Abstract
Psidium guajava L. (guava) is a small tree known for its fruit flavor that is cultivated almost around the globe in tropical areas. Its fruit is amazingly rich in antioxidants, vitamin C, potassium, and dietary fiber. In different parts of the world, this plant holds a special place with respect to fruit and nutritional items. Pharmacological research has shown that this plant has more potential than just a fruit source; it also has beneficial effects against a variety of chronic diseases due to its rich nutritional and phytochemical profile. The primary goal of this document is to provide an updated overview of Psidium guajava L. and its bioactive secondary metabolites, as well as their availability for further study, with a focus on the health benefits and potential industrial applications. There have been several studies conducted on Psidium guajava L. in relation to its use in the pharmaceutical industry. However, its clinical efficacy and applications are still debatable. Therefore, in this review a detailed study with respect to phytochemistry of the plant through modern instruments such as GC and LC-MS has been discussed. The biological activities of secondary metabolites isolated from this plant have been extensively discussed. In order to perform long-term clinical trials to learn more about their effectiveness as drugs and applications for various health benefits, a structure activity relationship has been established. Based on the literature, it is concluded that this plant has a wide variety of biopharmaceutical applications. As a whole, this article calls for long-term clinical trials to obtain a greater understanding of how it can be used to treat different diseases.
1. Introduction
The scientific community’s interest in plant growing is particularly with regard to the chemical components of bioactive compounds, their effect on pathogens and their application as functional foods and/or nutraceuticals to human health [1]. In addition, as various food sources, plant products provide several health benefits. Psidium guajava L., commonly known as guava is a small tree, and is grown in tropical areas of world due to its fruits. Guava leaf tea and some complementary items are available in many stores in Japan [2], as it is considered that phenolic compounds of guava leaves can resolve particular health issues such as the modulation of blood sugar levels [3]. In addition to being a healthy and tasty food, the fruit is an excellent source of dietary fiber. Eating guavas may aid healthy bowel movements and prevent constipation [4]. One guava per day can provide 12% of the recommended daily intake of fiber [5]. Additionally, P.guajava L. has many applications which make this plant very important from scientific point of view. Thus, a review covering the literature of recent 7 years has been accomplished. For this purpose, a comprehensive literature survey from 2015 to 2021 was performed with respect to its health benefits, GCMS and LC-MS based phytochemical profiling and bioactive secondary metabolites isolated from the different parts of the plant. The pharmacological activities of various plant parts are also discussed in this article. Further prior reviews of this amazing plant do not describe the plant’s full potential, and hence the gaps left by these reviews are filled by the present article where all-important aspects of P. guajava are describe comprehensively.
1.1. Review Methodology
The scientific literature has been extensively investigated by the use of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Databases have been searched from 2015 to 2020, including Ovid (Books, Journals, Cochrane, AMED, Embase, and MEDLINE), Scopus, Google Scholar, PubMed and Science Direct. To ensure the inclusion of corresponding works, search terminologies were “Psidium guajava L.”, “chemical profiling through GCMS and LC-MS”, “essential oils and GCMS analysis”, “phytochemicals”, “meroterpenoids”. Prime research was carried out for Psidium guajava L. It was later joined by pharmaceutical or biological activity; medicinal use or traditional or toxicity or cytotoxicity. To combine the search terms, Boolean operators were used. The literature was also searched manually using a backward and forward approach to the investigation of the gray field.
1.2. Morphology of P. guajava L.
P. guajava L. of the plant family Myrtaceae belongs to the genus Psidium, which consists of about 150 species. The P. guajava L. (guava) is the most important member of this genus [6]. History revealed that this plant is originated from southern Mexico or Central America [7]. It was believed that Spanish and Portuguese took this fruit to other parts of the world. Its adaptability to different environments in the world is important factor which makes it worldwide fruit in tropical and sub-tropical regions of the globe. The subcontinent, especially, is a great home for this fruit. Guava plant is an evergreen shrub or tree with 3–10 m height. Its leaves are 4–10 mm long and oval in shape. The flowers are pure white, having five petals and long, multiple central stamens. Its fruit is a berry and medium to large in size with a weight around 100–250 g. Fruit shape may be spherical, ovoid or pyriform depending on the type of plant. Skin of the fresh fruit is dark green which changes to light green-yellow, pale yellow and pure yellow based on the cultivar. The aroma of the fruit is pleasant, and a seed cavity is present in the center of the fruit. The fruit pulp is soft and white to red in color depending upon the type of cultivar.
1.3. Nutritional Assessment and Traditional Uses of P. guajava L.
P. guajava is cultivated as fruit crop and as medicinal plant. Its fruit has economic significance due to pleasant aroma and taste and is used in production of juice, nectar, paste, jam, jelly and candy bars. The nutritional composition of different parts of guava is given in Table 1, which indicates the high value of the fruit and seeds. Further, the fruit and leaves have great medicinal importance and are used to treat various aliments associated with the stomach. Its leaf extracts have strong antibacterial effect [8]. Guava plant is considered to be an excellent source of ascorbic acids, phenolic compounds and carotenoids with major role in the prevention of most the chronic diseases. Crude fiber in guava is 30.9%, which makes it a good source of antioxidant dietary fiber.
The major uses of the main producing countries for guava leaves are for treatment of diabetes mellitus, cardiovascular diseases, cancer and parasitic infections [4]. The application of the treatment is either oral or topical, depending on the disease. In India, China, Pakistan, and Bangladesh, ingestion through decoction, infusion and boiled preparations is the most common way to resolve many illnesses, such as rheumatism, diarrhea, gastrointestinal problems, diabetes mellitus, infections, inflammatory disorders and cough [8,9,10,11]. The decoction of leaves is used for mouth ulcers in Southeast Asia [8,10,11]. Poultice is externally used in Mexico, Brazil, Philippines, and Nigeria for skin and wound applications. In addition, in Nigeria, it is anti-bactericidal agent and chewing sticks are used for oral treatment [8,9,10,11].

Table 1.
Nutritional composition of different parts of P. guajava L.
Table 1.
Nutritional composition of different parts of P. guajava L.
| The Major Nutritional Composition of P. guajava L. Whole Fruit | ||
|---|---|---|
| Nutritional Components | % of Component/100 g | References |
| Sugars (g/100 g) | 8.92 | [12] |
| vitamin C (mg/100 g) | 228.3 | |
| vitamin A (IU/100 g) | 624 | |
| Vitamin E (mg/100 g) | 0.73 | |
| vitamin K (μg/100 g) | 2.6 | |
| Lycopene (in red-fleshed cultivars only); (mg/100 g) | 5.2 | |
| Potassium (mg/100 g) | 417 | |
| Phosphorus (mg/100 g) | 40 | |
| Magnesium (mg/100 g) | 22 | |
| Calcium (g/100 g) | 18 | |
| The major nutritional composition of P. guajava L. fruit Pulp | ||
| Protein (g/100 g) | 0.3 5.13 ± 0.26 0.88 | [13,14,15] |
| Carbohydrates (g/100 g) | 15 13.2 | |
| Vitamin A (IU/100 g) | 109 200–400 | |
| Thiamine (B1) (mg/100 g) | 0.06 0.046 | |
| Riboflavin (B2) (mg/100 g) | 0.06 0.03–0.04 | |
| Niacin (B3) (mg/100 g) | 1.3 0.6–1.068 | |
| Ascorbic acid (C) (mg/100 g) | 190 100 | |
| Calcium (mg/100 g) | 15 9.1–17 | |
| Phosphorus(mg/100 g) | 16 17.8–30 | |
| Iron (mg/100 g) | 0.3 0.30–0.70 | |
| Potassium (mg/100 g) | 292 | |
| Sodium (mg/100 g) | 6 | |
| Calories kcal/100 g | 54.97 36–50 | |
| The major nutritional composition of P. guajava L. seeds | ||
| Protein (g/100 g) | 11.19 4.8 ± 0.10 7.71 | [16,17,18,19,20] |
| Carbohydrates (g/100 g) | 22.2 ± 0.14 11.51 | |
| Vitamin A (IU/100 g) | 50.13 | |
| Niacin (B3) (mg/100 g) | 0.16 | |
| Ascorbic acid (C) (mg/100 g) | 87.44 0.20 | |
| Zinc (mg/100 g) | 3.31 | |
| Calcium (mg/100 g) | 0.05 ± 0.14 60.07 | |
| Phosphorus(mg/100 g) | 160.55 | |
| Iron (mg/100 g) | 13.8 3.32 | |
| Potassium (mg/100 g) | 300 | |
| Calories kcal/100 g | 182 | |
2. Phytochemistry of P. guajava L.
2.1. LCMS Analysis of the Leaves Extract of P. guajava L.
The literature revealed that UPLC-ESI-QTOF-MS analysis of the leaves extract of P. guajava disclosed fourteen compounds (Table 2) of phenolic class of secondary metabolites [21], while in another report, the chemical profiling by HPLC-PDA and LC-TOF-MS (negative and positive modes) showed the presence of altogether 21 compounds (Table 2) from seven P. guajava cultivars [22]. Study on pink guava (P. guajava L.), the phytochemical investigation through ultra-high performance liquid chromatography with diode array result in identification of 60 phenolics with different structural features, such as flavonoids, ellagitannins, flavones, flavonols, flavanols, proanthocyanidins, dihydrochalcones and anthocyanidins, stilbenes, acetophenones, and benzophenones. Out of all these identified compounds 42 polyphenols were reported for the first time in both peel and flesh, and 24 compounds were detected for the first time in P. guajava [23]. However, various chemical profiling of different cultivars of guava have shown the presence of phenolic compounds in major amounts. The dominant phenolic metabolites were assumed to be responsible for its strong antioxidant and antidiabetic activity.

Table 2.
Phytochemicals identified through LCMS studies of the Fresh Leaf of P. guajava (A).
2.2. GCMS Analysis of the Leaf Extracts of P. guajava L.
GCMS analysis of the ethanolic extract of guava leaves results in identification of 33 phytochemicals (Table 3). Further studies of ethanolic and aqueous extracts showed the presence of tannin, saponin, polyphenol, flavonoids, steroids, carbohydrate, terpenoids, triterpenoids and glycoside in both the extracts. The quantitative analysis showed phenolics as major constituents (9.33 mg/gm powder), followed by flavonoids (6.42 mg/gm powder), tannin (4.30 mg/gm powder) and saponin (3.67 mg/gm powder) and [24]. Other investigators reported nine compounds through GCMS analysis, which are presented in Table 3. The estimation of total phenolic showed that polyphenolic is present in high amount as in methanol fraction (261.4 ± 8.5) followed by the ethanol (146.7 ± 2.2), ethyl acetate (99.6 ± 2.4), acetone (84.2 ± 2.4), benzene (43.8 ± 2.3) and petrol ether (41.2 ± 1.9) fractions [25]. Ashraf et. al., in 2016 has published the chemical composition of the different extracts of leaves by GCMS and identified the 33 compounds. Further total phenolic contents (mg GAE/mg of plant extract) of methanol (83.34 ± 0.49), chloroform (71.49 ± 0.48) and hexane (53.24 ± 2.05) were determined. The total flavonoids contents (mg QE/mg of plant extract) of methanol, chloroform and hexane were 53.39 ± 0.89, 32.76 ± 1.15, 21.26 ± 1.49 respectively [26]. Another study conducted in 2018 for chemical profiling of the leaves of the guava showed the presence of total nine compounds as presented in Table 3 [27,28]. The analysis of GCMS studies showed interesting data as except some major components such as α-Copaene, Caryophyllene, Epiglobulol, Ledol, Copaene and γ-Muurolene, the extracts of different region possess the different compounds. The above contradictions in results from various groups may be attributed to agro-climatic conditions of the regions, type of cultivar, maturity stage at which the plant was collected, type of extraction technique and polarity of different solvents used.

Table 3.
GCMS analysis of the leaves extracts of Psidium guajava.
2.3. Chemical Composition of Essential Oil of P. guajava L.
During literature search, several reports were encountered highlighting essential oil compositions of guava fruit and leaves (Table 4). For example, the essential oil obtained through hydrodistillation from the leaves of P. guajava, collected from Kathmandu, Nepal, was analyzed by GC-MS. Out of 100 identified compounds 53 were major constituents and accounts for 100% of oil composition. The major phytochemicals identified from essential oil were (E)-nerolidol (35.6%) and (E)-caryophyllene (15.8%), (2Z,6E)-farnesol (6.7%), ledol (5.5%) and cubenol (3.99%) [32]. Another report describes leaf essential oil composition of P. guajava through GC-MS where 27 substances were identified with α-terpinyl acetate (23.57%), trans caryophyllene (17.65%), nerolidol (12.16%), αcadinol (6.71%), α-copaene (6.5%), α humulene (3.92%), (-)-caryphyllene oxide (3.66%), iso aroma-dendren epoxid (2.55%) and trans α-bisabolene (2.01%) as the major components. In this report, the overall % age yields of oils were obtained as 0.51% (v/w) [33]. GC-FID and GC-MS analyses of leaf essential oil identified even higher number of components i.e., 46 with major components have been reported as limonene (29.1%), (E)-caryophyllene (15.7%), caryophyllene oxide (8.8%), caryophylla-4(12),8(13)-dien-5-ol (6.5%), (E)-nerolidol (4.0%), α-cadinol (3.4%), muurola-4,10(14)-dien-1-β-ol (2.5%), 1,8-cineole (2.6%), α-copaene (2.3%) and α-humulene (2.0%) [34]., whereas, in other report it is observed that the examined essential oil from leaves of P. guajava, dominated by limonene, (E)-caryophyllene and (E)-nerolidol and their derivatives (Table 4). Since these studies were performed under different conditions and plants used were growing in different climatic conditions, it is reasonable to find varying compositions of essential oil. These variations (Table 4) correspond to climate, type of plant culture and stage at which this fruit was collected.

Table 4.
Phytochemicals identified from the leaf essential oil extracted from P. guajava.
3. Bioactive Compounds Isolated from P. guajava L. and Their Structure Activity Relationship
3.1. Phenolics/Phenolic Acids from P. guajava L.
The published literature revealed that different research groups have studied secondary metabolic profiles of guava through LCMS/UPLCMS and other related techniques, and have reported phenolics as the major components of guava. Further reports on isolation and structure elucidation of these secondary metabolites have substantiated the above analytical results, since variety of phenolics have been isolated from various parts of this plant. For example, six guavinosides A–F (1–6) were separated from the leaf extract of the P. guajava (Figure 1), which were identified due to spectroscopic means. Compounds 3 and 6 showed good cytotoxicity against HeLa, SGC-7901 and A549 cell lines, with IC50 values of 4.277, 7.288 and 3.246 µg/mL respectively (Table 5). Comparison with potential of reference drug (Adriamycin, IC50 = 1.359, 3.118 and 2.684 µg/mL) indicated significant anticancer property of these compounds. In variety of antioxidant assays, compound 3 inhibited the FRAP, DPPH and ABTS activity On the other hand, compound 1 showed a dose dependent FRAP, DPPH and ABTS activity with IC50 values of 11.54 (12.5 µg/mL), 14.00 (25 µg/mL), 23.73 (50 µg/mL) and 46.37 (100 µg/mL), followed by significant inhibition by compound 4, while other compounds also displayed varying potential (Table 5) [36].
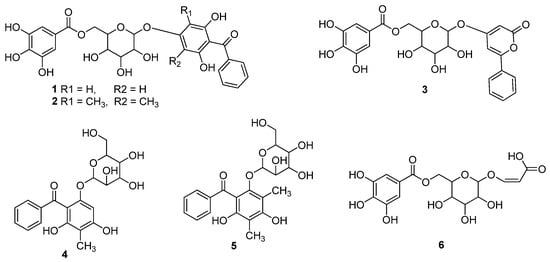
Figure 1.
Isolate from the leaves extract of the P. guajava L.

Table 5.
Antioxidant and anticancer activities of isolated compounds from P. guajava L.
Acylated Phenolic Glycosides (7–17) were isolated from the leaves of the P. guajava (Figure 2), which showed DPPH Scavenged Free Radicals activity with IC50 values in the range of 84.28 to 180.00 μM. Since the standard drug ascorbic acid exhibited IC50 value of 108.60 μM, it offers a strong candidature of compounds 7–17 to be further studied for their value as antioxidant drug [37]. Compounds 2, 5 and a benzophenone galloyl glycoside (18), were isolated from the leaves of the P. guajava L. (Figure 3) by High-Speed Counter-Current Chromatography and their structure was identified through details spectroscopic data. Compound 2, 5 and 18 were tested against HCT116 and HT29 cells. Compound 2 and 18 showed good inhibitory activity against HCT116 and HT29 cells in a dose dependent and time-dependent manner up to 81.4% at dose of 100 μM after 72 h of treatment, while under same conditions, compound 2 showed less inhibition by 66.2% (Table 5). Furthermore, compound 18 was tested for cellular apoptosis of HCT116 cells and showed good increased in the size of the apoptotic cell population by 1.50-fold (3.65%), 2.33-fold (5.67%), and 10.08-fold (24.53%) at concentration of 40, 60 and 80 μM respectively. It is very important that to mention here that chemical structure of the compound 2 and 18 was nearly similar to that of 5, and thus their inhibitory potential was compared, which indicated that a trihydroxybenzoate moiety (as in 2 and 18) is required for anticancer activity of these compounds [38]. Four flavonoids (19–22) were also isolated from leaves of the P. guajava, and were evaluated for their anticancer and antioxidant activity. Compound 19 showed the FRAP activity on dose dependent manner by a value of 333.26 ± 1.76 (12.5 µg/mL), 359.18 ± 15.14 (25 µg/mL), 379.40 ± 10.31 (50 µg/mL), 401.27 ± 12.23 (100 µg/mL), Compound 20 showed the inhibition with a value of 123.88 ± 14.95 (12.5 µg/mL), 269.00 ± 7.28 (25 µg/mL), 291.63 ± 32.79 (50 µg/mL), 324.58 ± 10.64 (100 µg/mL), Compound 21 displayed the inhibition with a value of 57.21 ± 4.94 (12.5 µg/mL), 175.59 ± 7.11(25 µg/mL), 220.51 ± 22.18 (50 µg/mL), 346.45 ± 25.61 (100 µg/mL) and Compound 22 showed the inhibition with a value of 68.06 ± 5.74 (12.5 µg/mL), 155.89 ± 17.90(25 µg/mL), 287.94 ± 2.26 (50 µg/mL) and 329.68 ± 17.72(100 µg/mL), while these compounds also showed good antioxidant activities on DPPH and ABTS free radicals. The compounds 19–22 displayed the DPPH free radical activity with IC50 values of 11.00 ± 0.26, 5 16.13 ± 0.32, 6 13.43 ± 0.12 and 27.03 ± 1.70, respectively and ABTS free radical activity with IC50 values of 4.40 ± 0.26, 6.90 ± 0.36, 6.70 ± 0.26, and 10.57 ± 0.51, respectively [36].
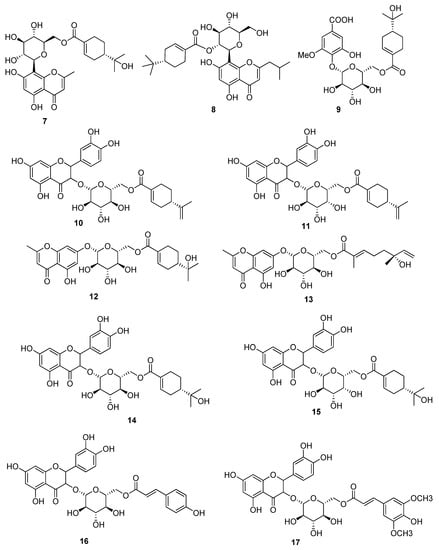
Figure 2.
Acylated Phenolic Glycosides (7–17) were isolated from the leaves of the P. guajava L.

Figure 3.
Benzophenone galloyl glycoside (18) and flavonoids (19–22) were isolated from the leaves of the P. guajava L.
3.2. Meroterpenoid
In addition to above phenolics mostly isolated from leaves of P. guajava L., hybrid compounds are noticed as a striking feature of this plant. Among these hybrid compounds, meroterpenoids (Table 4, Table 5, Table 6, Table 7 and Table 8) are the most attractive group of secondary metabolites, in which the terpenoidal part is bonded with other classes of secondary metabolites, especially phenolics, for example, guajavadimer A (23), a dimeric sesquiterpene-based meroterpenoid isolated from the leaves of P. guajava L. the chemical structure of the compound was established through detail spectroscopic studies and X-ray crystallography. It possessed two caryophyllenes, a flavonone-fused and a benzylphlorogulcinol complicated skeleton and showed moderate hepatoprotective activity against N-acetyl-p-aminophenol (APAP)- induced toxicity in HepG2 cells with value of OD (mean ± SD) 1.654 ± 0.094 [39]. Diformylphloroglucinol-derived meroterpenoids psiguajavadials A (24) and B (25), guadial A–C (26–28), psiguadial B–D (29–31), guajadial (32), psidial A (33), 4,5-diepipsidial A (34), guajadial B (35) and guajadials C–F (36–39) were isolated from P. guajava L. and their structure was identified through spectroscopic analysis and ECD calculations [40]. All compounds (24–39) showed antitumor activity (Table 5) against HCT116, CCRF-CEM, DU145, Huh7, and A549 with the cell viability rates less than 50% at 60 μM concentration. Compounds 34 and 35 were the most active against A549 cells with IC50 values of 160 and 150 μM, respectively. While compounds 24, 36, 37 and 39 have same potential at 50 μM, compounds 24, 25, 35 and 39 showed dose-dependent inhibition of Top1 activity. Mechanistic study for compounds 24 and 25 displayed potent antiproliferative effects against HCT-116 cells by inducing apoptosis in a dose-dependent manner and induced antagonized Top1-mediated DNA break [41]. Guajadial (32), inhibited endothelial cell proliferation and migration, and furthermore it suppresses tumor growth in human NSCLC (A549 and H1650 cells) xenograft mouse models. This potential has been reported as significant antineoplasmic activity of 32. Western blotting method to study the underlying mechanisms of VEGF receptor (VEGFR)2-mediated revealed that compound 32 inhibited A549 (IC50 = 3.58 μM) proliferation via blocking the Ras/MAPK pathway [42]. Moreover, guadial A–C (26–28) [43,44], psiguadial B–D (22–24) [41,43], guajadial (32) [45], psidial A (33) [46], 4,5-diepipsidial A (34) [47], guajadial B (35) [48] and guajadials C–F (36–39) [49] were also previously reported from P. guajava L. Among other anticancer meroterpenoids the Diformylphloroglucinol-based guajavadials A–C (40–42) were isolated from P. guajava L. and the structures of all compounds were determined through spectroscopic studies. All compounds displayed good cytotoxicity against HL-60, A-549, SMMC-7721, MCF-7, and SW480 cancer cell lines with IC50 values between 2.28–3.38 µM (Table 5). Compound 40 exhibited the highest potential with a value of IC50 = 3.54 µM against SMMC-7721 cell lines, and this activity level is higher than the control drug cisplatin (IC50 = 19.82 µM) [50]. The structures activity relationship showed that the arrangement of the isoprene units is responsible for the activity, and thus the terpenoidal skeleton plays key role in activity potential, as can be seen in compounds 41 and 42. Guajavadial A (40) is a 3,5-diformylbenzyl phloroglucinol-coupled monoterpenoid skeleton and guajavadials B (41) and C (42) are the adducts of the 3,5-diformylbenzyl phloroglucinol and a sesquiterpene with different coupling models [50]. More meroterpenoids namely psiguajadials A–K (43–53) along with psiguadial A (54), guapsidial A (55), and psiguajadial L (56) were also isolated from P. guajava. All these natural products (43–56) has shown significant inhibition of PDE4D-4 with IC50 values ranging from 1.34–7.26 μM (Table 6) [51]. This activity potential is comparable with the rolipram, a standard PDE4 inhibitor (IC50 0.62 μM). Since a little difference has been reported in activity level of all these compounds, which may lead to the conclusion that diformylphloroglucinol moiety is required for PDE4D2 inhibitory activities (Table 6). Compounds psiguadial A (54) [41], guapsidial A (55) [44], and psiguajadial L (56) [47] were previously also reported from P. guajava produced complex and diverse meroterpenoids bearing phloroglucinol-coupled to sesquiterpenoids or monoterpenes. Similarly, here the Phloroglucinol-coupled to cubebane sesquiterpenoid core in compounds 43 and 44, and compound 45 has globulane as terpene unit, 48 has caryolane, 49 has caryophyllane, and compounds 50–52 have cadinane unit as terpene unit [51]. One more meroterpenoid Guavadial (57) was isolated of P. guajava which has caryophyllene combined to diformyl phloroglucinol core [49]. Furthermore, meroterpenoids were obtained from P. guajava L. namely Psiguajdianone (58), Psiguajanone A (59), Psiguajanone B (60), Psiguajanone C (61), Psiguajanone D (62), Psiguajanol A (63) along with the already reported Guapsidial A (55) and Psiguajadial D (44). All compounds were tested for anticancer and anti-inflammatory activity on NO, TNF-α and PEG2 production in RAW264.7 cells. All isolates showed the inhibition with a value ranging from 2.86–11.82, 1.66–31.59 and 1.08–13.63 for NO. TNF-α and PEG2 respectively [52] (Figure 4 and Figure 5).

Table 6.
Anti-inflammatory and Enzyme inhibitory activities of isolated compounds from P. guajava L.
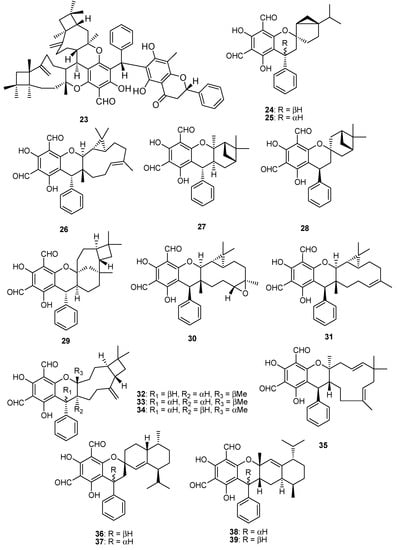
Figure 4.
Meroterpenoids isolated from P. guajava L.
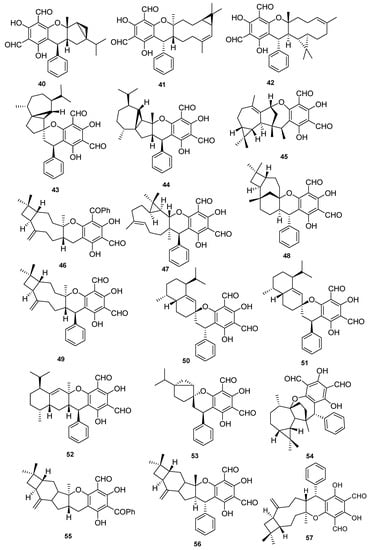
Figure 5.
Meroterpenoids isolated from P. guajava L.
Psiguadiols A–J (64–73), meroterpenoids were isolated from leaves of the P. guajava L., which has 6,8-diformyl-5,7-dihydroxy-4-phenylchromane-coupled sesquiterpenoids along with a C-8-spiro-fused 6/6/9/4 tetracyclic ring system. The chemical structure of the natural product was determined from spectroscopic data, ECD data, and single-crystal X-ray diffraction data. The protein tyrosine phosphatase 1B (PTP1B) is critically involved in insulin receptor signaling and an important target from treatment of DM type II. Interestingly guava meroterpenoids, psiguadiols A, G, H (64, 70 & 71) inhibited PTP1B with IC50 values of 4.7, 6.2 and 9.2 μM respectively [53], while all other compounds were found inactive. Three psiguamers A–C (74–76) sesquiterpene-based meroterpenoids bearing a rare methylated benzoylphloroglucinol and bicyclogermacrene units, were isolated from leaves of P. guajava L. Their structures were established through comprehensive analysis of spectroscopic data, electronic circular dichroism (ECD) and X-ray crystallographic data. All compounds were tested for anticancer activity but only compound (+)-74 showed strong cytotoxic activities against five human tumor cell lines; HCT-116, HepG2, BGC-823, A549, and U251 with values of IC50; 2.94, 9.01, 6.45, 5.42 and 5.33 μmol/L respectively [54]. Seventeen meroterpenoids were isolated from the leaves of the Psidium guajava including euglobal B1-1 (77), euglobal Ib (78), euglobal Ic (79), euglobal III (80), euglobal IIb (81), euglobal-Iva (82), euglobal Ivb (83), euglobal V (84), macrocarpal A (85), ecalrobusone E (86), guajadial C (36), guajadial D (37), guajadial E (38), guajudial (87), psiguajadial H (50), psiguajadial I (51), and psiguajadial J (52) were isolated from the leaves of guava [37]. Jejuguajavones A–J (88–97) isolated from the 95% EtOH extract of Jejuguava leaves and evaluated against the PTP1B enzyme. Compound 88–91 showed the good inhibitory activity with value ranging from 9.40–37.83 µM [55]. Eleven polycyclic phloroglucinol meroterpenoids guajamers A–I (98–106), along with guadial A (26) guadial C (28) were isolated from the leaves of P. guajava. All compounds were checked for antibacterial activity and 99–104, 26 and 28 showed moderate activity [56]. It is concluded from the bioactivity of the isolated compounds that P. guajava L. is excellent sources for the treatment of oxidative stress, diabetes and inflammation. Further compounds isolated from P. guajava L. leaves and their anti-cancer effects against human cancer cells, paving the way for these compounds and P. guajava L. leaves to be used as possible chemoprevention agents against cancer (Figure 6, Figure 7 and Figure 8).

Figure 6.
Meroterpenoids isolated from P. guajava L.

Figure 7.
Meroterpenoids isolated from P. guajava L.

Figure 8.
Meroterpenoids isolated from P. guajava L.
4. Pharmacological Activities
4.1. Antidiabetic Potential of Leaf Extract
Literature search revealed that several studies have been carried out to evaluate pharmacological potential of guava leaves. The guava leaves have an ability to increase glycogen synthesis and halt the process of hepatic gluconeogenesis by regulating AMPK/ACC pathway in streptozotocin-induced diabetic rats, when orally administered as 200 mg/kg by weight [57]. It is further reported that guava leaves reduced triglycerides, phospholipids, free fatty acids, total cholesterol and LDL levels while HDL level was raised in STZ induced rats [57]. To validate the role of guava leaves in treatment of Diabetes Mellitus (DM), a novel purified heteropolysaccharide GP70-3M from the leaves was tested in vitro against α-glucosidase, which showed outstanding inhibitory activity with an IC50 value of 2.539 µM. This potential has been reported to be 1867 times greater than control acarbose (IC50 value of 4.744 mM). Different parts of the plant were screened for their diabetic inhibition (Table 7) [58].

Table 7.
Anti-diabetic potential of different parts of P. guajava.
Table 7.
Anti-diabetic potential of different parts of P. guajava.
| Activity | Enzyme | IC50 μM | Plant Part Used | References |
|---|---|---|---|---|
| Anti-diabetic | α-glucosidase | 2.539 | Leaves | [58] |
| α-glucosidase | 1.0 | Leaves | [59] | |
| α-glucosidase | 0.5 | Bark | [59] | |
| α-amylase | 10.6 | Bark | [59] | |
| Glucose-6-Phosphatase | Significant | Leaves | [58] | |
| Control | Acarbose | 4.744 | [58] |

Table 8.
IC50 and selectivity index (SI) of essential oil of P. guajava leaves (PG-EO) against diferent cell lines.
Table 8.
IC50 and selectivity index (SI) of essential oil of P. guajava leaves (PG-EO) against diferent cell lines.
| Cell Line | Treatment (µg/mL) | |||
|---|---|---|---|---|
| PG-EO | DXR | |||
| IC50 | SI | IC50 | SI | |
| GM07492A | 126.4 ± 11.8 | - | 0.5 ± 0.2 | - |
| MCF-7 | 96.9 ± 8.4 a | 1.3 | 62.1 ± 2.0 | - |
| HeLa | 128.7 ± 1.5 | - | 5.3 ± 1.3 | - |
| M059J | 103.6 ± 5.1 a | 1.2 | 16.2 ± 2.5 | - |
The leaf extract of P. guajava increased the uptake of 2-deoxy-d-[1–3H]-glucose in C2C12 muscle cells with a value of 161.4 ± 10.1%, p = 0.0015 as compared to vehicle control (dimethyl sulfoxide), and standard drugs metformin (144.0 ± 7.7%, p = 0.0345) and insulin (141.5 ± 13.8%, p = 0.0495). Furthermore, it also noted that leaf extract of P. guajava considerably enhanced the triglyceride accumulation in 3T3-L1 cells compared to standard drug (rosiglitazone) [59].
4.2. Anticonvulsant Effects
The P. guajava leaf ethanolic extract has been found to afford anti-convulsant activity; since it exerted dose dependent (200 mg/kg and 400 mg/kg) effect on seizures induced mice using MES as suggested by some parameters for example reduced hind-limb tonic extension (HLTE), increased percentage protection from induced MES convulsions. Anti-convulsant effect on Maximal Electrocshock (MES) mice model suggests that the extract has produced dose dependent anticonvulsant effect in albino mice. While in pentylenetetrazole (PTZ) induced mice model P. guajava extract prolonged the clonic convulsion latency, reduced convulsion duration in a dose dependent manner along with reduction in seizure score [60].
4.3. Antiproliferative Potential of Essential Oils from P. guajava Leaves Extracts
Essential oil obtained from leaves of P. guajava was evaluated for its antiproliferative activity against human gliobastoma (M059J), human cervical adenocarcinoma (HeLa), breast adenocarcinoma (MCF-7) and normal human GM07492A cell lines, whereas lung fibroblasts cell line was used as control. The reported results showed that the oil exhibited significant IC50 values for M059J and MCF-7 compared to normal line as presented in Table 8 [61].
4.4. Antimutagenic Effect
The methanolic extract of P. guajava leaves tested against methyl sulfonate (MMS), sodium azide (NaN3), benzo(a)pyrene (BP) and 2-aminofluorene for its antimutagenic effect. The methanol extract of P. guajava leaf extract was found to inhibit 70% of mutagenesis at concentration of 80 mg/mL. This setup is on the basis that the phenolic contents present in P. guajava have broad-spectrum antimutagenic activity and could serve as potentially good Candidates for phytomedicine [25].
4.5. Antifungal Properties
Various published work revealed that P. guajava L. has also shown to possess some antifungal activities. Its tannins and flavonoid fraction were analyzed for antifungal activity using 21 different compounds in fraction of two, having phenolic compound in higher concentration. In an in vitro assay performed against three fungal strains of Candida i.e albicans, krusei and tropicalis through microdilution showed that IC50 values for these strains ranged from 69.29 to 3444.62 μg/mL for the isolated and combined fractions of flavonoids and tannins. While the reference compound fluconazole and combined fraction ranged from 1.57 to 925.56 μg/mL, which shows that natural products have some synergism with antifungal potential. The fractions were found to affect pleomorphism capacity as well along with inhibition of fungal strain in isolated form, enhancing the action potential of Fluconazole, reducing the concentration and hindering the morphological transition, one of the main virulence factor of Candida genus [62]. The P. guajava leaves extract was used for microbiological assays to determine IC50, inhibitory effect of associated fraction with Fluconazole against Candida species and cell viability curve through microdilution method. Antifungal bioassay performed on solid media by modifying morphological and fungicidal concentration and results ranged between 5.10 and 926.56 mg/mL revealed the effect of change in concentration effects the inhibition [21]. This suggests that P. brownianum and P. guajava can potentially be used to develop drugs to treat fungal infections [63,64].
4.6. Antiparasitic Potential
P. guajava L. and Psidium browninaum Mart ex DC leaf aqueous and hydroethanolic extracts were tested for their antiparasitic and cytotoxic potential against Leishmania braziliensis, Trypanosoma cruzi epimastigote forms, L. infantum promastigotes and fibroblasts at three different concentrations (250, 500 and 1000 μg/mL). The T. cruzi forms were not inhibited by the extracts from P. guajava L. P. guajava showed small amount of activity against both L. braziliensis and L. infantum. As for cytotoxicity aqueous decoction extract of P. browninaum showed highest percentage among all other extracts and showed mortality rate of 90.85% for fibroblast at 1000 μg/mL [65].
4.7. Anthelmintic Activity
The aqueous extract of P. guajava paralyzes the nematodes both Levamisole-sensitive and Levamisole-resistant strains of Caenorhabditis elegans, in a dose dependent manner. Different studies were carried out by applying concentration-dependent doses. At a concentration of 25 mg/mL of the P. guajava extract, 100% paralysis of the wild type worms was achieved with in 4 h. A similar effect was observed for N2 wild type and CB193 resistant worms and egg-laying ability was decreased by 40% at the same concentration. These reports disclose that P. guajava extracts have also potential anthelminic effect against nematodes [66]. Further these studies revealed the presence of triterpene responsible for anthelmintic activity. Therefore, it is also concluded that secondary metabolites from P. guajava leaves extract could serve as basis for antileishmanial drugs [67].
4.8. Antioxidant Potential
Phytochemistry of Guava showed the presence of flavonoids and phenolics, which is in agreement with antioxidant activity. The antioxidant activity for crude extract of peels, flesh and seed was 264.30 ± 5.39 μmol TE/g dw, 98.78 ± 3,40 μmol TE/g dw and 62.84 ± 2.81 μmol TE/g dw, respectively, which is nearly equal to the genotype known as Fan Retief and Advanced Selection. The crude extract of the peel part having highest antioxidant activity has a greater proportion of antioxidant compounds [68]. The products in P. guajava leaf tea (GLT) contain both phenolic forms i.e., soluble and insoluble-bound. The fermentation process via Saccharomyces cerevisiae and Monascus anka followed by hydrolysis through complex enzymes increases the soluble phenolic form. Free radical scavenging (DPPH) results of guava were found in close agreement with standard Trlox and ascorbic acid. The excellent IC50 in μM revealed the guava extract as potential anti-oxidant. Comparative studies on extracts and Trolox revealed the direct relationship with concentration. As the concentration was increased, the reducing ability was also enhanced and, like radical scavenging activity, SPFE reducing power was greater than rest of the extracts. The reducing power for SPFE = 97.86 mmol TE/g DM, SPF = 50.3 mmol TE/g DM, SPUF = 7.4 mmol TE/g DM. The reducing ability of soluble phenolics (SPF/SPFE) was higher than insoluble bound phenolics (IBF/IBFE) at same concentration. However, the reducing power for insoluble-bound phenolics of unfermented GLT (IBUF) was found to be 11.4 mmol TE/g DM which greater than IBF (2.9 mmol TE/g DM) and IBFE (3.5 mmol TE/g DM). When the extracts were tested for their inhibitory activity against α-glucosidase, the inhibitory effect was promisingly high for SPUF, but it was observed that it was much higher for fermented extract. The IC50 values for inhibition of α-glucosidase were in order SPFE (IC 50 = 11.8 µg/mL) > SPF (IC 50 = 19.2 µg/mL) > SPUF (IC 50 = 29.1 µg/mL). Moreover, insoluble-bound phenolics of fermented GLT (IBPF) and IBPFE have low inhibitory effect on α-glucosidase (i.e., IC50 = 104.4 µg/mL and IC 50 = 112.2 µg/mL respectively) as compared to insoluble-bound phenolics of unfermented GLT (IBPUF) having IC50 = 71.6 µg/mL. The IC50 value for positive control (acarbose) was significantly greater than all the extracts i.e., IC50 = 178.52 µg/mL [69].
4.9. Suppression of Osteoarthritis
The leaves extract from P. guajava and ellagic acid, a polyphenolic compound from the extract, have role in degeneration of aggrecan at the onset of osteoarthritis (OA) by halting activity of metalloproteinase and disintegrin with throbospondin-type-5. The efficacy of extract along with ellagic acid was determined on destruction of cartilage by giving extract as a constituent of diet of anterior cruciate ligament-transected rats (ACLT). The results suggested that P. guajava leaves extract have role in suppressing progression of OA in ACLT rats and inhibition of joint destruction at early stages through ellagic acid-mediation [70].
4.10. Antidiarrheal Activity
The antidiarrheal effect of P. guajava leaf extract carried out at normal rats and diarrheal rats suggested promising effect. Four groups were formed using normal rats: low-dose P. guajava leaves extract, high dose P. guajava leaves extract, control and gallic acid while 5 groups were formed using diarrheal rats: low-dose P. guajava leaves extract, high-dose P. guajava leaves extract, desmopressin, untreated control group and gallic acid. The low-dose P. guajava extract was equal to 50 mg/kg, high-dose P. guajava extract was equal to 100 mg/kg for both normal and diarrheal rats while desmopressin was used 0.2 mg/kg for a period of one month. The administration of P. guajava leaves extract to diarrheal rats stabilized all the parameters such as kidney weight decline, levels of potassium, sodium and chloride in serum, urine volume, serum urea etc. along with antidiarrheal effect P. guajava leaves extracts also aids in protein conservation [71]. According to survey, there is no single clinical trial found on intake of guava as active anti-diarrheal ingredient.
4.11. Antiestrogenic Activity
The Guajadial, meroterpenoids, from P. guajava leaves extract reported to have antiproliferative and antiestrogenic activity, the action mechanism is similar to tamoxifen which indicates it as promising therapeutic agent based on phytoestrogen. The enriched fraction of guajadial form crude P. guajava leaves extract has selectivity and antiproliferative activity in vitro against human breast cancer cell lines MCF-7 and MCF-7 BUS. The total growth inhibition for MCF-7 was 5.59 µg/mL and for MCF-7 BUS was 2.27 µg/mL. The in vivo analysis on uterus of pre-pubescent rats also confirmed the antiestrogenic activity as guajadial fraction halted the proliferative activity of estradiol [72].
4.12. Anticancer Potential
4.12.1. Anticancer Activity of Leaves Extract
The ethanolic extract of P. guajava leaves and quercetin isolated fractions reduce CCl4-induced cytotoxic effect on HepG2 cell lines. The levels of GSH, viability and cytotoxicity were reduced in CCl4 treated cell lines while lipid peroxidation, Lactate dehydrogenase (LDH), Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) were increased. The levels of all these parameters were regulated in a positive manner through the application of P. guajava leaves extract [73].
4.12.2. Cytotoxic Effect of P. guajava Fruit Extract
With the useful biological effects, it is necessary to determine the cytotoxicity of any drug, formulation and nutraceutical. The P. guajava extract administered orally at 2000 mg/kg and 5000 mg/kg b.w of mice did not make any noticeable change in number of kidney podocyte, liver hepatocyte and body weight. The extract from P. guajava leaves extract is safe to use as it non-toxic to both kidney and liver [74]. The aqueous extract of P. guajava leaves in the diet of Oreochromis niloticus not only increased body weight but also increased the villi surface area by increasing its length and width. The immune response and antioxidant activity were improved as total protein content (glutathione S-transferase, superoxide dismutase, and glutathione peroxidase) increased. The Aeromonas hydrophila, a pathogen towards O. niloticus, inhibited by the presence of P. guajava leaves extract, while the absence of extract in the diet of fish increased the mortality rate [75]. In 2019 Babatola et al. also evaluated the toxicity of aqueous leaves extract of three different species of guava i.e., pink, red and white. The rats were used as test animals for this study and extract was administered at a dose of 50, 500 and 5000 mg/kg bodyweight for a period of 14 days. Observation of experimental periods showed effect on parameters accounted and found no toxic effects with a slight increase in body weight but no deaths. The estimated LD50 was found to be 50–5000 mg/kg for these pink, red and white leaves extract of guava [76].
4.13. Antiviral Activity
The antiviral selectivity of P. guajava leaves extract was determined against herpes simplex virus 1 and human immunodeficiency virus, and median cytotoxicity and half-maximal effective concentration were obtained. The EC50 values for HIV-1 strains and HSV-1 were ranged between 0.05 and 3 mg/mL and below 0.2 mg/mL respectively. Antiviral activity of guava extract was found to be based on flavonoid and phenolic contents as HPLC analysis results revealed the presence of phenols (0.8 to 2.1 GAE mg/mL) and flavonoids (62.7 to 182.1 Rutin Eq mg/g DW) [77]. Some direct studies on P. guajava isolated compounds quercetin, catechin, and gallic acid have antiviral activity against Dengue virus. The catechin is best among the three of them as it showed 100% inhibition (pre-treatment) and 91.8% inhibition (post-treatment) depending upon the experimental strategies [78].
4.14. Antibacterial Activity
In 2019, a study conducted by E. A. J. Silva and his colleagues evaluated antibacterial activity for essential oil present in P. guajava leaves. The essential oil has shown moderate activity for various genus of Streptococcus. The activity was described in terms of MIC values S. sobrinus has MIC value of 100 µg/mL, S. mitis has MIC value of 200 µg/mL, S. mutans has MIC value of 200 µg/mL), S. salivarius has MIC value of 200 µg/mL and sanguinis has MIC value of 400 µg/mL [61]. The crude extracts obtained from P. guajava leaves were evaluated for antimicrobial activities by Priscilla Alexander et al. 2019 have evaluated the antimicrobial activities. Crude extractions have mixed fraction of saponins, flavonoids, tannins, glycosides, terpenoids and steroids. The antimicrobial screening showed that crude extracts were strongly active against S. faecalis, E. coli and S. aureus having MIC value of 5.00 mg/mL. In contrast, ethanolic extracts were more actively involved in inhibition and mean zone values were found out to be 6.72 ± 0.01 for S. faecalis and 10.44 ± 0.02 for E. coli [79]. P. guajava have also been tested for its antibacterial activity as a toothpaste. Three different formulations (F) were made F1, F2 and F3 having leaves powder of P. guajava 10, 15 and 20 mg respectively. Therefore, results revealed that the antimicrobial potential against Streptococcus mutants, Streptococcus oralis, Proteus vulgaris, Bacillus subtilis and Staphylococcus aureus strains concentration dependent and F3 formulation found best one. Among all bacterial strains, the best inhibition found for Proteus vulgaris i.e., 1.1 cm while lowest zone inhibition was found against Staphylococcus aureus i.e., 0.5 cm [28]. Therefore, Patel et al. 2019 investigate an important factor regarding the medium of extraction and he evaluated the water extract of P. guajava leaves as anti-infective against Staphylococcus aureus and Pseudomonas aeruginosa present in Caenorhabditis elegans nematode host. The extracts were prepared through three different methods i.e., Decoction, Vacuum Assisted Extraction (VAE) and Microwave Assisted Extraction (MAE). The proved that extracts prepared through MAE showed better activity while its anti-infective activity was than compared to hydroalcoholic extract against five pathogenic bacteria, which were obtained using same extraction technique. Both the extracts had capability to reduce the virulence of all strains (Serratia marcescens, S. aureus, Chromobacterium violaceum, and P. aeruginosa) except S. pyrogens towards C. elegans (Table 9). This lead to reveal the method of extraction effects the activity. According to them, it seems that anti-infective property of these extracts is somehow related to property of quorum modulation that can modulate production of pigments related to quorum sensing within these susceptible bacteria [80,81].

Table 9.
Minimal inhibitory concentration (MIC) values of P. guajava leaves extract.
4.15. Acute and Sub-Acute Toxicity of P. guajava Leaves Extract
The acute and subacute toxicity level of P. guajava bark extract was evaluated using Wistar rats. The extract was proved non-toxic and non-lethal and estimated LD50 was found to be >5000 mg/kg body weight for acute toxicity. The variations in relative weight of organs, body weight and other biochemical parameters that were significant were taken into account in treated animals and control group. Single dose administration at 5000 mg/kg body weight is non-toxic while repeated administration at 1000 mg/kg body weight produced sex-specific toxic effect i.e., minor liver inflammation was observed in females. Hence Psidium plant proved to have mild organ toxicity but have hepatoprotective and hematological potency [82].
4.16. Antimicrobial Activity of Essential Oils of P. guajava Leaves
The essential oils present in leaves of P. guajava have known to have cytotoxic and antimicrobial activities. Their antimicrobial activity was determined against three Gram-positive (Streptococcus aureus, Enterococcus faecalis and Staphylococcus aureus) and three Gram-negative strains (Escherichia coli, Pseudomonas aeruginosa and Haemophilus influenzae). The antimicrobial activity of oil was significant against both Gram-positive and Gram-negative strains and ranges between 0–13 mm while no cytotoxicity was observed using brine shrimp lethality bioassay [83]. The essential oil reportedly inhibit two bacterial human pathogens with MIC values that range from 0.065–0.261 mg/mL while it also inhibits some pathogenic fungi in plants i.e., 82.80% inhibition of Fusarium chlamydosporum and 86.02% inhibition of Curvularia lunata [34].
4.17. P. guajava Leaves Activity against Diarrhea
An antidiarrheal activity of P. guajava leaves was clinically measured and three different doses (6-leaf, 10-leaf, and 14-leaf) of P. guajava leaves decoction extracts were used to their ability against diarrhea. The 14-leaf (7.4 g) decoction proved to be the most successful in the testing. Patients who received the decoction three times per day were able to return to normalcy in 72 h as opposed to 120 h for controls. Haemoglobin, liver, and kidney indicators were all within normal limits, which demonstrated the intervention’s safety [84].
5. Industrial Applications of P. guajava
Dyes and pigments are used in numerous industries worldwide, although the discharge of these materials presents significant risks to the natural environment. Nowadays, water contamination is one of the main causes of environmental pollution. Different synthetic dyes are released directly into natural water resources that are potentially pollutant the resources and make it unfit for domestic and agricultural use. On the other hand, the aromatic structures of these dyes give them greater stability and their degradation process is very slow. Further their oxidation through different oxidizing agents is not easy. Thus, these materials become main pollutants to environments. So, there is a crucial need to find environmentally friendly and cost-effective materials and methods to remove these materials from environment. P. guajava L. leaves nanocomposites materials were widely studies for decontamination of these pollutants. In a recent study a silver: iron oxide (α-Fe2O3-Ag) nanocomposite was prepared for decontamination of chromium (VI) ions from water. Further it is observed that the Cr(VI) adsorption on Fe2O3-Ag surface is endothermic and spontaneous in nature. The adsorbed Cr(VI) can easily be recovered (α-Fe2O3-Ag) nanocomposite and used up to five times [85]. In another study P. guajava leaves were used as biosorbents for the removal of Brilliant Green (BG) [86]. Magnetic nanohybrid composite γ-Fe2O3@GL was prepared by incorporated the Maghemite nanoparticles into framework of P. guajava leaves. γ-Fe2O3@GL was developed for water purification and found efficient for adsorption of methylene blue [87].
Plant-derived proteases are widely used in food and pharmaceutical industries. The upward requirement for biologic-based enzymes, in the food and pharmaceutical industries, has made them an interesting topic for physiologists and biochemists. The existence of two pH optima of P. guajava leaves protease suggests that at least two major proteases are present in it [21]. An environmentally friendly and cost-effective material CuONPs was biosynthesized by using P. guajava L. leaf. It showed potential antibacterial activity against Gram-positive and Gram-negative bacteria. It is non-toxic and exhibited good photocatalytic degradation for Congo red (CR) and methylene blue (MB). The SnO2 nanoparticles within the size of 8 to 8 nm were synthesized by using P. guajava L. leaves extract. These nanoparticles photocatalytic activity was analyzed and found effective for photo degradation of reactive yellow 186. A novel, eco-friendly cotton gauze fabric was synthesized by using P. guajava leaves powder extract. The outer membrane of Biocompatible microcapsules was synthesized from P. guajava leaves powder extract, starch core and calcium-alginate (Ca-alginate). This product was found effective for medical uses. Another novel, eco-friendly and cost-effective material, tungsten oxide nanorods (WO3 NRs), was synthesized by using P. guajava leaves extract. These nanorods were found prodigious in photocatalytic degradation of reactive green 19 (RG 19) dye.
6. Conclusions and Future Prospects
The results demonstrated that almost all parts of P. guajava are rich in diverse secondary metabolites, especially phenolics, flavonoids, squalene and vitamin E. This feature makes the plant a potential source of antioxidants to be used in nutraceuticals and functional food products. Essential oil analysis of this plant indicated the presence of caryopyllene and a variety of its derivatives, which makes P. guajava an anti-inflammatory agent. Striking feature of P. guajava is that all its parts are rich in meroterpenoids specially derived from phloroglucinol, which are mainly produced by different fungi with immunosupressive activity. Leaf and bark extracts can be used as a natural source of α-glucosidase inhibitors. In addition, the bark extract of P. guajava was an effective α-amylase inhibitor. Moreover, P. guajava leaf extract improved glucose uptake in muscle cells, while both leaf and bark extracts enhanced the triglyceride content in adipocytes in culture. P. guajava leaf and bark extracts may thus hypothetically have future applications in the treatment of type 2 diabetes. Similar to this, meroterpenoids’ isolation and activity against many cancer cell lines make it a crucial source for the development of anticancer drugs. The domestic applications of leaves also indicate its important in the field of medicine. Additionally, its applications in industry for the development of numerous beneficial products makes it a significant source that demands special consideration from the scientific community. Overall, it can be said that P. guajava is a useful plant and a rich supplier of nutrients for human growth.
Author Contributions
Conceptualization, M.I.T., M.N. and M.S.; methodology, S.T., N.S. and H.H.; software, M.I.T. and H.H.; validation, M.I.T., N.S., H.H. and G.Z.; formal analysis, M.I.T. and I.A.; investigation, M.I.T., G.Z., D.M. and D.N.; resources, M.S., H.H. and I.A.; data curation, I.A.; writing—original draft preparation, M.I.T., M.N., M.S. and L.H.; writing—review and editing, H.H., D.M., D.N. and G.Z.; visualization, H.H.; supervision, I.A.; project administration, M.I.T.; funding acquisition, D.M. and D.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cornforth, J. Terpenoid biosynthesis. Chem. Br. 1968, 4, 102–106. [Google Scholar] [PubMed]
- Matsuda, Y.; Abe, I. Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 2016, 33, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef] [PubMed]
- Naseer, S.; Hussain, S.; Naeem, N.; Pervaiz, M.; Rahman, M. The phytochemistry and medicinal value of Psidium guajava (guava). Clin. Phytoscience 2018, 4, 32. [Google Scholar] [CrossRef]
- Kafle, A.; Mohapatra, S.S.; Reddy, I.; Chapagain, M. A review on medicinal properties on Psidium guajava. J. Med. Plants Stud. 2018, 6, 44–47. [Google Scholar]
- Pommer, C.V.; Murakami, K.R. Breeding guava (Psidium guajava L.). In Breeding Plantation Tree Crops: Tropical Species; Springer: Berlin/Heidelberg, Germany, 2009; pp. 83–120. [Google Scholar]
- Morton, J.F. Fruits of Warm Climates; JF Morton: Miami, FL, USA, 1987. [Google Scholar]
- Gutiérrez, R.M.P.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Dakappa, S.S.; Adhikari, R.; Timilsina, S.S.; Sajjekhan, S. A review on the medicinal plant Psidium guajava Linn.(Myrtaceae). J. Drug Deliv. Ther. 2013, 3, 162–168. [Google Scholar] [CrossRef]
- Morais-Braga, M.F.B.; Carneiro, J.N.P.; Machado, A.J.T.; Dos Santos, A.T.L.; Sales, D.L.; Lima, L.F.; Figueredo, F.G.; Coutinho, H.D.M. Psidium guajava L., from ethnobiology to scientific evaluation: Elucidating bioactivity against pathogenic microorganisms. J. Ethnopharmacol. 2016, 194, 1140–1152. [Google Scholar] [CrossRef]
- Sanda, K.; Grema, H.; Geidam, Y.; Bukar-Kolo, Y. Pharmacological aspects of Psidium guajava: An update. Int. J. Pharmacol. 2011, 7, 316–324. [Google Scholar] [CrossRef]
- USDA. Guavas, Common, Raw (sr Legacy, 173044) U.S. Department of Agriculture. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/173044/nutrients (accessed on 1 April 2019).
- da Silva Lima, R.; Ferreira, S.R.S.; Vitali, L.; Block, J.M. May the superfruit red guava and its processing waste be a potential ingredient in functional foods? Food Res. Int. 2019, 115, 451–459. [Google Scholar] [CrossRef]
- Verma, A.K.; Rajkumar, V.; Banerjee, R.; Biswas, S.; Das, A.K. Guava (Psidium guajava L.) powder as an antioxidant dietary fibre in sheep meat nuggets. Asian-Australas. J. Anim. Sci. 2013, 26, 886. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.G.; Cavalcanti, M.C.D.A.; Nobre, P.T.; Queiroga, R.D.C.R.D.E.; Medeiros, G.R.D.; Silva, N.V.D.; Batista, A.S.M.; Araújo Filho, J.T.D. Sensory quality of meat from Santa Inês lambs fed with guava (Psidium guajava L.) agroindustrial by-product. Food Sci. Technol. 2019, 40, 653–658. [Google Scholar] [CrossRef]
- Caballero, B.; Finglas, P.; Toldrá, F. Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Serna Cock, L.; Mera Ayala, J.D.; Angulo López, J.E.; Gómez Schouben, A.L. Kinetics of alcoholic fermentation using guava (Psidium guajava) seed flour and dry mycelium of Aspergillus niger as nitrogen sources. Dyna 2013, 80, 113–121. [Google Scholar]
- Uchôa-thomaz, A.M.A.; Sousa, E.C.; Carioca, J.O.B.; Morais, S.M.D.; Lima, A.D.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P. Chemical composition, fatty acid profile and bioactive compounds of guava seeds (Psidium guajava L.). Food Sci. Technol. 2014, 34, 485–492. [Google Scholar] [CrossRef]
- Fontanari, G.; Souza, G.; Batistuti, J.; Neves, V.; Pastre, I.; Fertonani, F. DSC studies on protein isolate of guava seeds Psidium guajava. J. Therm. Anal. Calorim. 2008, 93, 397–402. [Google Scholar] [CrossRef]
- Bezerra, C.F.; Rocha, J.E.; do Nascimento Silva, M.K.; de Freitas, T.S.; de Sousa, A.K.; Carneiro, J.N.P.; Leal, A.L.A.B.; dos Santos, A.T.L.; da Cruz, R.P.; Rodrigues, A.S. UPLC-MS-ESI-QTOF analysis and Anti-Candida activity of fractions from Psidium guajava L. S. Afr. J. Bot. 2020, 131, 421–427. [Google Scholar] [CrossRef]
- Flores, G.; Wu, S.-B.; Negrin, A.; Kennelly, E.J. Chemical composition and antioxidant activity of seven cultivars of guava (Psidium guajava) fruits. Food Chem. 2015, 170, 327–335. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Characterization of phenolic and other polar compounds in peel and flesh of pink guava (Psidium guajava L. cv.‘Criolla’) by ultra-high performance liquid chromatography with diode array and mass spectrometric detection. Food Res. Int. 2017, 100, 445–453. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Rajan, S. GC-MS analysis of bioactive compounds in Psidium guajava leaves. J. Pharm. Phytochem. 2015, 3, 162–166. [Google Scholar]
- Zahin, M.; Ahmad, I.; Aqil, F. Antioxidant and antimutagenic potential of Psidium guajava leaf extracts. Drug Chem. Toxicol. 2017, 40, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Mahmood, A.; Shahid, M.; Noor, N. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharm. Biol. 2016, 54, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.B.; Barkath, T.; Vijayaraghavan, P.; Rejiniemon, T. Gc-Ms Analysis of Phytochemical From Psidium guajava Linn Leaf Extract and Their Invitro Antimicrobial Activities. Int. J. Pharma Biol. Sci. 2018, 8, 583–589. [Google Scholar]
- Shaheena, S.; Chintagunta, A.D.; Dirisala, V.R.; Kumar, N.S. Extraction of bioactive compounds from Psidium guajava and their application in dentistry. AMB Express 2019, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Paudel, P.; Lamichhane, B.; Setzer, W.N. Leaf essential oil composition and bioactivity of Psidium guajava from Kathmandu, Nepal. Am. J. Essent. Oils Nat. Prod. 2015, 3, 11–14. [Google Scholar]
- Borah, A.; Pandey, S.K.; Haldar, S.; Lal, M. Chemical composition of leaf essential oil of Psidium guajava L. from North East India. J. Essent. Oil Bear. Plants 2019, 22, 248–253. [Google Scholar] [CrossRef]
- Chaturvedi, T.; Singh, S.; Nishad, I.; Kumar, A.; Tiwari, N.; Tandon, S.; Saikia, D.; Verma, R.S. Chemical composition and antimicrobial activity of the essential oil of senescent leaves of guava (Psidium guajava L.). Nat. Prod. Res. 2021, 35, 1393–1397. [Google Scholar] [CrossRef]
- Feng, X.-H.; Wang, Z.-H.; Meng, D.-L.; Li, X. Cytotoxic and antioxidant constituents from the leaves of Psidium guajava. Bioorgan. Med. Chem. Lett. 2015, 25, 2193–2198. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; An, Q.; Ma, H.; Mu, Y.; Qiao, W.; Zhang, Z.; Zhang, J.; Huang, X.; Li, L. New acylated phenolic glycosides with ROS-scavenging activity from Psidium guajava leaves. J. Agric. Food Chem. 2019, 67, 11089–11098. [Google Scholar] [CrossRef]
- Zhu, X.; Ouyang, W.; Pan, C.; Gao, Z.; Han, Y.; Song, M.; Feng, K.; Xiao, H.; Cao, Y. Identification of a new benzophenone from Psidium guajava L. leaves and its antineoplastic effects on human colon cancer cells. Food Funct. 2019, 10, 4189–4198. [Google Scholar] [CrossRef]
- Li, C.-J.; Ma, J.; Sun, H.; Zhang, D.; Zhang, D.-M. Guajavadimer A, a dimeric caryophyllene-derived meroterpenoid with a new carbon skeleton from the leaves of Psidium guajava. Org. Lett. 2016, 18, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-J.; Yu, Q.; Yan, H.; Khan, A.; Feng, M.-Y.; Li, P.-P.; Hao, X.-J.; An, L.-K.; Liu, H.-Y. Meroterpenoids with antitumor activities from guava (Psidium guajava). J. Agric. Food Chem. 2017, 65, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Wang, Y.; Liu, Z.; Zhang, D.-M.; Cao, H.-H.; Jiang, R.-W.; Fan, C.-L.; Zhang, X.-Q.; Chen, H.-R.; Yao, X.-S. Psiguadials A and B, two novel meroterpenoids with unusual skeletons from the leaves of Psidium guajava. Org. Lett. 2010, 12, 5040–5043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, M.; Zhao, L.; Ma, P. Guajadial inhibits NSCLC growth and migration following activation of the VEGF receptor-2. Fitoterapia 2018, 129, 73–77. [Google Scholar] [CrossRef]
- Shao, M.; Wang, Y.; Jian, Y.-Q.; Huang, X.-J.; Zhang, D.-M.; Tang, Q.-F.; Jiang, R.-W.; Sun, X.-G.; Lv, Z.-P.; Zhang, X.-Q. Guadial A and psiguadials C and D, three unusual meroterpenoids from Psidium guajava. Org. Lett. 2012, 14, 5262–5265. [Google Scholar] [CrossRef]
- Jian, Y.Q.; Huang, X.J.; Zhang, D.M.; Jiang, R.W.; Chen, M.F.; Zhao, B.X.; Wang, Y.; Ye, W.C. Guapsidial A and guadials B and C: Three new meroterpenoids with unusual skeletons from the leaves of Psidium guajava. Chem.-A Eur. J. 2015, 21, 9022–9027. [Google Scholar] [CrossRef]
- Yang, X.-L.; Hsieh, K.-L.; Liu, J.-K. Guajadial: An unusual meroterpenoid from guava leaves Psidium guajava. Org. Lett. 2007, 9, 5135–5138. [Google Scholar] [CrossRef]
- Fu, H.-Z.; Luo, Y.-M.; Li, C.-J.; Yang, J.-Z.; Zhang, D.-M. Psidials A−C, three unusual meroterpenoids from the leaves of Psidium guajava L. Org. Lett. 2010, 12, 656–659. [Google Scholar] [CrossRef]
- Lawrence, A.L.; Adlington, R.M.; Baldwin, J.E.; Lee, V.; Kershaw, J.A.; Thompson, A.L. A short biomimetic synthesis of the meroterpenoids guajadial and psidial A. Org. Lett. 2010, 12, 1676–1679. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, G.-Q.; Wei, K.; Hai, P.; Wang, F.; Liu, J.-K. Isolation and biomimetic synthesis of (±)-guajadial B, a novel meroterpenoid from Psidium guajava. Org. Lett. 2012, 14, 5936–5939. [Google Scholar] [CrossRef]
- Gao, Y.; Li, G.-T.; Li, Y.; Hai, P.; Wang, F.; Liu, J.-K. Guajadials CF, four unusual meroterpenoids from Psidium guajava. Nat. Prod. Bioprospect. 2013, 3, 14–19. [Google Scholar] [CrossRef]
- Qin, X.-J.; Yan, H.; Ni, W.; Yu, M.-Y.; Khan, A.; Liu, H.; Zhang, H.-X.; He, L.; Hao, X.-J.; Di, Y.-T. Cytotoxic meroterpenoids with rare skeletons from Psidium guajava cultivated in temperate zone. Sci. Rep. 2016, 6, 32748. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-H.; Dong, Z.; Guo, Y.-Q.; Cheng, Z.-B.; Zhou, C.-J.; Yin, S. Psiguajadials A–K: Unusual Psidium meroterpenoids as phosphodiesterase-4 inhibitors from the leaves of Psidium guajava. Sci. Rep. 2017, 7, 1047. [Google Scholar] [CrossRef]
- Ning, S.; Liu, Z.; Wang, Z.; Liao, M.; Xie, Z. Biomimetic synthesis of psiguajdianone guided discovery of the meroterpenoids from Psidium guajava. Org. Lett. 2019, 21, 8700–8704. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-Q.; Fan, C.-L.; Pei, X.; Zhang, P.-L.; Deng, F.; Jiang, W.-Q.; Wang, G.-C.; Zhang, X.-Q.; Ye, W.-C.; Wang, H. Psiguadiols A–J, rearranged meroterpenoids as potent PTP1B inhibitors from Psidium guajava. J. Nat. Prod. 2019, 82, 3267–3278. [Google Scholar] [CrossRef]
- Huang, J.; Li, C.; Ma, J.; Zang, Y.; Sun, X.; Chen, X.; Zhang, D. Psiguamers A–C, three cytotoxic meroterpenoids bearing a methylated benzoylphloroglucinol framework from Psidium guajava and total synthesis of 1 and 2. Chin. Chem. Lett. 2021, 32, 1721–1725. [Google Scholar] [CrossRef]
- Ryu, B.; Cho, H.M.; Zhang, M.; Lee, B.W.; Doan, T.P.; Park, E.J.; Lee, H.J.; Oh, W.K. Meroterpenoids from the leaves of Psidium guajava (guava) cultivated in Korea using MS/MS-based molecular networking. Phytochemistry 2021, 186, 112723. [Google Scholar] [CrossRef]
- Huang, J.W.; Li, C.J.; Yang, J.Z.; Li, C.; Zhang, Y.; Liu, K.; Yu, Y.; Jiang, J.D.; Zhang, D.M. Guajamers A—I, Rearranged Polycyclic Phloroglucinol Meroterpenoids from Psidium guajava Leaves and Their Antibacterial Activity. Chin. J. Chem. 2021, 39, 1129–1137. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Chung, S.S.M.; Xu, B. Guava leaf inhibits hepatic gluconeogenesis and increases glycogen synthesis via AMPK/ACC signaling pathways in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 1012–1017. [Google Scholar] [CrossRef]
- Jiao, Y.; Hua, D.; Huang, D.; Zhang, Q.; Yan, C. Characterization of a new heteropolysaccharide from green guava and its application as an α-glucosidase inhibitor for the treatment of type II diabetes. Food Funct. 2018, 9, 3997–4007. [Google Scholar] [CrossRef]
- Beidokhti, M.N.; Eid, H.M.; Villavicencio, M.L.; Jäger, A.K.; Lobbens, E.S.; Rasoanaivo, P.R.; McNair, L.M.; Haddad, P.S.; Staerk, D. Evaluation of the antidiabetic potential of Psidium guajava L.(Myrtaceae) using assays for α-glucosidase, α-amylase, muscle glucose uptake, liver glucose production and triglyceride accumulation in adipocytes. J. Ethnopharmacol. 2020, 257, 112877. [Google Scholar] [CrossRef] [PubMed]
- Lahon, J.; Phukan, S.; Lahkar, M.; Sharma, U. Anti convulsant potential of leaves of Psidium guajava Linn. In mes and ptz induced convulsion in experimental animals. Int. J. Pharm. Sci. Res. 2015, 6, 3946. [Google Scholar]
- Silva, E.; Estevam, E.; Silva, T.; Nicolella, H.; Furtado, R.; Alves, C.; Souchie, E.; Martins, C.; Tavares, D.; Barbosa, L. Antibacterial and antiproliferative activities of the fresh leaf essential oil of Psidium guajava L.(Myrtaceae). Braz. J. Biol. 2019, 79, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.F.; Rocha, J.E.; do Nascimento Silva, M.K.; de Freitas, T.S.; de Sousa, A.K.; Dos Santos, A.T.L.; da Cruz, R.P.; Ferreira, M.H.; da Silva, J.C.P.; Machado, A.J.T. Analysis by UPLC-MS-QTOF and antifungal activity of guava (Psidium guajava L.). Food Chem. Toxicol. 2018, 119, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Morais-Braga, M.F.B.; Sales, D.L.; Carneiro, J.N.P.; Machado, A.J.T.; Dos Santos, A.T.L.; de Freitas, M.A.; Martins, G.M.d.A.B.; Leite, N.F.; de Matos, Y.M.L.; Tintino, S.R. Psidium guajava L. and Psidium brownianum Mart ex DC.: Chemical composition and anti–Candida effect in association with fluconazole. Microb. Pathog. 2016, 95, 200–207. [Google Scholar] [CrossRef]
- Morais-Braga, M.F.; Carneiro, J.N.; Machado, A.J.; Sales, D.L.; Dos Santos, A.T.; Boligon, A.A.; Athayde, M.L.; Menezes, I.R.; Souza, D.S.; Costa, J.G. Phenolic composition and medicinal usage of Psidium guajava Linn.: Antifungal activity or inhibition of virulence? Saudi J. Biol. Sci. 2017, 24, 302–313. [Google Scholar] [CrossRef]
- Machado, A.J.; Santos, A.T.; Martins, G.M.; Cruz, R.P.; Costa, M.d.S.; Campina, F.F.; Freitas, M.A.; Bezerra, C.F.; Leal, A.L.; Carneiro, J.N. Antiparasitic effect of the Psidium guajava L.(guava) and Psidium brownianum MART. EX DC.(araçá-de-veado) extracts. Food Chem. Toxicol. 2018, 119, 275–280. [Google Scholar] [CrossRef]
- Piña-Vázquez, D.M.; Mayoral-Peña, Z.; Gómez-Sánchez, M.; Salazar-Olivo, L.A.; Arellano-Carbajal, F. Anthelmintic effect of Psidium guajava and Tagetes erecta on wild-type and Levamisole-resistant Caenorhabditis elegans strains. J. Ethnopharmacol. 2017, 202, 92–96. [Google Scholar] [CrossRef]
- Phakeovilay, C.; Bourgeade-Delmas, S.; Perio, P.; Valentin, A.; Chassagne, F.; Deharo, E.; Reybier, K.; Marti, G. Antileishmanial compounds isolated from Psidium guajava L. using a metabolomic approach. Molecules 2019, 24, 4536. [Google Scholar] [CrossRef]
- Liu, X.; Yan, X.; Bi, J.; Liu, J.; Zhou, M.; Wu, X.; Chen, Q. Determination of phenolic compounds and antioxidant activities from peel, flesh, seed of guava (Psidium guajava L.). Electrophoresis 2018, 39, 1654–1662. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Wu, Y.; Liu, Y.; Wu, Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem. 2018, 264, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Fushimi, T.; Nakamura, J.; Ota, N. Guava leaf extract suppresses osteoarthritis progression in a rat anterior cruciate ligament transection model. Food Sci. Nutr. 2018, 6, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.; Arbid, M.S.; Saleh, H.N. Antidiarrheal and protein conservative activities of Psidium guajava in diarrheal rats. J. Integr. Med. 2019, 17, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bazioli, J.M.; Costa, J.H.; Shiozawa, L.; Ruiz, A.L.T.G.; Foglio, M.A.; Carvalho, J.E.d. Anti-Estrogenic Activity of Guajadial Fraction, from Guava Leaves (Psidium guajava L.). Molecules 2020, 25, 1525. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, K.; Rengarajan, R.; Radhakrishnan, R.; Mathew, S.; Qadri, I.; Anand, A.V. Psidium guajava Leaf Extracts and Their Quercetin Protect HepG2 Cell Lines Against CCL 4 Induced Cytotoxicity. Indian J. Clin. Biochem. 2019, 34, 324–329. [Google Scholar] [CrossRef]
- Atik, N.; Muda, I.; Rahmadi, A.; Achadiyani, A.; Djunaedi, D. Phytochemical screening and histology appearance of acute oral toxicity study on ethanol extract of Psidium guajava Linn. fruit in mice. Asian J. Pharm. Clin. Res. 2019, 12, 351–355. [Google Scholar] [CrossRef]
- Omitoyin, B.O.; Ajani, E.K.; Orisasona, O.; Bassey, H.E.; Kareem, K.O.; Osho, F.E. Effect of guava Psidium guajava (L.) aqueous extract diet on growth performance, intestinal morphology, immune response and survival of Oreochromis niloticus challenged with Aeromonas hydrophila. Aquac. Res. 2019, 50, 1851–1861. [Google Scholar] [CrossRef]
- Babatola, L.J.; Oboh, G.; Ademiluyi, A.O. Toxicological evaluation of aqueous extract of different varieties of guava (Psidium guajava Linn.) leaves. Comp. Clin. Pathol. 2019, 28, 1689–1697. [Google Scholar] [CrossRef]
- Melo, C.; Cornejal, N.; Cruz, V.; Alsaidi, S.; Cruz Rodriguez, G.; Gomez Ramirez, A.; Sorel, V.; Bonnaire, T.; Zydowsky, T.M.; Priano, C. Antioxidant Capacity and Antimicrobial Activity of Commercial Samples of Guava Leaves (Psidium guajava). J. Med. Act. Plants 2020, 9, 2. [Google Scholar]
- Trujillo-Correa, A.I.; Quintero-Gil, D.C.; Diaz-Castillo, F.; Quiñones, W.; Robledo, S.M.; Martinez-Gutierrez, M. In vitro and in silico anti-dengue activity of compounds obtained from Psidium guajava through bioprospecting. BMC Complement. Altern. Med. 2019, 19, 298. [Google Scholar] [CrossRef]
- Alexander, P.; Sudi, I.Y.; Tizhe, M. Phytochemical and Antimicrobial Studies of the Crude Extracts of the Leaves of Carica papaya Linn (Pawpaw) and Psidium guajava Linn (Guava). Microbiol. Res. J. Int. 2019, 28, 1–7. [Google Scholar] [CrossRef]
- Patel, P.; Joshi, C.; Birdi, T.; Kothari, V. Anti-infective efficacy of Psidium guajava L. leaves against certain pathogenic bacteria. F1000Research 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Saleh, B.; Al-Mariri, A. Antibacterial Activity Evaluation of Psidium guajava L.(Myrtaceae) Crude Extracts Against Selected Bacterial Pathogens. Biol. Sci.-PJSIR 2020, 63, 119–126. [Google Scholar] [CrossRef]
- Manekeng, H.T.; Mbaveng, A.T.; Ntyam Mendo, S.A.; Agokeng, A.-J.D.; Kuete, V. Evaluation of Acute and Subacute Toxicities of Psidium guajava Methanolic Bark Extract: A Botanical with In Vitro Antiproliferative Potential. Evid.-Based Complement. Altern. Med. 2019, 2019, 8306986. [Google Scholar] [CrossRef] [PubMed]
- Weli, A.; Al-Kaabi, A.; Al-Sabahi, J.; Said, S.; Hossain, M.A.; Al-Riyami, S. Chemical composition and biological activities of the essential oils of Psidium guajava leaf. J. King Saud Univ.-Sci. 2019, 31, 993–998. [Google Scholar] [CrossRef]
- Birdi, T.; Krishnan, G.G.; Kataria, S.; Gholkar, M.; Daswani, P. A randomized open label efficacy clinical trial of oral guava leaf decoction in patients with acute infectious diarrhoea. J. Ayurveda Integr. Med. 2020, 11, 163–172. [Google Scholar] [CrossRef]
- Biswal, S.K.; Panigrahi, G.K.; Sahoo, S.K. Green synthesis of Fe2O3-Ag nanocomposite using Psidium guajava leaf extract: An eco-friendly and recyclable adsorbent for remediation of Cr (VI) from aqueous media. Biophys. Chem. 2020, 263, 106392. [Google Scholar] [CrossRef]
- Rehman, R.; Mahmud, T.; Irum, M. Brilliant green dye elimination from water using Psidium guajava leaves and Solanum tuberosum peels as adsorbents in environmentally benign way. J. Chem. 2015, 2015, 126036. [Google Scholar] [CrossRef]
- Abdulla, N.K.; Siddiqui, S.I.; Tara, N.; Hashmi, A.A.; Chaudhry, S.A. Psidium guajava leave-based magnetic nanocomposite γ-Fe2O3@ GL: A green technology for methylene blue removal from water. J. Environ. Chem. Eng. 2019, 7, 103423. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Veeramani, V.; Saravanan, M.; Rajalakshmi, G.; Kaliannan, T.; Al-Misned, F.A.; Pugazhendhi, A. Green chemistry route of biosynthesized copper oxide nanoparticles using Psidium guajava leaf extract and their antibacterial activity and effective removal of industrial dyes. J. Environ. Chem. Eng. 2021, 9, 105033. [Google Scholar] [CrossRef]
- Kumar, M.; Mehta, A.; Mishra, A.; Singh, J.; Rawat, M.; Basu, S. Biosynthesis of tin oxide nanoparticles using Psidium guajava leave extract for photocatalytic dye degradation under sunlight. Mater. Lett. 2018, 215, 121–124. [Google Scholar] [CrossRef]
- Rehan, M.; Ahmed-Farid, O.A.; Ibrahim, S.R.; Hassan, A.A.; Abdelrazek, A.M.; Khafaga, N.I.; Khattab, T.A. Green and sustainable encapsulation of Guava leaf extracts (Psidium guajava L.) into alginate/starch microcapsules for multifunctional finish over cotton gauze. ACS Sustain. Chem. Eng. 2019, 7, 18612–18623. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, H.; Rawat, M. A novel green approach for the synthesis of tungsten oxide nanorods and its efficient potential towards photocatalytic degradation of reactive green 19 dye. J. Mater. Sci. Mater. Electron. 2018, 29, 13715–13722. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).