In Vitro Activity of Essential Oils Distilled from Colombian Plants against Candidaauris and Other Candida Species with Different Antifungal Susceptibility Profiles
Abstract
1. Introduction
2. Results
2.1. Essential Oil Composition
2.2. Antifungal Activity of Essential Oils and Terpenes
2.3. Cytotoxic Activity
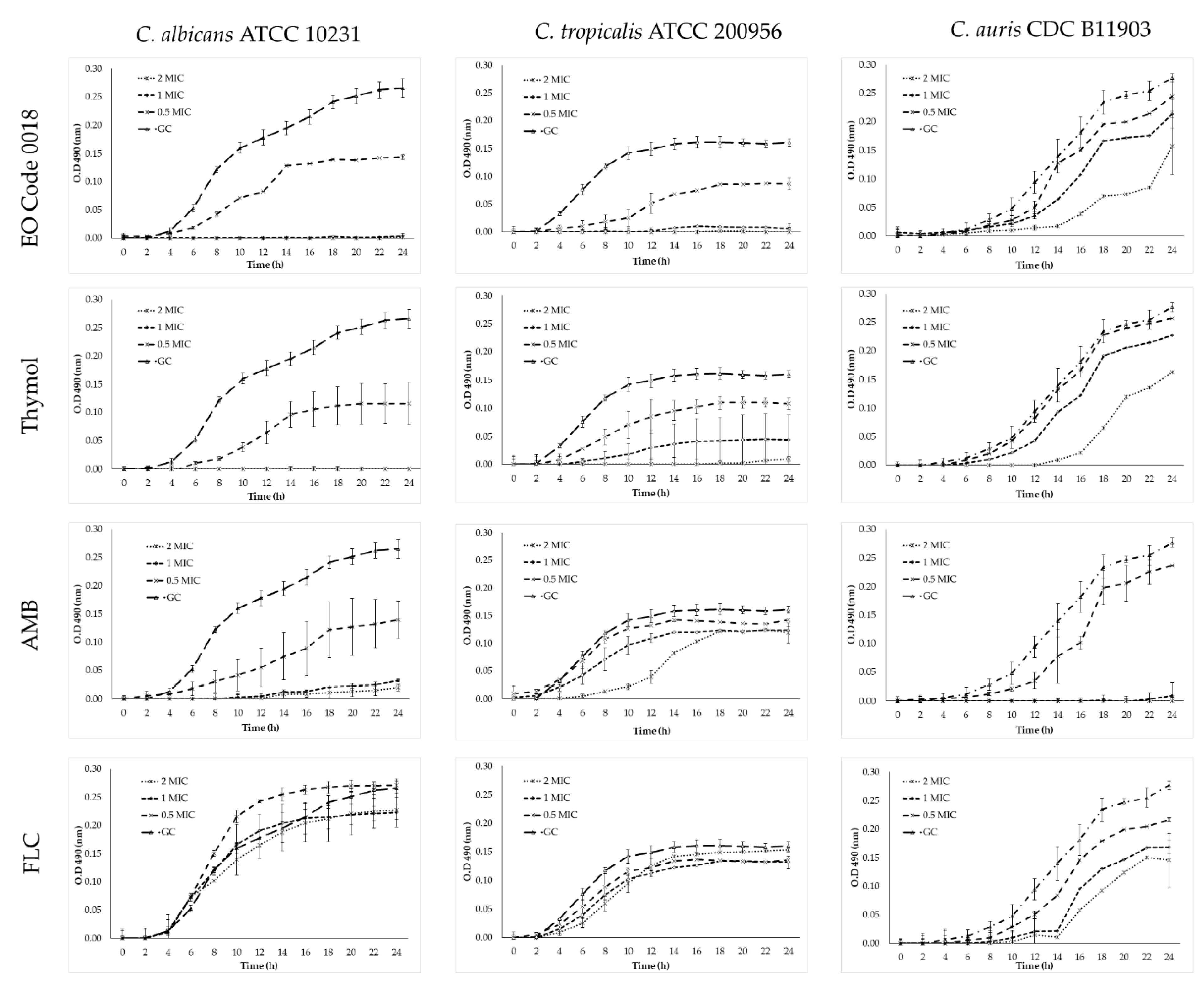
2.4. Time–Kill Assays
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Essential Oil Distillation
4.2. Sample Preparation
4.3. Chromatographic Analysis
4.4. Antifungals
4.5. Essential Oils and Terpenes
4.6. Fungi
4.7. Antifungal Susceptibility Testing (Antifungals, EOs, and Terpenes)
4.8. Cytotoxicity of the Essential Oils and Terpenes
4.9. Time–Kill Assays
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto Esoda, K.; et al. The emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef] [PubMed]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Brown, C.S.R. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar] [CrossRef]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A latent threat to critically ill patients with Coronavirus disease. Clin. Infect. Dis. 2020, 73, ciaa1595. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Improved efficacy of antifungal drugs in combination with monoterpene phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Kean, R.; Delaney, C.; Sherry, L.; Borman, A.; Johnson, M.J.; Richardson, M.D.; Rautemaa-Richardson, R.; Williams, C.; Ramage, G. Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere 2018, 3, e00334-18. [Google Scholar] [CrossRef]
- Romera, D.; Aguilera-Correa, J.J.; Gadea, I.; Viñuela-Sandoval, L.; García-Rodríguez, J.; Esteban, J. Candida auris: A comparison between planktonic and biofilm susceptibility to antifungal drugs. J. Med. Microbiol. 2019, 68, 1353–1358. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the antifungal activity and mode of action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus essential oils. Molecules 2018, 5, 1116. [Google Scholar] [CrossRef]
- Soares, I.H.; Loreto, É.S.; Rossato, L.; Mario, D.N.; Venturini, T.P.; Baldissera, F.; Santurio, J.M.; Alves, S.H. In vitro activity of essential oils extracted from condiments against fluconazole-resistant and -sensitive Candida glabrata. J. Mycol. Med. 2015, 25, 213–217. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Córdoba, S.; Vivot, W.; Szusz, W.; Albo, G. Antifungal activity of essential oils against Candida species isolated from clinical samples. Mycopathologia 2019, 184, 615–623. [Google Scholar] [CrossRef]
- Arbeláez-Cortés, E. Knowledge of Colombian biodiversity: Published and indexed. Biodivers. Conserv. 2013, 22, 2875–2906. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero Rincón, P.; Stashenko, E.E.; Olivero-Verbel, J. Photoprotective agents obtained from aromatic plants grown in Colombia: Total phenolic content, antioxidant activity, and assessment of cytotoxic potential in cancer cell lines of Cymbopogon flexuosus L. and Tagetes lucida Cav. essential oils. Plants 2022, 11, 1693. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.; Zapata, B.; Baena, A.; Bueno, J.; Ruiz-Nova, C.A.; Stashenko, E.; Mesa-Arango, A.C. Antifungal, cytotoxic and chemical analyses of essential oils of Lippia origanoides H.B.K grown in Colombia. Rev. La Univ. Ind. Santander Salud. 2011, 43, 141–148. [Google Scholar]
- Moreno, E.M.; Leal, S.M.; Stashenko, E.E.; García, L.T. Induction of programmed cell death in Trypanosoma cruzi by Lippia alba essential oils and their major and synergistic terpenes (citral, limonene and caryophyllene oxide). BMC Complement. Altern. Med. 2018, 18, 225. [Google Scholar] [CrossRef]
- Quintero, W.L.; Moreno, E.M.; Pinto, S.M.L.; Sanabria, S.M.; Stashenko, E.; García, L.T. Immunomodulatory, trypanocide, and antioxidant properties of essential oil fractions of Lippia alba (Verbenaceae). BMC Complement. Med. Ther. 2021, 21, 187. [Google Scholar] [CrossRef]
- García, L.T.; Lea, A.F.; Moreno, E.M.; Stashenko, E.E.; Arteaga, H.J. Differential anti-proliferative effect on K562 leukemia cells of Lippia alba (Verbenaceae) essential oils produced under diverse growing, collection and extraction conditions. Ind. Crops Prod. 2017, 96, 140–148. [Google Scholar] [CrossRef]
- CDC. Antifungal Susceptibility Testing-Candida Auris. Available online: https://www.cdc.gov/fungal/Candida-auris/C-auris-antifungal.html (accessed on 15 February 2022).
- Hokken, M.W.J.; Zwaan, B.J.; Melchers, W.J.G.; Verweij, P.E. Facilitators of adaptation and antifungal resistance mechanisms in clinically relevant fungi. Fungal Genet. Biol. 2019, 132, 103254. [Google Scholar] [CrossRef]
- Parikh, L.; Agindotan, B.O.; Burrows, M.E. Antifungal activity of plant-derived essential oils on pathogens of pulse crops. Plant Dis. 2021, 105, 1692–1701. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic −OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef]
- Pozzatti, P.; Scheid, L.A.; Spader, T.B.; Atayde, M.L.; Santurio, J.M.; Alves, S.H. In vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can. J. Microbiol. 2008, 54, 950–956. [Google Scholar] [CrossRef]
- Vale-Silva, L.A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L.; Pinto, E. Antifungal activity of the essential oil of Thymus x Viciosoi against Candida, Cryptococcus, Aspergillus and dermatophyte species. Planta Med. 2010, 76, 882–888. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Rossi, D.C.P.; Jabes, D.L.; Barbosa, D.A.; Cunha, F.F.M.; Nunes, L.R.; Arruda, D.C.; Taborda, C.P. In vitro and in vivo inhibitory activity of limonene against different isolates of Candida spp. J. Fungi 2020, 6, 183. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, A.; Shokri, H.; Katiraee, F. Anti-adherence and anti-fungal abilities of thymol and carvacrol against Candida species isolated from patients with oral candidiasis in comparison with fluconazole and voriconazole. Jundishapur. J. Nat. Pharm. Prod. 2021, 16, e65005. [Google Scholar] [CrossRef]
- Santamarina, M.P.; Ibáñez, M.D.; Marqués, M.; Roselló, J.; Giménez, S.; Blázquez, M.A. Bioactivity of essential oils in phytopathogenic and post-harvest fungi control. Nat. Prod. Res. 2017, 31, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Fontenelle, R.O.S.; Morais, S.M.; Brito, E.H.S.; Brilhante, R.S.N.; Cordeiro, R.A.; Lima, Y.C.; Brasil, N.V.G.P.S.; Monteiro, A.J.; Sidrim, J.J.C.; Rocha, M.F.G. Alkylphenol activity against Candida spp. and Microsporum canis: A focus on the antifungal activity of thymol, eugenol and o-methyl derivatives. Molecules 2011, 16, 6422–6431. [Google Scholar] [CrossRef]
- Guo, N.; Liu, J.; Wu, X.; Bi, X.; Meng, R.; Wang, X.; Xiang, H.; Deng, X.; Yu, L. Antifungal activity of thymol against clinical isolates of fluconazole-sensitive and -resistant Candida albicans. J. Med. Microbiol. 2009, 58, 1074–1079. [Google Scholar] [CrossRef]
- Thakre, A.; Zore, G.; Kodgire, S.; Kazi, R.; Mulange, S.; Patil, R.; Shelar, A.; Santhakumari, B.; Kulkarni, M.; Kharat, K.; et al. Limonene inhibits Candida albicans growth by inducing apoptosis. Med. Mycol. 2018, 56, 565–578. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. In CLSI Standard M27, 4th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.; Nakamura, C.V.; Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Forastiero, A.; Mesa-Arango, A.C.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Pelaez, T.; Lopez, J.F.; Grimalt, J.O.; Gomez-Lopez, A.; Cuesta, I.; et al. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob. Agents Chemother. 2013, 57, 4769–4781. [Google Scholar] [CrossRef]
- Dudiuk, C.; Gamarra, S.; Leonardeli, F.; Jimenez-Ortigosa, C.; Vitale, R.G.; Afeltra, J.; Perlin, D.S.; Garcia-Effron, G. Set of classical PCRs for detection of mutations in Candida glabrata FKS genes linked with echinocandin resistance. J. Clin. Microbiol. 2014, 52, 2609–2614. [Google Scholar] [CrossRef]
- Singh-Babak, S.D.; Babak, T.; Diezmann, S.; Hill, J.A.; Xie, J.L.; Chen, Y.L.; Poutanen, S.M.; Rennie, R.P.; Heitman, J.; Cowen, L.E. Global analysis of the evolution and mechanism of echinocandin resistance in Candida glabrata. PLoS Pathog. 2012, 8, e1002718. [Google Scholar] [CrossRef]
- Brennan, T.C.R.; Krömer, J.O.; Nielsen, L.K. Physiological and transcriptional responses of Saccharomyces cerevisiae to D-limonene show changes to the cell wall but not to the plasma membrane. Appl. Environ. Microbiol. 2013, 79, 3590–3600. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K.; et al. Carvacrol induces Candida albicans apoptosis associated with Ca2+/calcineurin pathway. Front. Cell. Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Baldim, I.; Paziani, M.H.; Grizante Barião, P.H.; Kress, M.R.V.Z.; Oliveira, W.P. Nanostructured lipid carriers loaded with Lippia sidoides essential oil as a strategy to combat the multidrug-resistant Candida auris. Pharmaceutics 2022, 14, 180. [Google Scholar] [CrossRef]
- Georgieva, A.; Ilieva, Y.; Kokanova-Nedialkova, Z.; Zaharieva, M.M.; Nedialkov, P.; Dobreva, A.; Kroumov, A.; Najdenski, H.; Mileva, M. Redox-modulating capacity and antineoplastic activity of wastewater obtained from the distillation of the essential oils of four Bulgarian oil-bearing roses. Antioxidants 2021, 10, 1615. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, Y.; Xu, F.; Gu, W.; Yan, G.J.; Dong, J.; Chen, J. Skin electrical resistance measurement of oxygen-containing terpenes as penetration enhancers: Role of stratum corneum lipids. Molecules 2019, 24, 523. [Google Scholar] [CrossRef]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The effect of ten essential oils on several cutaneous drug-resistant microorganisms and their cyto/genotoxic and antioxidant properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef]
- Borges, A.R.; Aires, J.R.; Higino, T.M.; de Medeiros, M.D.; Citó, A.M.; Lopes, J.A.; de Figueiredo, R.C. Trypanocidal and cytotoxic activities of essential oils from medicinal plants of Northeast of Brazil. Exp. Parasitol. 2012, 132, 123–128. [Google Scholar] [CrossRef]
- Carvalho, R.C.V.; Sousa, V.C.; Santos, L.P.; Santos, I.L.D.; Diniz, R.C.; Rodrigues, R.R.L.; Medeiros, M.D.G.F.; Rodrigues, K.A.D.F.; Alves, M.M.M.; Arcanjo, D.D.R.; et al. Limonene-carvacrol: A combination of monoterpenes with enhanced antileishmanial activity. Toxicol. In Vitro 2021, 74, 105158. [Google Scholar] [CrossRef]
- Miranda-Cadena, K.; Dias, M.; Costa-Barbosa, A.; Collins, T.; Marcos-Arias, C.; Eraso, E.; Pais, C.; Quindós, G.; Sampaio, P. Development and characterization of monoolein-based liposomes of carvacrol, cinnamaldehyde, citral, or thymol with anti-Candida activities. Antimicrob. Agents Chemother. 2021, 65, e01628-20. [Google Scholar] [CrossRef]
- Rayan, M.; Abdallah, Z.; Abu-Lafi, S.; Masalha, M.; Rayan, A. Indexing natural products for their antifungal activity by filters-based approach: Disclosure of discriminative properties. Curr. Comput. Aided Drug Des. 2019, 15, 235–242. [Google Scholar] [CrossRef]
- Masalha, M.; Rayan, M.; Adawi, A.; Abdallah, Z.; Rayan, A. Capturing antibacterial natural products with in silico techniques. Mol. Med. Rep. 2018, 18, 763–770. [Google Scholar] [CrossRef]
- Zhao, Y.; Tsang, C.C.; Xiao, M.; Chan, J.F.W.; Lau, S.K.P.; Kong, F.; Xu, Y.; Woo, P.C.Y. Yeast identification by sequencing, biochemical kits, MALDI-TOF MS and rep-PCR DNA fingerprinting. Med. Mycol. 2018, 56, 816–827. [Google Scholar] [CrossRef]
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of test conditions on antifungal time-kill curve results: Proposal for standardized methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef]
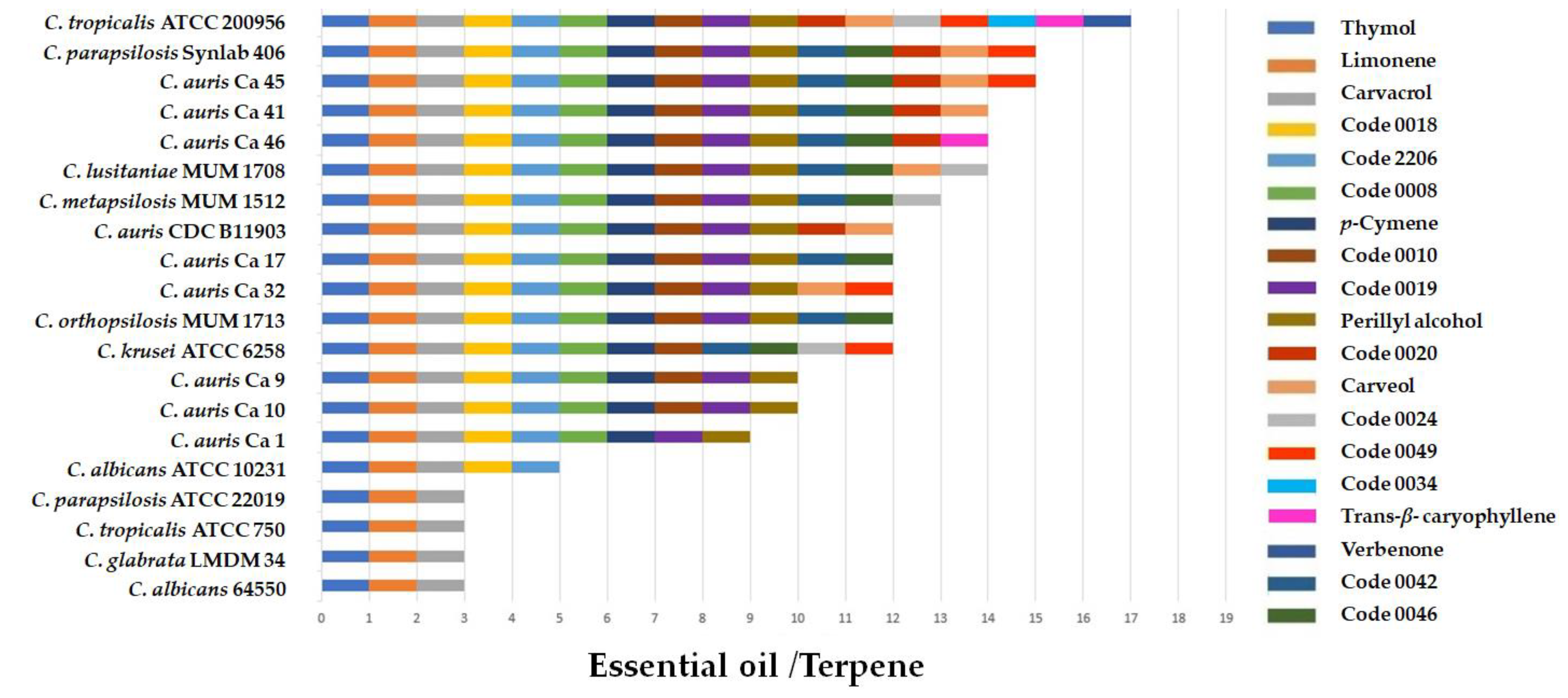
| Code | Plant Species and Chemotype | Collection Site | Voucher Number | Principal Compounds |
|---|---|---|---|---|
| 2206 | L. origanoides (Carvacrol + thymol) chemotype | Barbosa—Santander, Colombia | COL 587104 | Carvacrol (34.9%), thymol (23.3%), γ-terpinene (11.1%), p-cymene (9.0%), trans-β-caryophyllene (5.0%), α-humulene (2.5%), α-terpinene (1.8%), β-myrcene (1.7%), thymyl methyl ether (1.6%), and carvacryl acetate (0.8%). |
| 0008 | L. origanoides (Carvacrol + p-cymene) chemotype | Bucaramanga—Santander, Colombia | 22034 UIS Herbarium | Carvacrol (35.0%), p-cymene (14.4%), thymol (8.0%), γ-terpinene (5.3%), trans-β-caryophyllene (4.4%), β-myrcene (2.4%), carvacryl acetate (2.0%), thymyl methyl ether (1.9%), α-terpinene (1.7%), and α-thujene (1.6%). |
| 0010 | L. origanoides Thymol chemotype | Bucaramanga—Santander, Colombia | 22035 UIS Herbarium | Thymol (75.3%), trans-β-caryophyllene (5.4%), carvacrol (4.9%), α-humulene (3.2%), p-cymene (2.3%), thymyl acetate (1.6%), thymyl methyl ether (1.3%), caryophyllene oxide (1.3%), and trans-β-bergamotene (1.0%). |
| 0018 | L. origanoides (Thymol + p-cymene) chemotype | Bucaramanga—Santander, Colombia | 22039 UIS Herbarium | Thymol (49.4%), p-cymene (19.1%), γ-terpinene (9.2%), β-myrcene (5.2%), α-terpinene (2.9%), carvacrol (2.7%), thymyl methyl ether (1.8%), trans-β-caryophyllene (1.6%), cis-β-ocimene (1.2%), and limonene (0.9%). |
| 0019 | L. origanoides Thymol chemotype | Bucaramanga—Santander, Colombia | 22036 UIS Herbarium | Thymol (71.7%), p-cymene (10.5%), carvacrol (4.4%), β-myrcene (2.1%), γ-terpinene (2.0%), caryophyllene oxide (1.6%), thymyl methyl ether (0.9%), trans-β-caryophyllene (0.9%), humulene epoxide II (0.7%), and terpinen-4-ol (0.7%). |
| Species | MIC (µg/mL) | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AMB | ITC | FLC | CSF | ||||||
| Range | GM | Range | GM | Range | GM | Range | GM | ||
| C. albicans ATCC 64550 | 0.03–0.12 | 0.04 | 0.5–1 | 0.7 | 4–8 | 5.6 | 0.25–0.5 | 0.35 | Collection |
| C. albicans ATCC 10231 | 0.03–0.12 | 0.04 | 0.03–0.125 | 0.06 | 4 | 4 | 0.12–0.25 | 0.18 | Collection |
| C. parapsilosis ATCC 22019 | 0.06 | 0.06 | 0.25–0.50 | 0.35 | 0.5–1 | 0.70 | 1 | 1 | Collection |
| C. krusei ATCC 6258 | 0.12–0.25 | 0.15 | 0.12–0.50 | 0.28 | 8 | 8 | 1 | 1 | Collection |
| C. tropicalis ATCC 750 | 0.06–0.12 | 0.11 | 0.06–0.25 | 0.12 | 1–2 | 1.4 | 0.25–0.5 | 0.35 | Collection |
| C. tropicalis ATCC 200956 | 1–2 | 1.41 | >16 | >16 | >64 | >64 | 0.5 | 0.5 | Collection |
| C. glabrata LMDM 34 | 0.06–0.12 | 0.06 | 1 | 1 | 2–4 | 2.8 | 8 | 8 | Collection |
| C. metapsilosis MUM 15.12 | <0.03 | <0.03 | 0.06–0.12 | 0.09 | 1–2 | 1.4 | 1 | 1 | Collection |
| C. orthopsilosis MUM 17.13 | <0.03 | <0.03 | 0.12 | 0.12 | 1 | 1 | 1 | 1 | Collection |
| C. lusitaniae MUM 17.08 | 0.03 | 0.03 | 0.03–0.06 | 0.04 | 0.5 | 0.5 | 1 | 1 | Collection |
| C. auris CDCB11903 | 0.06–0.12 | 0.07 | 0.06–0.12 | 0.04 | 1–2 | 1.4 | 0.5 | 0.5 | Collection |
| C. auris Ca 1 | 0.12–0.25 | 0.20 | 0.03–0.12 | 0.06 | 4 | 4 | 0.5 | 0.5 | Subcutaneous tissue |
| C. auris Ca 9 | 0.12–0.5 | 0.25 | 0.03–0.12 | 0.06 | 4 | 4 | 0.5 | 0.5 | No data available |
| C. auris Ca 10 | 0.12–0.25 | 0.18 | 0.06–0.12 | 0.09 | 4 | 4 | 0.5–1 | 0.7 | Pleura tissue |
| C. auris Ca 32 | 0.12–0.5 | 0.19 | 0.25–0.50 | 0.35 | 8–16 | 11.3 | 0.5 | 0.5 | Parietal pleura |
| C. auris Ca 41 | 1 | 1 | 0.03–0.12 | 0.06 | 2 | 2 | 0.5 | 0.5 | Groin smear |
| C. auris Ca 45 | 1–2 | 1.41 | 0.06–0.12 | 0.04 | 2 | 2 | 0.5 | 0.5 | Axillary smear |
| C. auris Ca 46 | 1–2 | 1.4 | 0.06–0.12 | 0.04 | 2–4 | 2.8 | 0.5 | 0.5 | Axillary smear |
| C. auris Ca 17 | 0.06–0.25 | 0.15 | 0.12–0.5 | 0.25 | 32 | 32 | 0.5 | 0.5 | Urine culture |
| C. parapsilosis Synlab 406 | 0.06–0.25 | 0.15 | 0.5–1 | 0.70 | 8–16 | 11.3 | 2 | 2 | Blood culture |
| EO Code/Terpene | GM—Range MIC (μg/mL) | |||||
|---|---|---|---|---|---|---|
| C. krusei ATCC 6258 | C. tropicalis ATCC 200956 | C. parapsilosis Synlab 406 | C. metapsilosis MUM 17.13 | C. orthopsilosis MUM 15.12 | C. lusitaniae MUM 17.08 | |
| 2206 | 256 | 181 (128–256) | 16 | 128 | 128 | 181 (128–256) |
| 0008 | 256 | 181 (128–256) | 16 | 128 | 128 | 181 (128–256) |
| 0010 | 256 | 128 | 22.6 (16–32) | 90.5 (64–128) | 128 | 128 |
| 0018 | 256 | 128 | 22.6 (16–32) | 90.5 (64–128) | 128 | 90.5 (128–64) |
| 0019 | NA | 181 (128–256) | 64 | 128 | 128 | 181 (128–256) |
| Thymol | 181 (128–256) | 90.5 (64–128) | 64 | 128 | 128 | 90.5 (64–128) |
| Carvacrol | 256 | 128 | 45.3 (32–64) | 128 | 128 | 181 (128–256) |
| Perillyl alcohol | NA | 181 (128–256) | 90.5 (64–128) | 256 | 256 | 181 (128–256) |
| p-Cymene | 181 (128–256) | 181 (128–256) | 256 | 256 | 256 | 256 |
| Limonene | 32 | 64 | 64 | 64 | 16 | 22.6 (16–32) |
| EO Code/Terpene | GM—Range MIC (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C. auris CDC B11903 | C. auris Ca 1 | C. auris Ca 9 | C. auris Ca 13 | C. auris Ca 32 | C. auris Ca 17 | C. auris Ca 41 | C. auris Ca 45 | C. auris Ca 46 | |
| 2206 | 128 | 256 | 256 | 256 | 64 | 128 | 128 | 128 | 90.5 (64–128) |
| 0008 | 128 | 256 | 256 | 256 | 181 (128–256) | 128 | 128 | 128 | 90.5 (64–128) |
| 0010 | 64 | NA | 256 | 256 | 64 | 90.5 (64–128) | 64 | 64 | 64 |
| 0018 | 64 | 256 | 181 (128–256) | 256 | 128 | 64 | 64 | 64 | 64 |
| 0019 | 128 | 256 | 181 (128–256) | 256 | 90.5 (64–128) | 128 | 128 | 128 | 128 |
| Thymol | 64 | 181 (128–256) | 128 | 128 | 64 | 64 | 64 | 64 | 64 |
| Carvacrol | 90.5 (64–128) | 181 (128–256) | 256 | 181 (128–256) | 64 | 128 | 90.5 (64–128) | 90.5 (64–128) | 64 |
| Perillyl alcohol | 256 | 256 | 128 | 256 | 128 | 256 | 128 | 256 | 256 |
| p-Cymene | 256 | NA | 256 | 256 | 128 | 256 | 256 | 256 | 256 |
| Limonene | 64 | 64 | 22.6 (16–32) | 22.6 (16–32) | 22.6 (16–32) | 64 | 16 | 16 | 16 |
| EO Code/Terpene | HaCaT Cells Mean CC50 (µg/mL) | SI Range (CC50/MIC) |
|---|---|---|
| 2206 | 788.0 | 3.0–49.2 |
| 0008 | 877.9 | 3.4–54.8 |
| 0010 | 903.6 | 3.5–56 |
| 0018 | 665.9 | 2.6–41 |
| 0019 | 354.7 | 1.4–5.5 |
| Thymol | 427.5 | 3.3–6.7 |
| p-Cymene | 831.2 | 3.2–5.5 |
| Carvacrol | 410.7 | 1.6–12.8 |
| Limonene | 400.5 | 3.1–50 |
| Perillyl alcohol | 400.7 | 1.6–6.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata-Zapata, C.; Loaiza-Oliva, M.; Martínez-Pabón, M.C.; Stashenko, E.E.; Mesa-Arango, A.C. In Vitro Activity of Essential Oils Distilled from Colombian Plants against Candidaauris and Other Candida Species with Different Antifungal Susceptibility Profiles. Molecules 2022, 27, 6837. https://doi.org/10.3390/molecules27206837
Zapata-Zapata C, Loaiza-Oliva M, Martínez-Pabón MC, Stashenko EE, Mesa-Arango AC. In Vitro Activity of Essential Oils Distilled from Colombian Plants against Candidaauris and Other Candida Species with Different Antifungal Susceptibility Profiles. Molecules. 2022; 27(20):6837. https://doi.org/10.3390/molecules27206837
Chicago/Turabian StyleZapata-Zapata, Carolina, Manuela Loaiza-Oliva, María C. Martínez-Pabón, Elena E. Stashenko, and Ana C. Mesa-Arango. 2022. "In Vitro Activity of Essential Oils Distilled from Colombian Plants against Candidaauris and Other Candida Species with Different Antifungal Susceptibility Profiles" Molecules 27, no. 20: 6837. https://doi.org/10.3390/molecules27206837
APA StyleZapata-Zapata, C., Loaiza-Oliva, M., Martínez-Pabón, M. C., Stashenko, E. E., & Mesa-Arango, A. C. (2022). In Vitro Activity of Essential Oils Distilled from Colombian Plants against Candidaauris and Other Candida Species with Different Antifungal Susceptibility Profiles. Molecules, 27(20), 6837. https://doi.org/10.3390/molecules27206837






