Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management
Abstract
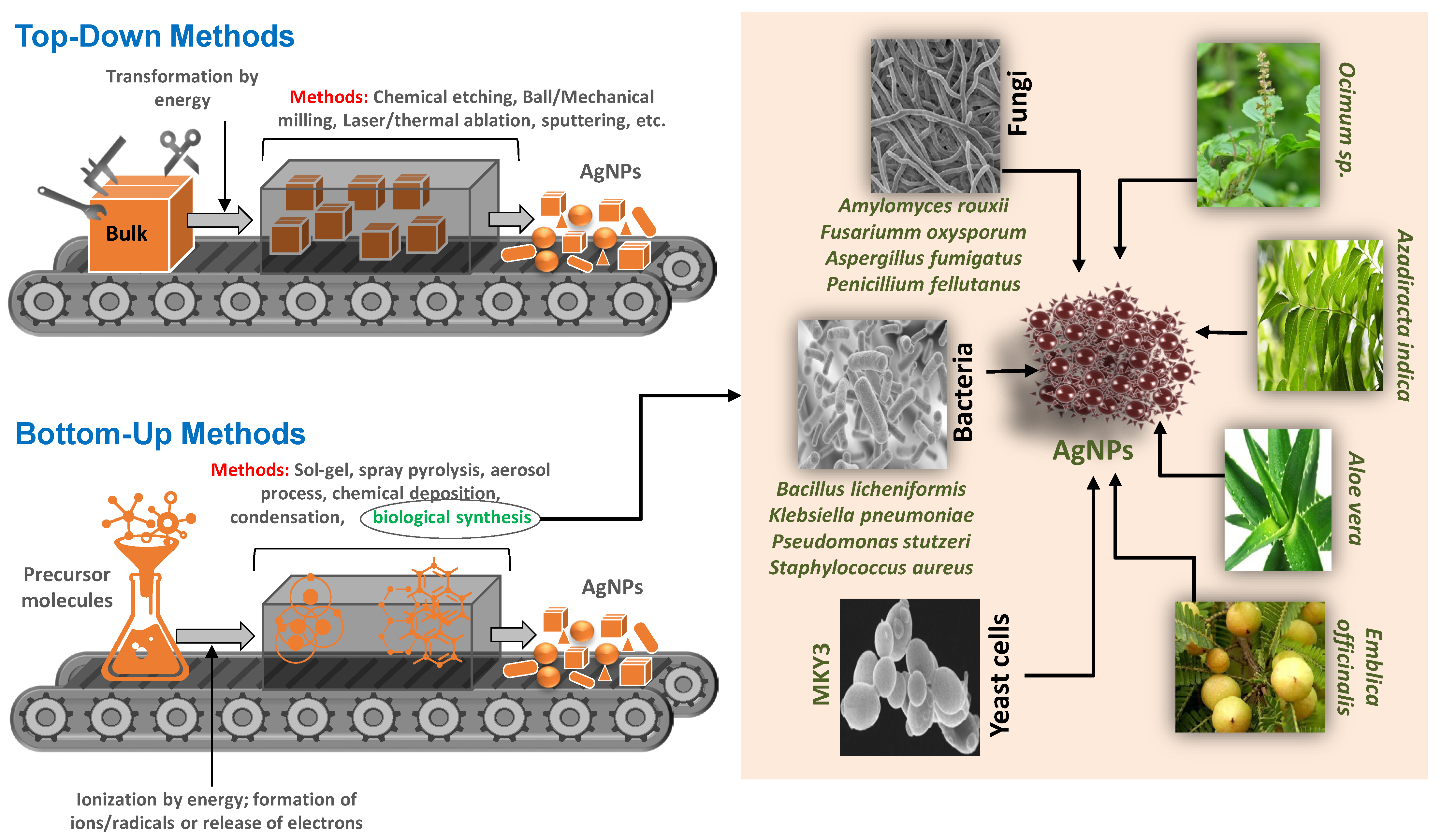
:1. Introduction
2. Biosynthesis of AgNPs
2.1. Biological Synthesis of AgNPs by Plant Parts
2.2. Biological Synthesis of AgNPs by Bacteria
2.3. Biological Synthesis of AgNPs by Algae and Fungi
3. Determining the Size, Shape, and Yield of Biological AgNPs
4. Silver Nanoparticles in Plant Disease Management
4.1. AgNPs in Bacterial Disease Management
4.2. AgNPs in Viral Disease Management
4.3. AgNPs in Fungal Disease Management
4.4. AgNPs in Nematode Disease Management
4.5. Mechanisms of AgNPs Mediated Inhibition or Killing of Phytopathogens
5. Biological AgNPs against Plant Pathogens: Possible Implications in Lab to Land Transfer
6. Environmental Relevance and Possible Toxicity of Biological AgNPs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savary, S.; Ficke, A.; Aubertot, J.-N.; Hollier, C. Crop Losses Due to Diseases and Their Implications for Global Food Production Losses and Food Security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Shahid, M.; Ahmed, B.; Zaidi, A.; Khan, M.S. Toxicity of Fungicides to: Pisum Sativum: A Study of Oxidative Damage, Growth Suppression, Cellular Death and Morpho-Anatomical Changes. RSC Adv. 2018, 8, 38483–38498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, M.; Ahmed, B.; Khan, M.S. Evaluation of Microbiological Management Strategy of Herbicide Toxicity to Greengram Plants. Biocatal. Agric. Biotechnol. 2018, 14, 96–108. [Google Scholar] [CrossRef]
- Rani, P.; Ahmed, B.; Singh, J.; Kaur, J.; Rawat, M.; Kaur, N.; Matharu, A.S.; AlKahtanie, M.; Alhomaidif, E.A.H.; Lee, J. Silver Nanostructures Prepared via Novel Green Approach as an Effective Platform for Biological and Environmental Applications. Saudi J. Biol. Sci. 2022, 29, 103296. [Google Scholar] [CrossRef]
- Kaur, K.; Ahmed, B.; Singh, J.; Rawat, M.; Kaur, G.; AlKahtani, M.; Alhomaidi, E.A.H.; Lee, J. Bryonia Laciniosa Linn Mediated Synthesized Green Au NPs for Catalytic and Antimicrobial Applications. J. King Saud Univ. 2022, 34, 102022. [Google Scholar] [CrossRef]
- Janani, B.; Syed, A.; Raju, L.L.; Bahkali, A.H.; Al-Rashed, S.; Elgorban, A.M.; Ahmed, B.; Thomas, A.M.; Khan, S.S. Designing Intimate Porous Al2O3 Decorated 2D CdO Nano-Heterojunction as Enhanced White Light Driven Photocatalyst and Antibacterial Agent. J. Alloys Compd. 2022, 896, 162807. [Google Scholar] [CrossRef]
- Sinha, K.; Ghosh, J.; Sil, P.C. New Pesticides: A Cutting-Edge View of Contributions from Nanotechnology for the Development of Sustainable Agricultural Pest Control. In New Pesticides and Soil Sensors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–79. [Google Scholar]
- Arya, S.S.; Sharma, M.M.; Das, R.K.; Rookes, J.; Cahill, D.; Lenka, S.K. Vanillin Mediated Green Synthesis and Application of Gold Nanoparticles for Reversal of Antimicrobial Resistance in Pseudomonas Aeruginosa Clinical Isolates. Heliyon 2019, 5, e02021. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Li, C.; Dag, Ö.; Abe, H.; Takei, T.; Imai, T.; Hossain, M.; Shahriar, A.; Islam, M.; Wood, K. Mesoporous Metallic Rhodium Nanoparticles. Nat. Commun. 2017, 8, 15581. [Google Scholar] [CrossRef]
- Elmer, W.H.; White, J.C. The Use of Metallic Oxide Nanoparticles to Enhance Growth of Tomatoes and Eggplants in Disease Infested Soil or Soilless Medium. Environ. Sci. Nano 2016, 3, 1072–1079. [Google Scholar] [CrossRef]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for Plant Disease Management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-José, N.; Abhilash, P.C.; Fraceto, L.F.; De Lima, R. Nanoparticles Based on Chitosan as Carriers for the Combined Herbicides Imazapic and Imazapyr. Sci. Rep. 2016, 6, 19768. [Google Scholar] [CrossRef]
- He, Y.; Qian, L.; Liu, X.; Hu, R.; Huang, M.; Liu, Y.; Chen, G.; Losic, D.; Zhu, H. Graphene Oxide as an Antimicrobial Agent Can Extend the Vase Life of Cut Flowers. Nano Res. 2018, 11, 6010–6022. [Google Scholar] [CrossRef]
- Saha, N.; Dutta Gupta, S. Promotion of Shoot Regeneration of Swertia Chirata by Biosynthesized Silver Nanoparticles and Their Involvement in Ethylene Interceptions and Activation of Antioxidant Activity. Plant Cell Tissue Organ Cult. 2018, 134, 289–300. [Google Scholar] [CrossRef]
- Thangavelu, R.M.; Gunasekaran, D.; Jesse, M.I.; SU, M.R.; Sundarajan, D.; Krishnan, K. Nanobiotechnology Approach Using Plant Rooting Hormone Synthesized Silver Nanoparticle as “Nanobullets” for the Dynamic Applications in Horticulture—An In Vitro and Ex Vitro Study. Arab. J. Chem. 2018, 11, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of Nano-Silicon Dioxide Improves Salt Stress Tolerance in Strawberry Plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Feregrino-Perez, A.A.; Magaña-López, E.; Guzmán, C.; Esquivel, K. A General Overview of the Benefits and Possible Negative Effects of the Nanotechnology in Horticulture. Sci. Hortic. 2018, 238, 126–137. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Juan, J.C.; Phang, S.-M. Production of New Cellulose Nanomaterial from Red Algae Marine Biomass Gelidium Elegans. Carbohydr. Polym. 2016, 151, 1210–1219. [Google Scholar] [CrossRef]
- Ali, K.; Ahmed, B.; Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Microwave Accelerated Green Synthesis of Stable Silver Nanoparticles with Eucalyptus Globulus Leaf Extract and Their Antibacterial and Antibiofilm Activity on Clinical Isolates. PLoS ONE 2015, 10, e0131178. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, L.; Khalil, A.T.; Ali, N.; Zia, D.; Ali, M.; Shinwari, Z.K. Endophyte-Mediated Synthesis of Silver Nanoparticles and Their Biological Applications. Appl. Microbiol. Biotechnol. 2019, 103, 2551–2569. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef]
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.-Z.M.A.; Husseiny, S.M. Extracellular Biosynthesis of Silver Nanoparticles Using Rhizopus Stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Phanjom, P.; Ahmed, G. Effect of Different Physicochemical Conditions on the Synthesis of Silver Nanoparticles Using Fungal Cell Filtrate of Aspergillus Oryzae (MTCC No. 1846) and Their Antibacterial Effect. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 45016. [Google Scholar] [CrossRef] [Green Version]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and Characterization of Silver Nanoparticles Using Trichoderma Longibrachiatum and Their Effect on Phytopathogenic Fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.C.; Chen, W.M.; Chen, C.Y.; Xiong, S.X.; Yu, A.B. Role of Temperature in the Growth of Silver Nanoparticles through a Synergetic Reduction Approach. Nanoscale Res. Lett. 2011, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Y.; Yu, H.; He, D.; Yang, H.; Wang, W.; Wan, X.; Wang, L. Biosynthesis of Silver Nanoparticles by the Endophytic Fungus Epicoccum Nigrum and Their Activity against Pathogenic Fungi. Bioprocess Biosyst. Eng. 2013, 36, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Xu, Q.; Huang, M.; Xian, L.; Feng, J.-X. Synthesis of Small Silver Nanoparticles under Light Radiation by Fungus Penicillium Oxalicum and Its Application for the Catalytic Reduction of Methylene Blue. Mater. Chem. Phys. 2015, 160, 40–47. [Google Scholar] [CrossRef]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS Mediated Destruction of Cell Membrane, Growth and Biofilms of Human Bacterial Pathogens by Stable Metallic AgNPs Functionalized from Bell Pepper Extract and Quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Panja, A.; Mishra, A.K.; Dash, M.; Pandey, N.K.; Singh, S.K.; Kumar, B. Silver Nanoparticles–A Review. Eurasian J. Med. Oncol. 2021, 5, 95. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Mehmood, A. Brief Overview of the Application of Silver Nanoparticles to Improve Growth of Crop Plants. IET Nanobiotechnol. 2018, 12, 701–705. [Google Scholar] [CrossRef]
- Haroon, M.; Zaidi, A.; Ahmed, B.; Rizvi, A.; Khan, M.S.M.S.; Musarrat, J. Effective Inhibition of Phytopathogenic Microbes by Eco-Friendly Leaf Extract Mediated Silver Nanoparticles (AgNPs). Indian J. Microbiol. 2019, 59, 273–287. [Google Scholar] [CrossRef]
- Parveen, A.; Rao, S. Effect of Nanosilver on Seed Germination and Seedling Growth in Pennisetum Glaucum. J. Clust. Sci. 2015, 26, 693–701. [Google Scholar] [CrossRef]
- Jasim, B.; Thomas, R.; Mathew, J.; Radhakrishnan, E.K. Plant Growth and Diosgenin Enhancement Effect of Silver Nanoparticles in Fenugreek (Trigonella Foenum-Graecum L.). Saudi Pharm. J. 2017, 25, 443–447. [Google Scholar] [CrossRef]
- Latif, H.H.; Ghareib, M.; Tahon, M.A. Phytosynthesis of Silver Nanoparticles Using Leaf Extracts from Ocimum Basilicum and Mangifira Indica and Their Effect on Some Biochemical Attributes of Triticum Aestivum. Gesunde Pflanz. 2017, 69, 39–46. [Google Scholar] [CrossRef]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M.Q. Nanosilver Particles in Medical Applications: Synthesis, Performance, and Toxicity. Int. J. Nanomed. 2014, 9, 2399. [Google Scholar]
- Biradar, D.; Lingappa, K.; Dayanand, A. Antibacterial Activity of Nano Goldparticles Synthesized by Bacillus Sps. J. Ecobiotechnol. 2012, 4, 43–45. [Google Scholar]
- Arokiyaraj, S.; Vincent, S.; Saravanan, M.; Lee, Y.; Oh, Y.K.; Kim, K.H. Green Synthesis of Silver Nanoparticles Using Rheum Palmatum Root Extract and Their Antibacterial Activity against Staphylococcus Aureus and Pseudomonas Aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017, 45, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Sumathi, S.; Thomas, A. Eco-Friendly and Antibacterial Finishes of Organic Fabrics Using Herbal Composite Microencapsules. Int. J. Pharma Bio Sci. 2017, 8, 310–321. [Google Scholar]
- Joseph, S.; Mathew, B. Microwave Assisted Biosynthesis of Silver Nanoparticles Using the Rhizome Extract of Alpinia Galanga and Evaluation of Their Catalytic and Antimicrobial Activities. J. Nanopart. 2014, 2014, 967802. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green Synthesis of Silver Nanoparticles Using Azadirachta Indica Aqueous Leaf Extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green Synthesis of Silver Nanoparticles Using Coffea Arabica Seed Extract and Its Antibacterial Activity. Mater. Sci. Eng. C 2016, 58, 36–43. [Google Scholar] [CrossRef]
- Fatimah, I.; Indriani, N. Silver Nanoparticles Synthesized Using Lantana Camara Flower Extract by Reflux, Microwave and Ultrasound Methods. Chem. J. Mold. 2018, 13, 95–102. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green Synthesis of Silver Nanoparticles Using Andean Blackberry Fruit Extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Smita, K.; Cumbal, L.; Angulo, Y. Fabrication of Silver Nanoplates Using Nephelium Lappaceum (Rambutan) Peel: A Sustainable Approach. J. Mol. Liq. 2015, 211, 476–480. [Google Scholar] [CrossRef]
- Ojha, S.; Sett, A.; Bora, U. Green Synthesis of Silver Nanoparticles by Ricinus Communis Var. Carmencita Leaf Extract and Its Antibacterial Study. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 35009. [Google Scholar] [CrossRef]
- Joshi, P.S.; Ramesh, G.; Packiyam, J.E.; Jayanna, S.K. Green Synthesis and Evaluation of Silver Nanoparticles Using Leaf Extract from Calotropis Gigantean. Int. J. Curr. Biotechnol. 2007, 5, 1–5. [Google Scholar]
- Ahmed, B.; Syed, A.; Ali, K.; Elgorban, A.M.; Khan, A.; Lee, J.; AL-Shwaiman, H.A. Synthesis of Gallotannin Capped Iron Oxide Nanoparticles and Their Broad Spectrum Biological Applications. RSC Adv. 2021, 11, 9880–9893. [Google Scholar] [CrossRef]
- Ali, K.; Saquib, Q.; Ahmed, B.; Siddiqui, M.A.; Ahmad, J.; Al-Shaeri, M.; Al-Khedhairy, A.A.; Musarrat, J. Bio-Functionalized CuO Nanoparticles Induced Apoptotic Activities in Human Breast Carcinoma Cells and Toxicity against Aspergillus Flavus: An in Vitro Approach. Process Biochem. 2020, 91, 387–397. [Google Scholar] [CrossRef]
- Ahmed, B.; Solanki, B.; Zaidi, A.; Khan, M.S.; Musarrat, J. Bacterial Toxicity of Biomimetic Green Zinc Oxide Nanoantibiotic: Insights into ZnONP Uptake and Nanocolloid-Bacteria Interface. Toxicol. Res. 2019, 8, 246–261. [Google Scholar] [CrossRef] [Green Version]
- Ali, K.; Ahmed, B.; Khan, M.S.; Musarrat, J. Differential Surface Contact Killing of Pristine and Low EPS Pseudomonas Aeruginosa with Aloe Vera Capped Hematite (α-Fe2O3) Nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 188, 146–158. [Google Scholar] [CrossRef]
- Jebakumar Immanuel Edison, T.N.; Sethuraman, M.G. Electrocatalytic Reduction of Benzyl Chloride by Green Synthesized Silver Nanoparticles Using Pod Extract of Acacia Nilotica. ACS Sustain. Chem. Eng. 2013, 1, 1326–1332. [Google Scholar] [CrossRef]
- Mallikarjuna, K.; Narasimha, G.; Dillip, G.R.; Praveen, B.; Shreedhar, B.; Lakshmi, C.S.; Reddy, B.V.S.; Raju, B.D.P. Green Synthesis of Silver Nanoparticles Using Ocimum Leaf Extract and Their Characterization. Dig. J. Nanomater. Biostruct. 2011, 6, 181–186. [Google Scholar]
- Vanaja, M.; Annadurai, G. Coleus Aromaticus Leaf Extract Mediated Synthesis of Silver Nanoparticles and Its Bactericidal Activity. Appl. Nanosci. 2013, 3, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Green Synthesis and Characterization of Silver Nanoparticles Using Lantana Camara Leaf Extract. Mater. Sci. Eng. C 2015, 49, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.J.; Vali, D.N.; Rani, M.; Rani, S.S. Evaluation of Antioxidant, Antibacterial and Cytotoxic Effects of Green Synthesized Silver Nanoparticles by Piper Longum Fruit. Mater. Sci. Eng. C 2014, 34, 115–122. [Google Scholar] [CrossRef]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer Activity of Moringa Oleifera Mediated Silver Nanoparticles on Human Cervical Carcinoma Cells by Apoptosis Induction. Colloids Surf. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef]
- Atale, N.; Saxena, S.; Nirmala, J.G.; Narendhirakannan, R.T.; Mohanty, S.; Rani, V. Synthesis and Characterization of Sygyzium Cumini Nanoparticles for Its Protective Potential in High Glucose-Induced Cardiac Stress: A Green Approach. Appl. Biochem. Biotechnol. 2017, 181, 1140–1154. [Google Scholar] [CrossRef]
- Anil Kumar, S.; Abyaneh, M.K.; Gosavi, S.W.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate Reductase-Mediated Synthesis of Silver Nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A Comprehensive Review on Green Nanomaterials Using Biological Systems: Recent Perception and Their Future Applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Malarkodi, C.; Rajeshkumar, S.; Paulkumar, K.; Gnanajobitha, G.; Vanaja, M.; Annadurai, G. Bacterial Synthesis of Silver Nanoparticles by Using Optimized Biomass Growth of Bacillus sp. Nanosci. Nanotechnol. 2013, 3, 26–32. [Google Scholar]
- Hallol, M. Studies on Bacterial Synthesis of Silver Nanoparticles Using Gamma Radiation and Their Activity against Some Pathogenic Microbes. 2013. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/46/066/46066330.pdf (accessed on 1 July 2022).
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Extracellular Synthesis of Silver Nanoparticles by the Bacillus Strain CS 11 Isolated from Industrialized Area. 3 Biotech 2014, 4, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, H.; Khan, S. Biological Synthesis of Silver Nanoparticles and Its Antibacterial Activity. J. Nanomed. Nanotechnol. 2016, 7, 1000366. [Google Scholar] [CrossRef] [Green Version]
- Parikh, R.Y.; Ramanathan, R.; Coloe, P.J.; Bhargava, S.K.; Patole, M.S.; Shouche, Y.S.; Bansal, V. Genus-Wide Physicochemical Evidence of Extracellular Crystalline Silver Nanoparticles Biosynthesis by Morganella spp. PLoS ONE 2011, 6, e21401. [Google Scholar] [CrossRef]
- Shivaji, S.; Madhu, S.; Singh, S. Extracellular Synthesis of Antibacterial Silver Nanoparticles Using Psychrophilic Bacteria. Process Biochem. 2011, 46, 1800–1807. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of Silver Nanoparticles from Bacillus Brevis (NCIM 2533) and Their Antibacterial Activity against Pathogenic Bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A Review of Green Synthesis of Metal Nanoparticles Using Algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef]
- Akther, T.; Mathipi, V.; Kumar, N.S.; Davoodbasha, M.; Srinivasan, H. Fungal-Mediated Synthesis of Pharmaceutically Active Silver Nanoparticles and Anticancer Property against A549 Cells through Apoptosis. Environ. Sci. Pollut. Res. 2019, 26, 13649–13657. [Google Scholar] [CrossRef]
- Barabadi, H.; Tajani, B.; Moradi, M.; Damavandi Kamali, K.; Meena, R.; Honary, S.; Mahjoub, M.A.; Saravanan, M. Penicillium Family as Emerging Nanofactory for Biosynthesis of Green Nanomaterials: A Journey into the World of Microorganisms. J. Clust. Sci. 2019, 30, 843–856. [Google Scholar] [CrossRef]
- Gade, A.; Ingle, A.; Whiteley, C.; Rai, M. Mycogenic Metal Nanoparticles: Progress and Applications. Biotechnol. Lett. 2010, 32, 593–600. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Dash, B.P.; Sukla, L.B.; Panda, P.K. Biosynthesis and Characterization of Silver Nanoparticles Using Microalga Chlorococcum Humicola and Its Antibacterial Activity. Int. J. Nanomater Biostruct 2013, 3, 1–8. [Google Scholar]
- Jena, J.; Pradhan, N.; Dash, B.P.; Panda, P.K.; Mishra, B.K. Pigment Mediated Biogenic Synthesis of Silver Nanoparticles Using Diatom Amphora Sp. and Its Antimicrobial Activity. J. Saudi Chem. Soc. 2015, 19, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green Synthesis of Silver Nanoparticles Using Marine Algae Caulerpa Racemosa and Their Antibacterial Activity against Some Human Pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Sahayaraj, K.; Rajesh, S.; Rathi, J.M. Silver nanoparticles biosynthesis using marine alga padina pavonica (linn.) and its microbicidal activity. Dig. J. Nanomater. Biostruct. 2012, 7, 1557–1567. [Google Scholar]
- Kannan, R.; Arumugam, R.; Ramya, D.; Manivannan, K.; Anantharaman, P. Green Synthesis of Silver Nanoparticles Using Marine Macroalga Chaetomorpha Linum. Appl. Nanosci. 2013, 3, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and Characterization of Silver Nanoparticles Using Gelidium Amansii and Its Antimicrobial Property against Various Pathogenic Bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Bhainsa, K.C.; D’souza, S.F. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Aspergillus Fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K.; Manivannan, S.; Nabeel, M.A.; Dhivya, B. Studies on Silver Nanoparticles Synthesized by a Marine Fungus, Penicillium Fellutanum Isolated from Coastal Mangrove Sediment. Colloids Surf. B Biointerfaces 2009, 71, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological Synthesis of Silver Nanoparticles Using the Fungus Aspergillus Flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Basavaraja, S.; Balaji, S.D.; Lagashetty, A.; Rajasab, A.H.; Venkataraman, A. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Activity against Pathogenic Fungi in Combination with Fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Rashidipour, M.; Heydari, R. Biosynthesis of Silver Nanoparticles Using Extract of Olive Leaf: Synthesis and in Vitro Cytotoxic Effect on MCF-7 Cells. J. Nanostruct. Chem. 2014, 4, 112. [Google Scholar] [CrossRef] [Green Version]
- Majeed Khan, M.A.; Kumar, S.; Ahamed, M.; Alrokayan, S.A.; AlSalhi, M.S. Structural and Thermal Studies of Silver Nanoparticles and Electrical Transport Study of Their Thin Films. Nanoscale Res. Lett. 2011, 6, 434. [Google Scholar] [CrossRef] [Green Version]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Suib, S.L. Biosynthesis of Iron and Silver Nanoparticles at Room Temperature Using Aqueous Sorghum Bran Extracts. Langmuir 2011, 27, 264–271. [Google Scholar] [CrossRef]
- Lukman, A.I.; Gong, B.; Marjo, C.E.; Roessner, U.; Harris, A.T. Facile Synthesis, Stabilization, and Anti-Bacterial Performance of Discrete Ag Nanoparticles Using Medicago Sativa Seed Exudates. J. Colloid Interface Sci. 2011, 353, 433–444. [Google Scholar] [CrossRef]
- Raju, D.; Mehta, U.J.; Hazra, S. Synthesis of Gold Nanoparticles by Various Leaf Fractions of Semecarpus Anacardium L. Tree. Trees 2011, 25, 145–151. [Google Scholar] [CrossRef]
- Marchiol, L. Synthesis of Metal Nanoparticles in Living Plants. Ital. J. Agron. 2012, 7, e37. [Google Scholar] [CrossRef]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of Silver Nanoparticles Using Mangosteen Leaf Extract and Evaluation of Their Antimicrobial Activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for Rapid Synthesis of Silver Nanoparticles and Its Effect on Phytopathogenic Fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Ali, M.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Sre, P.R.R.; Reka, M.; Poovazhagi, R.; Kumar, M.A.; Murugesan, K. Antibacterial and Cytotoxic Effect of Biologically Synthesized Silver Nanoparticles Using Aqueous Root Extract of Erythrina Indica Lam. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 1137–1144. [Google Scholar]
- Braithwaite, M.C.; Tyagi, C.; Tomar, L.K.; Kumar, P.; Choonara, Y.E.; Pillay, V. Nutraceutical-Based Therapeutics and Formulation Strategies Augmenting Their Efficiency to Complement Modern Medicine: An Overview. J. Funct. Foods 2014, 6, 82–99. [Google Scholar] [CrossRef]
- Bose, D.; Chatterjee, S. Biogenic Synthesis of Silver Nanoparticles Using Guava (Psidium Guajava) Leaf Extract and Its Antibacterial Activity against Pseudomonas Aeruginosa. Appl. Nanosci. 2016, 6, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-resistant Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Ahmed, B.; Ameen, F.; Rizvi, A.; Ali, K.; Sonbol, H.; Zaidi, A.; Khan, M.S.; Musarrat, J. Destruction of Cell Topography, Morphology, Membrane, Inhibition of Respiration, Biofilm Formation, and Bioactive Molecule Production by Nanoparticles of Ag, ZnO, CuO, TiO2, and Al2O3 toward Beneficial Soil Bacteria. ACS Omega 2020, 5, 7861–7876. [Google Scholar] [CrossRef] [Green Version]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Biosynthesis of Silver Nanoparticles Using Citrus Sinensis Peel Extract and Its Antibacterial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef]
- Palanisamy, S.; Rajasekar, P.; Vijayaprasath, G.; Ravi, G.; Manikandan, R.; Prabhu, N.M. A Green Route to Synthesis Silver Nanoparticles Using Sargassum Polycystum and Its Antioxidant and Cytotoxic Effects: An in Vitro Analysis. Mater. Lett. 2017, 189, 196–200. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; de Melo, A.P.Z.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; de Maciel, M.V.O.B.; Bertoldi, F.C.; Barreto, P.L.M. Application in Situ of Zein Nanocapsules Loaded with Origanum Vulgare Linneus and Thymus Vulgaris as a Preservative in Bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- Kang, Y.O.; Lee, T.S.; Park, W.H. Green Synthesis and Antimicrobial Activity of Silver Chloride Nanoparticles Stabilized with Chitosan Oligomer. J. Mater. Sci. Mater. Med. 2014, 25, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Tang, H.; Wu, D.; Liu, D.; Liu, Y.; Cao, A.; Wang, H. Enhanced Bactericidal Toxicity of Silver Nanoparticles by the Antibiotic Gentamicin. Environ. Sci. Nano 2016, 3, 788–798. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Liu, X. Silver Nanoparticles—The Real “Silver Bullet” in Clinical Medicine? Medchemcomm 2010, 1, 125–131. [Google Scholar] [CrossRef]
- Wu, J.; Yu, C.; Tan, Y.; Hou, Z.; Li, M.; Shao, F.; Lu, X. Effects of Prenatal Exposure to Silver Nanoparticles on Spatial Cognition and Hippocampal Neurodevelopment in Rats. Environ. Res. 2015, 138, 67–73. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Cui, L.; Xu, B.; Zhang, H.; Yu, C.P. Interaction of Silver Nanoparticles with Pure Nitrifying Bacteria. Chemosphere 2013, 90, 1404–1411. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial Properties of Nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and Their Role in Phytopathogens Management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef]
- Masum, M.; Islam, M.; Siddiqa, M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus Emblica Fruit Extract and Its Inhibitory Action against the Pathogen Acidovorax Oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusarium Graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef] [Green Version]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.; Fouad, H.; Zhang, M.; Zhang, Y.; Qiu, W.; Yan, C.; Li, B.; Mo, J.; Chen, J. Biosynthesis of Silver Nanoparticles Using Endophytic Bacteria and Their Role in Inhibition of Rice Pathogenic Bacteria and Plant Growth Promotion. RSC Adv. 2019, 9, 29293–29299. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green Synthesis of Metallic Nanoparticles as Effective Alternatives to Treat Antibiotics Resistant Bacterial Infections: A Review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J.C.; Duran, P.; Lourenço, I.M.; Seabra, A.B. Bactericidal and Virucidal Activities of Biogenic Metal-Based Nanoparticles: Advances and Perspectives. Antibiotics 2021, 10, 783. [Google Scholar] [CrossRef]
- Andleeb, S.; Tariq, F.; Muneer, A.; Nazir, T.; Shahid, B.; Latif, Z.; Abbasi, S.A.; ul Haq, I.; Majeed, Z.; Khan, S.U.-D. In Vitro Bactericidal, Antidiabetic, Cytotoxic, Anticoagulant, and Hemolytic Effect of Green-Synthesized Silver Nanoparticles Using Allium Sativum Clove Extract Incubated at Various Temperatures. Green Process. Synth. 2020, 9, 538–553. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, C.; Guzmán-Moreno, J.; Ángeles-Chávez, C.; Rodríguez-González, V.; Ortega-Sigala, J.J.; Ramírez-Santoyo, R.M.; Vidales-Rodríguez, L.E. Biosynthesis of Silver Nanoparticles by Fusarium Scirpi and Its Potential as Antimicrobial Agent against Uropathogenic Escherichia Coli Biofilms. PLoS ONE 2020, 15, e0230275. [Google Scholar] [CrossRef] [Green Version]
- Noshad, A.; Iqbal, M.; Hetherington, C.; Wahab, H. Biogenic AgNPs—a Nano Weapon against Bacterial Canker of Tomato (Bct). Adv. Agric. 2020, 2020, 9630785. [Google Scholar] [CrossRef]
- Le Thi, H.; Nguyen, T.P.H.; Le Trong, D.; Vu, T.H.; Le Thi, V.; Hoang, T.G.; Chu, D.H.; Nguyen, T.H.; Nguyen, D.P.; Le Huy, H. Synthesis of Chitosan Stabilized Silver Nanoparticles and Evaluation of the in Vitro Antibacterial Activity Against Xanthomonas Oryzae Pv. Oryzae Causing Blight Disease of Rice. VNU J. Sci. Nat. Sci. Technol. 2021, 6, 65–75. [Google Scholar]
- Cheng, H.-J.; Wang, H.; Zhang, J.-Z. Phytofabrication of Silver Nanoparticles Using Three Flower Extracts and Their Antibacterial Activities against Pathogen Ralstonia Solanacearum Strain YY06 of Bacterial Wilt. Front. Microbiol. 2020, 11, 2110. [Google Scholar] [CrossRef]
- Xing, Y.; Liao, X.; Liu, X.; Li, W.; Huang, R.; Tang, J.; Xu, Q.; Li, X.; Yu, J. Characterization and Antimicrobial Activity of Silver Nanoparticles Synthesized with the Peel Extract of Mango. Materials 2021, 14, 5878. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C. Silver Nanoparticles Synthesized by Using Bacillus Cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens 2020, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- del Carmen Travieso Novelles, M.; Ortega, A.R.; Pita, B.A.; López, M.C.; Pérez, L.D.; Medina, E.A.; Pérez, O.P. Biosynthesis of Fluorescent Silver Nanoparticles from Leea Coccinea Leaves and Their Antibacterial Potentialities against Xanthomonas Phaseoli Pv Phaseoli. Bioresour. Bioprocess. 2021, 8, 3. [Google Scholar] [CrossRef]
- Mazzon, M.; Marsh, M. Targeting Viral Entry as a Strategy for Broad-Spectrum Antivirals. F1000Research 2019, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Noha, K.; Bondok, A.M.; El-Dougdoug, K.A. Evaluation of Silver Nanoparticles as Antiviral Agent against ToMV and PVY in Tomato Plants. Sciences 2018, 8, 100–111. [Google Scholar]
- Elbeshehy, E.K.F.; Elazzazy, A.M.; Aggelis, G. Silver Nanoparticles Synthesis Mediated by New Isolates of Bacillus spp., Nanoparticle Characterization and Their Activity against Bean Yellow Mosaic Virus and Human Pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef] [Green Version]
- Shafie, R.M.; Salama, A.M.; Farroh, K.Y. Silver Nanoparticles Activity against Tomato Spotted Wilt Virus. Middle East J. Appl. Sci. 2018, 7, 1251–1267. [Google Scholar]
- El-Shazly, M.A.; Attia, Y.A.; Kabil, F.F.; Anis, E.; Hazman, M. Inhibitory Effects of Salicylic Acid and Silver Nanoparticles on Potato Virus Y-Infected Potato Plants in Egypt. Middle East J. Agric. Res. 2017, 6, 835–848. [Google Scholar]
- Jain, D.; Kothari, S.L. Green Synthesis of Silver Nanoparticles and Their Application in Plant Virus Inhibition. J. Mycol Plant Pathol 2014, 44, 21–24. [Google Scholar]
- Mahfouze, H.A.; El-Dougdoug, N.K.; Mahfouze, S.A. Virucidal Activity of Silver Nanoparticles against Banana Bunchy Top Virus (BBTV) in Banana Plants. Bull. Natl. Res. Cent. 2020, 44, 199. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.; Jia, H.; Liu, C.; Wang, D.; Sun, X. Foliar Exposure of Fe3O4 Nanoparticles on Nicotiana Benthamiana: Evidence for Nanoparticles Uptake, Plant Growth Promoter and Defense Response Elicitor against Plant Virus. J. Hazard. Mater. 2020, 393, 122415. [Google Scholar] [CrossRef]
- Vinković, T.; Novák, O.; Strnad, M.; Goessler, W.; Jurašin, D.D.; Parađiković, N.; Vrček, I.V. Cytokinin Response in Pepper Plants (Capsicum Annuum L.) Exposed to Silver Nanoparticles. Environ. Res. 2017, 156, 10–18. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, Accumulation and Toxicity of Silver Nanoparticle in Autotrophic Plants, and Heterotrophic Microbes: A Concentric Review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Hassan, S.A.M.; Mahfouze, H.A.; Mahfouze, S.A.; Abd-Allatif, A.M. Genotoxicity Assessment of Nano-Particles on Micropropagated Olive (Olea Europaea L.) Plants Using RAPD and DAMD Markers. Plant Arch. 2019, 19, 1985–1994. [Google Scholar]
- Elazzazy, A.M.; Elbeshehy, E.K.F.; Betiha, M.A. In Vitro Assessment of Activity of Graphene Silver Composite Sheets against Multidrug-Resistant Bacteria and Tomato Bushy Stunt Virus. Trop. J. Pharm. Res. 2017, 16, 2705–2711. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, C.; Xu, C.; Wang, Z.; Zhao, M.; Wang, C.; Liu, L.; Chen, F. Preliminary Experiments on Nano-Silver against Tobacco Mosaic Virus and Its Mechanism. Tob. Sci. Technol. 2016, 49, 22–30. [Google Scholar]
- Jo, Y.-K.; Cromwell, W.; Jeong, H.-K.; Thorkelson, J.; Roh, J.-H.; Shin, D.-B. Use of Silver Nanoparticles for Managing Gibberella Fujikuroi on Rice Seedlings. Crop Prot. 2015, 74, 65–69. [Google Scholar] [CrossRef]
- Nyilasi, I.; Kocsubé, S.; Krizsán, K.; Galgóczy, L.; Pesti, M.; Papp, T.; Vágvölgyi, C. In Vitro Synergistic Interactions of the Effects of Various Statins and Azoles against Some Clinically Important Fungi. FEMS Microbiol. Lett. 2010, 307, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Guilger, M.; Pasquoto-Stigliani, T.; Bilesky-Jose, N.; Grillo, R.; Abhilash, P.C.; Fraceto, L.F.; de Lima, R. Biogenic Silver Nanoparticles Based on Trichoderma Harzianum: Synthesis, Characterization, Toxicity Evaluation and Biological Activity. Sci. Rep. 2017, 7, 44421. [Google Scholar] [CrossRef]
- Auyeung, A.; Casillas-Santana, M.A.; Martínez-Castañón, G.A.; Slavin, Y.N.; Zhao, W.; Asnis, J.; Häfeli, U.O.; Bach, H. Effective Control of Molds Using a Combination of Nanoparticles. PLoS ONE 2017, 12, e0169940. [Google Scholar] [CrossRef] [Green Version]
- Fatima, F.; Verma, S.R.; Pathak, N.; Bajpai, P. Extracellular Mycosynthesis of Silver Nanoparticles and Their Microbicidal Activity. J. Glob. Antimicrob. Resist. 2016, 7, 88–92. [Google Scholar] [CrossRef]
- Mallmann, E.J.J.; Cunha, F.A.; Castro, B.N.M.F.; Maciel, A.M.; Menezes, E.A.; Fechine, P.B.A. Antifungal Activity of Silver Nanoparticles Obtained by Green Synthesis. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 165–167. [Google Scholar] [CrossRef]
- Gopinath, V.; Velusamy, P. Extracellular Biosynthesis of Silver Nanoparticles Using Bacillus sp. GP-23 and Evaluation of Their Antifungal Activity towards Fusarium Oxysporum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 170–174. [Google Scholar] [CrossRef]
- Hahn, M. The Rising Threat of Fungicide Resistance in Plant Pathogenic Fungi: Botrytis as a Case Study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of Bactericidal Action of Silver Zeolite and Its Comparison with That of Silver Nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia Coli and Staphylococcus Aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-Spectrum Bioactivities of Silver Nanoparticles: The Emerging Trends and Future Prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- Al-Thabaiti, S.A.; Khan, Z.; Manzoor, N. Biosynthesis of Silver Nanoparticles and Its Antibacterial and Antifungal Activities towards Gram-Positive, Gram-Negative Bacterial Strains and Different Species of Candida Fungus. Bioprocess Biosyst. Eng. 2015, 38, 1773–1781. [Google Scholar]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, Characterization and Antifungal Activity of Chemically and Fungal-produced Silver Nanoparticles against T Richophyton Rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.-K.; Ma, Q.-H.; Li, S.-Y.; Zhang, D.-Q.; Cong, L.; Tian, Y.-L.; Yang, R.-Y. The Antifungal Effect of Silver Nanoparticles on Trichosporon Asahii. J. Microbiol. Immunol. Infect. 2016, 49, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bera, R.K.; Mandal, S.M.; Raj, C.R. Antimicrobial Activity of Fluorescent Ag Nanoparticles. Lett. Appl. Microbiol. 2014, 58, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Balakumaran, M.D.; Ramachandran, R.; Kalaichelvan, P.T. Exploitation of Endophytic Fungus, Guignardia Mangiferae for Extracellular Synthesis of Silver Nanoparticles and Their in Vitro Biological Activities. Microbiol. Res. 2015, 178, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Hiradate, S. Form of Aluminium for Uptake and Translocation in Buckwheat (Fagopyrum Esculentum Moench). Planta 2000, 211, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal Effects of Silver Nanoparticles (AgNPs) against Various Plant Pathogenic Fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.J.; Rivera-Gil, P.; Montenegro, J.-M.; Parak, W.J.; De Smedt, S.C.; Braeckmans, K. Cellular Toxicity of Inorganic Nanoparticles: Common Aspects and Guidelines for Improved Nanotoxicity Evaluation. Nano Today 2011, 6, 446–465. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of Silver Nanoparticles Using Malva Parviflora and Their Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.-R.M.A.; Almunqedhi, B.M.A. Biosynthesis of Silver Nanoparticles Using Penicillium Verrucosum and Analysis of Their Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Vo, T.N.N.; Le, N.T.T.; Thi, D.P.N.; Thi, T.T.H. Evaluation of Saponin-Rich/Poor Leaf Extract-Mediated Silver Nanoparticles and Their Antifungal Capacity. Green Process. Synth. 2020, 9, 429–439. [Google Scholar] [CrossRef]
- Shafea, T.M.M.; Mahmoud, K.M. Green Synthesis, Characterization and Antimicrobial Activity of Silver Nanoparticles Using Locally Growing Allium Sativum Extract. Zanco J. Pure Appl. Sci. 2021, 33, 35–41. [Google Scholar]
- Anum, F.; Jabeen, K.; Javad, S.; Iqbal, S.; Tahir, A.; Javed, Z.; Cruz-Martins, N.; Ayatollahi, S.A.; Sharifi-Rad, J.; Alshehri, M.M. Green Synthesized Silver Nanoparticles as Potent Antifungal Agent against Aspergillus Terreus Thom. J. Nanomater. 2021, 2021, 2992335. [Google Scholar] [CrossRef]
- Le, N.T.T.; Nguyen, D.H.; Nguyen, N.H.; Ching, Y.C.; Pham Nguyen, D.Y.; Ngo, C.Q.; Nhat, H.N.T.; Hoang Thi, T.T. Silver Nanoparticles Ecofriendly Synthesized by Achyranthes Aspera and Scoparia Dulcis Leaf Broth as an Effective Fungicide. Appl. Sci. 2020, 10, 2505. [Google Scholar] [CrossRef] [Green Version]
- Ghojavand, S.; Madani, M.; Karimi, J. Green Synthesis, Characterization and Antifungal Activity of Silver Nanoparticles Using Stems and Flowers of Felty Germander. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2987–2997. [Google Scholar] [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Junior, A.G.O.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal Activity of Silver Nanoparticles and Simvastatin against Toxigenic Species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef]
- Nokkrut, B.; Pisuttipiched, S.; Khantayanuwong, S.; Puangsin, B. Silver Nanoparticle-Based Paper Packaging to Combat Black Anther Disease in Orchid Flowers. Coatings 2019, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.H.; Vo, T.N.N.; Nguyen, N.T.; Ching, Y.C.; Hoang Thi, T.T. Comparison of Biogenic Silver Nanoparticles Formed by Momordica Charantia and Psidium Guajava Leaf Extract and Antifungal Evaluation. PLoS ONE 2020, 15, e0239360. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of Silver Nanoparticles against Fungal Pathogens. Front. Nanotechnol. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Vrandečić, K.; Ćosić, J.; Ilić, J.; Ravnjak, B.; Selmani, A.; Galić, E.; Pem, B.; Barbir, R.; Vinković Vrček, I.; Vinković, T. Antifungal Activities of Silver and Selenium Nanoparticles Stabilized with Different Surface Coating Agents. Pest Manag. Sci. 2020, 76, 2021–2029. [Google Scholar] [CrossRef]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Maafi, Z.T. Current Nematode Threats to World Agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Berlin, Germany, 2011; pp. 21–43. [Google Scholar]
- Baronia, R.; Kumar, P.; Singh, S.P.; Walia, R.K. Silver Nanoparticles as a Potential Nematicide against Meloidogyne Graminicola. J. Nematol. 2020, 52, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fouda, M.M.G.; Abdelsalam, N.R.; Gohar, I.M.A.; Hanfy, A.E.M.; Othman, S.I.; Zaitoun, A.F.; Allam, A.A.; Morsy, O.M.; El-Naggar, M. Utilization of High Throughput Microcrystalline Cellulose Decorated Silver Nanoparticles as an Eco-Nematicide on Root-Knot Nematodes. Colloids Surf. B Biointerfaces 2020, 188, 110805. [Google Scholar] [CrossRef]
- Lim, D.; Roh, J.; Eom, H.; Choi, J.; Hyun, J.; Choi, J. Oxidative Stress-related PMK-1 P38 MAPK Activation as a Mechanism for Toxicity of Silver Nanoparticles to Reproduction in the Nematode Caenorhabditis Elegans. Environ. Toxicol. Chem. 2012, 31, 585–592. [Google Scholar] [CrossRef]
- Miura, N.; Shinohara, Y. Cytotoxic Effect and Apoptosis Induction by Silver Nanoparticles in HeLa Cells. Biochem. Biophys. Res. Commun. 2009, 390, 733–737. [Google Scholar] [CrossRef]
- Hassan, M.E.M.; Zawam, H.S.; El-Nahas, S.E.M.; Desoukey, A.F. Comparison Study between Silver Nanoparticles and Two Nematicides against Meloidogyne Incognita on Tomato Seedlings. Plant Pathol. J. 2016, 15, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Soliman, B.S.; Abbassy, M.A.; Abdel-Rasoul, M.A.; Nassar, A.M. Efficacy of Silver Nanoparticles of Extractives of Artemisia Judaica against Root-Knot Nematode. J. Environ. Stud. Res 2017, 7, 1–13. [Google Scholar]
- Nassar, A.M.K. Effectiveness of Silver Nano-Particles of Extracts of Urtica Urens (Urticaceae) against Root-Knot Nematode Meloidogyne Incognita. Asian J. Nematol. 2016, 5, 14–19. [Google Scholar] [CrossRef]
- Walters, D.R.; Newton, A.C.; Lyon, G.D. Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection; John Wiley & Sons: New York, NY, USA, 2014; ISBN 1118371836. [Google Scholar]
- Wuyts, N.; De Waele, D.; Swennen, R. Extraction and Partial Characterization of Polyphenol Oxidase from Banana (Musa Acuminata Grande Naine) Roots. Plant Physiol. Biochem. 2006, 44, 308–314. [Google Scholar] [CrossRef]
- Fabiyi, O.; Olatunji, G.; Atolani, O.; Olawuyi, O. Preparation of Bio-Nematicidal Nanoparticles of Eucalyptus Officinalis for the Control of Cyst Nematode (Heterodera Sacchari). J. Anim. Plant Sci. 2020, 30, 1172–1177. [Google Scholar]
- Kalaiselvi, D.; Mohankumar, A.; Shanmugam, G.; Nivitha, S.; Sundararaj, P. Green Synthesis of Silver Nanoparticles Using Latex Extract of Euphorbia Tirucalli: A Novel Approach for the Management of Root Knot Nematode, Meloidogyne Incognita. Crop Prot. 2019, 117, 108–114. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Abdel-Rasoul, M.A.; Nassar, A.M.K.; Soliman, B.S.M. Nematicidal Activity of Silver Nanoparticles of Botanical Products against Root-Knot Nematode, Meloidogyne Incognita. Arch. Phytopathol. Plant Prot. 2017, 50, 909–926. [Google Scholar] [CrossRef]
- El-Deen, A.H.N.; El-Deeb, B.A. Effectiveness of Silver Nanoparticles against Root-Knot Nematode, Meloidogyne Incognita Infecting Tomato under Greenhouse Conditions. J. Agric. Sci. 2018, 10, 148–156. [Google Scholar]
- Hamed, S.M.; Hagag, E.S.; El-Raouf, N.A. Green Production of Silver Nanoparticles, Evaluation of Their Nematicidal Activity against Meloidogyne Javanica and Their Impact on Growth of Faba Bean. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, K.F.; Abdelfattah, R.H.; El-Ansary, M.S.M. Green Nanoparticles Engineering on Root-Knot Nematode Infecting Eggplants and Their Effect on Plant DNA Modification. Iran. J. Biotechnol. 2016, 14, 250. [Google Scholar] [CrossRef] [Green Version]
- Cromwell, W.A.; Yang, J.; Starr, J.L.; Jo, Y.-K. Nematicidal Effects of Silver Nanoparticles on Root-Knot Nematode in Bermudagrass. J. Nematol. 2014, 46, 261. [Google Scholar]
- Ghareeb, R.Y.; EL-Saedy, M.A.M.; Fathy, M.M.; Hafez, E.E.; Basyony, A.B.A. Green Biosynthesized Silver Nanoparticles Using Macroalgae and Their Usage as a Bioagent on Meloidogyne incognita. Plant Archives. 2020, 20, 9682–9692. [Google Scholar]
- Ardakani, A.S. Toxicity of Silver, Titanium and Silicon Nanoparticles on the Root-Knot Nematode, Meloidogyne Incognita, and Growth Parameters of Tomato. Nematology 2013, 15, 671–677. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Attia, M.S.; Nofel, M.M.; El-Sayyad, G.S. Potential Nematicidal Properties of Silver Boron Nanoparticles: Synthesis, Characterization, in Vitro and in Vivo Root-Knot Nematode (Meloidogyne Incognita) Treatments. J. Clust. Sci. 2019, 30, 687–705. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Alfy, H.; Fahmy, A.A.; Ali, H.M.; Abdelsalam, N.R. Utilization of Cladophora Glomerata Extract Nanoparticles as Eco-Nematicide and Enhancing the Defense Responses of Tomato Plants Infected by Meloidogyne Javanica. Sci. Rep. 2020, 10, 19968. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Jian, Y.; Chen, X.; Ahmed, T.; Shang, Q.; Zhang, S.; Ma, Z.; Yin, Y. Toxicity and Action Mechanisms of Silver Nanoparticles against the Mycotoxin-Producing Fungus Fusarium Graminearum. J. Adv. Res. 2022, 38, 1–12. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Nayak, A.K. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.; Si, Y.; Shu, K. Size-Dependent Cytotoxicity of Silver Nanoparticles to Azotobacter Vinelandii: Growth Inhibition, Cell Injury, Oxidative Stress and Internalization. PLoS ONE 2018, 13, e0209020. [Google Scholar] [CrossRef] [Green Version]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Ciência Saúde Coletiva 2011, 16, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Bergeson, L.L. Nanosilver: US EPA’s pesticide office considers how best to proceed. Environ. Qual. Manag. 2010, 19, 79–85. [Google Scholar] [CrossRef]
- Gurav, A.S.; Kodas, T.T.; Wang, L.-M.; Kauppinen, E.I.; Joutsensaari, J. Generation of nanometer-size fullerene particles via vapor condensation. Chem. Phys. Lett. 1994, 218, 304–308. [Google Scholar] [CrossRef]
- Ahmed, B.; Rizvi, A.; Ali, K.; Lee, J.; Zaidi, A.; Khan, M.S.; Musarrat, J. Nanoparticles in the Soil–Plant System: A Review. Environ. Chem. Lett. 2021, 19, 1545–1609. [Google Scholar] [CrossRef]
- Morales-Díaz, A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of Nanoelements in Plant Nutrition and Its Impact in Ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 13001. [Google Scholar] [CrossRef]

| Plants | Family | Plant Part | Metabolites and Their Structures | Corresponding Particle Size | Refs. | ||
|---|---|---|---|---|---|---|---|
| Acacia nilotica | Fabaceae | Pod |  | 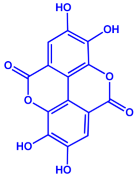 | 20–30 nm | [52] | |
| Gallic acid (C7H6O5) | Ellagic acid (C14H6O8) | ||||||
 |  | ||||||
| Epicatechin (C15H14O6) | Rutin (C27H30O16) | ||||||
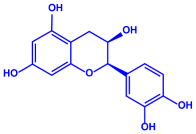
| Ocimum sanctum | Lamiaceae | Fresh leaves | 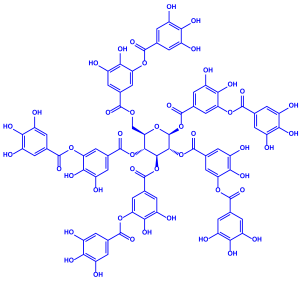 | 15.72 nm | [53] | ||
| Tannins (tannic acid; C76H52O46) | |||||||
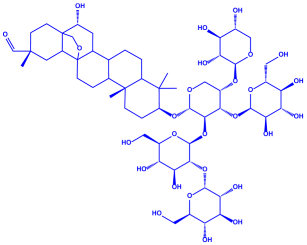 | |||||||
| Saponin (C58H94O27) | |||||||
| Coleus aromaticus | Lamiaceae | Fresh leaves | 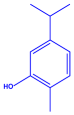 | 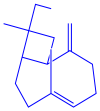 | 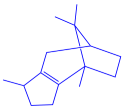 | 44 nm | [54] |
| Carvacrol (C10H14O) | Caryophyllen (C15H24) | Patchoulene (C15H24) | |||||
| Lantana camara | Verbenaceae | Fresh leaves | 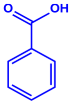 | 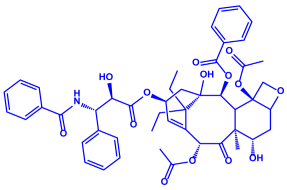 | 14–27 nm | [55] | |
| Phenolic acid | Terpenoid | ||||||
 | |||||||
| Lipid | |||||||
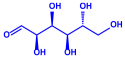 | |||||||
| Carbohydrate | |||||||
| Piper longum | Piperaceae | Dried fruit | 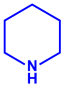 |  | 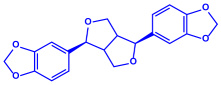 | 15–200 nm | [56] |
| Piperidine (C5H11N) | Terpinenes (C10H16) | Sesamin (C20H18O6) | |||||
| Moringa oleifera | Moringaceae | Fresh stem bark | 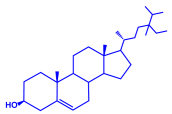 | 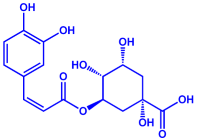 | 40 nm | [57] | |
| β-sitosterol (C29H50O) | Caffeoylquinic acid (C16H18O9) | ||||||
| Syzygium cumini | Myrtaceae | Air dried seeds |  | 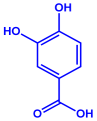 | 40–100 nm | [58] | |
| p-coumaric acid (C9H8O3) | 3,4-dihyroxybenzoic acid (C7H6O4) | ||||||
| Bacterial Strains | Metabolites | Structure | Size | References | ||
|---|---|---|---|---|---|---|
| Serratia nematodiphila |
|  | 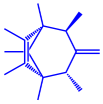 | 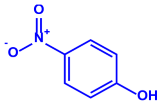 | 65–70 nm | [61] |
| Prodigiosin | Sodorifen | p-Nitrophenol | ||||
| Bacillus stearothermophilus |
| 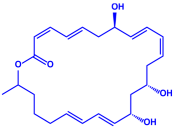 | 14 nm | [62] | ||
| Macrolactin-A | ||||||
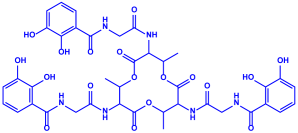 | ||||||
| Bacillibactin | ||||||
| Bacillus strain CS11 |
| 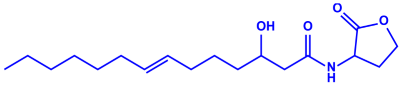 | 42–92 nm | [63] | ||
| Bacteriocin (small) | ||||||
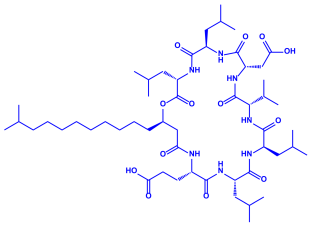 | ||||||
| Surfactin | ||||||
| Escherichia coli |
|  | NA | [64] | ||
| 13-Tetradecynoic acid | ||||||
 | ||||||
| Hexadecanol | ||||||
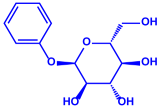
| Morganella morganii RP42 |
|  | 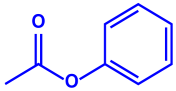 | 10–50 nm | [65] | |
| Phenyl-β-D-glucoside | Phenyl ester | |||||
| Pseudomonas proteolytica and Pseudomonas meridiana |
| 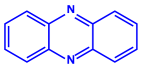 |  | 4–13 nm | [66] | |
| Phenazine | Hydrogen cyanide | |||||
 | ||||||
| Pseudomonine | ||||||
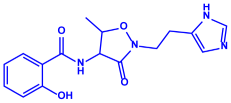
| Bacillus brevis (NCIM 2533) |
|  |  | 68 nm | [67] | |
| Bacilotetrin | Hetiamacin | |||||
| Algae | Metabolites | Structure | Size | References | |
|---|---|---|---|---|---|
| Sargassum plagiophyllum |
|  | 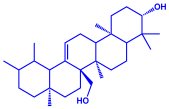 | 18–42 nm | [64] |
| Saponin | Anthocyanin | ||||
 | |||||
| Triterpene | |||||
| Chlorococcum humicola |
|  |  | 4–6 nm | [72] |
| Anthraquinone | 1-propene | ||||
 | |||||
| Steroid | |||||
| Amphora-46 |
|  | 5–70 nm | [73] | |
| β-carotene | |||||
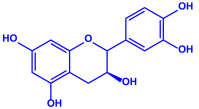 | |||||
| Catechin | |||||
 | |||||
| p-coumaric acid | |||||
| Caulerpa racemose |
| 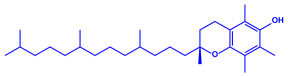 | 5–25 nm | [74] | |
| α-tocopherol | |||||
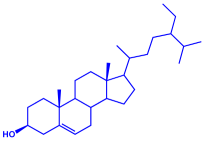 | |||||
| β-sitosterol | |||||
| Padina pavonica |
| 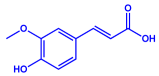 | 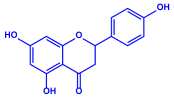 | 10–72 nm | [75] |
| Ferulic acid | Naringenin | ||||
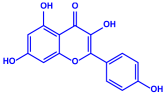 | |||||
| Kaempferol | |||||
| Chaetomorpha linum |
|  | 3–44 nm | [76] | |
| Linoleic acid | |||||
 | |||||
| Arachidonic acid | |||||
 | |||||
| Eicosapentaenoic acid | |||||
| Gelidiumamansii |
| 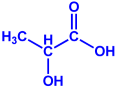 | 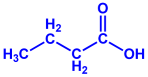 | 27–54 nm | [77] |
| Lactic acid | Butyric acid | ||||
| Fungi | |||||
| Aspergillus fumigates |
| 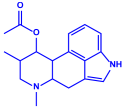 | 5–25 nm | [78] | |
| Fumigaclavine | |||||
 | |||||
| Fumagillins | |||||
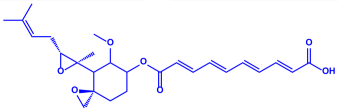
| Penicillium fellutanum |
|  | 5–25 nm | [79] | |
| Fellutamide | |||||
 | |||||
| Citrinin | |||||
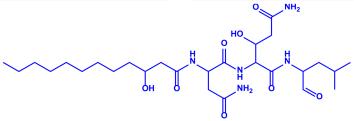
| Aspergillus flavus |
|  | 8.92 nm | [80] | |
| Aflavarin | |||||
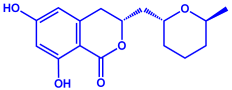 | |||||
| Cladosporin | |||||
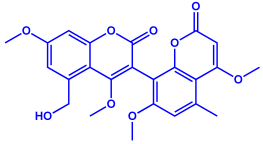
| Fusarium semitectum |
| 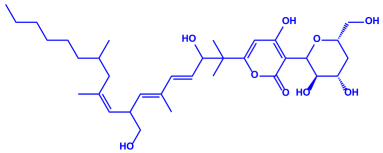 | 10–60 nm | [81] | |
| Fusapyrone | |||||
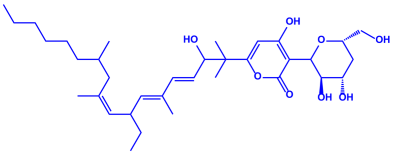 | |||||
| Deoxyfusapyrone | |||||
| Alternaria alternata |
| 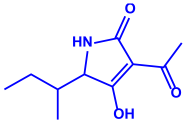 | 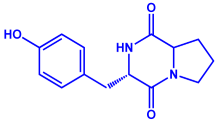 | 20–60 nm | [82] |
| Tenuazonic acid | Maculosin | ||||
| AgNP Types | Size (nm) | Pathogen(s) | Effect(s) | References |
|---|---|---|---|---|
| AgNPs | 25 to 50 | Xanthomonas oryzae pv. Oryzae strain LND0005 and Acidovorax oryzae strain RS-1 | Inhibited bacterial growth, biofilm formation | [115] |
| AgNPs | - | Bacillus subtilis and Escherichia coli | Suppressed the growth of pathogens | [116] |
| AgNPs | 7 and 25 | Edwardsiella tarda and E. coli | Antibacterial activity | [117] |
| AgNPs | 27 | P. aeruginosa and S. marcescens. | Antibacterial activity | [117] |
| AgNPs | - | Klebsiella pneumoniae, P. aeruginosa, S. marcescens, Streptococcus pyogenes, and Staphylococcus aureus | In vitro activity against bacterial pathogens | [118] |
| AgNPs | - | E. coli | Suppressed biofilm formation | [119] |
| AgNPs | 12 | Clavibacter michiganensis subsp. michiganensis (Cmm) | Inhibited bacterial canker in tomatoes | [120] |
| AgCSs | 15 to 25 | X. oryzae pv. oryzae | In vitro activity against blight disease of rice | [121] |
| AgNPs | 24.5 | R. solanacearum strain YY06 | Negatively affected bacterial growth, biofilm formation, swimming motility, induced cell membrane damage, and reactive oxygen species (ROS) | [122] |
| AgNPs | 25 to 75 | E. coli and S. aureus | Applied as antibacterial material for fruit and vegetable preservation | [123] |
| AgNPs | 18 to 39 | X. oryzae pv. oryzae | Increased the plant biomass with a decreased levels of cellular ROS | [124] |
| AgNPs | 470 | X. phaseoli pv. phaseoli | Growth inhibition | [125] |
| AgNP Types | Size (nm) | Plant | Pathogen | Effect(s) | References |
|---|---|---|---|---|---|
| AgNPs | 10–20 | Cymopsis tetragonaloba | Sunhemp Rosette Virus (SHRV) | Complete suppression of the disease | [132] |
| AgNPs | 77 | Vicia faba | Bean Yellow Mosaic Virus (BYMV) | Decrease in virus concentration, percentage of infection and disease severity, reduction in lesions on infected leaves | [129] |
| AgNPs | 12 | Solanum tuberosum | Potato Virus Y (PVY) | Resistance to virus infection | [131] |
| Graphene oxide-silver NPs (GO-AgNPs) | 30–50 | Lactuca sativa | Tomato Bushy Stunt Virus (TBSV) | Decrease in virus concentration, infection percentage, and disease severity | [138] |
| AgNPs | 12.6 | Solanum tuberosum L. cv. Spunta | Tomato Spotted Wilt Virus (TSWV) | Decrease in TSWV infectivity and produces an inhibitory effect in local lesions | [130] |
| AgNPs | - | Solanum lycopersicum | Tomato Mosaic Virus (ToMV) Potato Virus Y (PVY) | Reduction in disease severity and virus infection | [128] |
| AgNPs | - | Autotrophic plants | Banana Bunchy Top Virus (BBTV) | Inhibition of apoplastic trafficking by blocking pores and barriers in the cell wall or plasmodesmata | [136] |
| AgNPs | S. tuberosum | Potato Virus Y (PVY) | Induced resistance to virus | [138] | |
| Schiff base AgNPs | N. tabacum | Tobacco Mosaic Virus (TMV) | Induced resistance to virus by promoting plant immunity | [139] |
| Nanoparticles | Size (nm) | Pathogen | Effect | References |
|---|---|---|---|---|
| AgNPs | 50.6 | Helminthosporium rostratum, Fusarium solani, F. oxysporum and Alternaria alternata | Effectively mitigated the mycelial growth | [159] |
| AgNPs | 10–12 | F. chlamydosporum and Aspergillus flavus | Suppressed the growth of pathogens | [160] |
| OT-AgNPs | 5–61 | F. oxysporum, A. niger, and A. flavus | Antifungal ability | [161] |
| AgNPs | 15 | Candida albicans | Suppressed the growth of pathogens | [162] |
| AgNPs | 25.6 | A. terreus Thom | Retardation in fungus growth and biomass | [163] |
| AA.AgNPs and SD.AgNPs | 8–52 and 5–45 | A. niger, A. flavus and F. oxysporum | Highly antifungal effect against pathogens | [164] |
| AgNPs | - | F. oxysporum | Antifungal activity | [165] |
| AgCSs | - | Aspergillus sp. | Abnormal spore germination and distorted hyphae | [166] |
| AgNPs | 47 | Colletotrichum gloeosporioides | Controlled black anther infection during storage of cut orchid flowers | [167] |
| MC.AgNPs and PG.AgNPs | 5–29 and 5–53 | A. niger, A. flavus and F. oxysporum | Inhibitory action | [168] |
| AgNPs | A. fumigates, A. niger, A. flavus, Trichophyton rubrum, C. albicans, and Penicillium sp. | Inhibition of fungal growth and biofilm | [169] | |
| AgNPs | 100 | M. phaseolina, S. sclerotiorum, and D. longicolla | Inhibited the growth of fungi | [170] |
| Nanoparticles | Size (nm) | Target Nematode | Test Crop | Effect | References |
|---|---|---|---|---|---|
| AgNPs | 100 | Heterodera sacchari | Oryza sativa | Decreased nematode population in the root and soil, improved vegetative development of the rice plant | [181] |
| AgNPs | 15 | Meloidogyne incognita | Solanum nigrum | Apart from nematode movement, impacts on production, embryogenesis, hatchability percentage, and larval stages were evident | [173] |
| Et-AgNPs | 20–30 | M. incognita | S. lycopersicum | Inhibition of J2 worms and prevention of egg hatching (in vitro). In vivo infestation of tomato roots was considerably decreased when a root dip therapy with AgNPs was used | [182] |
| AgNPs | 30–100 | M. incognita | S. melongena | Inhibition of eggs and 2nd juvenile (J2) stage of M. incognita | [183] |
| AgNPs | 2 | M. incognita | Arachis hypogea | Vegetative growth and fruit weight were increased to varying degrees when the nematode population was diminished | [181] |
| AgNPs | 50–150 | M. incognita | S. lycopersicum | Antagonistic effect on the nematode eggs and larval stages | [177] |
| AgNPs | 5–50 | M. incognita | S. lycopersicum | Highest increase in growth parameters, as well as the minimum galls and egg masses | [184] |
| AgNPs | 20 | M. graminicola | O. sativa | A substantial reduction in the formation of root galls | [172] |
| AgNPs | 16 | M. javanica | Faba bean | Drastically decreased egg hatching, increased larval mortality, diminished root galling, and J2 population in soils | [185] |
| Green Silver Nanoparticles (GSN) | 8–19 | M. javanica | S. melongena | Reduced second-stage juveniles (J2s), nematode population in soil, and enhanced growth characteristics | [186] |
| AgNPs | - | M. incognita | Bermuda grass | Increased turfgrass productivity in one year and reduced root gall development in two years without phytotoxicity | [187] |
| AgNPs | 5–10 | M. incognita | S. lycopersicum | Significant reduction in the number of galls, egg masses, developmental stage, rate of build up, and nematode population in soil | [176] |
| AgNPs | 13.09 and 10.51 | M. javanica | S. lycopersicum | Increased the plant defense gene’s expression (chitinase gene) | [188] |
| AgNPs | 20 | M. incognita | S. lycopersicum | Second-stage juvenile immobility and mortality | [189] |
| Ag-BNPs | 29.55 | M. incognita | S. lycopersicum | Reduced the level of second-stage juveniles, females, and developmental stages while improving the host plant’s resistance and immunity | [190] |
| AgNPs | 25 to 55 | M. incognita | S. lycopersicum | Galls, egg masses, females per root system/plant, and juvenile mortality were all reduced, and the immune system was induced to resist against nematode infection | [191] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management. Molecules 2022, 27, 4754. https://doi.org/10.3390/molecules27154754
Tariq M, Mohammad KN, Ahmed B, Siddiqui MA, Lee J. Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management. Molecules. 2022; 27(15):4754. https://doi.org/10.3390/molecules27154754
Chicago/Turabian StyleTariq, Moh, Khan Nazima Mohammad, Bilal Ahmed, Mansoor A. Siddiqui, and Jintae Lee. 2022. "Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management" Molecules 27, no. 15: 4754. https://doi.org/10.3390/molecules27154754
APA StyleTariq, M., Mohammad, K. N., Ahmed, B., Siddiqui, M. A., & Lee, J. (2022). Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management. Molecules, 27(15), 4754. https://doi.org/10.3390/molecules27154754








