Highly Dispersed Vanadia Anchored on Protonated g-C3N4 as an Efficient and Selective Catalyst for the Hydroxylation of Benzene into Phenol
Abstract
1. Introduction
2. Results and Discussion
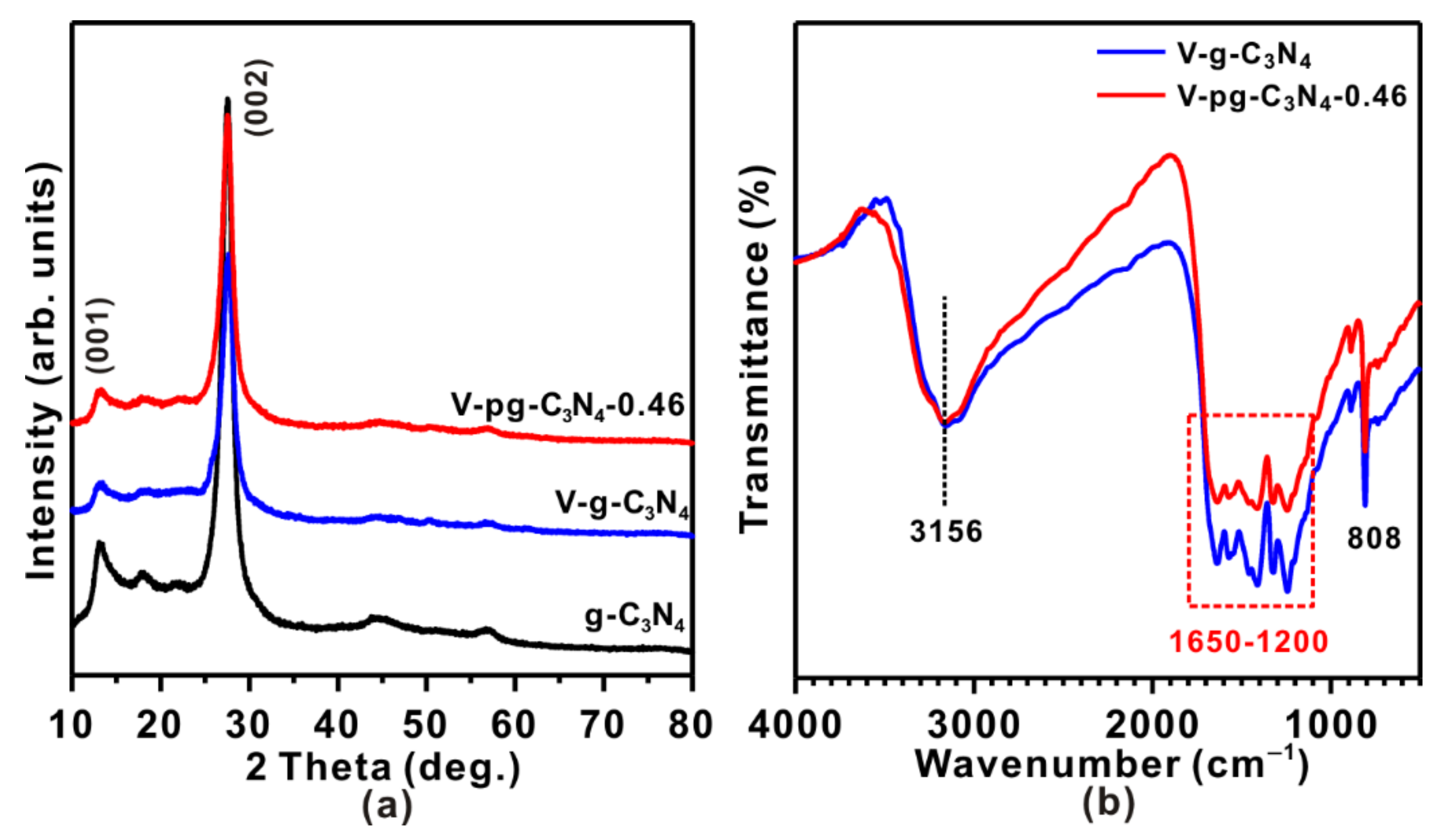
2.1. Bulk Structures and Surface Structures of V-g-C3N4 and V-pg-C3N4
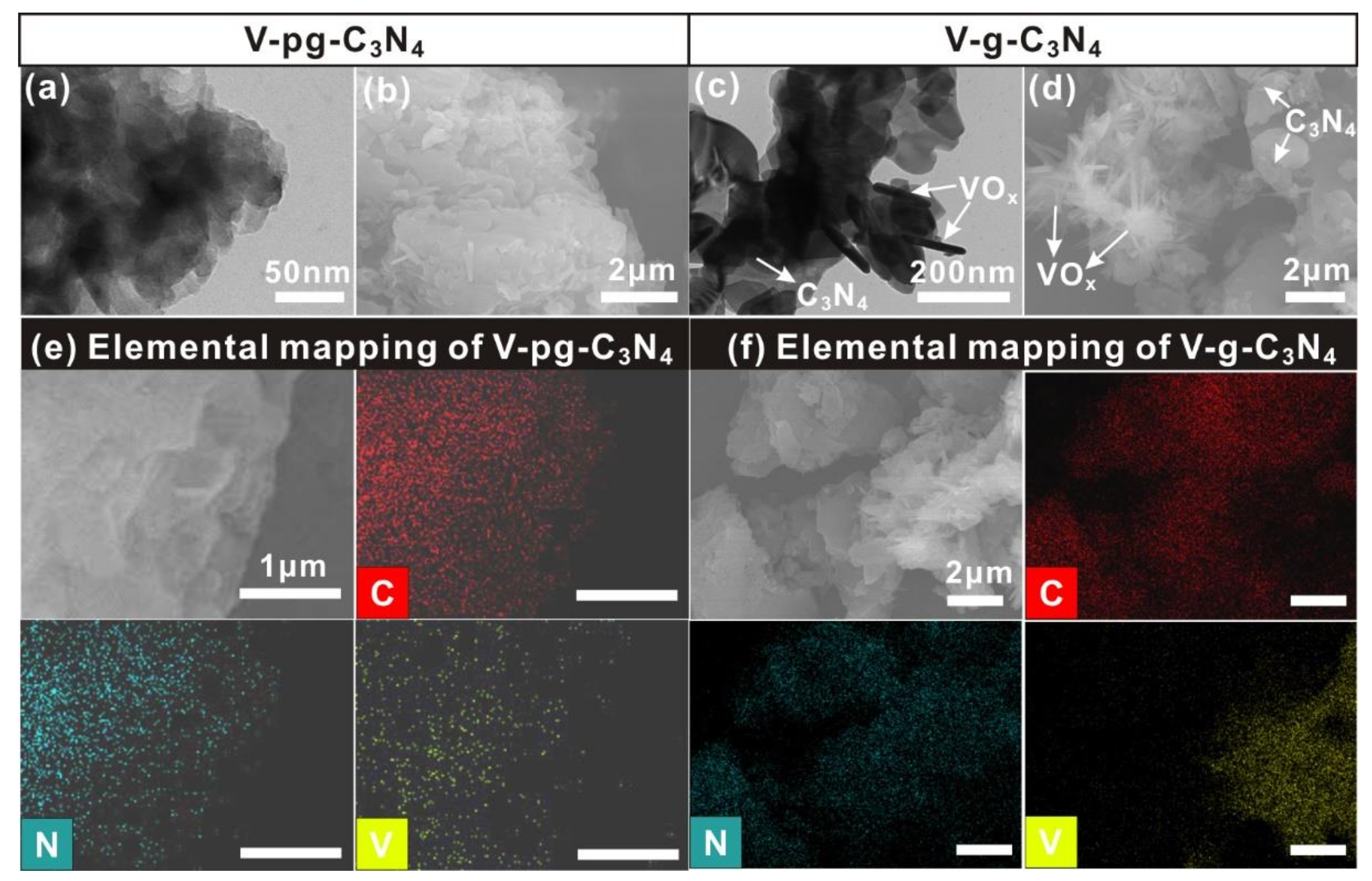
2.2. Microstructures of V-g-C3N4 and V-pg-C3N4
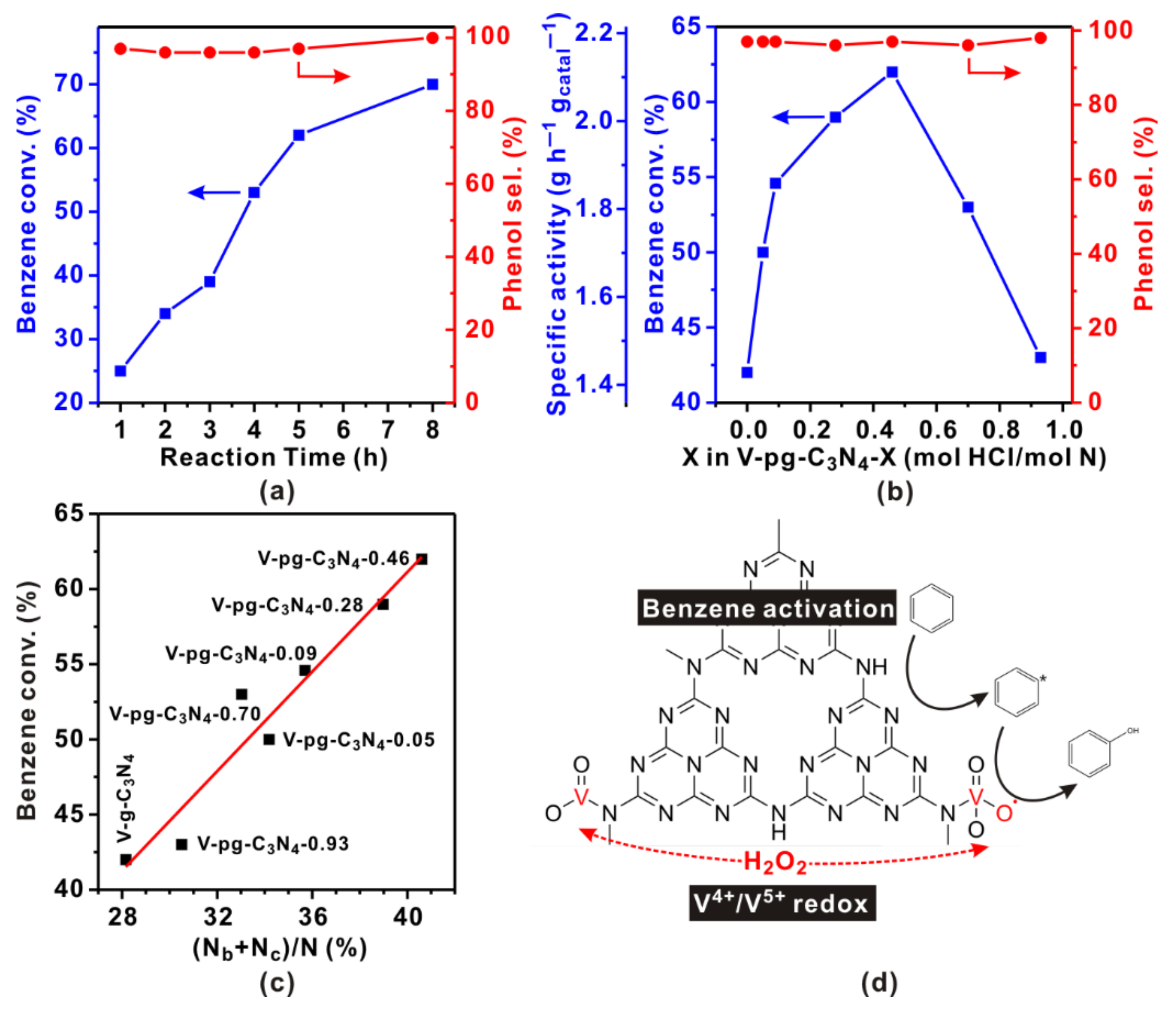
2.3. Catalytic Performance of V-pg-C3N4
2.4. Reaction Mechanism of Benzene Hydroxylation over V-pg-C3N4
3. Materials and Methods
3.1. Preparation of g-C3N4
3.2. Preparation of V-pg-C3N4
3.3. Characterizations
3.4. Catalytic Performance Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Niwa, S.-i.; Eswaramoorthy, M.; Nair, J.; Raj, A.; Itoh, N.; Shoji, H.; Namba, T.; Mizukami, F. A One-Step Conversion of Benzene to Phenol with a Palladium Membrane. Science 2002, 295, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Zaoputra, A.A.; Hitomi, Y.; Mieda, K.; Ogura, T.; Shiota, Y.; Yoshizawa, K.; Sato, H.; Kodera, M. Specific Enhancement of Catalytic Activity by a Dicopper Core: Selective Hydroxylation of Benzene to Phenol with Hydrogen Peroxide. Angew. Chem. Int. Ed. 2017, 56, 7779–7782. [Google Scholar] [CrossRef]
- Borah, P.; Ma, X.; Nguyen, K.T.; Zhao, Y. A vanadyl complex grafted to periodic mesoporous organosilica: A green catalyst for selective hydroxylation of benzene to phenol. Angew. Chem. Int. Ed. 2012, 51, 7756–7761. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wang, W.; Jiang, T.; Han, B.; Fan, H.; Yang, G. Highly Selective Synthesis of Phenol from Benzene over a Vanadium-Doped Graphitic Carbon Nitride Catalyst. ChemCatChem 2013, 5, 192–200. [Google Scholar] [CrossRef]
- Guo, B.; Zhu, L.; Hu, X.; Zhang, Q.; Tong, D.; Li, G.; Hu, C. Nature of vanadium species on vanadium silicalite-1 zeolite and their stability in hydroxylation reaction of benzene to phenol. Catal. Sci. Technol. 2011, 1, 1060–1067. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. 2+ ions immobilized on functionalized surface of mesoporous silica and their activity toward the hydroxylation of benzene. J. Phys. Chem. B 2003, 107, 2543–2551. [Google Scholar] [CrossRef]
- Verma, S.; Nasir Baig, R.; Nadagouda, M.N.; Varma, R.S. Hydroxylation of Benzene via C–H activation using bimetallic CuAg@ g-C3N4. ACS Sustain. Chem. Eng. 2017, 5, 3637–3640. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, L.; Wang, M.; Yue, B.; He, H. Cerium promoted Vg-C3N4 as highly efficient heterogeneous catalysts for the direct benzene hydroxylation. Roy. Soc. Open Sci. 2018, 5, 180371. [Google Scholar] [CrossRef]
- Long, Z.; Zhou, Y.; Chen, G.; Ge, W.; Wang, J. C3N4-H5PMo10V2O40: A dual-catalysis system for reductant-free aerobic oxidation of benzene to phenol. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef]
- Borah, P.; Datta, A.; Nguyen, K.T.; Zhao, Y. VOPO4·2H2O encapsulated in graphene oxide as a heterogeneous catalyst for selective hydroxylation of benzene to phenol. Green Chem. 2016, 18, 397–401. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Li, H.; Chen, Z.; Wang, Y. Selective oxidation of benzene to phenol by FeCl3/mpg-C3N4 hybrids. RSC Adv. 2013, 3, 5121–5126. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Stabilizing CuPd bimetallic alloy nanoparticles deposited on holey carbon nitride for selective hydroxylation of benzene to phenol. J. Catal. 2019, 379, 154–163. [Google Scholar] [CrossRef]
- Shah Bacha, R.u.; Li, L.; Guo, Y.-R.; Jing, L.; Pan, Q.-J. Actinyl-modified g-C3N4 as CO2 activation materials for chemical conversion and environmental remedy via an artificial photosynthetic route. Inorg. Chem. 2020, 59, 8369–8379. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Balasubramanian, V.V.; Selvan, S.T.; Sawant, D.P.; Chari, M.A.; Lu, G.e.Q.; Vinu, A. Highly ordered mesoporous carbon nitride nanoparticles with high nitrogen content: A metal-free basic catalyst. Angew. Chem. Int. Ed. 2009, 48, 7884–7887. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, K.; Xue, B.; Li, Y.-X. Microporous carbon nitride as an effective solid base catalyst for Knoevenagel condensation reactions. J. Mol. Catal. A 2013, 372, 105–113. [Google Scholar] [CrossRef]
- Xu, J.; Chen, T.; Jiang, Q.; Li, Y.X. Utilization of Environmentally Benign Dicyandiamide as a Precursor for the Synthesis of Ordered Mesoporous Carbon Nitride and its Application in Base-Catalyzed Reactions. Chem. Asian J. 2014, 9, 3269–3277. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Li, H.; Su, D.; Antonietti, M. Highly Selective Hydrogenation of Phenol and Derivatives over a Pd@Carbon Nitride Catalyst in Aqueous Media. J. Am. Chem. Soc. 2011, 133, 2362–2365. [Google Scholar] [CrossRef]
- Wang, C.; Hu, L.; Wang, M.; Ren, Y.; Yue, B.; He, H. Vanadium supported on graphitic carbon nitride as a heterogeneous catalyst for the direct oxidation of benzene to phenol. Chin. J. Catal. 2016, 37, 2003–2008. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Q.; Chen, T.; Wu, F.; Li, Y.-X. Vanadia supported on mesoporous carbon nitride as a highly efficient catalyst for hydroxylation of benzene to phenol. Catal. Sci. Technol. 2015, 5, 1504–1513. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Q.; Shang, J.-K.; Wang, Y.; Li, Y.-X. A Schiff-base-type vanadyl complex grafted on mesoporous carbon nitride: A new efficient catalyst for hydroxylation of benzene to phenol. RSC Adv. 2015, 5, 92526–92533. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Hong, Y.; Zheng, H.; Ma, D.; Xue, B.; Li, Y.-X. Direct catalytic hydroxylation of benzene to phenol catalyzed by vanadia supported on exfoliated graphitic carbon nitride. Appl. Catal. A 2018, 549, 31–39. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, M.; Zhao, Q.; Yang, Y.; Wang, C.; Wang, Y. In-situ immobilization of H5PMo10V2O40 on protonated graphitic carbon nitride under hydrothermal conditions: A highly efficient and reusable catalyst for hydroxylation of benzene. Ind. Eng. Chem. Res. 2017, 56, 2711–2721. [Google Scholar] [CrossRef]
- Xue, B.; Chen, Y.; Hong, Y.; Ma, D.-Y.; Xu, J.; Li, Y.-X. Facile synthesis of Fe-containing graphitic carbon nitride materials and their catalytic application in direct hydroxylation of benzene to phenol. Chin. J. Catal. 2018, 39, 1263–1271. [Google Scholar] [CrossRef]
- Qin, L.; Feng, Z.; Zhang, Q.; Mao, H.; Cheng, F.; Shi, S. Enhanced Hydroxylation of Benzene to Phenol with Hydrogen Peroxide over g-C3N4 Quantum Dots-Modified Fe-SBA-15 Catalysts: Synergistic Effect Among Fe Species, g-C3N4 QDs, and Porous Structure. Ind. Eng. Chem. Res. 2021, 60, 13876–13885. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Y.; Zhao, L.; Yuan, F. Preparation and characterization of Mesopoous VOx/SBA-16 and their application for the direct catalytic hydroxylation of benzene to phenol. J. Mol. Catal. A 2010, 315, 205–212. [Google Scholar] [CrossRef]
- Nemati Kharat, A.; Moosavikia, S.; Tamaddoni Jahromi, B.; Badiei, A. Liquid phase hydroxylation of benzene to phenol over vanadium substituted Keggin anion supported on amine functionalized SBA-15. J. Mol. Catal. A 2011, 348, 14–19. [Google Scholar] [CrossRef]
- Xu, D.; Jia, L.; Guo, X. Direct Hydroxylation of Benzene to Phenol Over Mixed-Crystal Particles of Mesoporous VOx/TiO2 Catalyst Mixed-Crystal VOx/TiO2 for Benzene Hydroxylation. Catal. Lett. 2012, 142, 1251–1261. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Chen, R.; Wang, Y.; Xing, W.; Wang, J.; Huang, J. The hydroxylation of benzene to phenol over heteropolyacid encapsulated in silica. Catal. Commun. 2014, 55, 34–37. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.; Ye, L.; Wu, Y.; Yue, B.; Chen, X.; He, H. Direct hydroxylation of benzene to phenol using H2O2 as an oxidant over vanadium-containing mesoporous carbon catalysts. Appl. Catal. A 2015, 504, 440–447. [Google Scholar] [CrossRef]
- Wang, C.; Hu, L.; Hu, Y.; Ren, Y.; Chen, X.; Yue, B.; He, H. Direct hydroxylation of benzene to phenol over metal oxide supported graphene oxide catalysts. Catal. Commun. 2015, 68, 1–5. [Google Scholar] [CrossRef]
- Muniandy, L.; Adam, F.; Mohamed, A.R.; Iqbal, A.; Rahman, N.R.A. Cu2+ coordinated graphitic carbon nitride (Cu-g-C3N4) nanosheets from melamine for the liquid phase hydroxylation of benzene and VOCs. Appl. Surf. Sci. 2017, 398, 43–55. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.-P.; Zou, S.; Dai, Y.; Xiao, L.; Gong, X.-Q.; Fan, J. Origin of the High Activity of Mesoporous CeO2 Supported Monomeric VOx for Low-Temperature Gas-Phase Selective Oxidative Dehydrogenation of Benzyl Alcohol: Role As an Electronic “Hole”. J. Phys. Chem. C 2014, 118, 24950–24958. [Google Scholar] [CrossRef]
- Maity, N.; Barman, S.; Minenkov, Y.; Ould-Chikh, S.; Abou-Hamad, E.; Ma, T.; Qureshi, Z.S.; Cavallo, L.; D’Elia, V.; Gates, B.C.; et al. A Silica-Supported Monoalkylated Tungsten Dioxo Complex Catalyst for Olefin Metathesis. ACS Catal. 2018, 8, 2715–2729. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Anandan, S.; Mane, G.P.; Anand, C.; Dhawale, D.S.; Varghese, S.; Mano, A.; Mori, T.; Vinu, A. Facile synthesis and basic catalytic application of 3D mesoporous carbon nitride with a controllable bimodal distribution. J. Mater. Chem. 2012, 22, 9831–9840. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Müller, J.O.; Antonietti, M.; Thomas, A. Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chem. Eur. J. 2008, 14, 8177–8182. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (001) vs (101) facets of TiO2. Appl. Catal. B 2015, 164, 420–427. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Zhu, Z.; Pan, H.; Murugananthan, M.; Gong, J.; Zhang, Y. Visible light-driven photocatalytically active g-C3N4 material for enhanced generation of H2O2. Appl. Catal. B 2018, 232, 19–25. [Google Scholar] [CrossRef]
- Su, Q.; Sun, J.; Wang, J.; Yang, Z.; Cheng, W.; Zhang, S. Urea-derived graphitic carbon nitride as an efficient heterogeneous catalyst for CO2 conversion into cyclic carbonates. Catal. Sci. Technol. 2014, 4, 1556–1562. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Lu, T.; Yuan, Y.; Yuan, G. Well-dispersed g-C3N4 nanophases in mesoporous silica channels and their catalytic activity for carbon dioxide activation and conversion. Appl. Catal. B 2013, 136, 269–277. [Google Scholar] [CrossRef]
- Lan, D.-H.; Wang, H.-T.; Chen, L.; Au, C.-T.; Yin, S.-F. Phosphorous-modified bulk graphitic carbon nitride: Facile preparation and application as an acid-base bifunctional and efficient catalyst for CO2 cycloaddition with epoxides. Carbon 2016, 100, 81–89. [Google Scholar] [CrossRef]
- Xu, J.; Long, K.-Z.; Wang, Y.; Xue, B.; Li, Y.-X. Fast and facile preparation of metal-doped g-C3N4 composites for catalytic synthesis of dimethyl carbonate. Appl. Catal. A 2015, 496, 1–8. [Google Scholar] [CrossRef]
- Barman, S.; Maity, N.; Bhatte, K.; Ould-Chikh, S.; Dachwald, O.; Haeßner, C.; Saih, Y.; Abou-Hamad, E.; Llorens, I.; Hazemann, J.-L.; et al. Single-Site VOx Moieties Generated on Silica by Surface Organometallic Chemistry: A Way To Enhance the Catalytic Activity in the Oxidative Dehydrogenation of Propane. ACS Catal. 2016, 6, 5908–5921. [Google Scholar] [CrossRef]
- Zou, S.; Liu, J.; Kobayashi, H.; Chen, C.; Qiao, P.; Li, R.; Xiao, L.; Fan, J. Boosting Hydrogen Evolution Activities by Strong Interfacial Electronic Interaction in ZnO@Bi(NO3)3 Core–Shell Structures. J. Phys. Chem. C 2017, 121, 4343–4351. [Google Scholar] [CrossRef]
- Liu, J.; Zou, S.; Lou, B.; Chen, C.; Xiao, L.; Fan, J. Interfacial electronic interaction induced engineering of ZnO-BiOI heterostructures for efficient visible-light photocatalysis. Inorg. Chem. 2019, 58, 8525–8532. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Lou, B.; Yang, K.; Yuan, W.; Zhu, C.; Zhu, Y.; Du, Y.; Lu, L.; Liu, J.; Huang, W. Grafting nanometer metal/oxide interface towards enhanced low-temperature acetylene semi-hydrogenation. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, C.; Zou, S.; Liu, J.; Xiao, L.; Fan, J. High H2O2 Utilization Promotes Selective Oxidation of Methane to Methanol at Low Temperature. Front. Chem. 2020, 8, 252. [Google Scholar] [CrossRef]
- Nomiya, K.; Nemoto, Y.; Hasegawa, T.; Matsuoka, S. Multicenter active sites of vanadium-substituted polyoxometalate catalysts on benzene hydroxylation with hydrogen peroxide and two reaction types with and without an induction period. J. Mol. Catal. A 2000, 152, 55–68. [Google Scholar] [CrossRef]

| Catalyst | Vbenzene (mL) | VH2O2 (mL) | Wcatal. (mg) | T (K) | t (h) | Conv. (%) | Sel. (%) | Specific Activity (h−1) a | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 0.4V-g-C3N4 | 1.0 | 3 | 60 | 333 | 6 | 17.7 | 100 | 0.52 | [4] |
| H5PMo10V2O40/pg-C3N4 | 1.0 | 4.1 | 100 | 333 | 8 | 25.8 | 99.7 | 0.34 | [22] |
| VO/MCM-41-NH2 | 0.9 | 1 | 100 | 333 | 1 | 58.6 | 18.5 | 1.03 | [6] |
| 8V-g-C3N4 | 1.0 | 3.5 | 40 | 343 | 4 | 24.6 | 99.2 | 1.61 | [18] |
| Fe-g-C3N4 | 1 | 3 | 50 | 333 | 2 | 17.5 | 99.0 | 1.84 | [23] |
| V2O5-mp-C3N4 | 1.5 | 3 | 60 | 333 | 3 | 18.7 | 95.9 | 1.59 | [19] |
| VO-peg-C3N4 | 1.0 | 3 | 75 | 333 | 4 | 11.7 | 97.9 | 0.40 | [21] |
| g-C3N4 QD/Fe-SBA-15 | 3 | 6 | 120 | 333 | 3 | 41,7 | 98.8 | 3.01 | [24] |
| VOx-SBA-16 | 0.3 | 1.5 | 10 | 333 | 4 | 13.8 | 97.5 | 1.06 | [25] |
| HPMoV/NH2-SBA-15 | 1.0 | 3.0 | 100 | 333 | 6 | 20.0 | 95.0 | 0.33 | [26] |
| VOx-TiO2 | 2.6 | 6 | 180 | 333 | 5 | 26.3 | 90.0 | 0.72 | [27] |
| PMoV2/SiO2 | 1.0 | 3.0 | 150 | 333 | 6 | 21.6 | 100 | 0.25 | [28] |
| V-C-600 | 0.4 | 1.4 | 20 | 343 | 3 | 31.8 | 94.9 | 2.12 | [29] |
| VOx-GO | 1.0 | 3.5 | 40 | 338 | 3 | 23.1 | 98.4 | 1.99 | [30] |
| Ce0.07-0.07 V-g-C3N4 | 1.0 | 3.5 | 40 | 343 | 4 | 33.7 | 95.9 | 2.13 | [8] |
| V-pg-C3N4-0.46 | 1.0 | 3.0 | 60 | 333 | 5 | 62.0 | 97.0 | 2.11 | p.w. b |
| V-pg-C3N4-0.46 | 1.0 | 3.0 | 60 | 333 | 1 | 25.0 | 97.0 | 4.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yin, H.; Nie, Q.; Zou, S. Highly Dispersed Vanadia Anchored on Protonated g-C3N4 as an Efficient and Selective Catalyst for the Hydroxylation of Benzene into Phenol. Molecules 2022, 27, 6965. https://doi.org/10.3390/molecules27206965
Liu J, Yin H, Nie Q, Zou S. Highly Dispersed Vanadia Anchored on Protonated g-C3N4 as an Efficient and Selective Catalyst for the Hydroxylation of Benzene into Phenol. Molecules. 2022; 27(20):6965. https://doi.org/10.3390/molecules27206965
Chicago/Turabian StyleLiu, Juanjuan, Haoyong Yin, Qiulin Nie, and Shihui Zou. 2022. "Highly Dispersed Vanadia Anchored on Protonated g-C3N4 as an Efficient and Selective Catalyst for the Hydroxylation of Benzene into Phenol" Molecules 27, no. 20: 6965. https://doi.org/10.3390/molecules27206965
APA StyleLiu, J., Yin, H., Nie, Q., & Zou, S. (2022). Highly Dispersed Vanadia Anchored on Protonated g-C3N4 as an Efficient and Selective Catalyst for the Hydroxylation of Benzene into Phenol. Molecules, 27(20), 6965. https://doi.org/10.3390/molecules27206965






