Nanozyme Based on Dispersion of Hemin by Graphene Quantum Dots for Colorimetric Detection of Glutathione
Abstract
1. Introduction
2. Results and Discussion
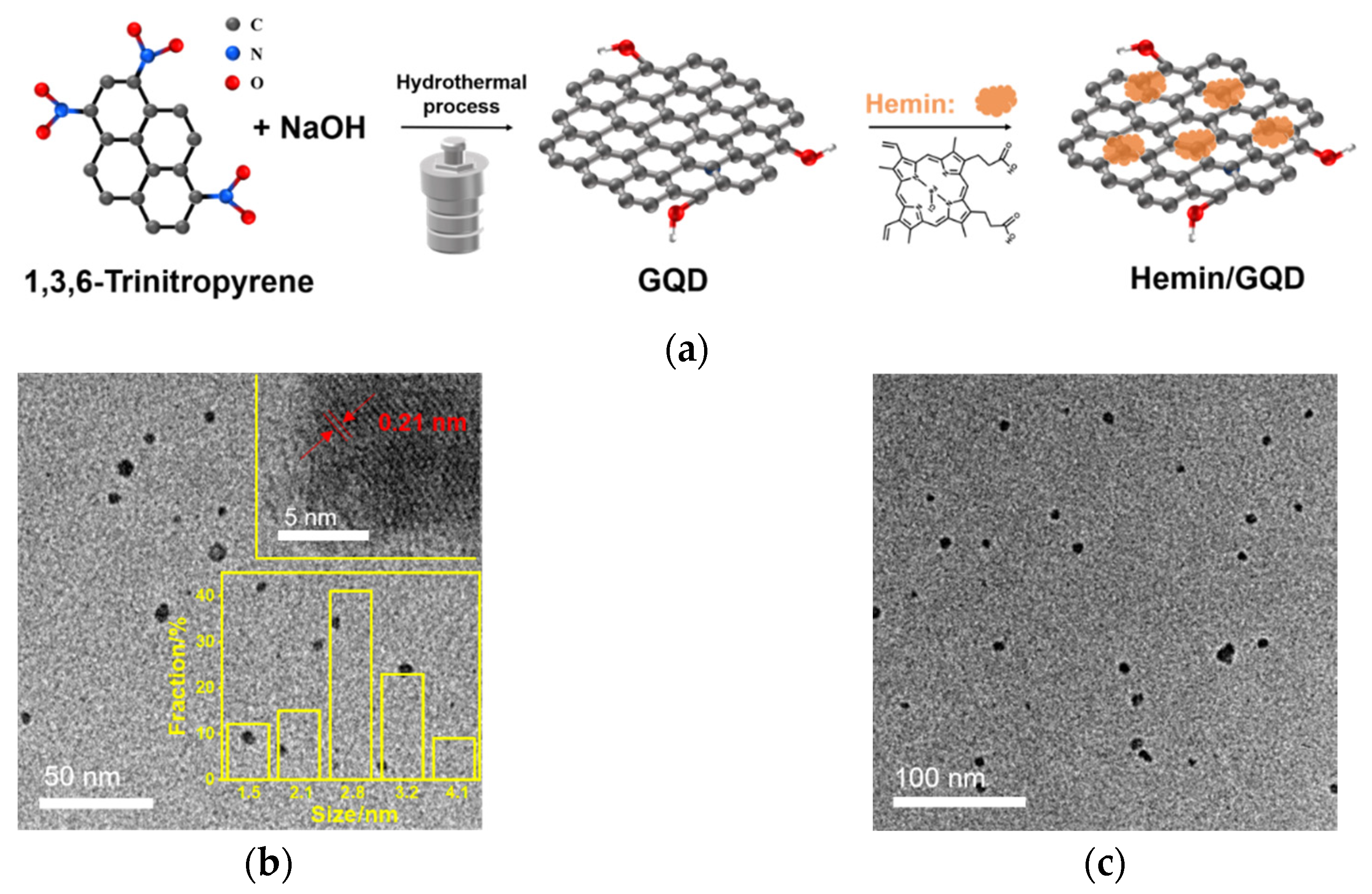
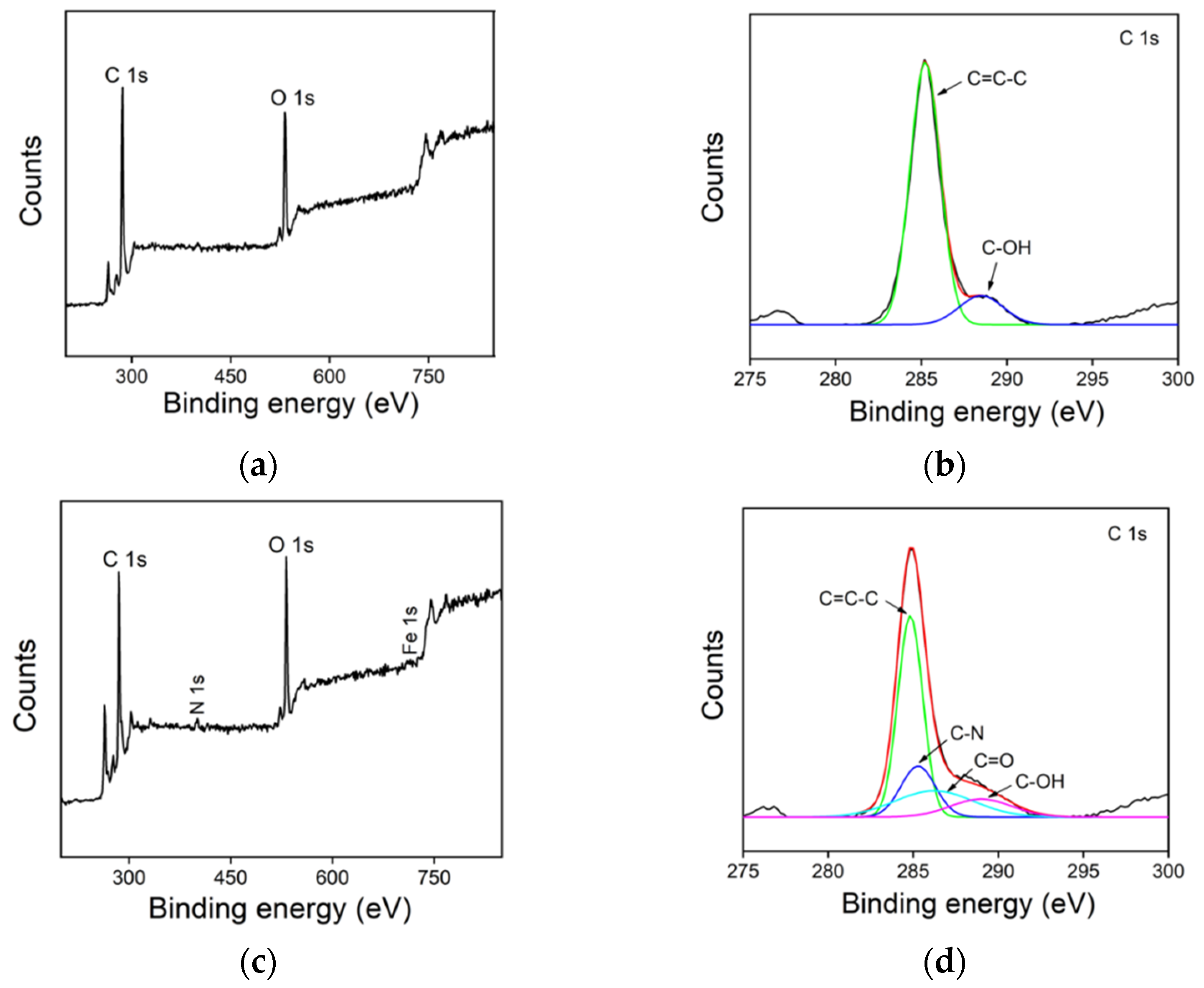
2.1. Synthesis and Characterization of GQD and Hemin/GQD
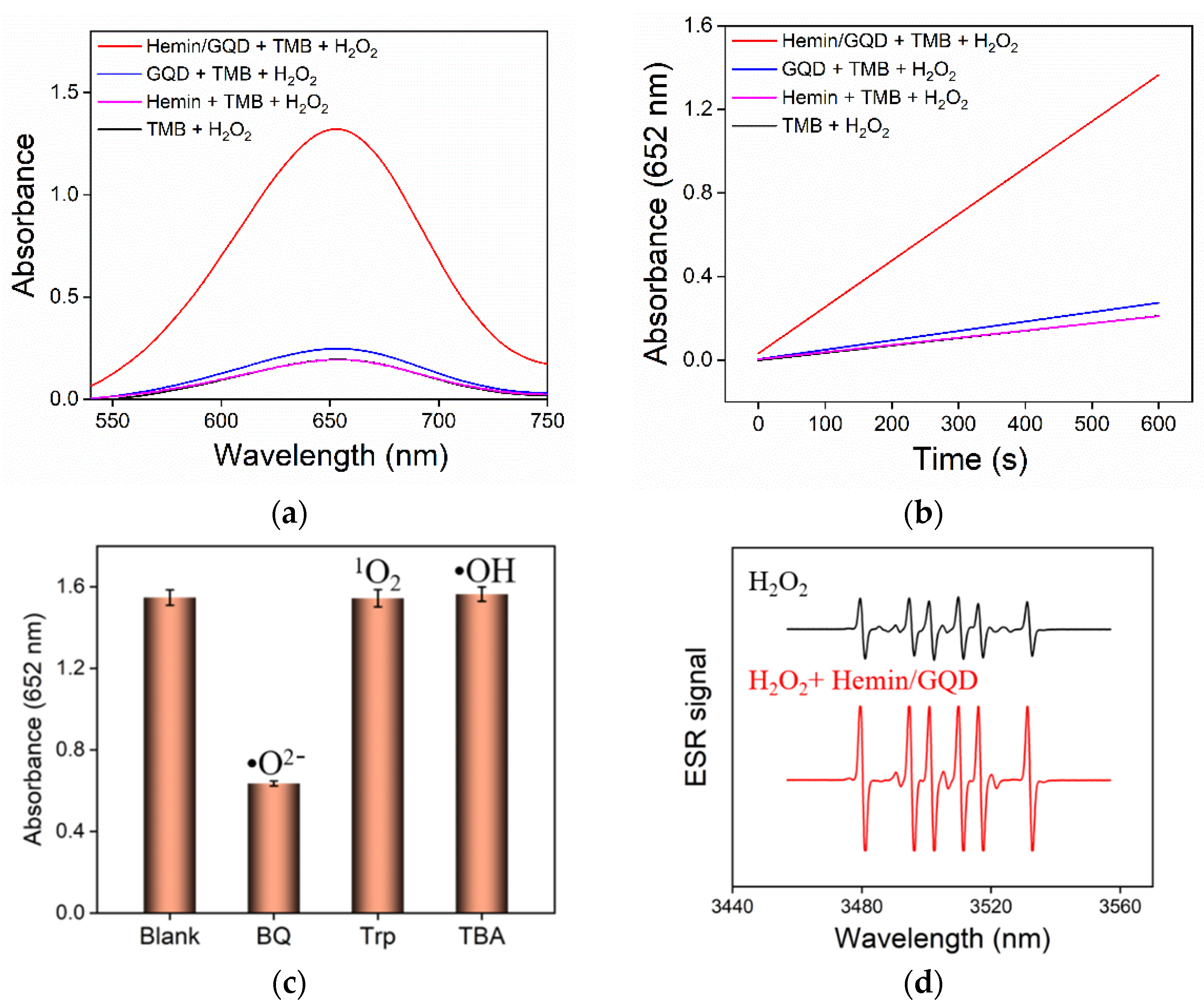
2.2. Peroxidase-Mimicking Activity of Hemin/GQDs
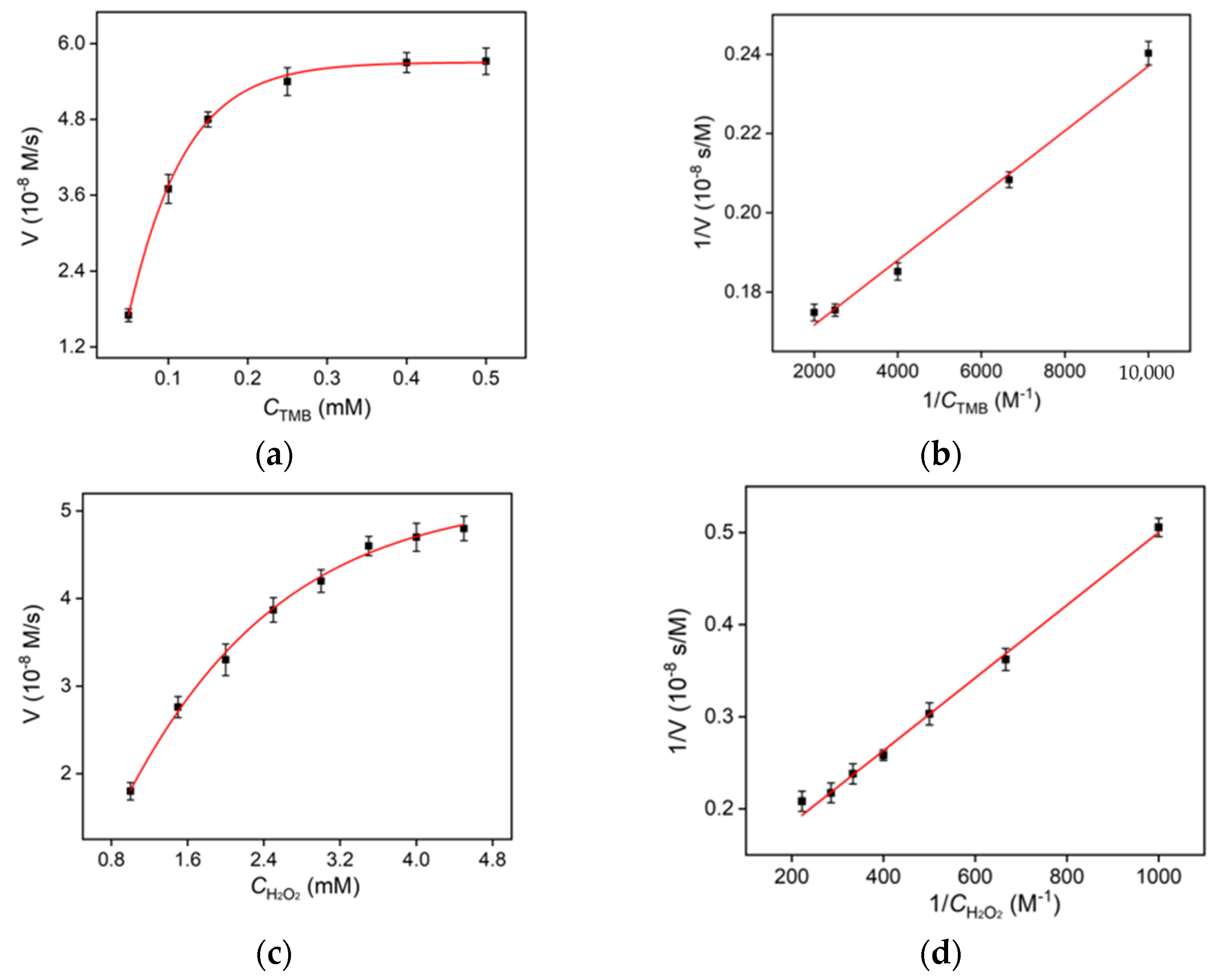
2.3. Steady-State Kinetics of Hemin/GQD Nanozyme
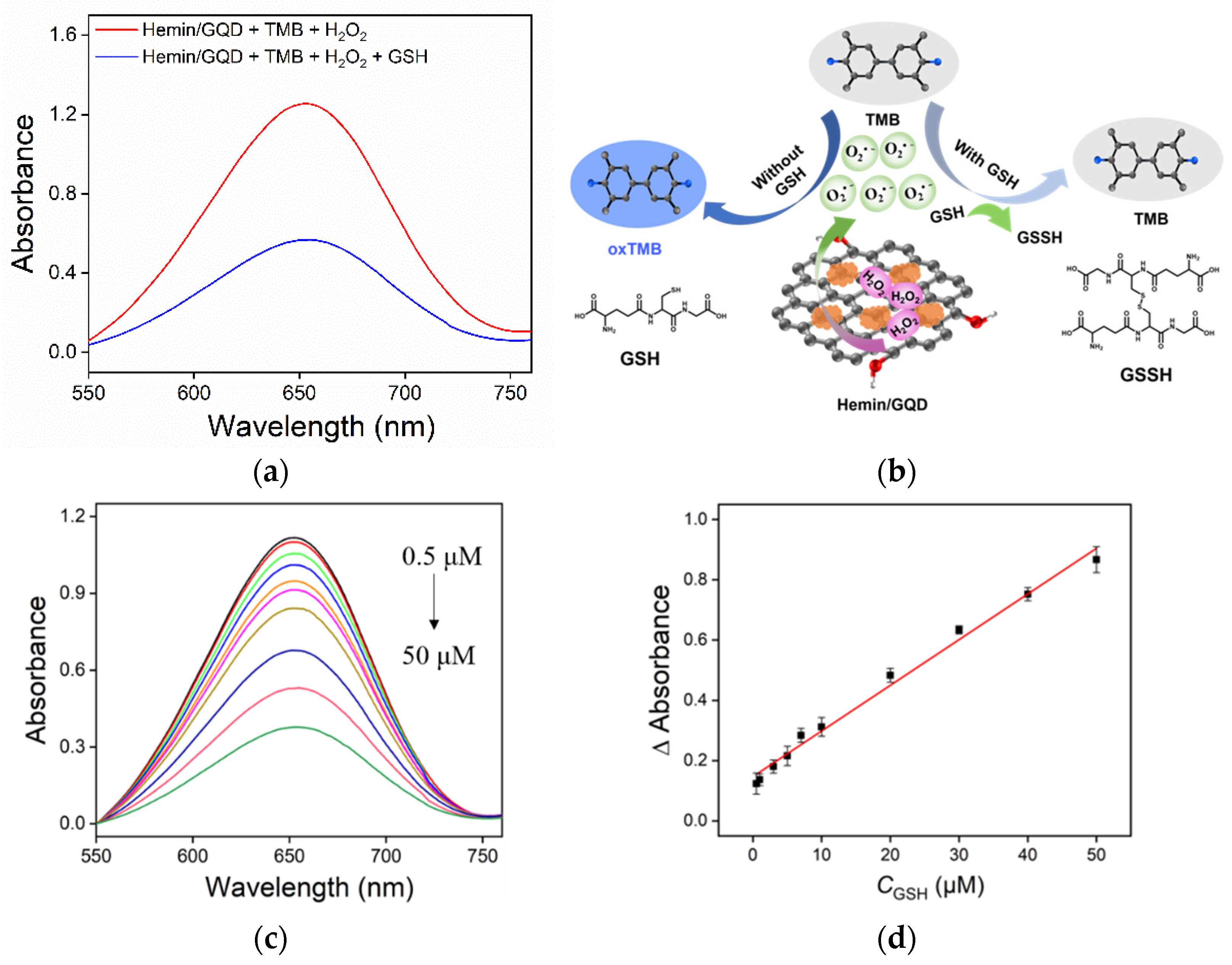
2.4. Colorimetric Detection of GSH
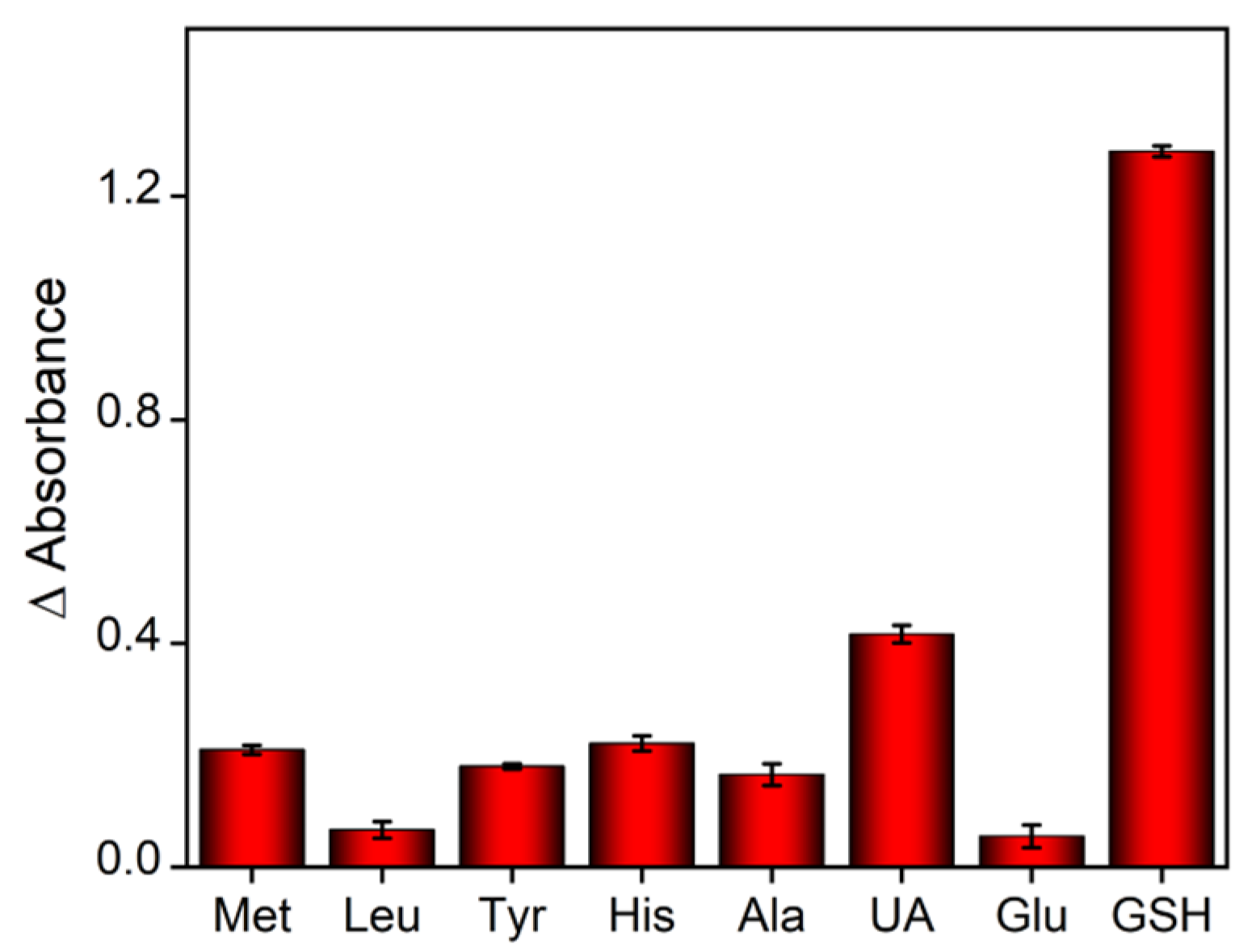
2.5. Detection Selectivity and Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of GQD and Hemin/GQD
3.3. Experiments and Instrumentations
3.4. Peroxidase-Mimicking Activity of Hemin/GQD
3.5. Determination of Steady-State Kinetic Constants of Nanozyme
3.6. Colorimetric Detection of GSH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yan, X. Nanozymes: An emerging field bridging nanotechnology and biology. Sci. China Life Sci. 2016, 59, 400–402. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lou, R.; Pan, W.; Li, N.; Tang, B. Nanozymes in disease diagnosis and therapy. Chem. Commun. 2020, 56, 15513–15524. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhou, Y.; Yan, X.; Fan, K. Advances in chiral nanozymes: A review. Microchim. Acta 2019, 186, 782. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon nanozymes: Enzymatic properties, catalytic mechanism, and applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, X.; Zhu, Y.; Muhammad, F.; Tan, S.; Cao, W.; Lin, S.; Jin, Z.; Gao, X.; Wei, H. Nitrogen-doped carbon nanomaterials as highly active and specific peroxidase mimics. Chem. Mater. 2018, 30, 6431–6439. [Google Scholar] [CrossRef]
- Jin, S.; Wu, C.; Ye, Z.; Ying, Y. Designed inorganic nanomaterials for intrinsic peroxidase mimics: A review. Sens. Actuators B Chem. 2019, 283, 18–34. [Google Scholar] [CrossRef]
- Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. When nanozymes meet single-atom catalysis. Angew. Chem. Int. Ed. 2020, 59, 2565–2576. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T.; Ling, C. Graphene-based nanomaterials as efficient peroxidase mimetic catalysts for biosensing applications: An overview. Molecules 2015, 20, 14155–14190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, R.; Chen, G.; Wang, L.; Yu, P.; Shu, H.; Bashir, K.; Fu, Q. Hemin-porous g-C3N4 hybrid nanosheets as an efficient peroxidase mimic for colorimetric and visual determination of glucose. Microchim. Acta 2019, 186, 446. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Zha, J.; Han, H.; Chen, Y.; Jia, Z. Enhancing the peroxidase-mimicking activity of hemin by covalent immobilization in polymer nanogels. Polym. Chem. 2021, 12, 858–866. [Google Scholar] [CrossRef]
- Li, J.; Yuan, T.; Yang, T.; Xu, L.; Zhang, L.; Huang, L.; Cheng, W.; Ding, S. DNA-grafted hemin with preferable catalytic properties than G-quadruplex/hemin for fluorescent miRNA biosensing. Sens. Actuators B Chem. 2018, 271, 239–246. [Google Scholar] [CrossRef]
- Xue, T.; Jiang, S.; Qu, Y.; Su, Q.; Cheng, R.; Dubin, S.; Chiu, C.; Kaner, R.; Huang, Y.; Duan, X. Graphene-supported hemin as a highly active biomimetic oxidation catalyst. Angew. Chem. Int. Ed. 2012, 51, 3822–3825. [Google Scholar] [CrossRef]
- Guo, Y.; Deng, L.; Li, J.; Guo, S.; Wang, E.; Dong, S. Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano 2011, 5, 1282–1290. [Google Scholar] [CrossRef]
- Xi, F.; Zhao, J.; Shen, C.; He, J.; Chen, J.; Yan, Y.; Li, K.; Liu, J.; Chen, P. Amphiphilic graphene quantum dots as a new class of surfactants. Carbon 2019, 153, 127–135. [Google Scholar] [CrossRef]
- Ge, S.; He, J.; Ma, C.; Liu, J.; Xi, F.; Dong, X. One-step synthesis of boron-doped graphene quantum dots for fluorescent sensors and biosensor. Talanta 2019, 199, 581–589. [Google Scholar] [CrossRef]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Graphene quantum dot-decorated luminescent porous silicon dressing for theranostics of diabetic wounds. Acta Biomater. 2021, 131, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, X.; Qian, Y.; Liu, J.; Li, L.; Liu, J.; Chen, J. Direct and sensitive detection of sulfide ions based on one-step synthesis of ionic liquid functionalized fluorescent carbon nanoribbons. RSC Adv. 2019, 9, 37484–37490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, L.; Pei, J.; Tian, Y.; Liu, J. A reagentless electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on the interface with redox probe-modified electron transfer wires and effectively immobilized antibody. Front. Chem. 2022, 10, 939736. [Google Scholar] [CrossRef]
- Yan, F.; Wang, M.; Jin, Q.; Zhou, H.; Xie, L.; Tang, H.; Liu, J. Vertically-ordered mesoporous silica films on graphene for anti-fouling electrochemical detection of tert-butylhydroquinone in cosmetics and edible oils. J. Electroanal. Chem. 2021, 881, 114969. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Luo, X.; Zhou, H.; Lin, X.; Wang, K.; Fei, Y.; Xi, F.; Liu, J. Vertically ordered mesoporous silica-nanochannel film-equipped three-dimensional macroporous graphene as sensitive electrochemiluminescence platform. Front. Chem. 2021, 9, 770512. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, X.; Sailjoi, A.; Zou, Y.; Lin, X.; Yan, F.; Su, B.; Liu, J. Vertical silica nanochannels supported by nanocarbon composite for simultaneous detection of serotonin and melatonin in biological fluids. Sens. Actuators B Chem. 2022, 353, 131101. [Google Scholar] [CrossRef]
- Yan, F.; Luo, T.; Jin, Q.; Zhou, H.; Sailjoi, A.; Dong, G.; Liu, J.; Tang, W. Tailoring molecular permeability of vertically-ordered mesoporous silica-nanochannel films on graphene for selectively enhanced determination of dihydroxybenzene isomers in environmental water samples. J. Hazard.Mater. 2021, 410, 124636. [Google Scholar] [CrossRef]
- Yan, F.; Chen, J.; Jin, Q.; Zhou, H.; Sailjoi, A.; Liu, J.; Tang, W. Fast one-step fabrication of a vertically-ordered mesoporous silica-nanochannel film on graphene for direct and sensitive detection of doxorubicin in human whole blood. J. Mater. Chem. C 2020, 8, 7113–7119. [Google Scholar] [CrossRef]
- Lin, J.; Li, K.; Wang, M.; Chen, X.; Liu, J.; Tang, H. Reagentless and sensitive determination of carcinoembryonic antigen based on a stable Prussian blue modified electrode. RSC Adv. 2020, 10, 38316–38322. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile pretreatment of three-dimensional graphene through electrochemical polarization for improved electrocatalytic performance and simultaneous electrochemical detection of catechol and hydroquinone. Nanomaterials 2022, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent advances on graphene quantum dots: From chemistry and physics to applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, J.; Xie, L.; Tang, H.; Wang, K.; Liu, J. One-step preparation of nitrogen-doped graphene quantum dots with anodic electrochemiluminescence for sensitive detection of hydrogen peroxide and glucose. Front. Chem. 2021, 9, 688358. [Google Scholar]
- Liu, X.; Chen, Z.; Wang, T.; Jiang, X.; Qu, X.; Duan, W.; Xi, F.; He, Z.; Wu, J. Tissue imprinting on 2D nanoflakes-capped silicon nanowires for lipidomic mass spectrometry imaging and cancer diagnosis. ACS Nano 2022, 16, 6916–6928. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Duan, W.; Jin, Y.; Wo, F.; Xi, F.; Wu, J. Ratiometric fluorescent nanohybrid for noninvasive and visual monitoring of sweat glucose. ACS Sens. 2020, 5, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, Z.; Zeng, Z.; Wang, W.; Kong, L.; Liu, J.; Chen, P. Graphene quantum dots assisted exfoliation of atomically-thin 2D materials and as-formed 0D/2D van der Waals heterojunction for HER. Carbon 2021, 184, 554–561. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Liu, J.; Tian, Q.; Chen, P. Graphene quantum dot engineered nickel-cobalt phosphide as highly efficient bifunctional catalyst for overall water splitting. Nano Energy 2018, 48, 284–291. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic water splitting and CO2 reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef]
- Huang, B.; He, J.; Bian, S.; Zhou, C.; Li, Z.; Xi, F.; Liu, J.; Dong, X. S-doped graphene quantum dots as nanophotocatalyst for visible light degradation. Chinese Chem. Lett. 2018, 29, 1698–1701. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, L.; Chen, J.; Yan, F.; Liu, J.; Dong, X.; Xi, F.; Chen, P. Nanochannel-confined graphene quantum dots for ultrasensitive electrochemical analysis of complex samples. ACS Nano 2018, 12, 12673–12681. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Han, Y.; Zhu, X.; Gong, J.; Luo, T.; Zhao, C.; Liu, J.; Liu, J.; Li, X. Sensitive detection of sulfide ion based on fluorescent ionic liquid-graphene quantum dots nanocomposite. Front. Chem. 2021, 9, 658045. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Z.; Zhao, R.; Lu, Y.; Shi, L.; Liu, J.; Dong, X.; Xi, F. Aqueous synthesis of amphiphilic graphene quantum dots and their application as surfactants for preparing of fluorescent polymer microspheres. Colloid Surface A 2019, 563, 77–83. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, J.; Deng, X.; Chen, J.; Xi, F.; Wang, X. Colorimetric and fluorescent dual-modality sensing platform based on fluorescent nanozyme. Front. Chem. 2021, 9, 774486. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Yan, Y.; Min, Y.; Li, H.; Xi, F.; Liu, J.; Chen, P. Quasi-homogeneous carbocatalysis for one-pot selective conversion of carbohydrates to 5-hydroxymethylfurfural using sulfonated graphene quantum dots. Carbon 2018, 136, 224–233. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, C.; Ge, S.; Luo, T.; Chen, J.; Liu, J.; Xi, F.; Liu, J. Gram-scale synthesis of nitrogen doped graphene quantum dots for sensitive detection of mercury ions and l-cysteine. RSC Adv. 2019, 9, 32977–32983. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, R.; Lu, Y.; Liu, J.; Dong, X.; Xi, F. Facile preparation of N-doped graphene quantum dots as quick-dry fluorescent ink for anti-counterfeiting. New J. Chem. 2018, 42, 17091–17095. [Google Scholar] [CrossRef]
- Li, N.; Than, A.; Chen, J.; Xi, F.; Liu, J.; Chen, P. Graphene quantum dots based fluorescence turn-on nanoprobe for highly sensitive and selective imaging of hydrogen sulfide in living cells. Biomater. Sci. 2018, 6, 779–784. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, J.; Ding, Y.; Tang, H.; Xi, F. Iron and nitrogen co-doped graphene quantum dots as highly active peroxidases for the sensitive detection of L-cysteine. New J. Chem. 2021, 45, 19056–19064. [Google Scholar] [CrossRef]
- Yan, F.; Ma, X.; Jin, Q.; Tong, Y.; Tang, H.; Lin, X.; Liu, J. Phenylboronic acid-functionalized vertically ordered mesoporous silica films for selective electrochemical determination of fluoride ion in tap water. Microchim. Acta 2020, 187, 470. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, N.; Khan, S.; Nandi, C. Carbon dots for naked eye colorimetric ultrasensitive arsenic and glutathione detection. Biosens. Bioelectron. 2016, 81, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Liu, W.; Zhang, P.; Wu, J.; Zhang, H.; Ren, H.; Ge, J.; Wang, P. A colorimetric and ratiometric fluorescent probe for highly selective detection of glutathione in the mitochondria of living cells. Sens. Actuators B Chem. 2018, 270, 459–465. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, Y.; Zhao, S.; Zhang, P.; Zhang, Y.; Bi, J.; Liu, G.; Yue, Z. Carbon dots based photoelectrochemical sensors for ultrasensitive detection of glutathione and its applications in probing of myocardial infarction. Biosens. Bioelectron. 2018, 99, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, Y.; Pang, Y.; Chen, J.; Zhang, Z.; Xi, F.; Chen, P. Graphene quantum dots as full-color and stimulus responsive fluorescence ink for information encryption. J. Colloid Interface Sci. 2020, 579, 307–314. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, X.; Routh, P.; Sana, B.; Lim, S.; Kim, D.; Lim, K.; Li, J.; Chen, P. Facile synthesis of graphene quantum dots from 3d graphene and their application for Fe3+ sensing. Adv. Funct. Mater. 2014, 24, 3021–3026. [Google Scholar] [CrossRef]

| Sample a | Added (μM) | Found (μM) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Fetal bovine Serum a | 1.00 | 1.11 | 0.3 | 111 |
| 5.00 | 4.96 | 2.6 | 99.2 | |
| 10.0 | 10.2 | 3.3 | 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Deng, X.; Hong, X.; Zhao, S. Nanozyme Based on Dispersion of Hemin by Graphene Quantum Dots for Colorimetric Detection of Glutathione. Molecules 2022, 27, 6779. https://doi.org/10.3390/molecules27206779
Li Z, Deng X, Hong X, Zhao S. Nanozyme Based on Dispersion of Hemin by Graphene Quantum Dots for Colorimetric Detection of Glutathione. Molecules. 2022; 27(20):6779. https://doi.org/10.3390/molecules27206779
Chicago/Turabian StyleLi, Zhaoshen, Xiaochun Deng, Xiaoping Hong, and Shengfa Zhao. 2022. "Nanozyme Based on Dispersion of Hemin by Graphene Quantum Dots for Colorimetric Detection of Glutathione" Molecules 27, no. 20: 6779. https://doi.org/10.3390/molecules27206779
APA StyleLi, Z., Deng, X., Hong, X., & Zhao, S. (2022). Nanozyme Based on Dispersion of Hemin by Graphene Quantum Dots for Colorimetric Detection of Glutathione. Molecules, 27(20), 6779. https://doi.org/10.3390/molecules27206779




