Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials
Abstract
:1. Introduction
2. Results and Discussion
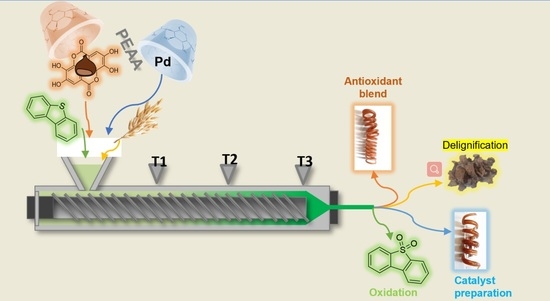
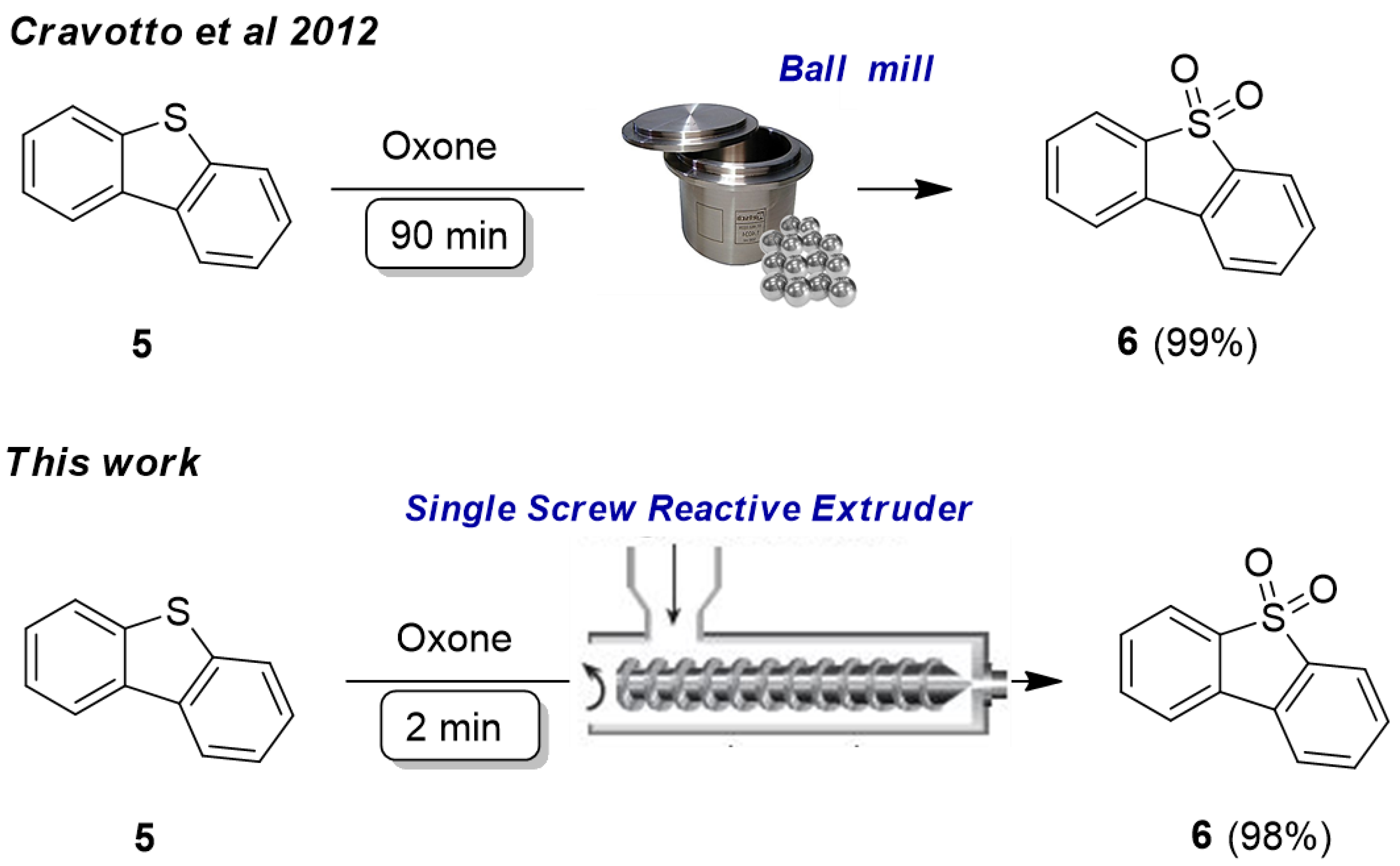
2.1. Single Screw Reactive Extruder (SSRE): Organic Synthesis Applications
2.2. SSRE: Polymer Applications
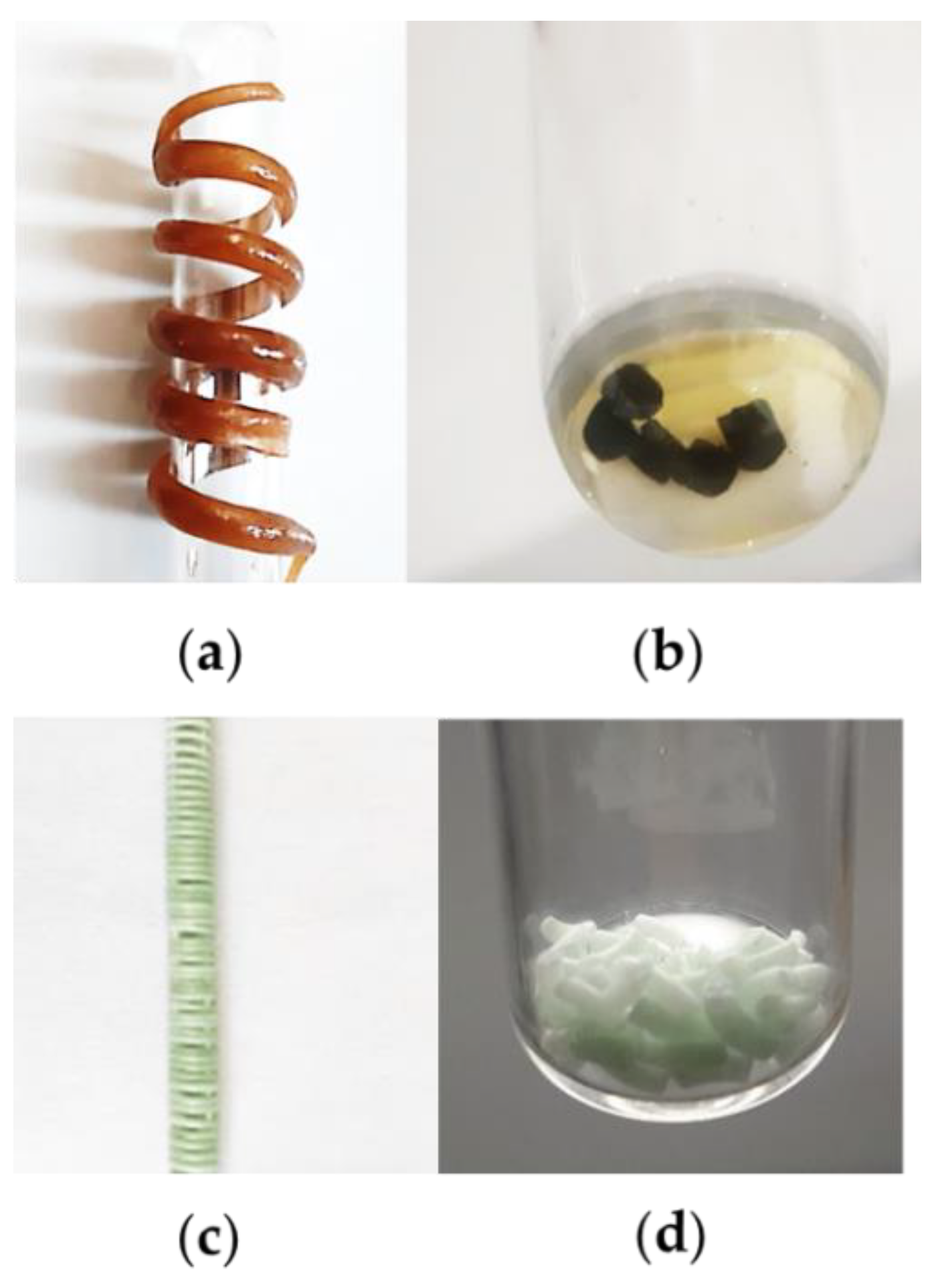
2.2.1. Blended Polymer with Polyphenols Extracted from Residual Agri-Food Waste
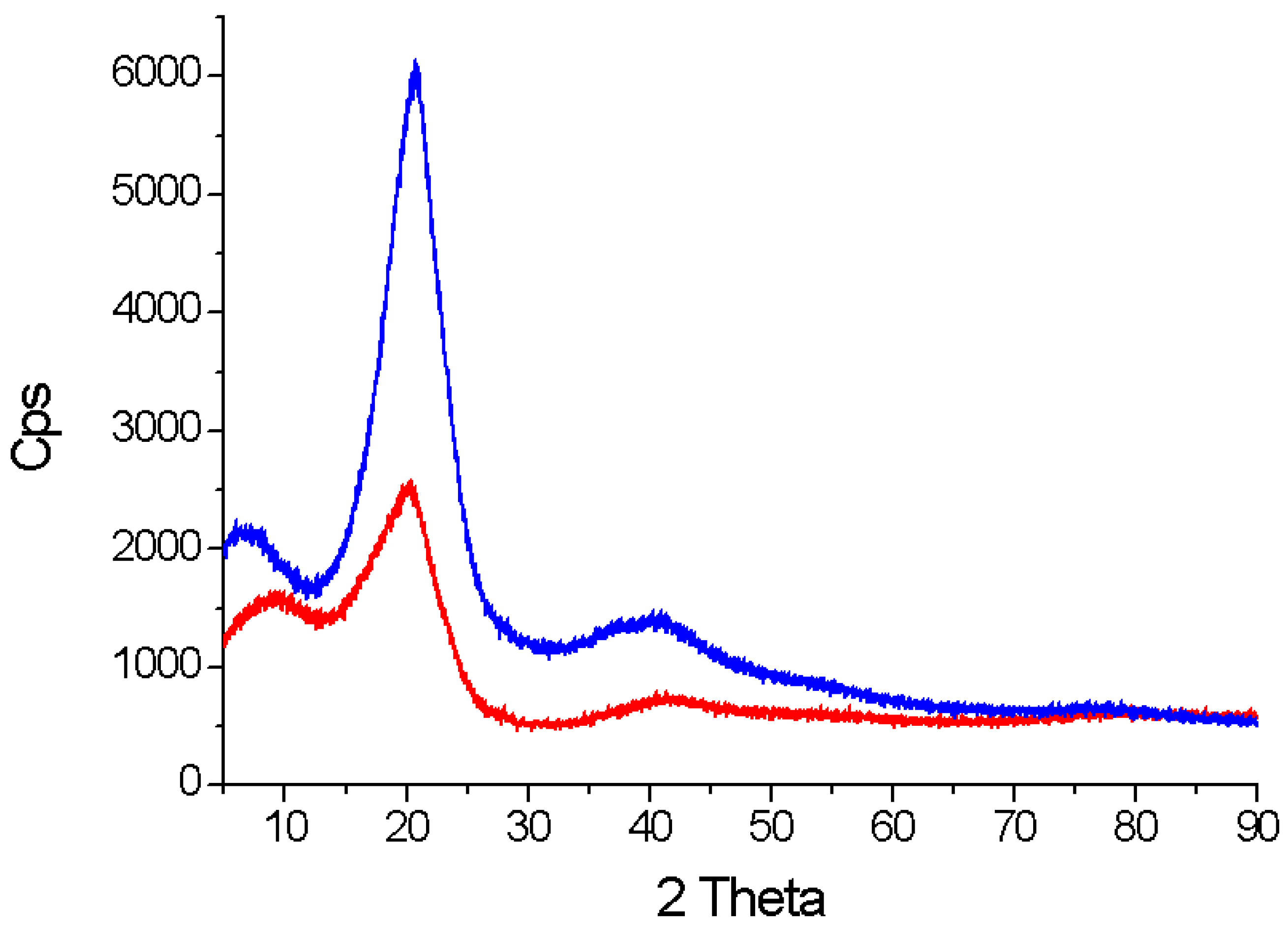
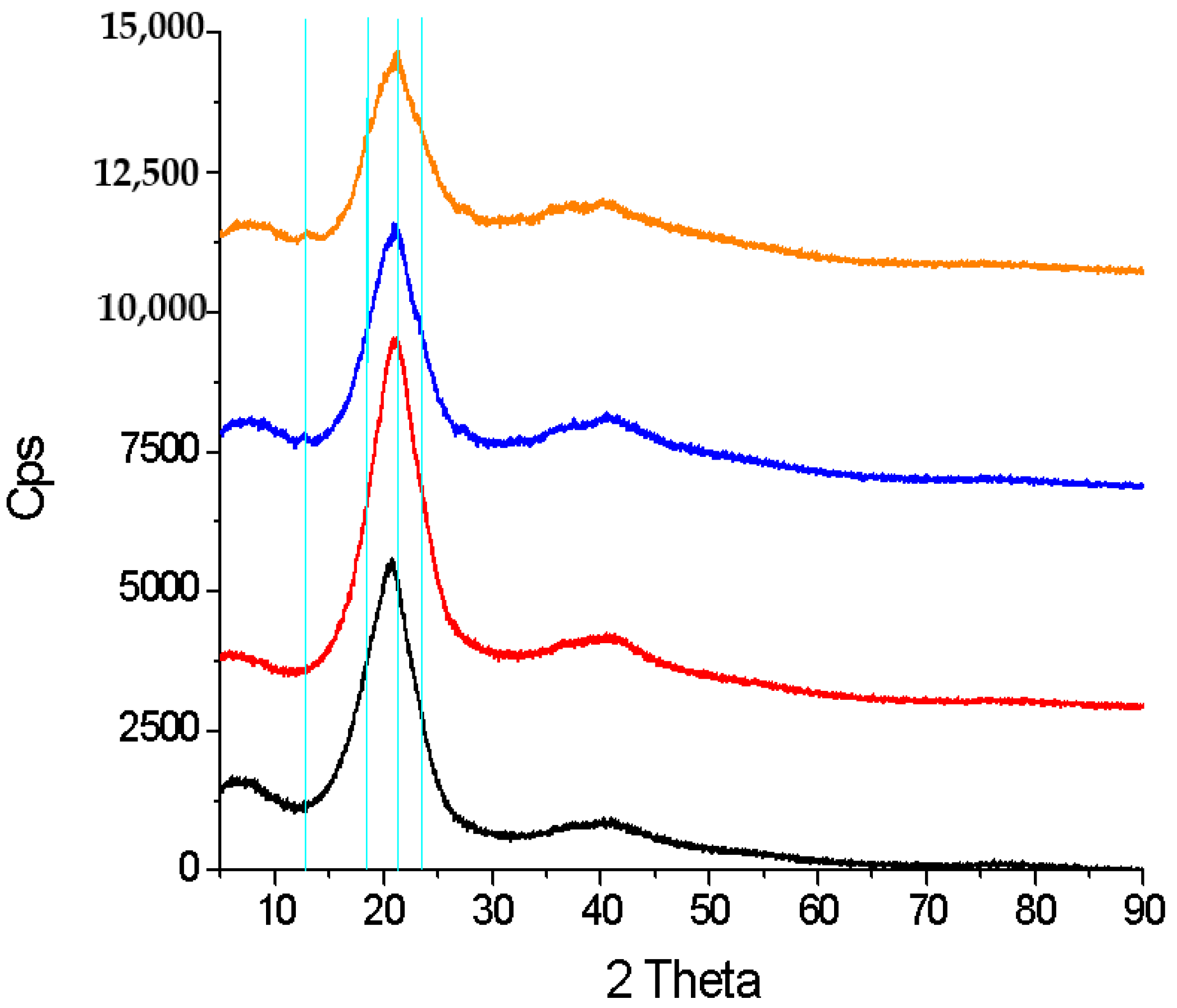
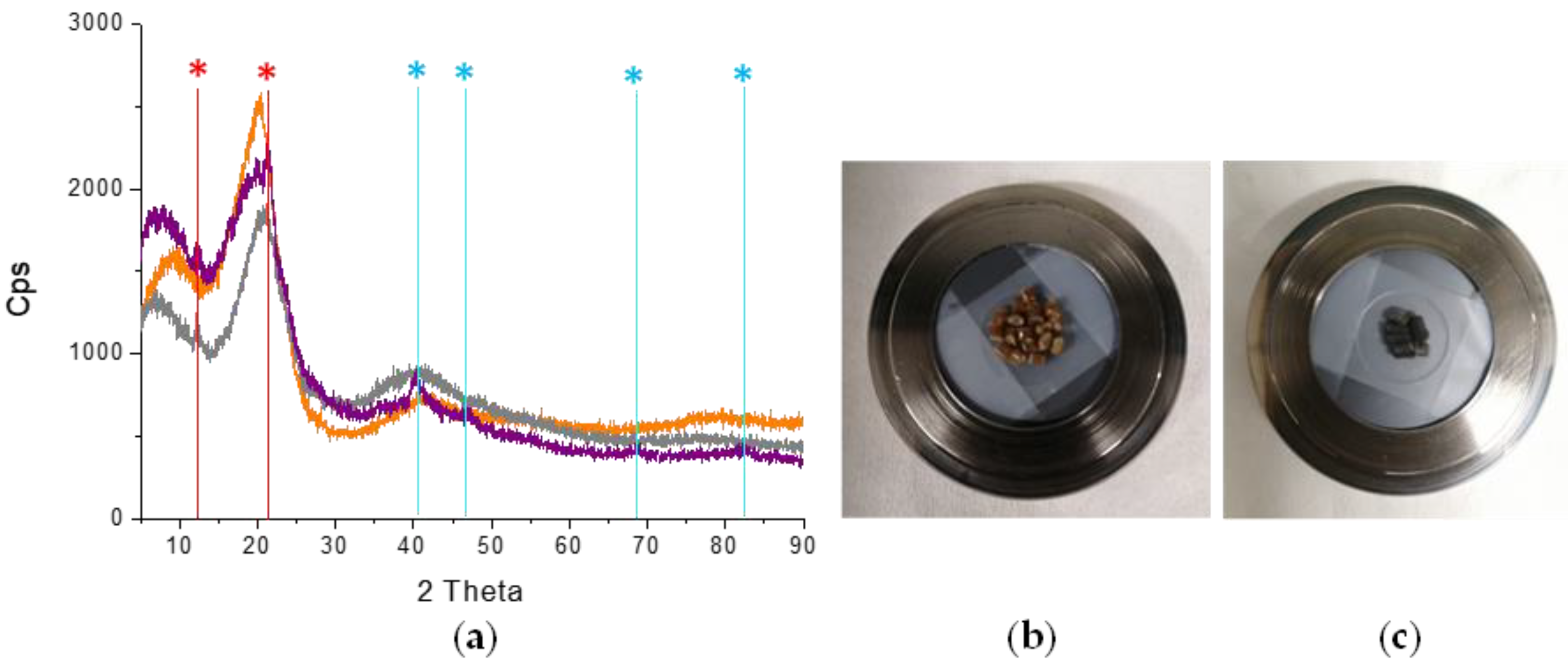
2.2.2. SSRE Preparation of Palladium Catalytic Polymer
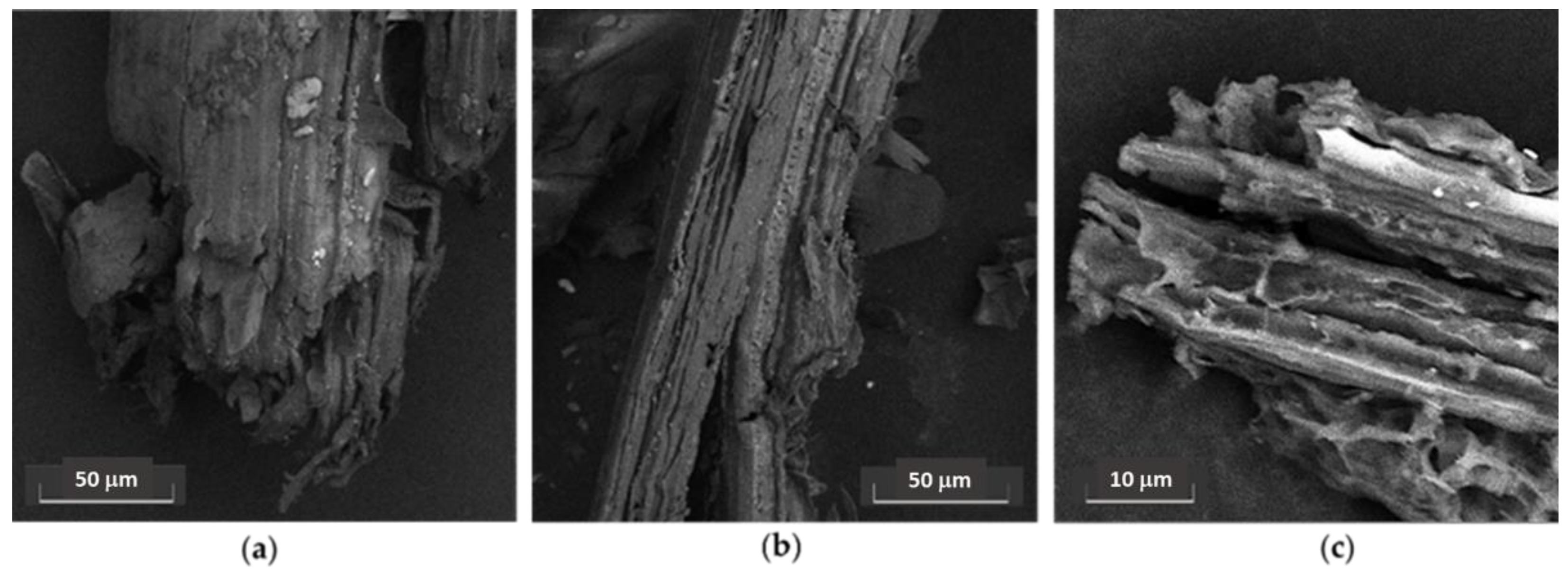
2.3. SSRE Biomass Delignification
3. Material and Methods
3.1. Materials
3.2. General Procedure for the Solvent-Free Oxidation of Organic Compounds
3.3. Preparation of the β-CD/CPPE Inclusion Complex
3.4. MW-Assisted Nitrobenzene Hydrogenation Using the Pd/B-CD-PEAA Polymer
3.5. Biomass Pretreatment
3.6. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Crawford, D.; Casaban, J.; Haydon, R.; Giri, N.; McNally, T.; James, S.L. Synthesis by extrusion: Continuous, large-scale preparation of MOFs using little or no solvent. Chem. Sci. 2015, 6, 1645–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; García, F. Main group mechanochemistry: From curiosity to established protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef] [Green Version]
- Howard, J.L.; Cao, Q.; Browne, D.B. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, J.G.; Bolm, C. Altering Product Selectivity by Mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. [Google Scholar] [CrossRef]
- Andersen, J.; Mack, J. Mechanochemistry and organic synthesis: From mystical to practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Crawford, D.E.; Casaban, J. Recent Developments in Mechanochemical Materials Synthesis by Extrusion. Adv. Mater. 2016, 28, 5747–5754. [Google Scholar] [CrossRef]
- Xu, C.P.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [Green Version]
- Cova, C.M.; Luque, R. Advances in mechanochemical processes for biomass valorization. BMC Chem. Eng. 2019, 1, 16. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono- and mechanochemical technologies in the catalytic conversion of biomass. Chem. Soc. Rev. 2021, 50, 1785–1812. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. Metal- Mediated and Metal-Catalyzed Reactions under Mechanochemical Conditions. ACS Catal. 2020, 10, 8344–8394. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical Synthesis of Catalytic Materials. Chem. Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Gajda, M.; Nartowski, K.P.; Pluta, J.; Karolewicz, B. Continuous, one-step synthesis of pharmaceutical cocrystals via hot melt extrusion from neat to matrix-assisted processing–State of the art. Int. J. Pharm. 2019, 558, 426–440. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward green synthesis of metal–organic frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Hasa, D.; Jones, W. Screening for new pharmaceutical solid forms using mechanochemistry: A practical guide. Adv. Drug Deliv. Rev. 2017, 117, 147–161. [Google Scholar] [CrossRef]
- Gomollón-Bel, F. Ten Chemical Innovations That Will Change Our World: IUPAC identifies emerging technologies in Chemistry with potential to make our planet more sustainable. Chem. Int. 2019, 41, 12–17. [Google Scholar] [CrossRef]
- Stolle, A.; Schmidt, R.; Jacob, K. Scale-up of organic reactions in ball mills: Process intensification with regard to energy efficiency and economy of scale. Faraday Discuss. 2014, 170, 267–286. [Google Scholar] [CrossRef]
- Maurice, D.R.; Courtney, T.H. The physics of mechanical alloying: A first report. Metall. Trans. A 1990, 21, 289–303. [Google Scholar] [CrossRef]
- Rippin, D.W.T. Design and operation of multiproduct and multipurpose batch chemical plants.—An analysis of problem structure. Comput. Chem. Eng. 1983, 7, 463–481. [Google Scholar] [CrossRef]
- Wagner, J.R., Jr.; Mount, E.M., III; Giles, H.F., Jr. Extrusion the Definative Processing Guide and Handbook, 2nd ed.; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Martín-Matute, B.; Meier, M.A.R.; Métro, T.X.; Koenig, S.G.; Sneddon, H.F.; Sudarsanam, P.; Watts, P. Sustainable Chemistry and Engineering in Pharma. ACS Sustain. Chem. Eng. 2021, 9, 13395–13398. [Google Scholar] [CrossRef]
- Crawford, D.E.; Miskimmin, C.K.G.; Albadarin, A.B.; Walker, G.; James, S.L. Organic synthesis by Twin Screw Extrusion (TSE): Continuous, scalable and solvent-free. Green Chem. 2017, 19, 1507–1518. [Google Scholar] [CrossRef] [Green Version]
- Blackmore, H.L. Christie’s Dictionary of London Gunmakers; Phaidon: London, UK, 1986. [Google Scholar]
- Rauwendaal, C.J. Understanding Extrusion, 2nd ed.; Hanser Gardner Publications: London, UK, 2010. [Google Scholar]
- Tumuluri, V.S.; Kemper, M.S.; Lewis, I.R.; Prodduturi, S.; Majumdar, S.; Avery, B.A.; Repka, M.A. Off-line and on-line measurements of drug-loaded hot-melt extruded films using Raman spectroscopy. Int. J. Pharm. 2008, 357, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Ezat, G.S.; Kelly, A.L.; Youseffi, M.; Coates, P.D. Effect of screw configuration on the dispersion and properties of polypropylene/multiwalled carbon nanotube composite. Polym. Compos. 2019, 40, 4196–4204. [Google Scholar] [CrossRef]
- Li, C.C.; Pan, S.J.; Xu, W.J.; Lu, Y.B.; Wang, P.P.; Zhang, F.M.; Gross, R.A. Lipase-catalyzed ring-opening copolymerization of omega-pentadecalactone and delta-valerolactone by reactive extrusion. Green Chem. 2020, 22, 662–668. [Google Scholar] [CrossRef]
- Li, T.T.; Feng, L.F.; Gu, X.P.; Zhang, C.L.; Wang, P.; Hu, G.H. Intensification of Polymerization Processes by Reactive Extrusion. Ind. Eng. Chem. Res. 2021, 60, 2791–2806. [Google Scholar] [CrossRef]
- Franzoso, F.; Causone, D.; Tabasso, S.; Antonioli, D.; Montoneri, E.; Persico, P.; Laus, M.; Mendichi, R.; Negre, M. Films Made from Poly Ethylene-Co-Acrylic Acid and Soluble Biopolymers Sourced from Agricultural and Municipal Biowaste. J. Appl. Polym. Sci. 2015, 132, 41909. [Google Scholar] [CrossRef]
- Brennan, M.A.; Monro, J.A.; Brennan, C.S. Effect of inclusion of soluble and insoluble fibres into extruded breakfast cereal products made with reverse screw configuration. Int. J. Food Sci. Technol. 2008, 43, 2278–2288. [Google Scholar] [CrossRef]
- Ye, J.; Luo, S.; Huang, A.; Chen, J.; Liu, C.; McClements, D.J. Synthesis and characterization of citric acid esterified rice starch by reactive extrusion: A new method of producing resistant starch. Food Hydrocoll. 2019, 92, 135–142. [Google Scholar] [CrossRef]
- Simões, M.F.; Pinto, R.M.A.; Simões, S. Hot-melt extrusion in the pharmaceutical industry: Toward filing a new drug application. Drug Discov. Today 2019, 24, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.E.; Miskimmin, C.K.; Cahir, J.; James, S.L. Continuous multi-step synthesis by extrusion–telescoping solvent-free reactions for greater efficiency. Chem. Commun. 2017, 53, 13067–13070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, D.E.; Porcheddu, A.; McCalmont, A.S.; Delogu, F.; James, S.-L.; Colacino, E. Solvent-Free, Continuous Synthesis of Hydrazone-Based Active Pharmaceutical Ingredients by Twin-Screw Extrusion. ACS Sustain. Chem. Eng. 2020, 8, 12230–12238. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.; Battu, S.K.; James, W.; McGinity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Jeremias, F.; Frhlich, D.; Janiak, C.; Henniger, S.K. Advancement of sorption-based heat transformation by a metal coating of highly-stable, hydrophilic aluminium fumarate MOF. RSC Adv. 2014, 4, 24073–24082. [Google Scholar] [CrossRef] [Green Version]
- Cravotto, G.; Garella, D.; Carnaroglio, D.; Calcio Gaudino, E.; Rosati, O. Solvent-free chemoselective oxidation of thioethers and thiophenes by mechanical milling. Chem. Commun. 2012, 48, 11632–11634. [Google Scholar] [CrossRef]
- Bolm, C.; Magnus, A.S.; Hildebrand, J.P. Catalytic Synthesis of Aldehydes and Ketones under Mild Conditions Using TEMPO/Oxone. Org. Lett. 2000, 2, 1173–1175. [Google Scholar] [CrossRef]
- Fields, J.D.; Kropp, P.J. Surface-Mediated Reactions. 9. Selective Oxidation of Primary and Secondary Amines to Hydroxylamines. J. Org. Chem. 2000, 65, 5937–5941. [Google Scholar] [CrossRef]
- Uyanik, M.; Akakura, M.; Ishihara, K. 2-Iodoxybenzenesulfonic Acid as an Extremely Active Catalyst for the Selective Oxidation of Alcohols to Aldehydes, Ketones, Carboxylic Acids, and Enones with Oxone. J. Am. Chem. Soc. 2009, 131, 251–262. [Google Scholar] [CrossRef]
- Hashimoto, N.; Kanda, A. Practical and Environmentally Friendly Epoxidation of Olefins Using Oxone. Org. Process Res. Dev. 2002, 6, 405–406. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.G.; Wong, E.L.M.; Xiang, S.; Che, C.M. Iron oligopyridine complexes as efficient catalysts for practical oxidation of arenes, alkanes, tertiary amines and N-acyl cyclic amines with Oxone. Chem. Sci. 2011, 2, 2187–2195. [Google Scholar] [CrossRef]
- Collom, S.L.; Anastas, P.T.; Beach, E.S.; Crabtree, R.H.; Hazari, N.; Sommer, T.J. Differing selectivities in mechanochemical versus conventional solution oxidation using Oxone. Tetrahedron Lett. 2013, 54, 2344–2347. [Google Scholar] [CrossRef]
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating Antioxidant Functionality into Polymer Materials: Fundamentals, Strategies, and Applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395. [Google Scholar] [CrossRef] [PubMed]
- Fanta, G.F.; Swanson, C.L.; Doane, W.M. Composites of starch and poly(ethylene-co-acrylic acid). Complexing between polymeric components. J. Appl. Polym. Sci. 1990, 40, 811–821. [Google Scholar] [CrossRef]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Thangavel, K. Synthesis and characterization of nano-encapsulated catechin by molecular inclusion with beta-cyclodextrin. J. Food. Eng. 2012, 111, 255–264. [Google Scholar] [CrossRef]
- Rubin Pedrazzo, A.; Cecone, C.; Trotta, F.; Zanetti, M. Mechanosynthesis of β-Cyclodextrin Polymers Based on Natural Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 14881–14889. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Carnaroglio, D.; Martina, K.; Palmisano, G.; Penoni, A.; Cravotto, G. Highly Efficient Microwave-Assisted CO Aminocarbonylation with a Recyclable Pd(II)/TPP-β-Cyclodextrin Cross-Linked Catalyst. Org. Process Res. Dev. 2015, 19, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Calcio Gaudino, E.; Tagliapietra, S.; Palmisano, G.; Martina, K.; Carnaroglio, D.; Cravotto, G. Microwave-Assisted, Green Synthesis of 4(3H)-Quinazolinones under CO Pressure in γ-Valerolactone and Reusable Pd/β-Cyclodextrin Cross-Linked Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 9233–9243. [Google Scholar] [CrossRef]
- Tabasso, S.; Calcio Gaudino, E.; Acciardo, E.; Manzoli, M.; Bonelli, B.; Cravotto, G. Microwave-Assisted Protocol for Green Functionalization of Thiophenes with a Pd/β-Cyclodextrin Cross-Linked Nanocatalyst. Front. Chem. 2020, 8, 253. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Backhouse, T.; Bradford, A.; Saunders, F.; Bourne, R.A.; Chamberlain, T.W.; Stones, R.; Clayton, A.; Lovelock, K. Highly efficient aqueous phase reduction of nitroarenes catalyzed by phosphine-decorated polymer immobilized ionic liquid stabilized PdNPs. Catal. Sci. Technol. 2018, 8, 1454–1467. [Google Scholar] [CrossRef]
- Tocco, D.; Carucci, C.; Monduzzi, M.; Salis, A.; Sanjust, E. Recent Developments in the Delignification and Exploitation of Grass Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 2412–2432. [Google Scholar] [CrossRef]
- Verdini, F.; Calcio Gaudino, E.; Grillo, G.; Tabasso, S.; Cravotto, G. Cellulose Recovery from Agri-Food Residues by Effective Cavitational Treatments. Appl. Sci. 2021, 11, 4693. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a pretreatment for lignocellulosic biomass: Fundamentals and applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Ali, M.F.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G. Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for improving enzymatic hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef]
- Genevini, P.; Adani, F.; Villa, C. Rice hull degradation by co-composting with dairy cattle slurry. Soil Sci. Plant. Nutr. 1997, 43, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Lauberte, L.; Telysheva, G.; Cravotto, G.; Andersone, A.; Janceva, S.; Dizhbite, T.; Arshanitsa, A.; Jurkjane, V.; Vevere, L.; Grillo, G.; et al. Lignin-Derived antioxidants as value-added products obtained under cavitation treatments of the wheat straw processing for sugar production. J. Clean. Prod. 2021, 303, 126369–126380. [Google Scholar] [CrossRef]
- Grillo, G.; Calcio Gaudino, E.; Rosa, R.; Leonelli, C.; Timonina, A.; Grygiškis, S.; Tabasso, S.; Cravotto, G. Green Deep Eutectic Solvents for Microwave-Assisted Biomass Delignification and Valorisation. Molecules 2021, 26, 798. [Google Scholar] [CrossRef]



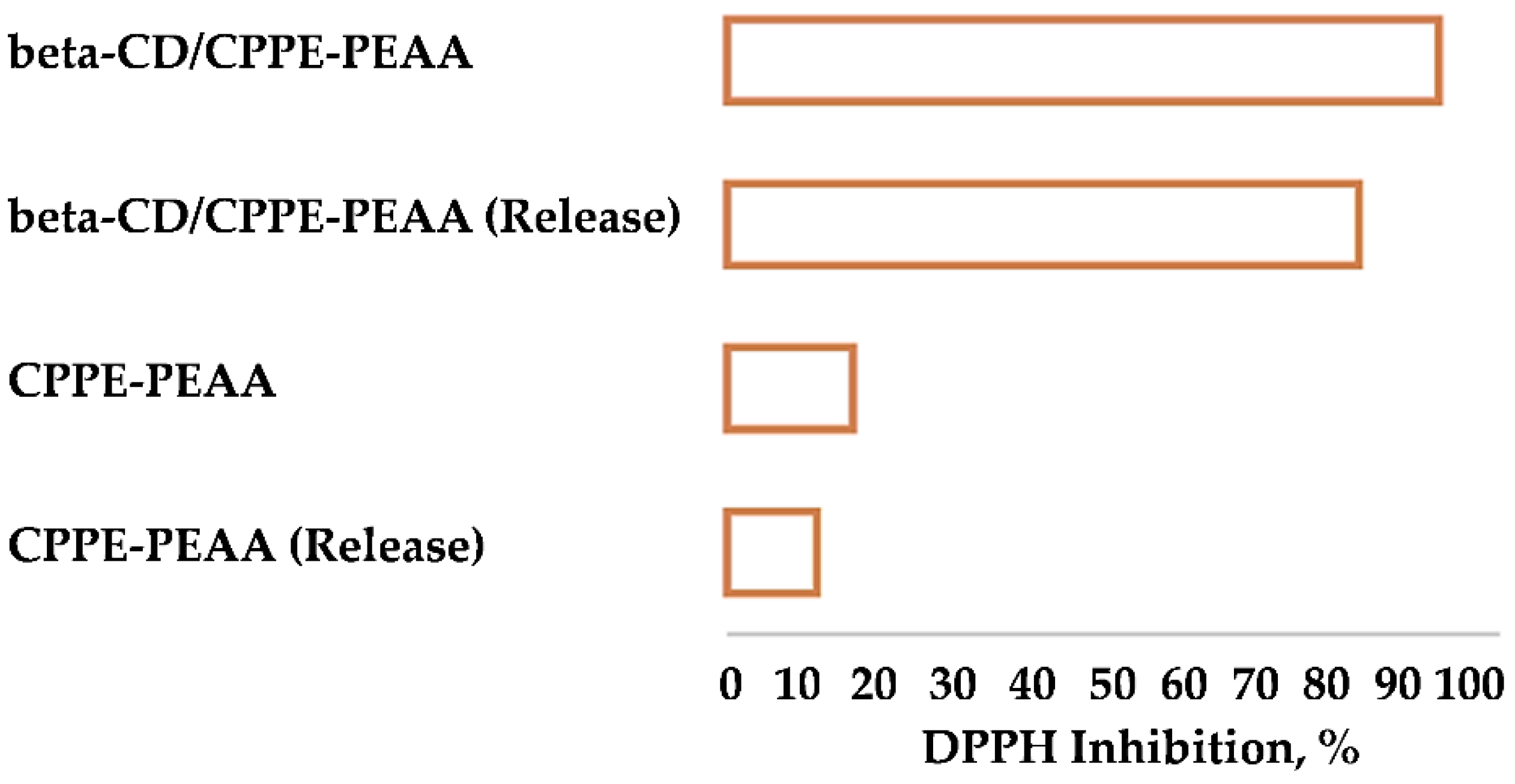
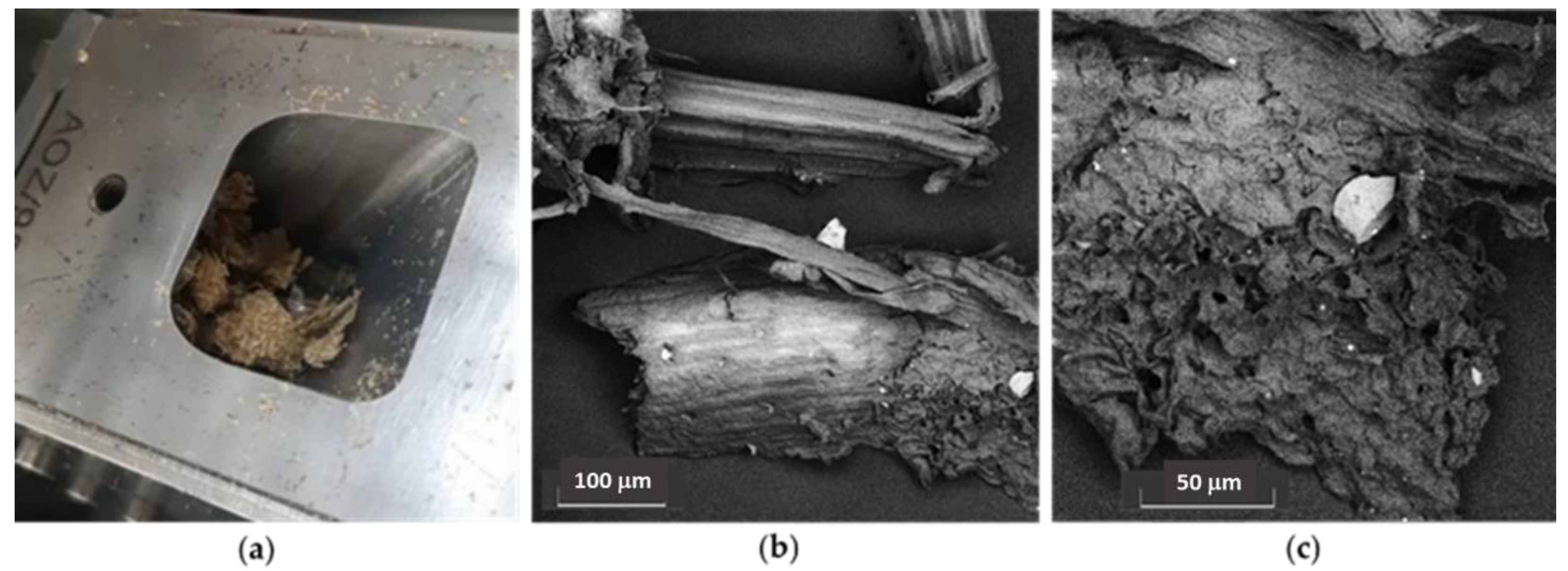

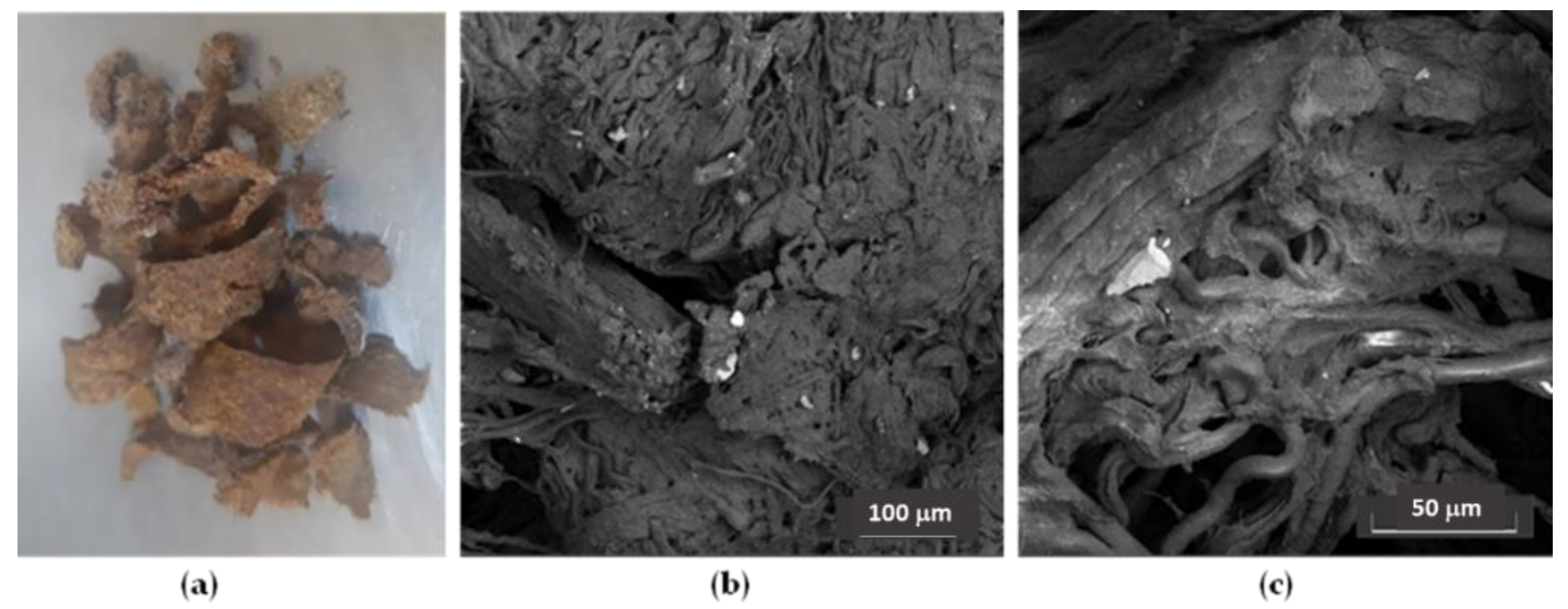
| Samples | EC50 (mg/mL) | Trolox eq. (µmol/g EXT) |
|---|---|---|
| CPPE | 0.14 | 112.44 |
| β-CD/CPPE-PEAA | 0.48 | 32.80 |
 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Time (min) | T (°C) | Yield (%) a | Selectivity (%) |
| 1 | EtOH | 18 h | RT | 100 | 100 |
| 2 | i-PrOH | 18 h | RT | 100 | 100 |
| 3 | Toluene | 18 h | RT | 100 | 100 |
| 4 c | EtOH | 15 | 60 | 29.91 | 91.8 |
| 5 c | EtOH | 30 | 60 | 65.2 | 65.2 b |
| 6 c | i-PrOH | 15 | 60 | 10.0 | 100 |
| 7 c | i-PrOH | 30 | 60 | 22.1 | 99 |
| 8 c | Toluene | 15 | 60 | 10.3 | 100 |
| 9 c | Toluene | 30 | 60 | 88.3 | 98.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcio Gaudino, E.; Grillo, G.; Manzoli, M.; Tabasso, S.; Maccagnan, S.; Cravotto, G. Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules 2022, 27, 449. https://doi.org/10.3390/molecules27020449
Calcio Gaudino E, Grillo G, Manzoli M, Tabasso S, Maccagnan S, Cravotto G. Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules. 2022; 27(2):449. https://doi.org/10.3390/molecules27020449
Chicago/Turabian StyleCalcio Gaudino, Emanuela, Giorgio Grillo, Maela Manzoli, Silvia Tabasso, Simone Maccagnan, and Giancarlo Cravotto. 2022. "Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials" Molecules 27, no. 2: 449. https://doi.org/10.3390/molecules27020449
APA StyleCalcio Gaudino, E., Grillo, G., Manzoli, M., Tabasso, S., Maccagnan, S., & Cravotto, G. (2022). Mechanochemical Applications of Reactive Extrusion from Organic Synthesis to Catalytic and Active Materials. Molecules, 27(2), 449. https://doi.org/10.3390/molecules27020449