Influence of Enzymatic Hydrolysis and Molecular Weight Fractionation on the Antioxidant and Lipase / α-Amylase Inhibitory Activities In Vitro of Watermelon Seed Protein Hydrolysates
Abstract
1. Introduction
2. Results and Discussion
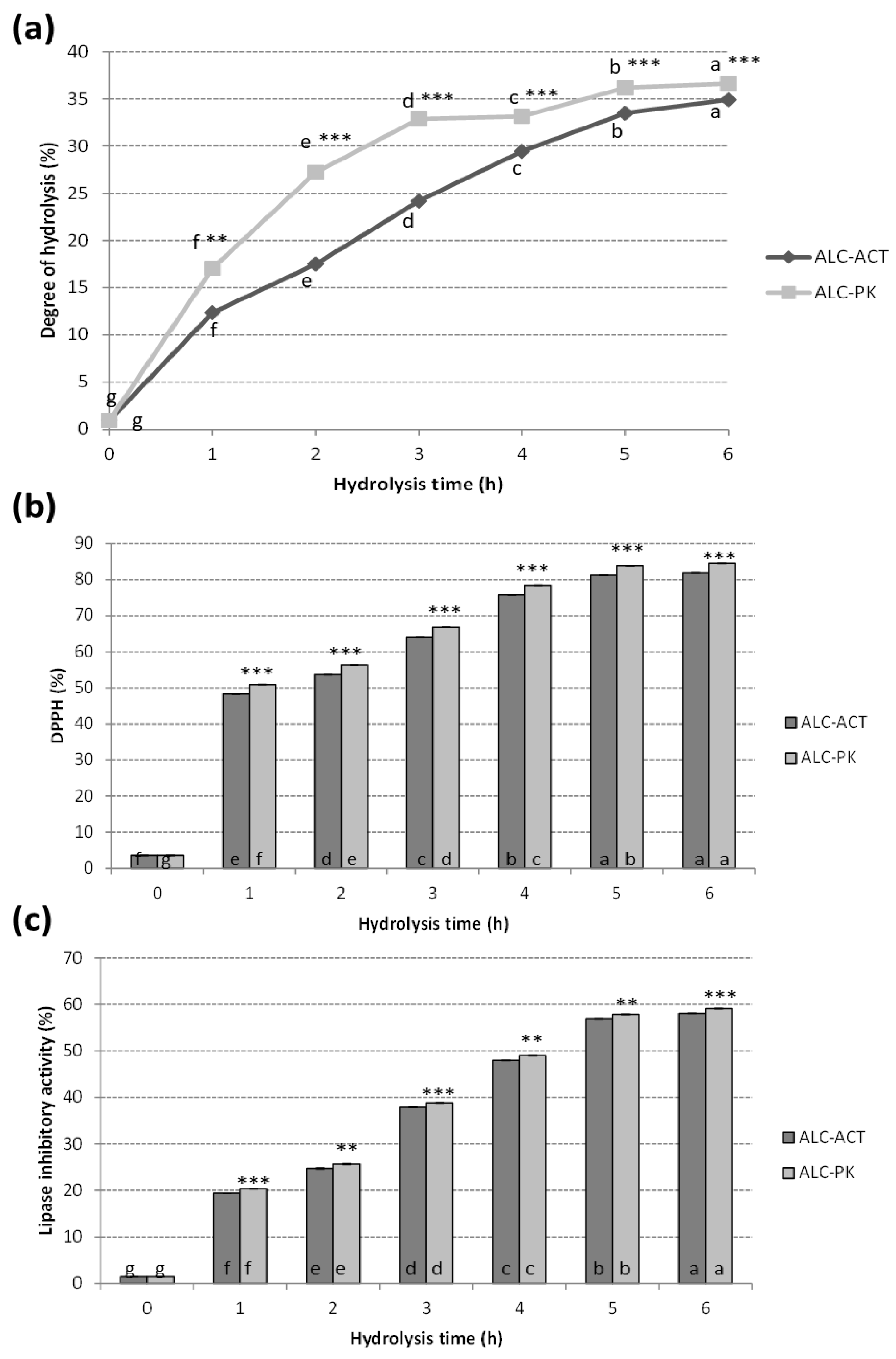
2.1. Degree of Hydrolysis
2.2. Antioxidant Activity
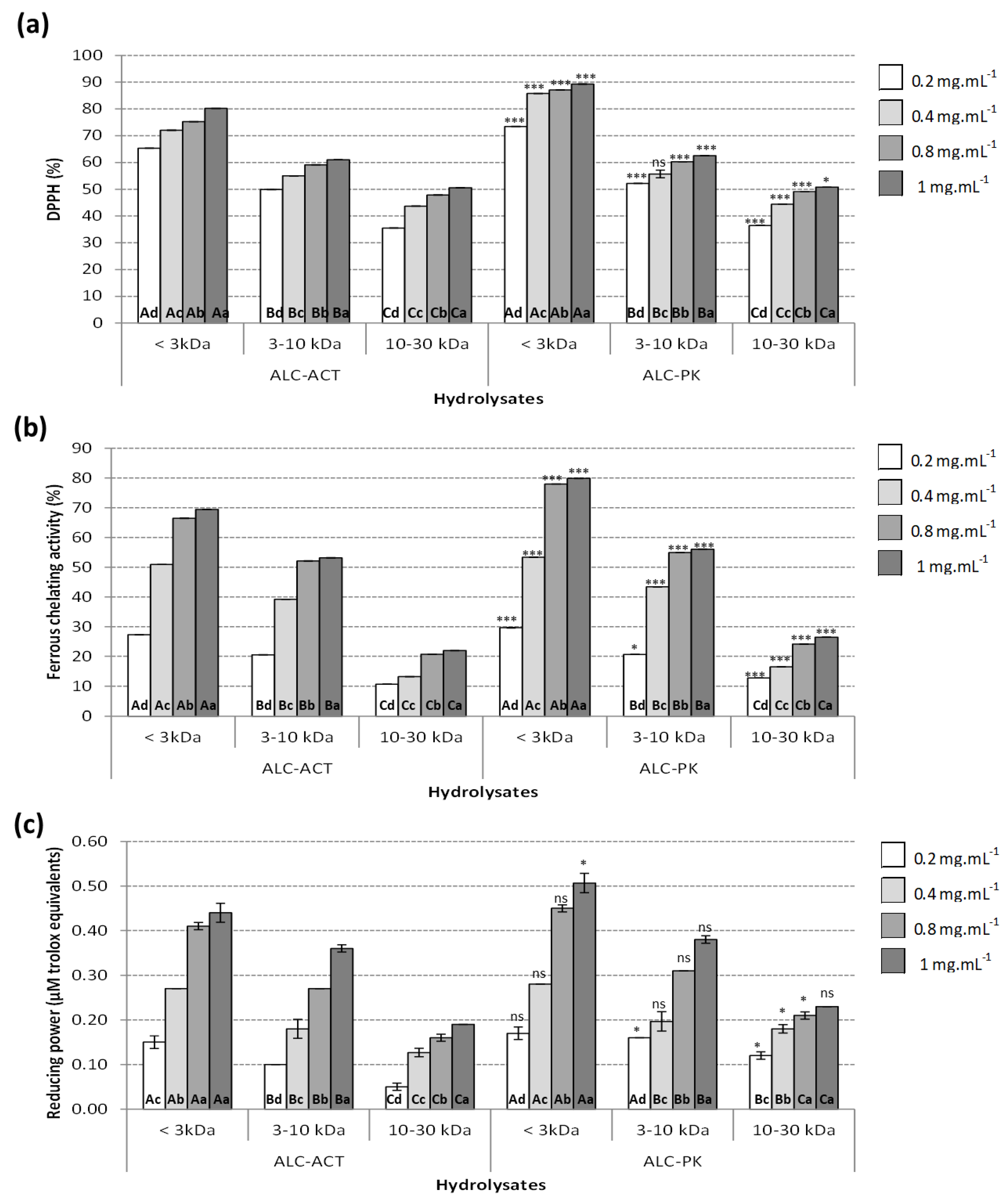
2.2.1. DPPH-Radical Scavenging Activity
2.2.2. Ferrous Chelating (FC) Activity
2.2.3. Reducing Power (RP) Activity
2.3. Anti-Obesity Activities
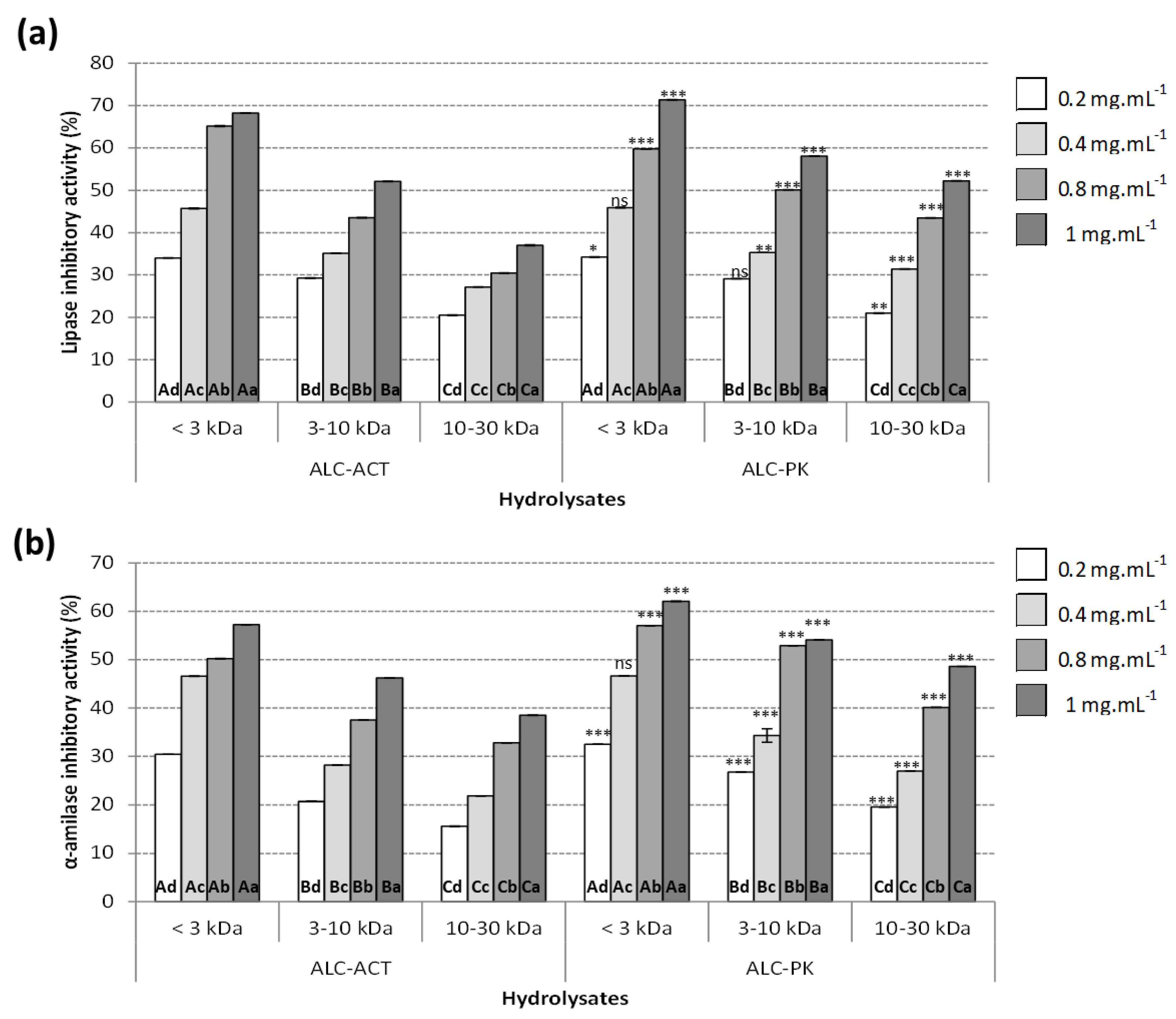
2.3.1. Lipase Inhibitory Activity
2.3.2. α-Amylase Inhibitory Activity
3. Materials and Methods
3.1. Biological Material and Chemical Reagents
3.2. Protein Extraction and Hydrolysis
3.3. Degree of Hydrolysis (DH)
3.4. Molecular-Weight Cut-Off Filtration (MWCO)
3.5. Antioxidant Activity
3.5.1. DPPH Radical Scavenging Activity
3.5.2. Ferrous Chelating (FC) Activity
3.5.3. Reducing Power (RP)
3.6. Anti-Obesity Activities
3.6.1. Lipase Inhibitory Activity
3.6.2. α-Amylase Inhibitory Activity
3.7. Amino Acid Analysis
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Garcia-Vaquero, M.; Mora, L.; Hayes, M. In vitro and in silico approaches to generating and identifying angiotensin-converting enzyme I inhibitory peptides from green macroalga Ulva lactuca. Mar. Drugs 2019, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzade, L.; Hosseini, S.F.; Nikkhah, M. Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides. Food Chem. 2017, 234, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Cumby, N.; Zhong, Y.; Naczk, M.; Shahidi, F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008, 109, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhou, J.; Feng, Y.; Duan, Y.; Zhang, H.; Ma, H. Slit divergent ultrasound pretreatment assisted watermelon seed protein enzymolysis and the antioxidant activity of its hydrolysates in vitro and in vivo. Food Chem. 2020, 328, 127135. [Google Scholar] [PubMed]
- Famuwagun, A.A.; Alashi, A.M.; Gbadamosi, S.O.; Taiwo, K.A.; Oyedele, D.; Adebooye, O.C.; Aluko, R.E. Effect of protease type and peptide size on the in vitro antioxidant, antihypertensive and anti-diabetic activities of eggplant leaf protein hydrolysates. Foods 2021, 10, 1112. [Google Scholar] [CrossRef]
- Mudgil, P.; Kilari, B.P.; Kamal, H.; Olalere, O.A.; FitzGerald, R.J.; Gan, C.-Y.; Maqsood, S. Multifunctional bioactive peptides derived from quinoa protein hydrolysates: Inhibition of α-glucosidase, dipeptidyl peptidase-IV and angiotensin I converting enzymes. J. Cereal Sci. 2020, 96, 103130. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Hanafi, M.A.; Chay, S.Y.; Hussin, F.S.; Auwal, S.M.; Zarei, M.; Sarbini, S.R.; Wan Ibadullah, W.Z.; Karim, R.; Saari, N. Multifunctional hydrolysates from kenaf (Hibiscus cannabinus L.) seed protein with high antihypertensive activity in vitro and in vivo. J. Food Meas. Charact. 2021, 15, 652–663. [Google Scholar]
- Sonklin, C.; Alashi, M.A.; Laohakunjit, N.; Kerdchoechuen, O.; Aluko, R.E. Identification of antihypertensive peptides from mung bean protein hydrolysate and their effects in spontaneously hypertensive rats. J. Funct. Foods 2020, 64, 103635. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Lee, C.W.; Yun, H.; Eun, J.-B. Structure-function engineering of novel fish gelatin-derived multifunctional peptides using high-resolution peptidomics and bioinformatics. Sci. Rep. 2021, 11, 7401. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, M.; Wu, T.; Fang, L.; Liu, C.; Min, W. Novel anti-obesity peptide (RLLPH) derived from hazelnut (Corylus heterophylla Fisch) protein hydrolysates inhibits adipogenesis in 3T3-L1 adipocytes by regulating adipogenic transcription factors and adenosine monophosphate-activated protein kinase (AMPK) activation. J. Biosci. Bioeng. 2020, 129, 259–268. [Google Scholar]
- Coronado-Cáceres, L.J.; Rabadán-Chávez, G.; Mojica, L.; Hernández-Ledesma, B.; Quevedo-Corona, L.; Lugo Cervantes, E. Cocoa (Theobroma cacao L.) seed proteins’ anti-obesity potential through lipase inhibition using in silico, in vitro and in vivo models. Foods 2020, 9, 1359. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Waterhouse, D.-S.; Liu, P.; Waterhouse, G.I.; Li, J.; Cui, C. Pancreatic lipase-inhibiting protein hydrolysate and peptides from seabuckthorn seed meal: Preparation optimization and inhibitory mechanism. LWT 2020, 134, 109870. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.; Deering, A.; Liceaga, A. New insights into the antimicrobial properties of hydrolysates and peptide fractions derived from chia seed (Salvia hispanica L.). Probiotics Antimicrob. Proteins 2020, 12, 1571–1581. [Google Scholar] [PubMed]
- Borrajo, P.; Pateiro, M.; Gagaoua, M.; Franco, D.; Zhang, W.; Lorenzo, J.M. Evaluation of the antioxidant and antimicrobial activities of porcine liver protein hydrolysates obtained using alcalase, bromelain, and papain. Appl. Sci. 2020, 10, 2290. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M. Shelf-life extension of whole shrimp using an active coating containing fish skin gelatin hydrolysates produced by a natural protease. Food Sci. Nutr. 2020, 8, 214–223. [Google Scholar] [PubMed]
- Moosavi-Nasab, M.; Oliyaei, N.; Eun, J.-B.; Mirzapour-Kouhdasht, A. Innovation in the Seafood Sector through the Valorization of By-Products. In Innovation in the Food Sector Through the Valorization of Food and Agro-Food By-Products; IntechOpen: London, UK, 2020. [Google Scholar]
- Adnyana, I.K.; Abuzaid, A.S.; Iskandar, E.Y.; Kurniati, N.F. Pancreatic lipase and α-amylase inhibitory potential of mangosteen (Garcinia mangostana Linn.) pericarp extract. Int. J. Med. Res. Health Sci. 2016, 5, 23–28. [Google Scholar]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complementary Altern. Med. 2012, 12, 110. [Google Scholar]
- Fathi, P.; Moosavi-Nasab, M.; Mirzapour-Kouhdasht, A.; Khalesi, M. Generation of hydrolysates from rice bran proteins using a combined ultrasonication-Alcalase hydrolysis treatment. Food Biosci. 2021, 42, 101110. [Google Scholar] [CrossRef]
- Singh, N.; Matta, N. Levels of seed proteins in Citrullus and Praecitrullus accessions. Plant Syst. Evol. 2010, 290, 47–56. [Google Scholar] [CrossRef]
- Arise, R.; Yekeen, A.; Ekun, O.; Olatomiwa, O. Angiotensin-I converting enzyme-inhibitory, antiradical and hydrogen peroxide-scavenging properties of Citrullus lanatus seed protein hydrolysates. Ceylon J. Sci 2016, 45, 39–52. [Google Scholar] [CrossRef]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Wei, C.-K.; Thakur, K.; Liu, D.-H.; Zhang, J.-G.; Wei, Z.-J. Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products. Food Chem. 2018, 263, 186–193. [Google Scholar] [PubMed]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from Mung bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [PubMed]
- Mirzapour-Kouhdasht, A.; Lee, C.W.; Yun, H.; Eun, J.-B. Structure–function relationship of fermented skate skin gelatin-derived bioactive peptides: A peptidomics approach. Food Sci. Biotechnol. 2021, 30, 1685–1693. [Google Scholar]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Cermeño, M.; Kleekayai, T.; Harnedy-Rothwell, P.A.; Fernandes, E.; Alves, R.C.; Oliveira, M.B.P.; FitzGerald, R.J. Enzymatic modification of Porphyra dioica-derived proteins to improve their antioxidant potential. Molecules 2020, 25, 2838. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fraguas, J.; Mirón, J.; Valcárcel, J.; Pérez-Martín, R.I.; Antelo, L.T. Valorisation of fish discards assisted by enzymatic hydrolysis and microbial bioconversion: Lab and pilot plant studies and preliminary sustainability evaluation. J. Clean. Prod. 2020, 246, 119027. [Google Scholar]
- Jafar, S.; Kamal, H.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel whey protein hydrolysates displayed enhanced cholesteryl esterase and lipase inhibitory, anti-hypertensive and anti-haemolytic properties. LWT 2018, 98, 212–218. [Google Scholar]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Kim, Y.-M.; Eun, J.-B. Antioxidant mechanism, antibacterial activity, and functional characterization of peptide fractions obtained from barred mackerel gelatin with a focus on application in carbonated beverages. Food Chem. 2021, 342, 128339. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Krishnaswamy, K.; Khalesi, M. Optimization of gelatin production from Barred mackerel by-products: Characterization and hydrolysis using native and commercial proteases. Food Hydrocoll. 2020, 108, 105970. [Google Scholar] [CrossRef]
- Shaik, M.I.; Noor, S.N.A.A.M.; Sarbon, N.M. In-vitro angiotensin converting enzyme (ACE), antioxidant activity and some functional properties of silver catfish (Pangasius sp.) protein hydrolysate by ultrafiltration. Biocatal. Agric. Biotechnol. 2021, 35, 102100. [Google Scholar] [CrossRef]
- Sonklin, C.; Laohakunjit, N.; Kerdchoechuen, O. Assessment of antioxidant properties of membrane ultrafiltration peptides from mungbean meal protein hydrolysates. PeerJ 2018, 6, e5337. [Google Scholar] [PubMed]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of protein hydrolysates of fish waste using membrane ultrafiltration: Investigation of antibacterial and antioxidant activities. Probiotics Antimicrob. Proteins 2019, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar]
- Kim, J.M.; Liceaga, A.M.; Yoon, K.Y. Purification and identification of an antioxidant peptide from perilla seed (Perilla frutescens) meal protein hydrolysate. Food Sci. Nutr. 2019, 7, 1645–1655. [Google Scholar]
- Paul, A.A.; Eghianruwa, Q.A.; Oparinde, O.G.; Adesina, A.S.; Osoniyi, O. Enzymatic protein hydrolysates, and ultrafiltered peptide fractions from two molluscs: Tympanotonus fuscatus var. radula (L.) and Pachymelania aurita (M.), with angiotensin-I-converting enzyme inhibitory and DPPH radical scavenging activities. Int. J. Appl. Basic Med. Res. 2021, 11, 70. [Google Scholar]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Inhibitory properties of kidney bean protein hydrolysate and its membrane fractions against renin, angiotensin converting enzyme, and free radicals. Austin J. Nutr. Food Sci. 2014, 2, 1008–1019. [Google Scholar]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar]
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J. Funct. Foods 2017, 35, 418–427. [Google Scholar]
- Halim, N.R.A.; Azlan, A.; Yusof, H.M.; Sarbon, N.M. Antioxidant and anticancer activities of enzymatic eel (Monopterus sp.) protein hydrolysate as influenced by different molecular weight. Biocatal. Agric. Biotechnol. 2018, 16, 10–16. [Google Scholar] [CrossRef]
- Farvin, K.S.; Andersen, L.L.; Nielsen, H.H.; Jacobsen, C.; Jakobsen, G.; Johansson, I.; Jessen, F. Antioxidant activity of Cod (Gadus morhua) protein hydrolysates: In vitro assays and evaluation in 5% fish oil-in-water emulsion. Food Chem. 2014, 149, 326–334. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y.; Sun, N.; Bao, Z.; Lin, S. Food protein-derived iron-chelating peptides: The binding mode and promotive effects of iron bioavailability. Food Res. Int. 2020, 131, 108976. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef]
- Safari, R.; Yaghoubzadeh, Z. Antioxidant activity of bioactive peptides extracted from sea cucumber (Holothuria leucospilata). Int. J. Pept. Res. Ther. 2020, 26, 2393–2398. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Szymanowska, U.; Karaś, M.; Złotek, U.; Kowalczyk, D. Potential anti-inflammatory and lipase inhibitory peptides generated by in vitro gastrointestinal hydrolysis of heat treated millet grains. CyTA-J. Food 2019, 17, 324–333. [Google Scholar] [CrossRef]
- Ketprayoon, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. An in vitro study of lipase inhibitory peptides obtained from de-oiled rice bran. RSC Adv. 2021, 11, 18915–18929. [Google Scholar]
- Siow, H.-L.; Choi, S.-B.; Gan, C.-Y. Structure–activity studies of protease activating, lipase inhibiting, bile acid binding and cholesterol-lowering effects of pre-screened cumin seed bioactive peptides. J. Funct. Foods 2016, 27, 600–611. [Google Scholar]
- Siow, H.-L.; Lim, T.S.; Gan, C.-Y. Development of a workflow for screening and identification of α-amylase inhibitory peptides from food source using an integrated Bioinformatics-phage display approach: Case study–Cumin seed. Food Chem. 2017, 214, 67–76. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Rupasinghe, S.G.; Schuler, M.A.; Gonzalez de Mejia, E. Peptides from purified soybean β-conglycinin inhibit fatty acid synthase by interaction with the thioesterase catalytic domain. FEBS J. 2010, 277, 1481–1493. [Google Scholar] [CrossRef]
- Stefanucci, A.; Luisi, G.; Zengin, G.; Macedonio, G.; Dimmito, M.P.; Novellino, E.; Mollica, A. Discovery of arginine-containing tripeptides as a new class of pancreatic lipase inhibitors. Future Med. Chem. 2019, 11, 5–19. [Google Scholar]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Malomo, S.A.; Girgih, A.T.; Aluko, R.E. Blood pressure lowering effects of Australian canola protein hydrolysates in spontaneously hypertensive rats. Food Res. Int. 2014, 55, 281–287. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: Barking, Essex, UK, 1986. [Google Scholar]
- Ambigaipalan, P.; Shahidi, F. Date seed flour and hydrolysates affect physicochemical properties of muffin. Food Biosci. 2015, 12, 54–60. [Google Scholar]
- Yen, G.-C.; Chen, H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 2017, 221, 1883–1894. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Faloye, Y.M. Effect of combination on the antioxidant and inhibitory properties of tropical pepper varieties against α-amylase and α-glucosidase activities in vitro. J. Med. Food 2011, 14, 1152–1158. [Google Scholar]
- Siswoyo, T.A.; Mardiana, E.; Lee, K.O.; Hoshokawa, K. Isolation and characterization of antioxidant protein fractions from melinjo (Gnetum gnemon) seeds. J. Agric. Food Chem. 2011, 59, 5648–5656. [Google Scholar] [CrossRef]

| Amino Acid | ALC-PK Hydrolysate | <3 kDa | 3–10 kDa | 10–30 kDa |
|---|---|---|---|---|
| Asp | 9.2 ± 0.1 c | 12.5 ± 0.1 a | 11.4 ± 0.2 b | 11.3 ± 0.2 b |
| Thr | 3.5 ± 0.1 b | 4.7 ± 0.0 a | 4.0 ± 0.1 b | 3.7 ± 0.1 b |
| Ser | 4.9 ± 0.0 b | 5.1 ± 0.1 a | 4.9 ± 0.0 b | 4.8 ± 0.1 b |
| Glu | 17.7 ± 0.1 c | 20.3 ± 0.2 a | 19.1 ± 0.0 b | 17.8 ± 0.0 c |
| Gly | 4.9 ± 0.1 b | 6.3 ± 0.0 a | 5.0 ± 0.2 b | 5.0 ± 0.1 b |
| Ala | 4.9 ± 0.0 b | 5.5 ± 0.2 a | 4.8 ± 0.1 b | 4.8 ± 0.2 b |
| Cys | 6.3 ± 0.1 c | 6.3 ± 0.2 c | 7.8 ± 0.1 a | 6.7 ± 0.1 b |
| Val | 4.1 ± 0.0 c | 5.5 ± 0.1 a | 4.1 ± 0.0 c | 5.0 ± 0.1 b |
| Met | 0.9 ± 0.1 c | 1.6 ± 0.2 a | 1.0 ± 0.1 b | 1.0 ± 0.1 b |
| Ile | 5.2 ± 0.0 b | 6.7 ± 0.1 a | 5.4 ± 0.0 b | 5.2 ± 0.1 b |
| Leu | 7.4 ± 0.1 b | 7.2 ± 0.0 a | 1.1 ± 0.0 b | 1.1 ± 0.1 b |
| Tyr | 3.3 ± 0.0 b | 5.1 ± 0.2 a | 3.3 ± 0.0 b | 3.3 ± 0.1 b |
| Phe | 3.4 ± 0.1 c | 5.1 ± 0.0 a | 4.0 ± 0.2 b | 4.0 ± 0.0 b |
| His | 1.7 ± 0.0 b | 3.0 ± 0.2 a | 1.8 ± 0.1 b | 1.7 ± 0.1 b |
| Lys | 3.2 ± 0.1 c | 6.1 ± 0.3 a | 4.5 ± 0.1 b | 3.3 ± 0.2 c |
| Arg | 14.6 ± 0.1 b | 18.4 ± 0.2 a | 14.7 ± 0.2 b | 14.6 ± 0.2 b |
| Pro | 4.1 ± 0.0 b | 4.9 ± 0.1 a | 4.4 ± 0.1 ab | 4.1 ± 0.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M.; Eun, J.-B.; Simal-Gandara, J. Influence of Enzymatic Hydrolysis and Molecular Weight Fractionation on the Antioxidant and Lipase / α-Amylase Inhibitory Activities In Vitro of Watermelon Seed Protein Hydrolysates. Molecules 2022, 27, 7897. https://doi.org/10.3390/molecules27227897
Mirzapour-Kouhdasht A, Garcia-Vaquero M, Eun J-B, Simal-Gandara J. Influence of Enzymatic Hydrolysis and Molecular Weight Fractionation on the Antioxidant and Lipase / α-Amylase Inhibitory Activities In Vitro of Watermelon Seed Protein Hydrolysates. Molecules. 2022; 27(22):7897. https://doi.org/10.3390/molecules27227897
Chicago/Turabian StyleMirzapour-Kouhdasht, Armin, Marco Garcia-Vaquero, Jong-Bang Eun, and Jesus Simal-Gandara. 2022. "Influence of Enzymatic Hydrolysis and Molecular Weight Fractionation on the Antioxidant and Lipase / α-Amylase Inhibitory Activities In Vitro of Watermelon Seed Protein Hydrolysates" Molecules 27, no. 22: 7897. https://doi.org/10.3390/molecules27227897
APA StyleMirzapour-Kouhdasht, A., Garcia-Vaquero, M., Eun, J.-B., & Simal-Gandara, J. (2022). Influence of Enzymatic Hydrolysis and Molecular Weight Fractionation on the Antioxidant and Lipase / α-Amylase Inhibitory Activities In Vitro of Watermelon Seed Protein Hydrolysates. Molecules, 27(22), 7897. https://doi.org/10.3390/molecules27227897










