts, J.T.; Girshick, S.L.; Zhang, B.; Nakamura, T.; Mochizuki, A. SiO2 coating of silver nanoparticles by photoinduced chemical vapor deposition. Nanotechnology 2009, 20, 295604. [Google Scholar] [CrossRef]
- Fei, L.; Naeemi, M.; Zou, G.; Luo, H. Chemical solution deposition of epitaxial metal-oxide nanocomposite thin films. Chem. Rec. 2013, 13, 85–101. [Google Scholar] [CrossRef]
- Alti, D.; Veeramohan Rao, M.; Rao, D.N.; Maurya, R.; Kalangi, S.K. Gold–silver bimetallic nanoparticles reduced with herbal leaf extracts induce ROS-mediated death in both promastigote and amastigote stages of leishmania donovani. ACS Omega 2020, 5, 16238–16245. [Google Scholar] [CrossRef]
- Acharya, D.; Mohanta, B.; Pandey, P. Green synthesis of Silver and Silver-gold core-shell nanoparticles using Pineapple leaf extract (Ananas comosus) and study of their antibacterial properties. Int. J. Nano Dimens. 2021, 12, 203–210. [Google Scholar]
- Bhuyan, B.; Arijita, P.; Bappi, P.; Siddhartha S., D.; Pranab, D. Paederia foetida Linn. promoted biogenic gold and silver nanoparticles: Synthesis, characterization, photocatalytic and in vitro efficacy against clinically isolated pathogen. J. Photochem. Photobio. B 2017, 173, 210–215. [Google Scholar] [CrossRef]
- Khan, M.; Al-Hamoud, K.; Liaqat, Z.; Shaik, M.R.; Adil, S.F.; Kuniyil, M. Synthesis of Au, Ag, and Au–Ag bimetallic nanoparticles using Pulicaria undulata extract and their catalytic activity for the reduction of 4-nitrophenol. Nanomaterials 2020, 10, 1885. [Google Scholar] [CrossRef]
- Krishnan Sundarrajan, S.; Pottail, L. Green synthesis of bimetallic Ag@ Au nanoparticles with aqueous fruit latex extract of Artocarpus heterophyllus and their synergistic medicinal efficacies. Appl. Nanosci. 2021, 11, 971–981. [Google Scholar] [CrossRef]
- Ramakritinan, C.; Kaarunya, E.; Shankar, S.; Kumaraguru, A. Antibacterial effects of Ag, Au and bimetallic (Ag-Au) nanoparticles synthesized from red algae. In Solid State Phenomena; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; pp. 211–230. [Google Scholar]
- Dahoumane, S.A.; Wijesekera, K.; Filipe, C.D.; Brennan, J.D. Stoichiometrically controlled production of bimetallic gold-silver alloy colloids using micro-alga cultures. J. Colloid Interface Sci. 2014, 416, 67–72. [Google Scholar] [CrossRef]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular biosynthesis of bimetallic Au–Ag alloy nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef]
- Maliszewska, I.; Wanarska, E.; Tylus, W. Sulfonated hydroxyaluminum phthalocyanine-biogenic Au/Ag alloy nanoparticles mixtures for effective photo-eradication of Candida albicans. Photodiagnosis Photodyn. Ther. 2020, 32, 102016. [Google Scholar] [CrossRef]
- Ding, X.; Yuan, P.; Gao, N.; Zhu, H.; Yang, Y.Y.; Xu, Q.H. Au-Ag core-shell nanoparticles for simultaneous bacterial imaging and synergistic antibacterial activity. Nanomedicine 2017, 13, 297–305. [Google Scholar] [CrossRef]
- Narendra; Mehata, A.K.; Viswanadh, M.K.; Sonkar, R.; Pawde, D.M.; Priya, V.; Singh, M.; Koch, B.; Muthu, M. Formulation and in vitro evaluation of upconversion nanoparticle-loaded liposomes for brain cancer. Ther. Deliv. 2020, 11, 557–571. [Google Scholar] [CrossRef]
- Nieto-Argüello, A.; Medina-Cruz, D.; Pérez-Ramírez, Y.S.; Pérez-García, S.A.; Velasco-Soto, M.A.; Jafari, Z. Composition-dependent cytotoxic and antibacterial activity of biopolymer-capped ag/au bimetallic nanoparticles against melanoma and multidrug-resistant pathogens. Nanomaterials 2022, 12, 779. [Google Scholar] [CrossRef]
- Rao, C.V.; Muttalik, S. Exploring bimetallic Au-Ag Core Shell Nanoparticles Reduced Using Leaf Extract of Ocimum Tenuiflorum as a Potential Antibacterial and Nanocatalytic Agent. Preprint. 2022. Available online: https://www.researchsquare.com/article/rs-1175999/v1 (accessed on 16 September 2022).
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.M. Kinetics of monodisperse iron oxide nanocrystal formation by “heating-up” process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef]
- Banerjee, M.; Sharma, S.; Chattopadhyay, A.; Ghosh, S.S. Enhanced antibacterial activity of bimetallic gold-silver core–shell nanoparticles at low silver concentration. Nanoscale 2011, 3, 5120–5125. [Google Scholar] [CrossRef]
- Ray, P.; Clément, M.; Martini, C.; Abdellah, I.; Beaunier, P.; Rodriguez-Lopez, J.-L. Stabilisation of small mono-and bimetallic gold–silver nanoparticles using calix [8] arene derivatives. New J. Chem. 2018, 42, 14128–14137. [Google Scholar] [CrossRef]
- Kariuki, N.N.; Luo, J.; Hassan, S.A.; Lim, I.-I.S.; Wang, L.; Zhong, C.J. Assembly of bimetallic gold-silver nanoparticles via selective interparticle dicarboxylate-silver linkages. Chem. Mater. 2006, 18, 123–132. [Google Scholar] [CrossRef]
- Anandan, S.; Grieser, F.; Ashokkumar, M. Sonochemical synthesis of Au–Ag core-shell bimetallic nanoparticles. J. Phys. Chem. C 2008, 112, 15102–15105. [Google Scholar] [CrossRef]
- Luchini, A.; Tidemand, F.G.; Johansen, N.T.; Campana, M.; Sotres, J.; Ploug, M. Peptide disc mediated control of membrane protein orientation in supported lipid bilayers for surface-sensitive investigations. Anal. Chem. 2020, 92, 1081–1088. [Google Scholar] [CrossRef]
- Haldar, K.K.; Kundu, S.; Patra, A. Core-size-dependent catalytic properties of bimetallic Au/Ag core-shell nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 21946–21953. [Google Scholar] [CrossRef]
- Berahim, N.; Basirun, W.J.; Leo, B.F.; Johan, M.R. Synthesis of bimetallic gold-silver (Au-Ag) nanoparticles for the catalytic reduction of 4-nitrophenol to 4-aminophenol. Catalysts 2018, 8, 412. [Google Scholar] [CrossRef]
- Rakshitha, R.; Gurupadayya, B.; Devi, S.H.K.; Pallavi, N. Coprecipitation aided synthesis of bimetallic silver tungstate: A response surface simulation of sunlight-driven photocatalytic removal of 2,4-dichlorophenol. Environ. Sci. Pollut. Res. Int. 2022, 29, 59433–59443. [Google Scholar] [CrossRef]
- Sun, L.; Guan, J.; Xu, Q.; Yang, X.; Wang, J.; Hu, X. Synthesis and Applications of Molecularly Imprinted Polymers Modified TiO2 Nanomaterials: A Review. Polymers 2018, 10, 1248. [Google Scholar] [CrossRef]
- Fu, X.; Cai, J.; Zhang, X.; Li, W.D.; Ge, H.; Hu, Y. Top-down fabrication of shape-controlled, monodisperse nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2018, 132, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Slepičková Kasálková, N.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of gold and silver nanoparticles preparation. Materials 2019, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, K.A.; Alomari, M.; Drmosh, Q.; Alheshibri, M.; Al Baroot, A.; Kayed, T.S.; Manda, A.A.; Al-Alotaibi, A.L. Fabrication of ZnO-Ag bimetallic nanoparticles by laser ablation for anticancer activity. Alex. Eng. J. 2022, 61, 1449–1457. [Google Scholar] [CrossRef]
- Anugop, B.; Kailasnath, M. Effect of Au/Ag bimetallic nanoparticles in the lasing characteristics of dye doped microring embedded hollow polymer optical fiber. Mater. Today Proc. 2022, 64, 27–31. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Batista, L.M.F.; John, M.G.; Rodrigues, C.J.; Tibbetts, K.M. Mechanism of gold–silver alloy nanoparticle formation by laser coreduction of gold and silver ions in solution. J. Phys. Chem. B 2021, 125, 907–917. [Google Scholar] [CrossRef]
- Alkhayatt, A.; University of Kufa; Moheel, M.H.; Abood, M.M. Antibacterial activity of mono and bimetallic au:ag colloidal nanoparticles prepared by pulse laser ablation PLA. J. Kufa-Physics 2018, 10, 8–19. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef]
- Heinz, M.; Srabionyan, V.V.; Avakyan, L.A.; Bugaev, A.L.; Skidanenko, A.V.; Kaptelinin, S.Y.; Ihlemann, J.; Meinertz, J.; Patzig, C.; Dubiel, M.; et al. Formation of bimetallic gold-silver nanoparticles in glass by UV laser irradiation. J. Alloy. Compd. 2018, 767, 1253–1263. [Google Scholar] [CrossRef]
- Abed, M.A.; Mutlak, F.A.; Ahmed, A.F.; Nayef, U.M.; Abdulridha, S.K.; Jabir, M.S. Synthesis of Ag/Au (core/shell) nanoparticles by laser ablation in liquid and study of their toxicity on blood human components. J. Phys. Conf. Ser. 2021, 1795, 12013. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Lugarà, P.M.; Palazzo, G.; Cioffi, N. The pros and cons of the use of laser ablation synthesis for the production of silver nano-antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef]
- Mehata, A.K.; Suseela, M.N.L.; Gokul, P.; Malik, A.K.; Viswanadh, M.K.; Singh, C.; Selvin, J.; Muthu, M.S. Fast and highly efficient liquid chromatographic methods for qualification and quantification of antibiotic residues from environmental waste. Microchem. J. 2022, 179, 107573. [Google Scholar] [CrossRef]
- Bankura, K.; Rana, D.; Mollick, M.M.; Pattanayak, S.; Bhowmick, B.; Saha, N.R. Dextrin-mediated synthesis of Ag NPs for colorimetric assays of Cu(2+) ion and Au NPs for catalytic activity. Int. J. Biol. Macromol. 2015, 80, 309–316. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Zaka, M.; Hashmi, S.S.; Khan, Z. Biogenic synthesis of Au, Ag and Au–Ag alloy nanoparticles using Cannabis sativa leaf extract. IET Nanobiotechnol. 2018, 12, 277–284. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and immunomodulatory potentials of biosynthesized Ag, Au, Ag-Au bimetallic alloy nanoparticles using the asparagus racemosus root extract. Nanomaterials 2020, 10, 2453. [Google Scholar] [CrossRef]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef]
- Tabrizi, N.; Tazikeh, M.; Shahgholi, N. Antibacterial properties of Au-Ag alloy nanoparticles. Int. J. Green Nanotechnol. 2012, 4, 489–494. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, Y.; Liu, P.; Li, Y.; Yue, J.; Jiang, L. Atomic-engineering Au-Ag nanoalloys for screening antimicrobial agents with low toxicity towards mammalian cells. Colloids Surf. B Biointerfaces 2021, 204, 111831. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Biosynthesis of Au and Au/Ag alloy nanoparticles using Coleus aromaticus essential oil and evaluation of their catalytic, antibacterial and antiradical activities. J. Mol. Liq. 2016, 221, 179–189. [Google Scholar] [CrossRef]
- Huo, D.; He, J.; Li, H.; Yu, H.; Shi, T.; Feng, Y.; Zhou, Z.; Hu, Y. Fabrication of Au@ Ag core–shell NPs as enhanced CT contrast agents with broad antibacterial properties. Coll. Surf. B Biointerfaces 2014, 117, 29–35. [Google Scholar] [CrossRef]
- Panicker, S.; Ahmady, I.; Han, C.; Chehimi, M.; Mohamed, A. On demand release of ionic silver from gold-silver alloy nanoparticles: Fundamental antibacterial mechanisms study. Mater. Today Chem. 2020, 16, 100237. [Google Scholar] [CrossRef]
- Yang, L.; Yan, W.; Wang, H.; Zhuang, H.; Zhang, J. Shell thickness-dependent antibacterial activity and biocompatibility of gold@ silver core–shell nanoparticles. RSC Adv. 2017, 7, 11355–11361. [Google Scholar] [CrossRef]
- Padmos, J.D.; Langman, M.; MacDonald, K.; Comeau, P.; Yang, Z.; Filiaggi, M.; Zhang, P. Correlating the atomic structure of bimetallic silver–gold nanoparticles to their antibacterial and cytotoxic activities. J. Phys. Chem. C 2015, 119, 7472–7482. [Google Scholar] [CrossRef]
- Zhang, J.; Chaker, M.; Ma, D. Pulsed laser ablation based synthesis of colloidal metal nanoparticles for catalytic applications. J. Coll. Interface Sci. 2017, 489, 138–149. [Google Scholar] [CrossRef]
- Villalobos-Noriega, J.M.A.; Rodríguez-León, E.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Plascencia-Jatomea, M.; Martínez-Higuera, A.; Martínez-Higuera, A.; Acuña-Campa, H.; García-Galaz, A.; Mora-Monroy,, B.; et al. Au@Ag Core@Shell nanoparticles synthesized with Rumex hymenosepalus as antimicrobial agent. Nanoscale Res. Lett. 2021, 16, 118. [Google Scholar] [CrossRef]
- Singh, R.; Nawale, L.; Arkile, M.; Wadhwani, S.; Shedbalkar, U.; Chopade, S.; Sarkar, D.; Chopade, B.A. Phytogenic silver, gold, and bimetallic nanoparticles as novel antitubercular agents. Int. J. Nanomed. 2016, 11, 1889. [Google Scholar]
- Liu, C.; Im, S.H.; Yu, T. Synthesis of au–cu alloy nanoparticles as peroxidase mimetics for H2O2 and glucose colorimetric detection. Catalysts 2021, 11, 343. [Google Scholar] [CrossRef]
- Kalwar, K.; Xi, J.; Ren, C.; Shen, M. Coating of Au@Ag on electrospun cellulose nanofibers for wound healing and antibacterial activity. Korean J. Chem. Eng. 2022, 39, 2165–2171. [Google Scholar] [CrossRef]
- Samal, A.K.; Polavarapu, L.; Rodal-Cedeira, S.; Liz-Marzan, L.M.; Perez-Juste, J.; Pastoriza-Santos, I. Size Tunable Au@ Ag core-shell nanoparticles: Synthesis and surface-enhanced raman scattering properties. Langmuir 2013, 29, 15076–15082. [Google Scholar] [CrossRef]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-resistant bacteria and alternative methods to control them: An overview. Microb. Drug Resist. 2019, 25, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, P.; Sun, H.; Wang, Z. Superhydrophobic surface with hierarchical architecture and bimetallic composition for enhanced antibacterial activity. ACS Appl. Mater. Interfaces 2014, 6, 22108–22115. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Exp. Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Yang, X.; Hou, Q.; Liu, S.; Zheng, W.; Long, Y.; Jiang, X. Mercaptophenylboronic acid-activated gold nanoparticles as nanoantibiotics against multidrug-resistant bacteria. ACS Appl. Mater. Interfaces 2020, 12, 51148–51159. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, Y.; Dong, R.; Deng, J.; Tang, H.; Hu, F.; Liu, S.; Jiang, X. Bimetallic nanoparticles against multi-drug resistant bacteria. Chem Commun. 2020, 56, 10918–10921. [Google Scholar] [CrossRef]
- Argueta-Figueroa, L.; Morales-Luckie, R.A.; Scougall-Vilchis, R.J.; Olea-Mejía, O.F. Synthesis, characterization and antibacterial activity of copper, nickel and bimetallic Cu–Ni nanoparticles for potential use in dental materials. Prog. Nat. Sci. Mater. Int. 2014, 24, 321–328. [Google Scholar] [CrossRef]
- He, J.; Qiao, Y.; Zhang, H.; Zhao, J.; Li, W.; Xie, T.; Zhong, D.; Wei, Q.; Hua, S.; Yu, Y.; et al. Gold-silver nanoshells promote wound healing from drug-resistant bacteria infection and enable monitoring via surface-enhanced Raman scattering imaging. Biomaterials 2020, 234, 119763. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Zhang, Q.; Zhu, S.; Liu, L.; Jiang, X.; Wei, D.H.; Liu, R.S.; Li, L. Gelatin sponge functionalized with gold/silver clusters for antibacterial application. Nanotechnology 2020, 31, 134004. [Google Scholar] [CrossRef]
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peștean, C.; Nagy, A.; Gal, A.F.; Tăbăran, F.; Purdoiu, R.C.; Licărete, E.; et al. The impact of composites with silicate-based glasses and gold nanoparticles on skin wound regeneration. Molecules 2021, 26, 620. [Google Scholar] [CrossRef]
- Mârza, S.M.; Magyari, K.; Bogdan, S.; Moldovan, M.; Peştean, C.; Nagy, A.; Tăbăran, F.; Licarete, E.; Suarasan, S.; Dreanca, A.; et al. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biomed. Mater. 2019, 14, 025011. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.; Prabu Poorani, G.; Jegatheeswaran, S.; Balakumar, C.; Gurumallesh Prabu, H.; Anand, K.; Prabhu, N.M.; Jeyakanthan, J.; Saravanan, M. Phyto-Engineered Gold Nanoparticles (AuNPs) with Potential Antibacterial, Antioxidant, and Wound Healing Activities Under in vitro and in vivo Conditions. Int. J. Nanomed. 2020, 15, 7553–7568. [Google Scholar] [CrossRef]
- Khan, M.I.; Behera, S.K.; Paul, P.; Das, B.; Suar, M.; Jayabalan, R.; Fawcett, D.; Poinern, G.E.J.; Tripathy, S.K.; Mishra, A. Biogenic Au@ZnO core-shell nanocomposites kill Staphylococcus aureus without provoking nuclear damage and cytotoxicity in mouse fibroblasts cells under hyperglycemic condition with enhanced wound healing proficiency. Med. Microbiol. Immunol. 2019, 208, 609–629. [Google Scholar] [CrossRef]
- Kantipudi, S.; Sunkara, J.R.; Rallabhandi, M.; Thonangi, C.V.; Cholla, R.D.; Kollu, P.; Parvathaneni, M.K.; Pammi, S.V.N. Enhanced wound healing activity of Ag-ZnO composite NPs in Wistar Albino rats. IET Nanobiotechnol. 2018, 12, 473–478. [Google Scholar] [CrossRef]
- Bayrami, M.; Bayrami, A.; Habibi-Yangjeh, A.; Shafeeyan, M.S.; Feizpoor, S.; Arvanagh, F.M.; Nourani, M.R.; Taheri, R.A. Biologically-synthesised ZnO/CuO/Ag nanocomposite using propolis extract and coated on the gauze for wound healing applications. IET Nanobiotechnol. 2020, 14, 548–554. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Sharma, N.; Gupta, N.; Kumar, A.; Chatterjee, S.; Nimesh, S. Catalytic, antibacterial and antibiofilm efficacy of biosynthesised silver nanoparticles using Prosopis juliflora leaf extract along with their wound healing potential. J. Photochem. Photobiol. B 2019, 190, 50–58. [Google Scholar] [CrossRef]
- Sood, R.; Chopra, D.S. Optimization of reaction conditions to fabricate Ocimum sanctum synthesized silver nanoparticles and its application to nano-gel systems for burn wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 575–589. [Google Scholar] [CrossRef]
- Ye, H.; Cheng, J.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef]
- Hajji, S.; Khedir, S.B.; Hamza-Mnif, I.; Hamdi, M.; Jedidi, I.; Kallel, R.; Boufi, S.; Nasri, M. Biomedical potential of chitosan-silver nanoparticles with special reference to antioxidant, antibacterial, hemolytic and in vivo cutaneous wound healing effects. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 241–254. [Google Scholar] [CrossRef]
- Bai, M.Y.; Ku, F.Y.; Shyu, J.F.; Hayashi, T.; Wu, C.C. Evaluation of Polyacrylonitrile Nonwoven Mats and Silver-Gold Bimetallic Nanoparticle-Decorated Nonwoven Mats for Potential Promotion of Wound Healing In Vitro and In Vivo and Bone Growth In Vitro. Polymers 2021, 13, 516. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, X.; Xu, W.; Zhang, R.; Wu, G. Biosynthesis of bimetallic Au-Ag nanoparticles using escherichia coli and its biomedical applications. ACS Biomater. Sci. Eng. 2020, 6, 680–689. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Neal, A.L. What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology 2008, 17, 362–371. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Coll. Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Shubha, P.; Gowda, M.L.; Namratha, K.; Shyamsunder, S.; Manjunatha, H.; Byrappa, K. Ex-situ fabrication of ZnO nanoparticles coated silk fiber for surgical applications. Mater. Chem. Phys. 2019, 231, 21–26. [Google Scholar] [CrossRef]
| Material | Method | Microbial Strain | Inhibition Zone (mm) or Rate % | MIC | Other Performance Indicators | References |
|---|---|---|---|---|---|---|
| Au–Ag NPs | Two-photon photoluminescence (2PPL) | S. aureus | - | MIC 7.5 ppm against S. aureus | Au–Ag NPs showed antibacterial activity by inhibiting biofilm | [81] |
| Au–Ag alloy NPs | Sodium borohydrate was used for the core reduction of the metal salts | E. coli ATCC 25922 | - | The antibacterial activity of alloy nano particles was higher than that of pure metals. At a higher molar ratio of silver, the antibacterial activity was improved. | [112] | |
| Au–Ag nanoalloy NPs | Laser ablation | E. coli and S. aureus | Significant bactericidal | [113] | ||
| Au–Ag NPs | Essential oil from Coleus aromaticus leaves was used as the reducing agent | E. coli and S. aureus | E. coli with an inhibition zone of 28 mm | E. coli with an MIC of NPs with 150 μL essential oil | - | [114] |
| Ag-Au bimetallic alloy NPs | Microwave-assisted green synthesis of NPs in the presence of Asparagus racemosus root extract | P. eurgnosia and S. aureus strains | - | - | [109] | |
| Au–Ag core–shell NPs | - | E. coli and S. aureus | - | - | [115] | |
| Au–Ag alloy NPs | - | E. coli ATCC strain 25,922 and S. aureus | - | - | [116] | |
| Au–Ag core–shell NPs | - | E. coli and S. aureus | 5 µg mL−1 for E. coli and 7.5 µg mL−1 for S. aureus | - | [117] | |
| Ag–Au NPs | - | S. aureus | [118] | |||
| Ag–Au NPs | Aqueous leaf extract of Gloriosa superba | B. subtilis ATCC 6633 and E. coli (MTCC 40) | 6.33 ± 0.33 mm for B. subtilis and 5.33 ± 0.33 mm for E. coli | - | Antibiofilm activity | [74] |
| Au–Ag colloidal NPs | Pulsed laser ablation | Gram-positive isolate (S. aureus) and Gram-negative isolate (E. coli) | - | - | [119] | |
| Au–Ag NPs | On pre-synthesized carboxy methyl tamarind, Au–Ag NPs were synthesized by reducing AgNO3 and KAuCl4 simultaneously to produce polysaccharide hydrogel-stabilized Au NPs | E. coli and Enterobacter cloacae | The MIC value of Au–Ag NP for clinical isolate Ec18 was found to be 6 μg/mL and for ATCC MDR isolates BAA-1143 and BAA-2469 was 6 μg/mL | - | [35] | |
| Au–Ag core–shell NPs | Catechins and stilbenes containing root extract of Rumex hymenosepalus were used as reducing agents | E. coli and Candida albicans | S. aureus did not grow at a concentration of 100 µg/mL. | - | [120] | |
| Au–Ag NPs | Phytogenic synthesis from a medicinal plant | M. tuberculosis and M. bovis | Anti TB activity, with MIC of <2.56 μg/mL, | [121] |
| Material | Animal Model | Performance Indicator | References |
|---|---|---|---|
| AuNPs | |||
| Bioactive glass (Silicate based) with spherical gold nanocages in vaseline ointment | In vivo study in rats | Bioactive glass with spherical gold nanocages supported by silver had a high antibacterial activity and wound-healing activity. | [135] |
| Bioactive glass–gold nanoparticles | Experimental rat model | Bioactive glasses led to the promotion of the growth of granulation tissue, while the gold nanoparticles (AuNPs) could induce the acceleration of wound healing by connective tissue formation and angiogenesis. | [136] |
| Green Au NPs | BALB/c mice | BALB/c mice model with infected diabetic wounds. The re-epithelialization layer was fully covered by green-synthesized Au NPs on the wound area and also collagen filled in the scar tissue when compared with the control group. | [137] |
| Au–ZnO core–shell | Mice model | Fabrication of Au–ZnO core–shell nanocomposite. Zinc oxide was overlaid on biogenic gold nanoparticles decorated by Hibiscus Sabdariffa plant extract. | [138] |
| Ag NPs | |||
| Ag–ZnO | In Wistar albino rat | Ag–ZnO composite NPs exhibited rapid healing within 10 days when compared with pure Ag NPs and the standard drug dermazin in rat. | [139] |
| Biosynthesized ZnO/CuO/Ag nanocomposite | Wistar rat | Four metallic NPs were synthesized by biological compounds (Propolis). The results strongly suggested the possibility of adopting this unique ZnO/Ag/Ext material dressing compared to other NPs. | [140] |
| Ag/Ag@AgCl/ZnO hybrid nanostructures (embedded in a hydrogel) | Wistar rat | Ag/Ag@AgCl (hydrogel synthesized via ultraviolet light chemical reduction) followed by the incorporation of ZnO nanostructures by NaOH precipitation). In vivo wound healing showed that Ag+ and Zn2+ release stimulated the immune system to recruit a high number of WBCs and neutrophils (2–4 times more than the control), resulting in faster wound healing. | [141] |
| Green Ag NPs | Mice | Green Ag NPs synthesized using Prosopis juliflora leaf extract were evaluated for the wound rate of healing activity. The wound closure in the treated mice was much faster than in the other two groups (Carbopol only and Povidone–iodine). | [142] |
| Green Ag NPs | Rat model | Green-Ag NP-reduction of AgNO3 using Ocimum sanctum. Skin wound healing was used to test the gel in vivo and the percentage of wound closures was calculated. The gel demonstrated 96% wound-healing activity by the 14th day compared to the standard and control bases. | [143] |
| Porous silver nanoparticle/chitosan composites | Gelatin-reduced AgNP | Wound healing was accelerated by gelatin/chitosan/Ag, which also had good biocompatibility. | [144] |
| Chitosan–PVA–silver nanoparticles (CS–Ag NPs) | PVA and chitosan acting as reducing and stabilizing agent | The wound contraction ratio and histological analysis both showed that the CS–Ag NPs promoted wound healing considerably. | [145] |
| Bimetallic Au–Ag NPs | |||
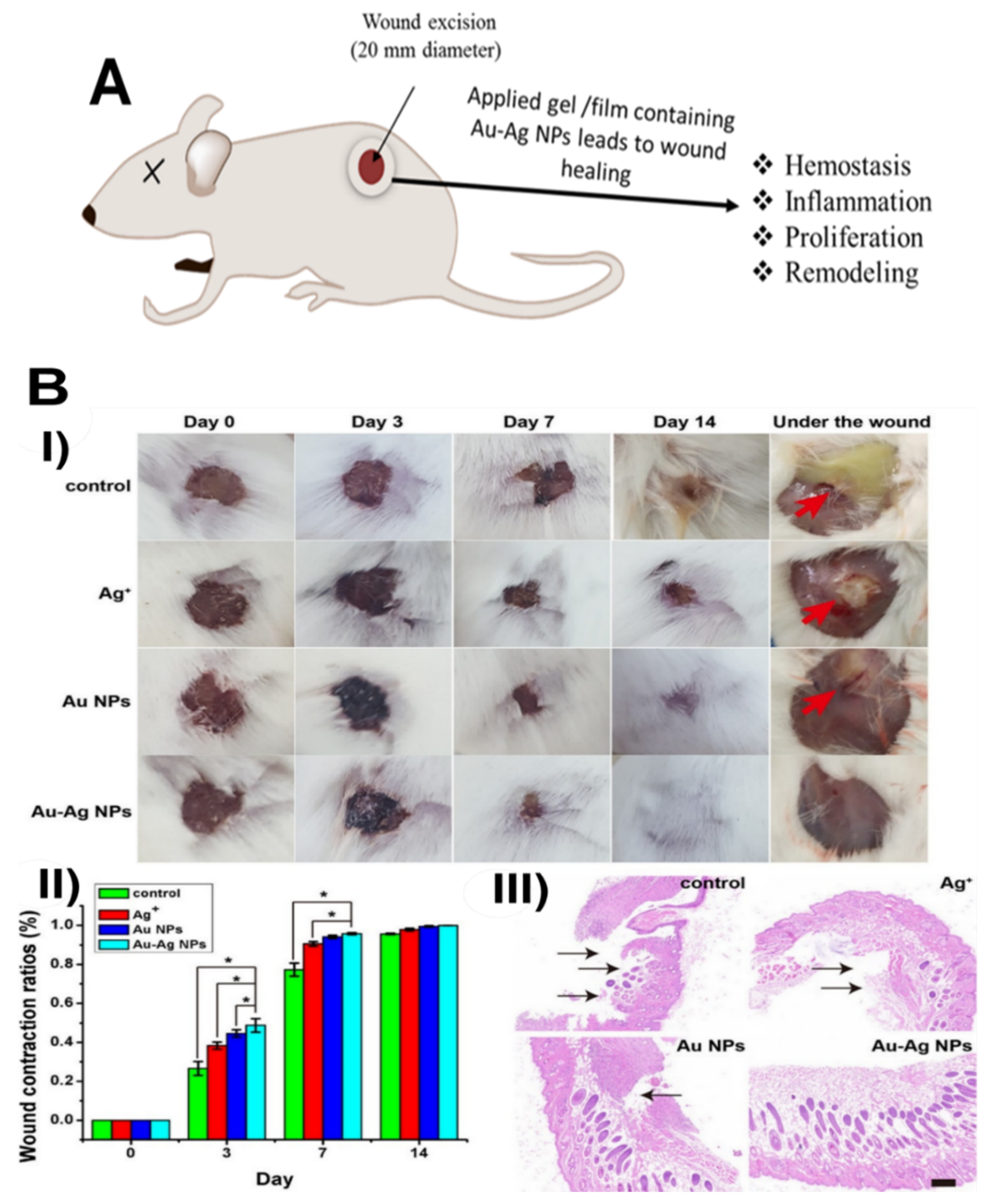
| Au–Ag NPs | Balb/c mice | A wound (5 mm) was created on the back of mice. The results showed that the Au–Ag NPs were highly effective against MDR-infected wounds in mice with no side effects. | [82] |
| Silver–gold bimetallic nanoparticles | Silver–gold bimetallic-nanoparticle-decorated nonwoven mats | Showed significant wound healing recovery. | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, C.; Mehata, A.K.; Priya, V.; Malik, A.K.; Setia, A.; Suseela, M.N.L.; Vikas; Gokul, P.; Samridhi; Singh, S.K.; et al. Bimetallic Au–Ag Nanoparticles: Advanced Nanotechnology for Tackling Antimicrobial Resistance. Molecules 2022, 27, 7059. https://doi.org/10.3390/molecules27207059
Singh C, Mehata AK, Priya V, Malik AK, Setia A, Suseela MNL, Vikas, Gokul P, Samridhi, Singh SK, et al. Bimetallic Au–Ag Nanoparticles: Advanced Nanotechnology for Tackling Antimicrobial Resistance. Molecules. 2022; 27(20):7059. https://doi.org/10.3390/molecules27207059
Chicago/Turabian StyleSingh, Chandrashekhar, Abhishesh Kumar Mehata, Vishnu Priya, Ankit Kumar Malik, Aseem Setia, M. Nikitha Lakshmi Suseela, Vikas, Patharaj Gokul, Samridhi, Sanjeev K. Singh, and et al. 2022. "Bimetallic Au–Ag Nanoparticles: Advanced Nanotechnology for Tackling Antimicrobial Resistance" Molecules 27, no. 20: 7059. https://doi.org/10.3390/molecules27207059
APA StyleSingh, C., Mehata, A. K., Priya, V., Malik, A. K., Setia, A., Suseela, M. N. L., Vikas, Gokul, P., Samridhi, Singh, S. K., & Muthu, M. S. (2022). Bimetallic Au–Ag Nanoparticles: Advanced Nanotechnology for Tackling Antimicrobial Resistance. Molecules, 27(20), 7059. https://doi.org/10.3390/molecules27207059