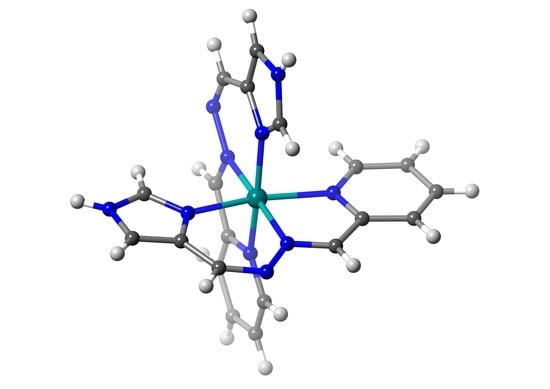
Selective Formation of Unsymmetric Multidentate Azine-Based Ligands in Nickel(II) Complexes
Abstract
1. Introduction
2. Results and Discussion
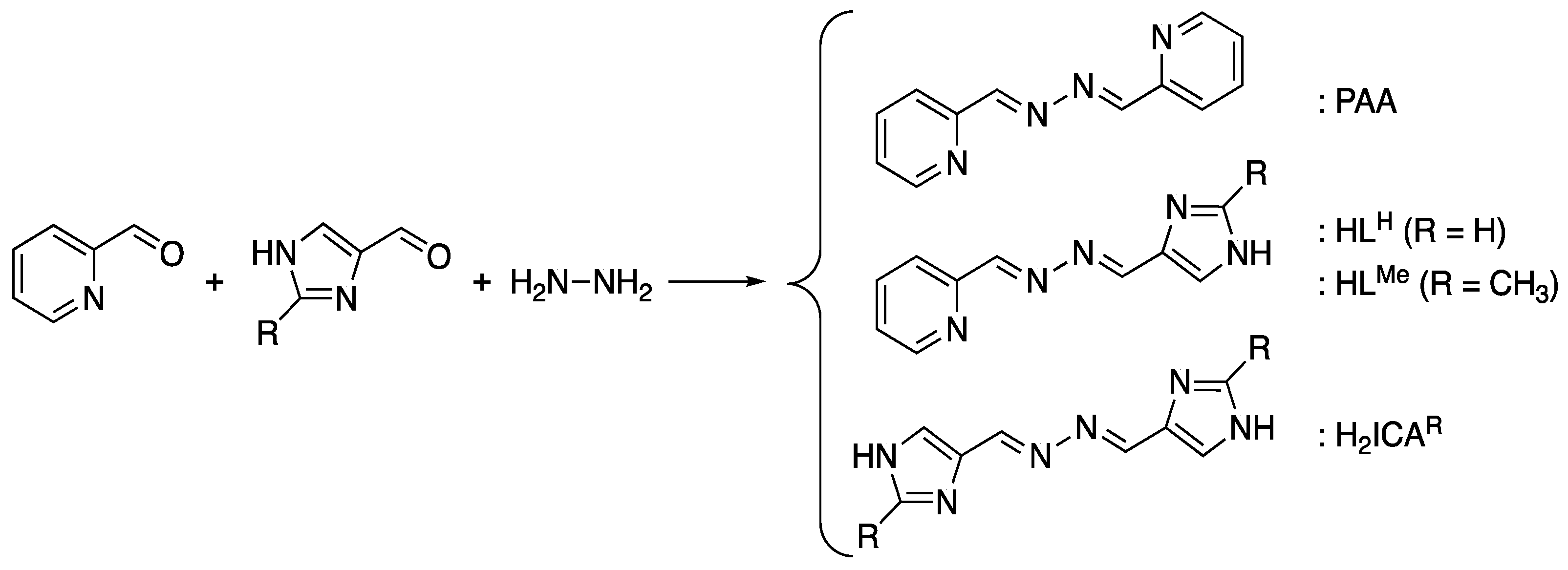
2.1. Preparation of Unsymmetric (2-Pyridyl)(4-Imidazolyl)azines and Their Nickel(II) Complexes
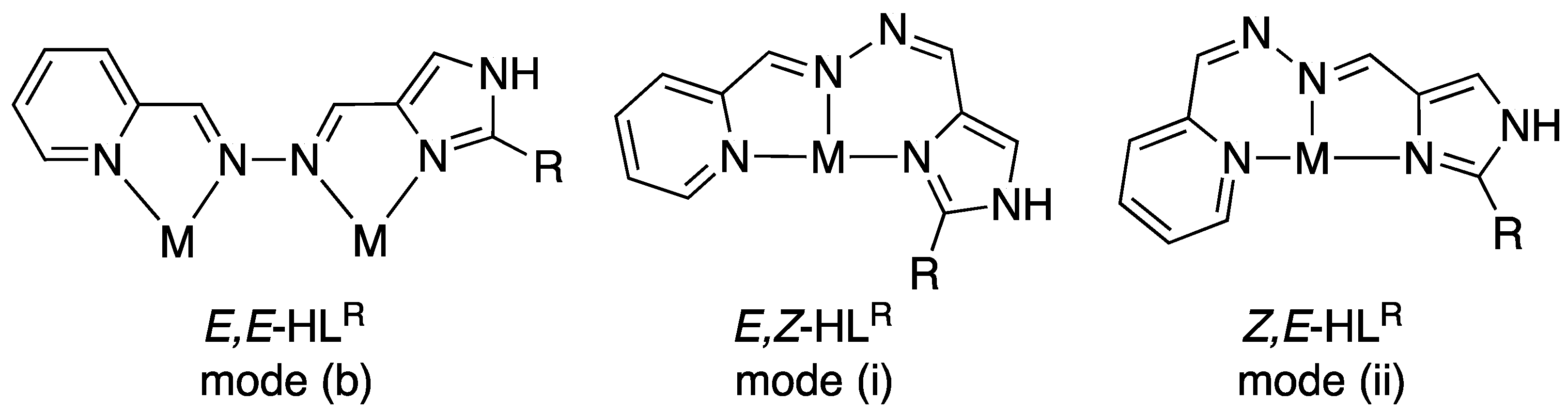
2.2. Crystal Structures of the Nickel(II) Complexes
3. Materials and Methods
3.1. Chemicals and Physical Methods
3.2. Preparation of Nickel(II) Complexes
3.2.1. [Ni(HLH)2](PF6)2 (1)
3.2.2. [Ni(HLMe)2](PF6)2 (2)
3.3. Structure Determination by X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safari, J.; Gandomi-Ravandi, S. Structure, synthesis and application of azines: A historical perspective. RSC Adv. 2014, 4, 46224–46249. [Google Scholar] [CrossRef]
- Chourasiya, S.S.; Kathuria, D.; Wani, A.A.; Bharatam, P.V. Azines: Synthesis, structure, electronic structure and their applications. Org. Biomol. Chem. 2019, 17, 8486–8521. [Google Scholar] [CrossRef]
- Hopkins, J.M.; Bowdridge, M.; Robertson, K.N.; Cameron, T.S.; Jenkins, H.A.; Clyburne, J.A.C. Generation of Azines by the Reaction of a Nucleophilic Carbene with Diazoalkanes: A Synthetic and Crystallographic Study. J. Org. Chem. 2001, 66, 5713–5716. [Google Scholar] [CrossRef]
- Padwa, A. 1,3-Dipolar Cycloaddition Chemistry; John Wiley and Sons: New York, NY, USA, 1984; Volumes 1 and 2. [Google Scholar]
- El-Alali, A.; Al-Kamali, A.S. Reactions of 1,3-dipolar aldazines and ketazines with the dipolarophile dimethyl acetylenedicarboxylate. Can. J. Chem. 2002, 80, 1293–1301. [Google Scholar] [CrossRef]
- Meth-Cohn, O.; Smalley, R.K. Heterocyclic chemistry. Annu. Rep. Prog. Chem. Sect. B Org. Chem. 1976, 73, 239–277. [Google Scholar] [CrossRef]
- Godara, M.; Maheshwari, R.; Varshney, S.; Varshney, A.K. Synthesis and characterization of some new coordination compounds of boron with mixed azines. J. Serb. Chem. Soc. 2007, 72, 367–374. [Google Scholar] [CrossRef]
- Sheng, R.; Wang, P.; Liu, W.; Wu, X.; Wu, S. A new colorimetric chemosensor for Hg2+ based on coumarin azine derivative. Sens. Actuators B Chem. 2008, 128, 507–511. [Google Scholar] [CrossRef]
- Kim, S.-H.; Gwon, S.-Y.; Burkinshaw, S.M.; Son, Y.-A. The synthesis and proton-induced spectral switching of a novel azine dye and its boron complex. Dye. Pigment. 2010, 87, 268–271. [Google Scholar] [CrossRef]
- Bodtke, A.; Pfeiffer, W.-D.; Ahrens, N.; Langer, P. Horseradish peroxidase (HRP) catalyzed oxidative coupling reactions using aqueous hydrogen peroxide: An environmentally benign procedure for the synthesis of azine pigments. Tetrahedron 2005, 61, 10926–10929. [Google Scholar] [CrossRef]
- Dolezal, M.; Kralov, K. Synthesis and Evaluation of Pyrazine Derivatives with Herbicidal Activity. In Herbicides, Theory, and Applications; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen Limited: London, UK, 2011. [Google Scholar]
- Moreland, D.E. Biochemical Mechanisms of Action of Herbicides and the Impact of Biotechnology on the Development of Herbicides. J. Pestic. Sci. 1999, 24, 299–307. [Google Scholar] [CrossRef]
- Manigandan, S.; Muthusamy, A.; Nandhakumar, R.; David, C.I.; Anand, S. Synthesis, characterization, theoretical investigations and fluorescent sensing behavior of oligomeric azine-based Fe3+ chemosensors. High Perform. Polym. 2022, 34, 321–336. [Google Scholar] [CrossRef]
- Sawminathan, S.; Munusamy, S.; Manickam, S.; Jothi, D.; KulathuIyer, S. Azine based fluorescent rapid “off-on” chemosensor for detecting Th4+ and Fe3+ ions and its real-time application. Dye. Pigment. 2021, 196, 109755. [Google Scholar] [CrossRef]
- Irmi, N.M.; Purwono, B.; Anwar, C. Synthesis of Symmetrical Acetophenone Azine Derivatives as Colorimetric and Fluorescent Cyanide Chemosensors. Indones. J. Chem. 2021, 21, 1337–1347. [Google Scholar] [CrossRef]
- Acker, P.; Speer, M.E.; Wössner, J.S.; Esser, B. Azine-based polymers with a two-electron redox process as cathode materials for organic batteries. J. Mater. Chem. A 2020, 8, 11195–11201. [Google Scholar] [CrossRef]
- Lyu, H.; Sun, X.G.; Dai, S. Organic Cathode Materials for Lithium-Ion Batteries: Past, Present, and Future. Adv. Energy Sustain. Res. 2021, 2, 2000044. [Google Scholar] [CrossRef]
- Hager, M.D.; Esser, B.; Feng, X.; Schuhmann, W.; Theato, P.; Schubert, U.S. Polymer-Based Batteries—Flexible and Thin Energy Storage Systems. Adv. Mater. 2020, 32, 2000587. [Google Scholar] [CrossRef]
- Tantardini, C.; Kvashnin, A.G.; Gatti, C.; Yakobson, B.I.; Gonze, X. Computational Modeling of 2D Materials under High Pressure and Their Chemical Bonding: Silicene as Possible Field-Effect Transistor. ACS Nano 2021, 15, 6861–6871. [Google Scholar] [CrossRef]
- Singh, A.; Kociok-Köhn, G.; Chauhan, R.; Muddassir, M.; Gosavi, S.W.; Kumar, A. Ferrocene Appended Asymmetric Sensitizers with Azine Spacers with phenolic/nitro anchors for Dye-Sensitized Solar Cells. J. Mol. Struct. 2021, 1249, 131630. [Google Scholar] [CrossRef]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A.; Jiang, D. An Azine-Linked Covalent Organic Framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. [Google Scholar] [CrossRef]
- Konavarapu, S.K.; Biradha, K. Luminescent Triazene-Based Covalent Organic Frameworks Functionalized with Imine and Azine: N2 and H2 Sorption and Efficient Removal of Organic Dye Pollutants. Cryst. Growth Des. 2019, 19, 362–368. [Google Scholar] [CrossRef]
- Sęk, D.; Siwy, M.; Małecki, J.G.; Kotowicz, S.; Golba, S.; Nowak, E.M.; Sanetra, J.; Schab-Balcerzak, E. Polycyclic aromatic hydrocarbons connected with Schiff base linkers: Experimental and theoretical photophysical characterization and electrochemical properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-F.; Cui, X.-B.; Yu, J.-H.; Lu, J.; Yu, X.-Y.; Zhang, X.; Xu, J.-Q.; Hou, Q.; Wang, T.-G. A series of metal–organic complexes constructed from in situ generated organic amines. CrystEngComm 2008, 10, 1534–1541. [Google Scholar] [CrossRef]
- Stratton, W.J.; Busch, D.H. The Complexes of Pyridinaldazine with Iron(II) and Nickel(II). J. Am. Chem. Soc. 1958, 80, 1286–1289. [Google Scholar] [CrossRef]
- Stratton, W.J.; Rettig, M.F.; Drury, R.F. Metal complexes with azine ligands. I. Ligand hydrolysis and template synthesis in the iron(II)-2-Pyridinaldazine system. Inorg. Chim. Acta 1969, 3, 97–102. [Google Scholar] [CrossRef]
- Stratton, W.J. Metal complexes with azine ligands. II. Iron(II), cobalt(II), and nickel(II) complexes with 2-pyridyl methyl ketazine. Inorg. Chem. 1970, 9, 517–520. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Kawamoto, R.; Fujita, K.; Maruyama, H.; Suzuki, T.; Ishida, H.; Kojima, M.; Iijima, S.; Matsumoto, N. Structures and Spin States of Bis(tridentate)-Type Mononuclear and Triple Helicate Dinuclear Iron(II) Complexes of Imidazole-4-carbaldehyde azine. Inorg. Chem. 2009, 48, 8784–8795. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Maruyama, H.; Fujita, K.; Suzuki, T.; Kojima, M.; Matsumoto, N. Mononuclear Bis(tridentate)-Type and Dinuclear Triple Helicate Iron(II) Complexes Containing 2-Ethyl-5-methylimidazole-4-carbaldehyde Azine. Bull. Chem. Soc. Jpn. 2009, 82, 1497–1505. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Fujita, K.; Maruyama, H.; Suzuki, T.; Ishida, H.; Kojima, M.; Glaser, R. Chiral Crystal Structure of a P212121 Kryptoracemate Iron(II) Complex with an Unsymmetric Azine Ligand and the Observation of Chiral Single Crystal Circular Dichroism. Cryst. Growth Des. 2014, 14, 3692–3695. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. Highly Efficient Practical Procedure for the Synthesis of Azine Derivatives Under Solvent-Free Conditions. Synth. Commun. 2011, 41, 645–651. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S.; Monemi, M. Novel and selective synthesis of unsymmetrical azine derivatives via a mild reaction. Monatsh. Chem. 2013, 144, 1375–1380. [Google Scholar] [CrossRef]
- Tang, B.; Ye, J.-H.; Ju, X.-H. Computational Study of Coordinated Ni(II) Complex with High Nitrogen Content Ligands. ISRN Org. Chem. 2011, 2011, 920753. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colpas, G.J.; Kumar, M.; Day, R.O.; Maroney, M.J. Structural investigations of nickel complexes with nitrogen and sulfur donor ligands. Inorg. Chem. 1990, 29, 4779–4788. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polodori, G. Crystal structure determination, and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Rigaku Co., Ltd. CrystalStructure; Rigaku Co., Ltd.: Akishima, Tokyo, 2000–2016. [Google Scholar]
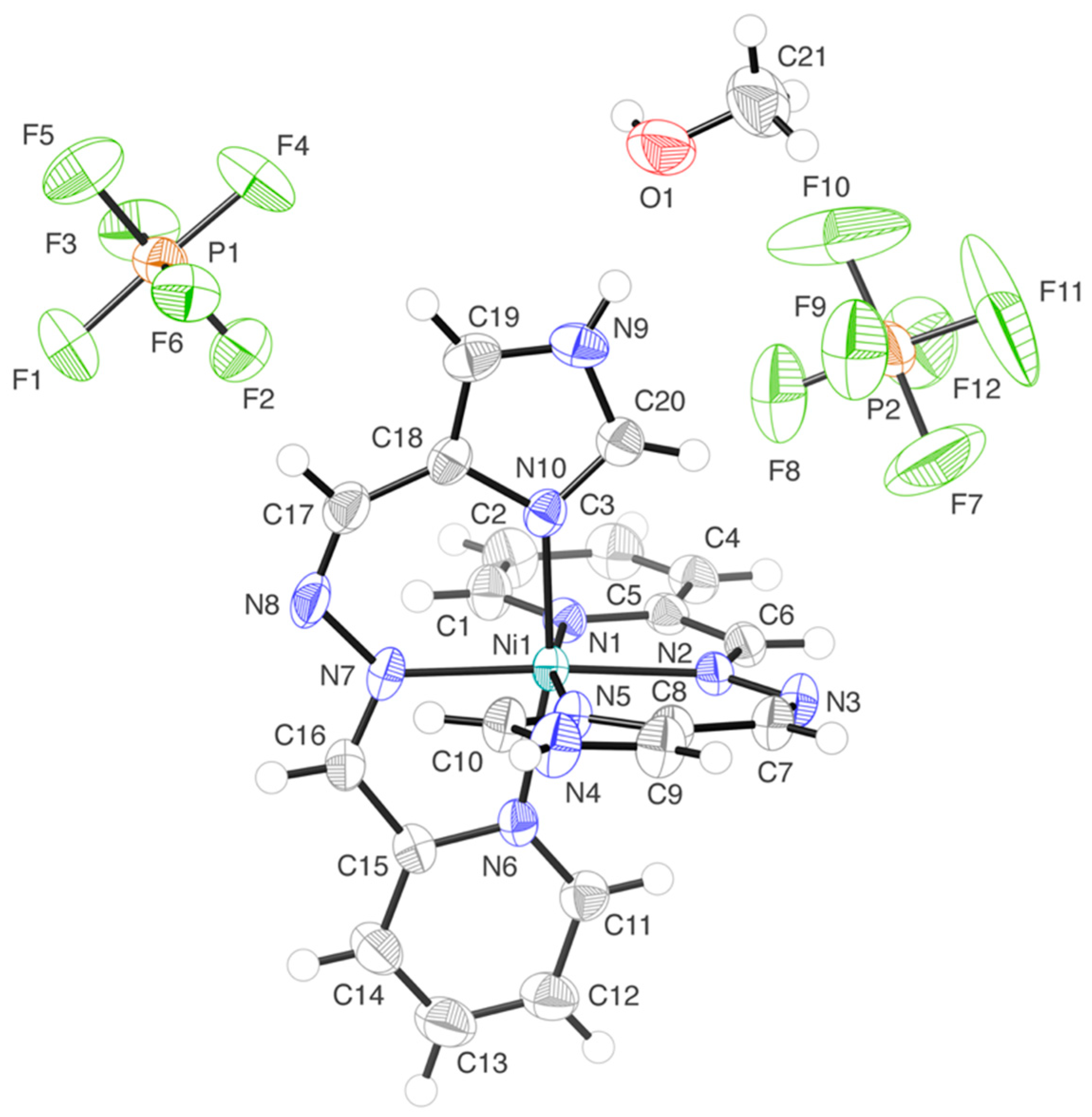


| Compound | 1·MeOH | 2·H2O·Et2O |
|---|---|---|
| Chemical formula | C21H22F12N10NiOP2 | C26H34F12N10NiO2P2 |
| Formula weight | 779.10 | 867.25 |
| T/K | 188(2) | 188(2) |
| Crystal color and shape | orange, block | orange, platelet |
| Size of specimen/mm | 0.30 × 0.26 × 0.25 | 0.45 × 0.30 × 0.29 |
| Crystal system | Orthorhombic | Monoclinic |
| Space group, Z | P212121, 4 | P21/n, 4 |
| a/Å | 8.6212(4) | 12.5812(12) |
| b/Å | 10.3267(6) | 14.6221(12) |
| c/Å | 33.20894(17) | 19.9359(18) |
| β/° | 90 | 98.664(3) |
| U/Å3 | 2956.6(2) | 3625.6(6) |
| Dcalc/g cm−3 | 1.750 | 1.589 |
| µ(Mo Kα)/mm−1 | 0.8783 | 0.7270 |
| Rint | 0.0547 | 0.0628 |
| No. reflns/params. | 6767/426 | 8299/478 |
| R1 [F2: Fo2 > 2σ(Fo2)] | 0.0522 | 0.0903 |
| wR2 (F2: all data) | 0.1351 | 0.2966 |
| GoF | 0.872 | 1.084 |
| Flack param. | 0.022(7) | — |
| Compound | 1·MeOH | 2·H2O·Et2O |
|---|---|---|
| Ni1–N1, Ni–N6 | 2.094(4), 2.095(5) | 2.110(4), 2.126(4) |
| Ni1–N2, Ni–N7 | 2.084(4), 2.082(4) | 2.078(4), 2.093(4) |
| Ni1–N5, Ni–N10 | 2.047(5), 2.039(5) | 2.068(4), 2.065(4) |
| N1–Ni1–N2, N6–Ni1–N7 | 79.22(19), 78.55(19) | 78.80(16), 78.94(17) |
| N2–Ni1–N5, N7–Ni1–N10 | 87.99(18), 88.63(19) | 88.95(16), 89.00(16) |
| N1–Ni1–N5, N6–Ni1–N10 | 167.20(18), 166.57(18) | 166.81(16), 167.93(16) |
| N1–Ni1–N6, N5–Ni1–N10 | 88.85(18), 91.3(2) | 91.91(18), 89.59(16) |
| N2–Ni1–N7 | 175.74(19) | 165.77(16) |
| N1–Ni1–N7, N2–Ni1–N6 | 99.83(19), 97.26(17) | 90.25(15), 92.25(16) |
| N1–Ni1–N10, N5–Ni1–N6 | 89.45(19), 93.27(19) | 87.64(16), 93.46(16) |
| N2–Ni1–N10, N5–Ni1–N7 | 95.50(19), 92.97(19) | 99.48(16), 102.60(16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayibor, K.M.; Sunatsuki, Y.; Suzuki, T. Selective Formation of Unsymmetric Multidentate Azine-Based Ligands in Nickel(II) Complexes. Molecules 2022, 27, 6788. https://doi.org/10.3390/molecules27206788
Hayibor KM, Sunatsuki Y, Suzuki T. Selective Formation of Unsymmetric Multidentate Azine-Based Ligands in Nickel(II) Complexes. Molecules. 2022; 27(20):6788. https://doi.org/10.3390/molecules27206788
Chicago/Turabian StyleHayibor, Kennedy Mawunya, Yukinari Sunatsuki, and Takayoshi Suzuki. 2022. "Selective Formation of Unsymmetric Multidentate Azine-Based Ligands in Nickel(II) Complexes" Molecules 27, no. 20: 6788. https://doi.org/10.3390/molecules27206788
APA StyleHayibor, K. M., Sunatsuki, Y., & Suzuki, T. (2022). Selective Formation of Unsymmetric Multidentate Azine-Based Ligands in Nickel(II) Complexes. Molecules, 27(20), 6788. https://doi.org/10.3390/molecules27206788