Analysis of Volatile Components of Jasmine and Jasmine Tea during Scenting Process
Abstract
:1. Introduction
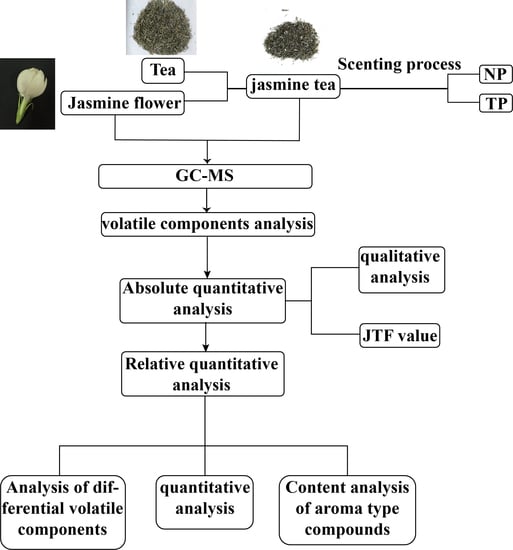
2. Results
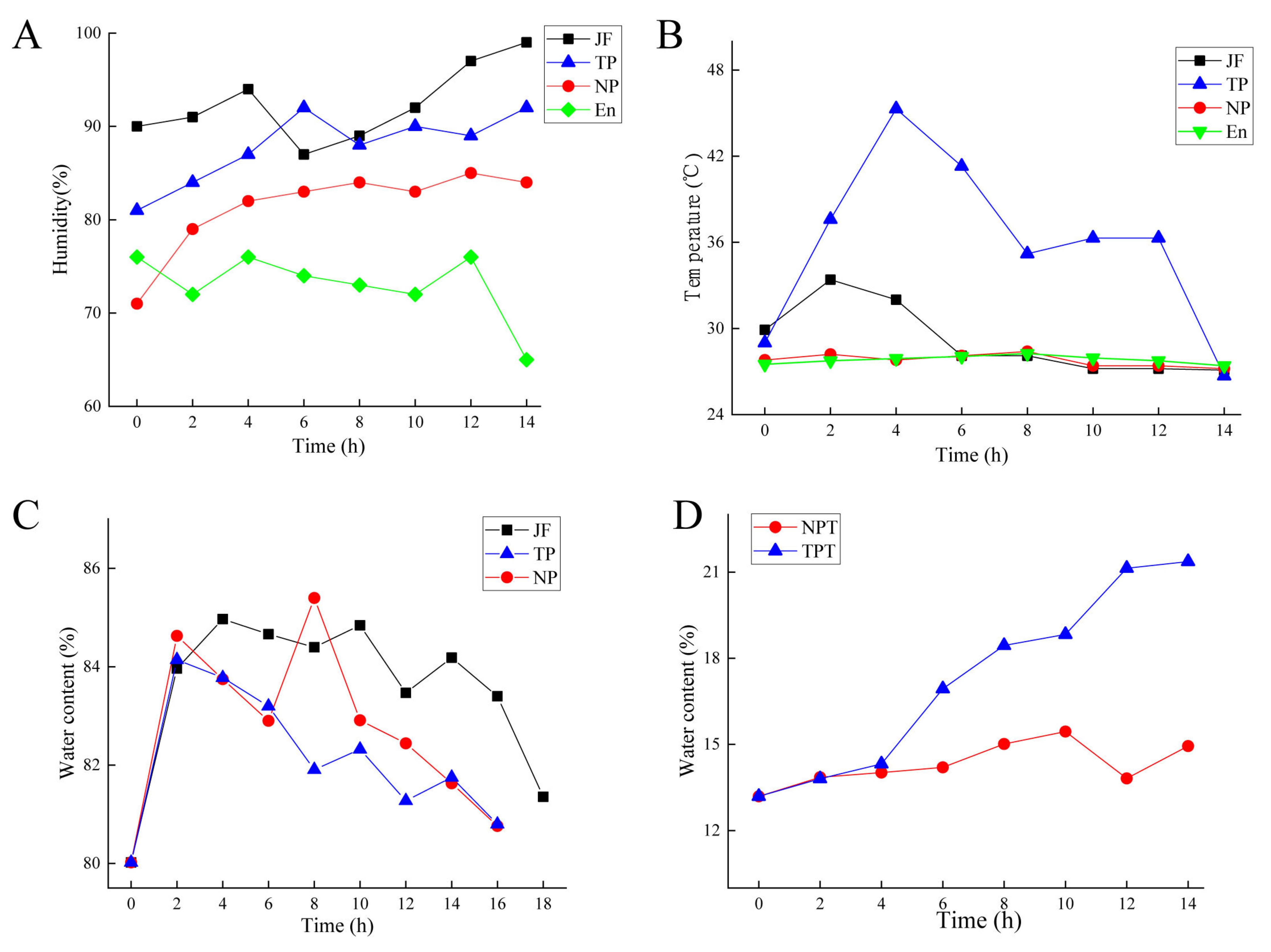
2.1. Changes in Water, Temperature, and Humidity during Scenting Processes
2.2. Qualitative Analysis of Volatile Components in Jasmine and Jasmine Tea
2.2.1. Qualitative Analysis of Volatile Components in Jasmine
2.2.2. Qualitative Analysis of Volatile Components in Jasmine Tea
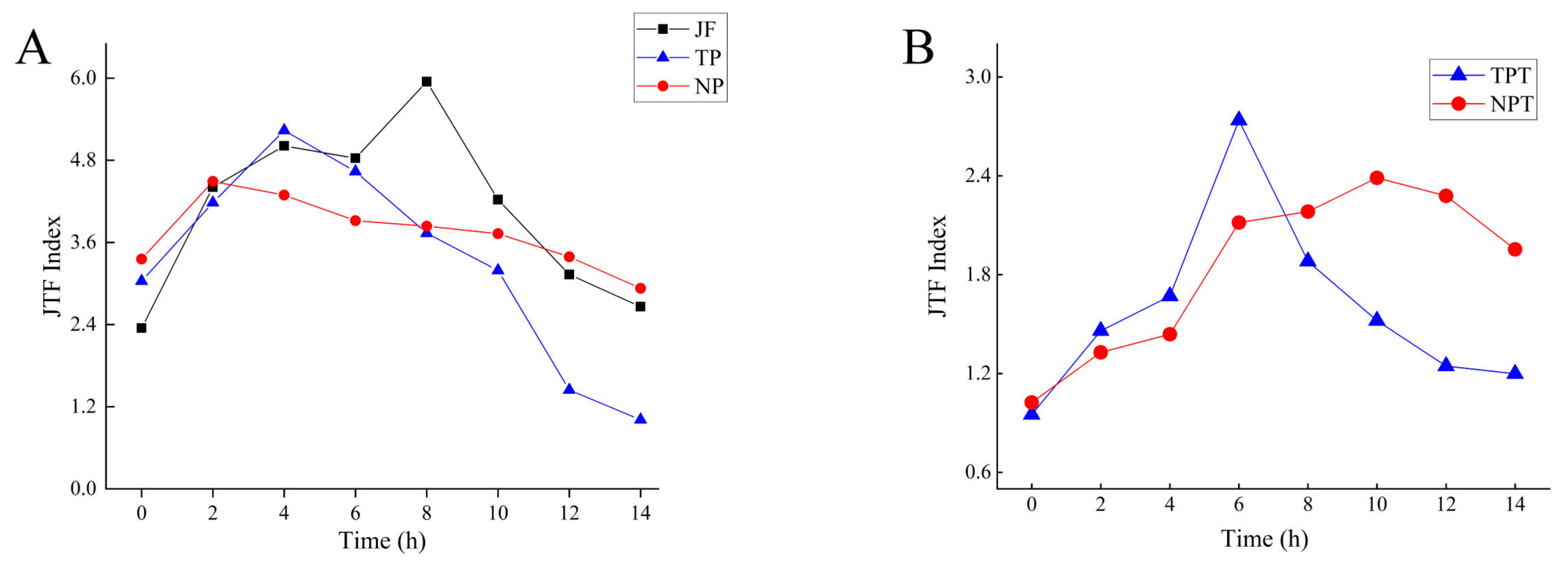
2.2.3. Fragrance Evaluation Index (JTF)
2.3. Quantitative Analysis of Volatile Components
2.3.1. Quantitative Analysis of Volatile Components in Jasmine
Establishment of Standard Curve of Jasmine
Quantitative Results of Jasmine
2.3.2. Quantitative Analysis of the Volatile Components of Jasmine Tea
Establishment of Standard Curve of Jasmine Tea
Quantitative Results of Jasmine Tea
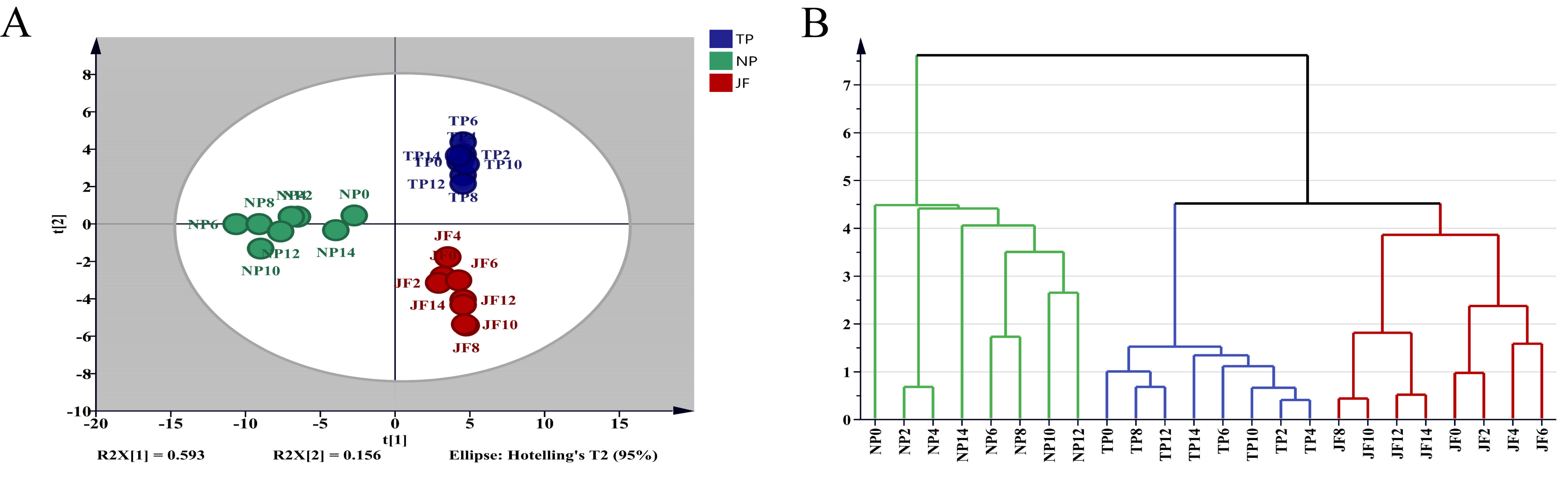
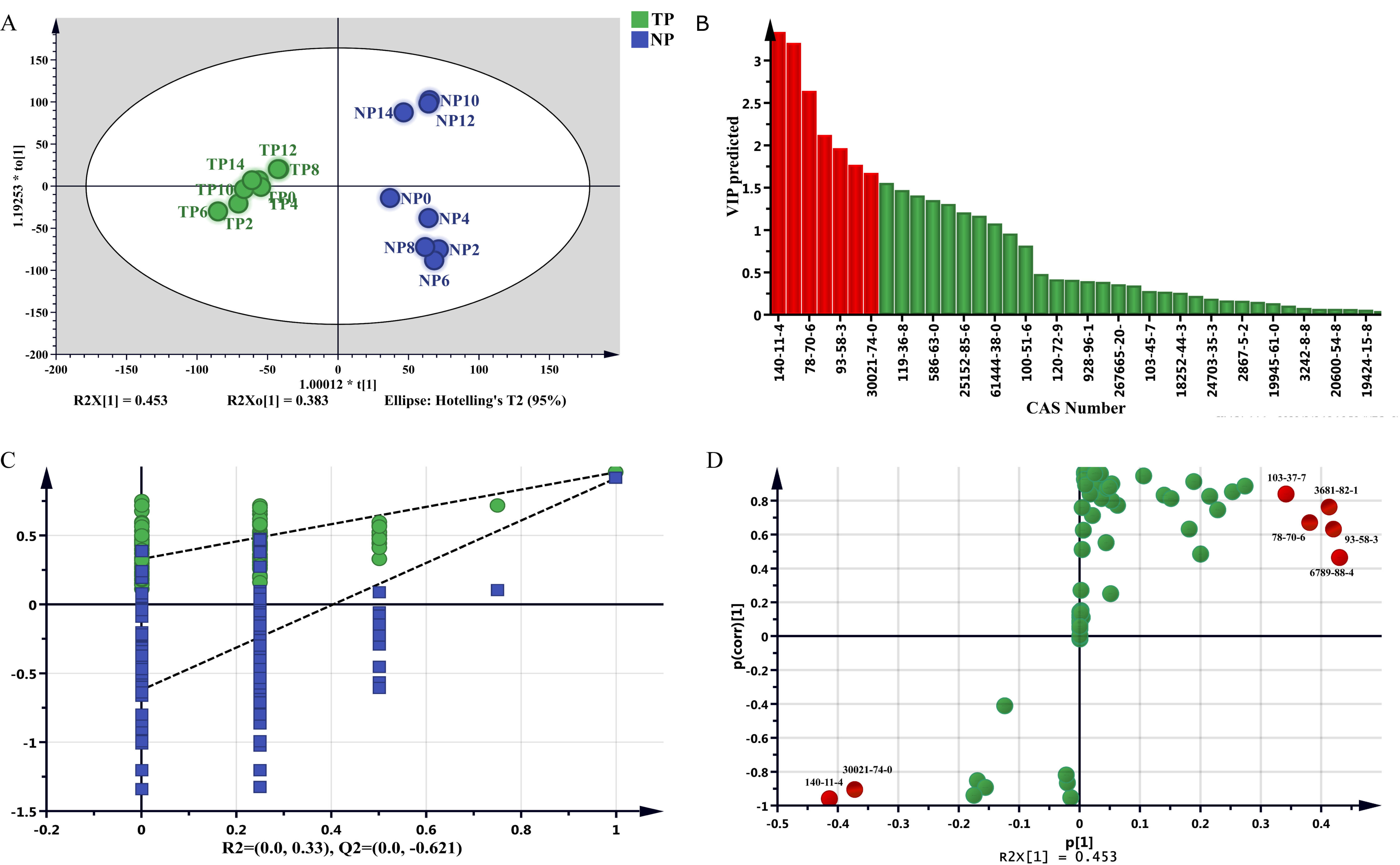
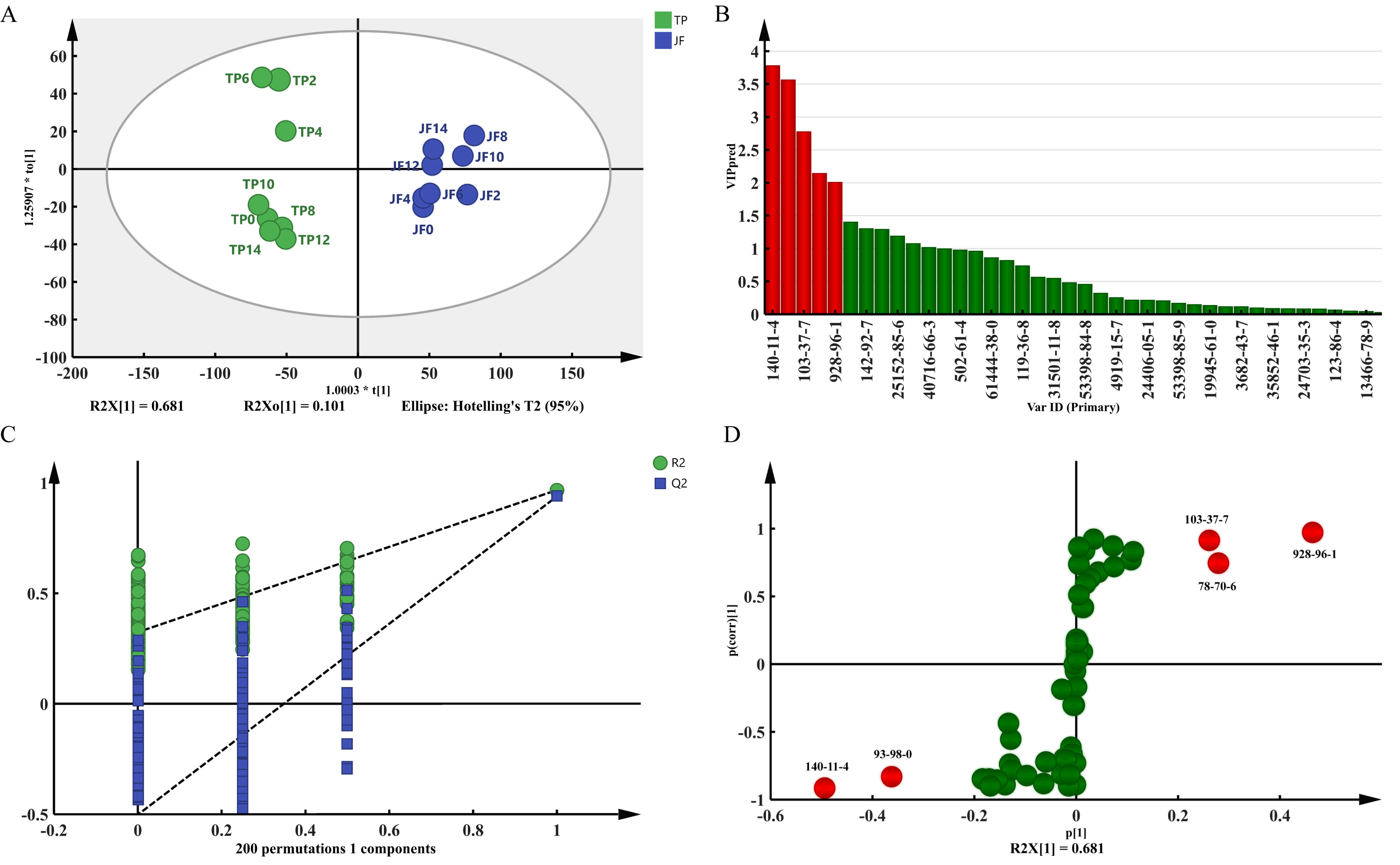
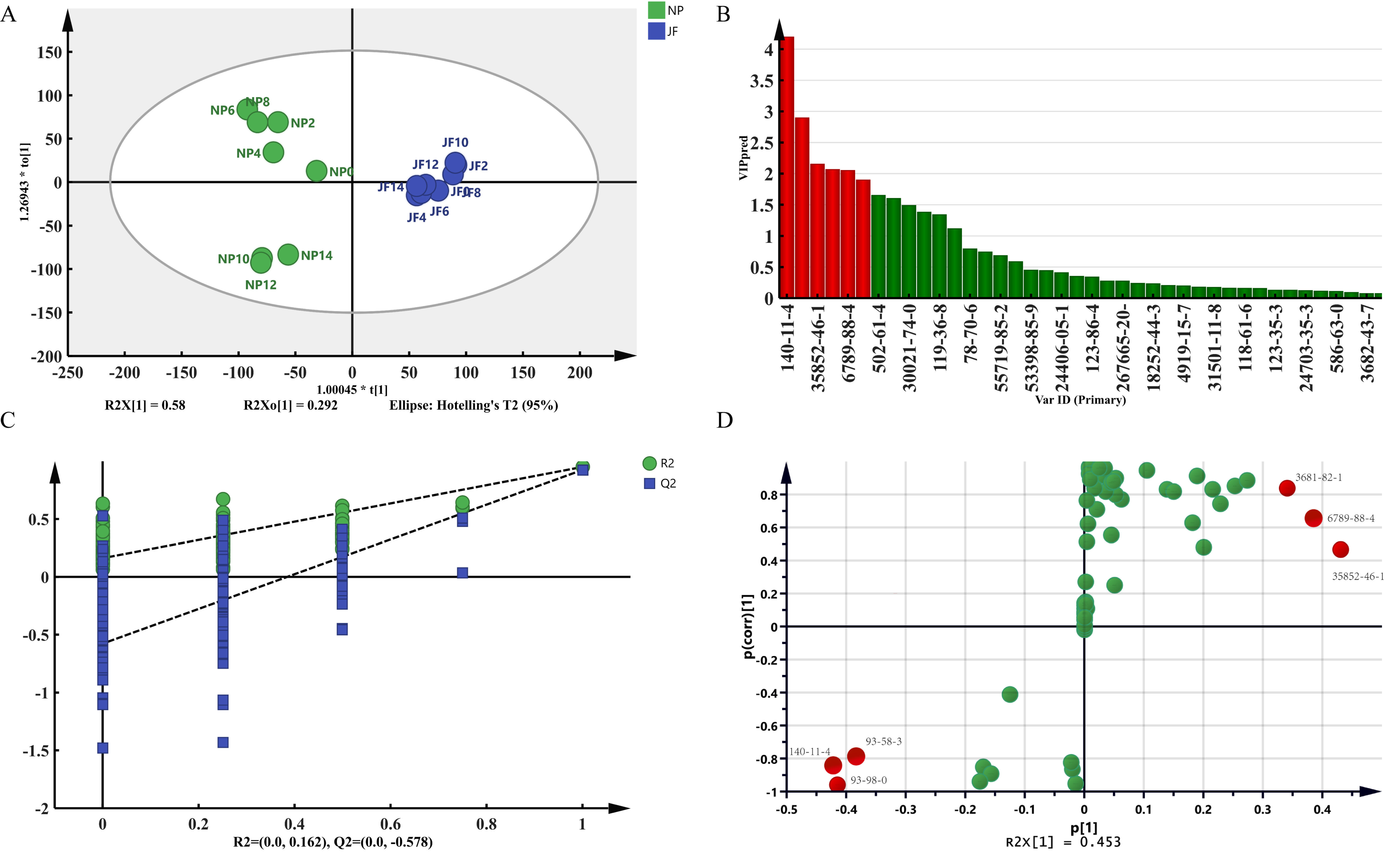
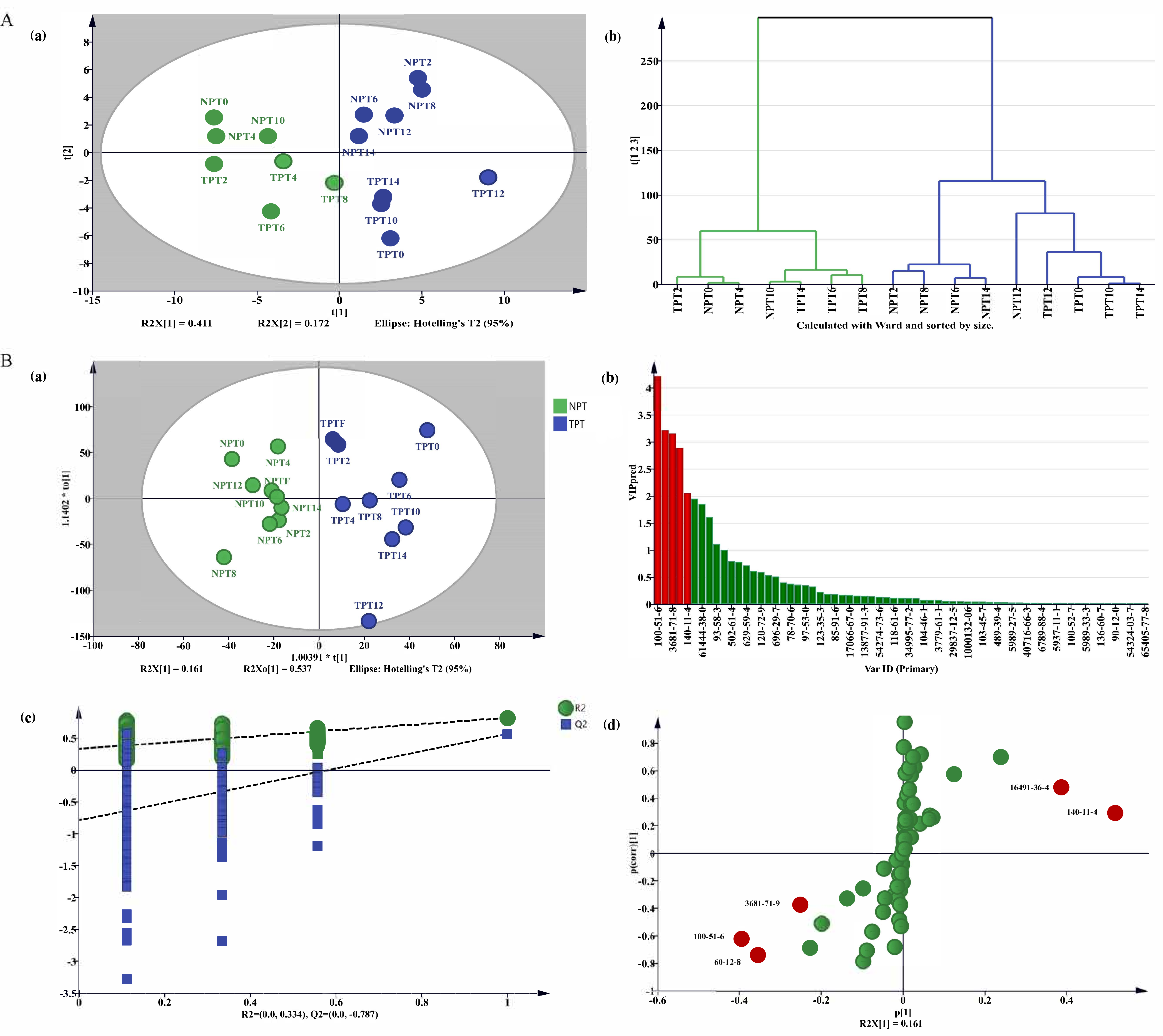
2.4. Analysis of the Difference in Volatile Components of Jasmine and Jasmine Tea
2.4.1. Analysis of the Difference in Volatile Components of Jasmine
PCA and HCA Analysis
Analysis of the Volatile Components between JF and TP
2.4.2. Analysis of the Difference in Volatile Components of Jasmine Tea
2.5. Analysis of the Main Differential Volatile Components
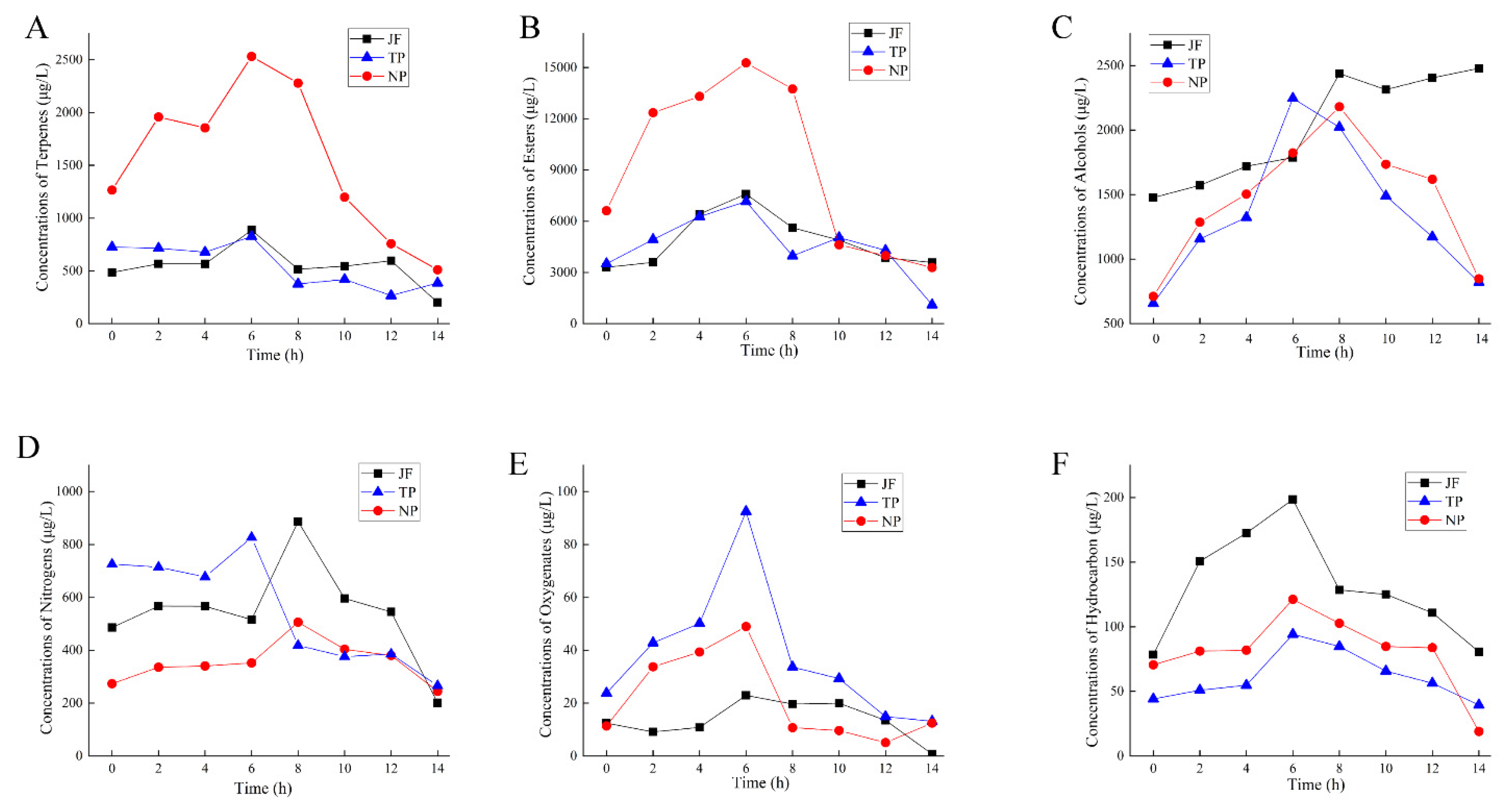
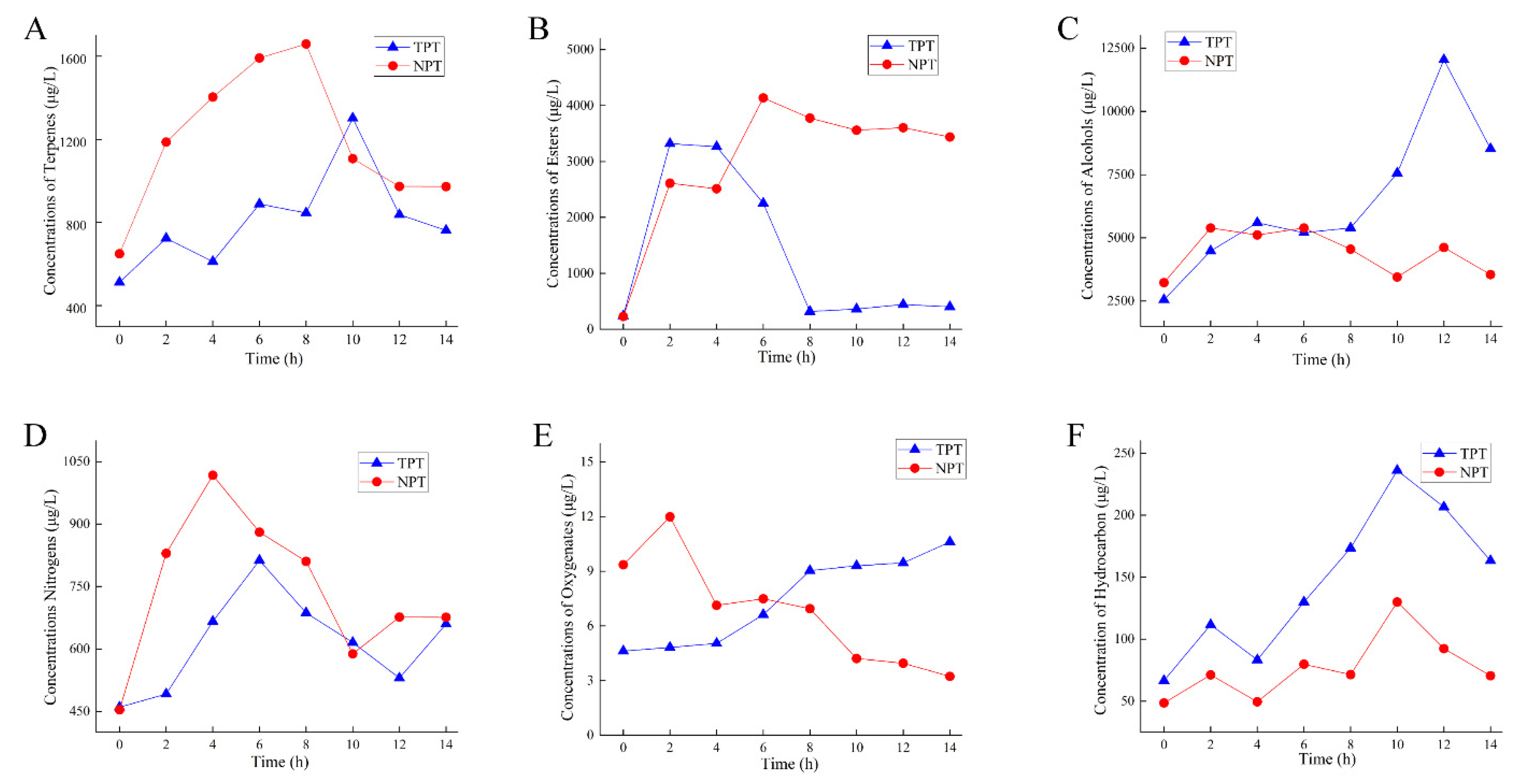
2.6. Changes in the Main Aroma Components of Jasmine and Jasmine Tea
2.6.1. Changes in Main Aroma Substances of Jasmine
2.6.2. Changes in Main Aroma Substances of Jasmine Tea
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Material
4.1.2. Reagents
4.1.3. Instrumentation
4.2. Process Parameters of Different Scenting Processes of Jasmine Tea
4.3. Optimization of Aroma Extraction Conditions
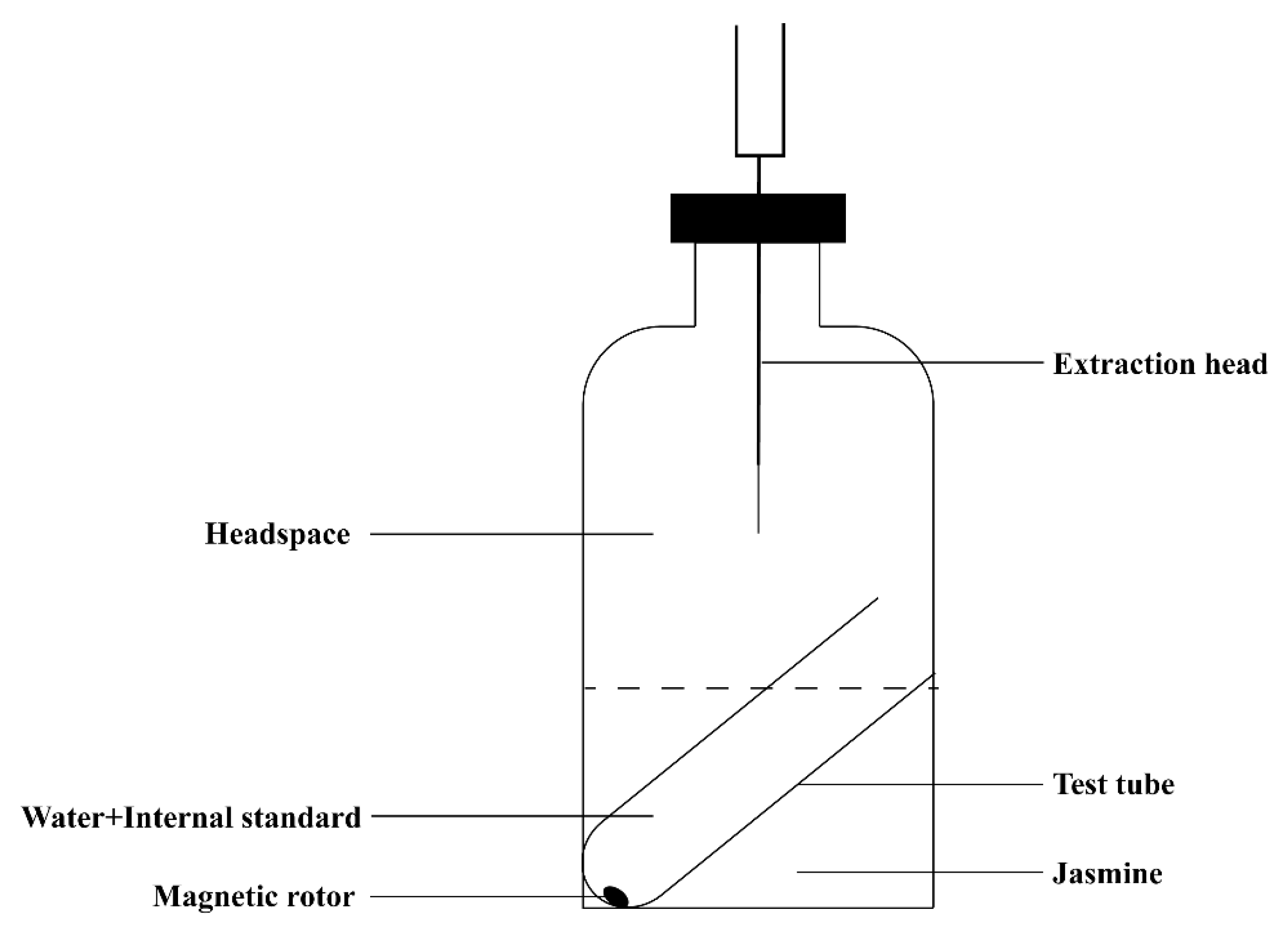
4.3.1. HS-HPME Extraction Process of Jasmine
4.3.2. HS-HPME Extraction Process of Jasmine Tea
4.3.3. Analysis of GC-MS Condition
4.4. Qualitative Analysis Data Processing Methods
4.5. Establishment of Quantitative Method
4.5.1. Preparation of Blank Matrix
4.5.2. Establishment of a Standard Curve
4.6. Calculation of Aroma Activity Threshold (OAV)
4.7. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| JF | Jasmine flower |
| NP | Jasmine flower in new scenting process |
| TP | Jasmine flower in traditional scenting process |
| NPT | Jasmine tea in new scenting process |
| TPT | Jasmine tea in traditional scenting process |
| En | Environment |
| JTF | Jasmine tea flavor index |
| RI | Retention index |
| QC | Quality control |
| LOQ | Limit of quantitation |
| LOD | Limit of detection |
| PCA | Principal component analysis |
| HCA | Hierarchical cluster analysis |
| OPLS-DA | Discriminant analysis of orthogonal partial least squares |
References
- Inoue, N.; Kuroda, K.; Sugimoto, A.; Kakuda, T.; Fushiki, T. Autonomic nervous responses according to preference for the odor of jasmine tea. Biosci. Biotechnol. Biochem. 2003, 67, 1206–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Seo, H.S. A Study on the Preference Degree of Jasmine Tea Package Design in China: Focused on China’s Top 10 Jasmine Tea Brands. J. Korea Contents Assoc. 2019, 19, 304–313. [Google Scholar]
- Yang, Q.T.; Yang, Q.B. Rearch on health effect of jasmine tea. Tea Fujian 2020, 42, 3–5. [Google Scholar]
- Cong, T.; Zhao, L.; Li, Z.; Zou, H.M. The effect of three kind of jasmine tea on nutritional physiological functions of growing rats. Mod. Prev. Med. 2011, 38, 456–460. [Google Scholar]
- Chen, M.; Zhu, Y.; Liu, B.; Chen, Z.; Zheng, J.; Guan, M.; Shi, H.; Wang, Y.; Yang, W. Changes in the volatiles, chemical components, and antioxidant activities of Chinese jasmine tea during the scenting processes. Int. J. Food Prop. 2017, 20, 681–693. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, H.R. Analysis on aromatic components of Longjing tea using SPME-GC/MS methods. Tea 2008, 2, 85–88. [Google Scholar]
- Lin, J.; Chen, Y.; Zhang, P.; Ren, M.; Xu, H.; Wang, X. A novel quality evaluation index and strategies to identify scenting quality of jasmine tea based on headspace volatiles analysis. Food Sci. Biotechnol. 2013, 22, 331–340. [Google Scholar] [CrossRef]
- Kuroda, K.; Inoue, N.; Ito, Y.; Ito, Y.; Kubota, K.; Sugimoto, A.; Kakuda, T.; Fushiki, T. Sedative effects of the jasmine tea odor and (R)-(-)-linalool, one of its major odor components, on autonomic nerve activity and mood states. Eur. J. Appl. Physiol. 2005, 95, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, L.; Ma, M.; Zeng, L. Characterization of Volatile Compounds and Sensory Analysis of Jasmine Scented Black Tea Produced by Different Scenting Processes. J. Food Sci. 2018, 83, 2718–2732. [Google Scholar] [CrossRef]
- Tang, X.N.; Xia, Y.M.; Lei, Y.H.; Wang, X.C.; Lin, J. The construction of jasmine tea flavor and decision tree model in identification scenting quality. J. Tea Sci. 2016, 36, 646–654. [Google Scholar]
- Zhang, R.L.; Yin, J.H.; Yuan, H.B.; Xu, Y.Q.; Chen, J.X.; Ye, G.Z.; Chen, S.Q. Using principal component analysis for investigating the effects of processing conditions on color stability of tea infusion. Food Sci. 2010, 31, 82–87. [Google Scholar]
- Ye, Q.; Jin, X.; Zhu, X.; Lin, T.; Hao, Z.; Yang, Q. An Efficient Extraction Method for Fragrant Volatiles from Jasminum sambac (L.). Ait. J. Oleo Sci. 2015, 64, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Pawliszyn, J. Sampling and Sample Preparation for Field and Laboratory: Fundamentals and New Directions in Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Pragadheesh, V.S.; Yadav, A.; Chanotiya, C.S.; Rout, P.K.; Uniyal, G.C. Monitoring the Emission of Volatile Organic Compounds from Flowers of Jasminum sambac Using Solid-Phase Micro-extraction Fibers and Gas Chromatography with Mass Spectrometry Detection. Nat. Prod. Commun. 2011, 6, 1333–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.X.; Hu, Z.H.; Leng, P.S.; Wang, W.H.; Fang, X.U.; Zhao, J. Qualitative and quantitative analysis of floral volatile components from different varieties of Lilium spp. Sci. Agric. Sin. 2013, 46, 790–799. [Google Scholar]
- Chen, W.W. Jasmine tea scenting process. Mod. Agric. Sci. Technol. 2020, 10, 190–193. [Google Scholar]
- Pei, X.J. Research on New Jasmine Tea Process for Improving Jasmine Aroma Utilization and Its Process Effect Evaluation. Master’s Thesis, Sichuan Agricultural University, Sichuan, China, 2017. [Google Scholar]
- An, H.M.; Ou, X.C.; Xiong, Y.F.; Zhang, Y.B.; Li, S.; Huang, J.A. Study on the changes of volatile components in jasmine tea during scenting process. J. Tea Commun. 2020, 47, 67–74. [Google Scholar]
- An, H.M.; Ou, X.C.; Xiong, Y.F.; Zhang, Y.B.; Li, J.; Li, Q.; Li, Q.; Li, S.; Huang, J.A. Study on the characteristic aroma components of jasmine tea. J. Tea Sci. 2020, 40, 225–237. [Google Scholar]
- Fang, S.H.; Xu, G.Q.; Xia, T.; Li, L.X.; Wan, X.C. Study on the correlation between the principal factors during scenting process for jasmine tea. J. Tea Sci. 2003, 23, 21–26. [Google Scholar]
- Chen, G.J. Key technology of scenting jasmine tea. China Tea 2011, 33, 19–20. [Google Scholar]
- Zheng, N.H.; Ye, N.X.; Wang, Z.K.; Yang, G.; Yang, J.F. Effect of wet fumigation on the quality of jasmine tea. J. Fujian Agric. For. Univ. 2006, 35, 372–376. [Google Scholar]
- Ye, N.X.; Yang, G.; Zheng, N.H.; Yang, J.F.; Wang, Z.K.; Liang, X.X. Effect of wet-scenting process and RJF on the aroma constituent of jasmine scented tea. J. Tea Sci. 2006, 26, 65–71. [Google Scholar]
- Di, Y.J. Research progress on the Quality formation mechanism of jasmine tea. Acta Tea Sin. 2008, 2, 1–4. [Google Scholar]
- Chen, M.C.; Zhu, Y.J.; Liu, X.G.; Chen, Z.; Liu, B. Characteristic of aroma compounds of Jasminum sambac (L.) Aiton. Chin. J. Trop. Crops 2017, 38, 747–751. [Google Scholar]
- Sheibani, E.; Duncan, S.E.; Kuhn, D.D.; Dietrich, A.M.; O’Keefe, S.F. SDE and SPME Analysis of Flavor Compounds in Jin Xuan Oolong Tea. J. Food Sci. 2016, 81, C348–C358. [Google Scholar] [CrossRef]
- Lu, J.; Fu, D.J.; Wu, D.F.; Liu, Y.; Luo, Y.P. Analysis of Volatile Compounds of Jasmine Scented Tea during the Orthodox Manufacturing Process using HS-SPME/GC-MS. J. Korean Tea Soc. 2015, 21, 132–140. [Google Scholar]
- As, Y.I.; Kakuda, T.; Kubota, K. Identification of Potent Odorants in Chinese Jasmine Green Tea Scented with Flowers of Jasminum sambac. J. Agric. Food Chem. 2002, 50, 4878–4884. [Google Scholar]
- Lu, J. Research on the Changes of Volatile Compounds of Jasmine Tea during the Scenting Process and Technology Improvement. Master’s Thesis, Zhejiang University, Zhejiang, China, 2015. [Google Scholar]
- Lu, J.; Wang, D.; Zhu, J.J.; Fu, D.J.; Wu, D.F.; Liu, Y.; Luo, Y.P. Effects main factors on the volatile compounds of jasmine scented tea during the isolated scenting process. J. Chin. Inst. Food Sci. Technol. 2019, 19, 65–74. [Google Scholar]
- Li, X.S. Optimization of the Processing Technology for Jasmine Tea from Rongxian County. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2013. [Google Scholar]
- Huang, B.Y. Discussion on technical problems of continuous scenting of jasmine tea. China Tea Process. 2003, 2, 14–15. [Google Scholar]
- Deng, Y.; Jia, X.; Liang, L.; Gu, C.; Sun, X. Morphological anatomy, sporogenesis and gametogenesis in flowering process of jasmine (Jasminum sambac Aiton). Sci. Hortic. 2016, 198, 257–266. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, X.; Gu, C.; Jia, X.; Liang, L.; Su, J. Identification of pre-fertilization reproductive barriers and the underlying cytological mechanism in crosses among three petal-types of Jasminum sambac and their relevance to phylogenetic relationships. PLoS ONE 2017, 12, e0176026. [Google Scholar] [CrossRef]
- Deng, Y.; Shao, Q.; Li, C.; Ye, X.; Tang, R. Differential responses of double petal and multi petal jasmine to shading: II. Morphology, anatomy and physiology. Sci. Hortic. 2012, 144, 19–28. [Google Scholar] [CrossRef]
- Zhang, L.X.; Wang, R.W. Research progress on the aroma of jasmine. Tea Fujian 1999, 2, 4–7. [Google Scholar]
- Ross, J.R.; Nam, K.H.; D’Auria, J.C.; Pichersky, E. S-Adenosyl-l-Methionine:Salicylic Acid Carboxyl Methyltransferase, an Enzyme Involved in Floral Scent Production and Plant Defense, Represents a New Class of Plant Methyltransferases. Arch. Biochem. Biophys. 1999, 367, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.; Ping, Z.; Honglei, T.; Peng, W.; Cong, L.; Yu, Z.; Ruijie, N.; Yuyu, Z. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC–MS, OAV, and multivariate analysis. Food Chem. 2021, 345, 128748. [Google Scholar]
- De la Fuente-Blanco, A.; Saenz-Navajas, M.P.; Valentin, D.; Ferreira, V. Fourteen ethyl esters of wine can be replaced by simpler ester vectors without compromising quality but at the expense of increasing aroma concentration. Food Chem. 2020, 307, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, H.H.; Zhou, S.H. Analysis of fragrance characterisric in three Congou black teas of highly fragrant species using odor active values. Food Res. Dev. 2020, 41, 184–191. [Google Scholar]
| No. | Time | Benzyl Butyrate | Benzyl Acetate | N-Hexyl benzoate | Methyl Benzoate | Ethyl Benzoate | Methyl 2-Aminobenzoate | Phytol Acetate | α- Farnesene | Linalool | Cis-3-hexanol | Benzyl Alcohol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JF | 0 | — | ↑ | ↑ | ↑ | — | ↑ | ↑ | — | — | ↑ | ↑ |
| 2 | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | |
| 4 | ↓ | ↓ | — | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ↑ | ↓ | |
| 6 | ↓ | ↓ | ↑ | ↓ | — | ↓ | ↓ | ↑ | ↓ | ↑ | ↓ | |
| 8 | ↓ | ↓ | ↑ | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ↑ | — | |
| 10 | — | ↓ | ↑ | — | ↓ | — | ↓ | ↓ | ↓ | ↓ | — | |
| 12 | — | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | — | |
| 14 | — | ↓ | ↑ | ↓ | ↓ | ↓ | — | ↓ | ↓ | ↓ | — | |
| NP | 0 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | / | ↑ | ↑ | ↑ | ↑ |
| 2 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | / | ↑ | ↑ | ↑ | ↑ | |
| 4 | ↑ | ↑ | ↑ | ↓ | — | ↑ | / | ↑ | ↑ | ↑ | ↓ | |
| 6 | ↓ | ↑ | ↓ | — | ↑ | ↑ | / | ↑ | ↑ | ↑ | ↓ | |
| 8 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | / | ↓ | ↑ | ↓ | — | |
| 10 | ↓ | ↓ | — | ↓ | ↓ | ↑ | / | ↓ | ↑ | ↓ | — | |
| 12 | ↓ | ↓ | — | ↓ | ↓ | ↓ | / | ↓ | ↑ | ↓ | — | |
| 14 | ↓ | ↓ | — | ↓ | ↓ | ↓ | / | ↓ | ↑ | ↓ | ↓ | |
| TP | 0 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↑ | ↓ |
| 2 | ↑ | ↑ | — | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | — | ↓ | |
| 4 | ↑ | — | ↑ | — | — | ↑ | ↑ | — | ↓ | ↑ | — | |
| 6 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | — | ↑ | ↓ | |
| 8 | ↓ | ↓ | ↓ | — | — | ↑ | — | ↓ | ↓ | — | ↓ | |
| 10 | ↓ | ↓ | ↓ | — | ↑ | ↑ | ↑ | ↓ | — | ↑ | ↓ | |
| 12 | ↓ | — | ↓ | — | ↓ | — | ↑ | — | — | ↑ | ↓ | |
| 14 | — | — | ↓ | ↓ | ↓ | ↓ | ↓ | — | ↓ | ↓ | ↓ | |
| NPT | 0 | — | — | — | — | — | — | — | — | — | / | — |
| 2 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | / | ↑ | |
| 4 | ↑ | — | ↑ | ↑ | — | ↑ | ↑ | — | ↑ | / | — | |
| 6 | ↑ | ↑ | — | ↑ | ↑ | ↑ | ↑ | — | ↑ | / | ↑ | |
| 10 | — | ↑ | — | ↑ | — | ↑ | ↑ | — | — | / | — | |
| 12 | ↑ | ↓ | — | ↑ | — | ↑ | ↑ | ↓ | ↓ | / | — | |
| 14 | ↓ | — | — | ↓ | — | ↑ | ↑ | — | ↓ | / | ↑ | |
| F | ↓ | — | — | ↓ | ↑ | — | ↓ | ↓ | — | / | ↓ | |
| TPT | 0 | — | — | — | ↑ | — | — | — | ↑ | ↑ | / | ↑ |
| 2 | ↑ | ↑ | ↑ | — | ↑ | ↑ | — | ↓ | ↓ | / | ↑ | |
| 4 | ↑ | ↑ | ↑ | — | ↑ | ↑ | ↑ | ↓ | ↓ | / | ↑ | |
| 6 | ↑ | — | — | — | ↑ | ↑ | ↑ | ↓ | ↓ | / | ↑ | |
| 8 | ↑ | — | ↑ | — | ↑ | ↑ | ↑ | ↓ | ↓ | / | ↓ | |
| 10 | ↓ | — | ↑ | — | ↑ | ↑ | ↑ | ↓ | ↓ | / | ↓ | |
| 12 | ↓ | ↓ | ↑ | ↓ | ↑ | ↑ | ↓ | ↓ | ↓ | / | ↓ | |
| 14 | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | / | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xiong, Y.; An, H.; Li, J.; Li, Q.; Huang, J.; Liu, Z. Analysis of Volatile Components of Jasmine and Jasmine Tea during Scenting Process. Molecules 2022, 27, 479. https://doi.org/10.3390/molecules27020479
Zhang Y, Xiong Y, An H, Li J, Li Q, Huang J, Liu Z. Analysis of Volatile Components of Jasmine and Jasmine Tea during Scenting Process. Molecules. 2022; 27(2):479. https://doi.org/10.3390/molecules27020479
Chicago/Turabian StyleZhang, Yangbo, Yifan Xiong, Huimin An, Juan Li, Qin Li, Jianan Huang, and Zhonghua Liu. 2022. "Analysis of Volatile Components of Jasmine and Jasmine Tea during Scenting Process" Molecules 27, no. 2: 479. https://doi.org/10.3390/molecules27020479
APA StyleZhang, Y., Xiong, Y., An, H., Li, J., Li, Q., Huang, J., & Liu, Z. (2022). Analysis of Volatile Components of Jasmine and Jasmine Tea during Scenting Process. Molecules, 27(2), 479. https://doi.org/10.3390/molecules27020479