New Analogs of Polyamine Toxins from Spiders and Wasps: Liquid Phase Fragment Synthesis and Evaluation of Antiproliferative Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
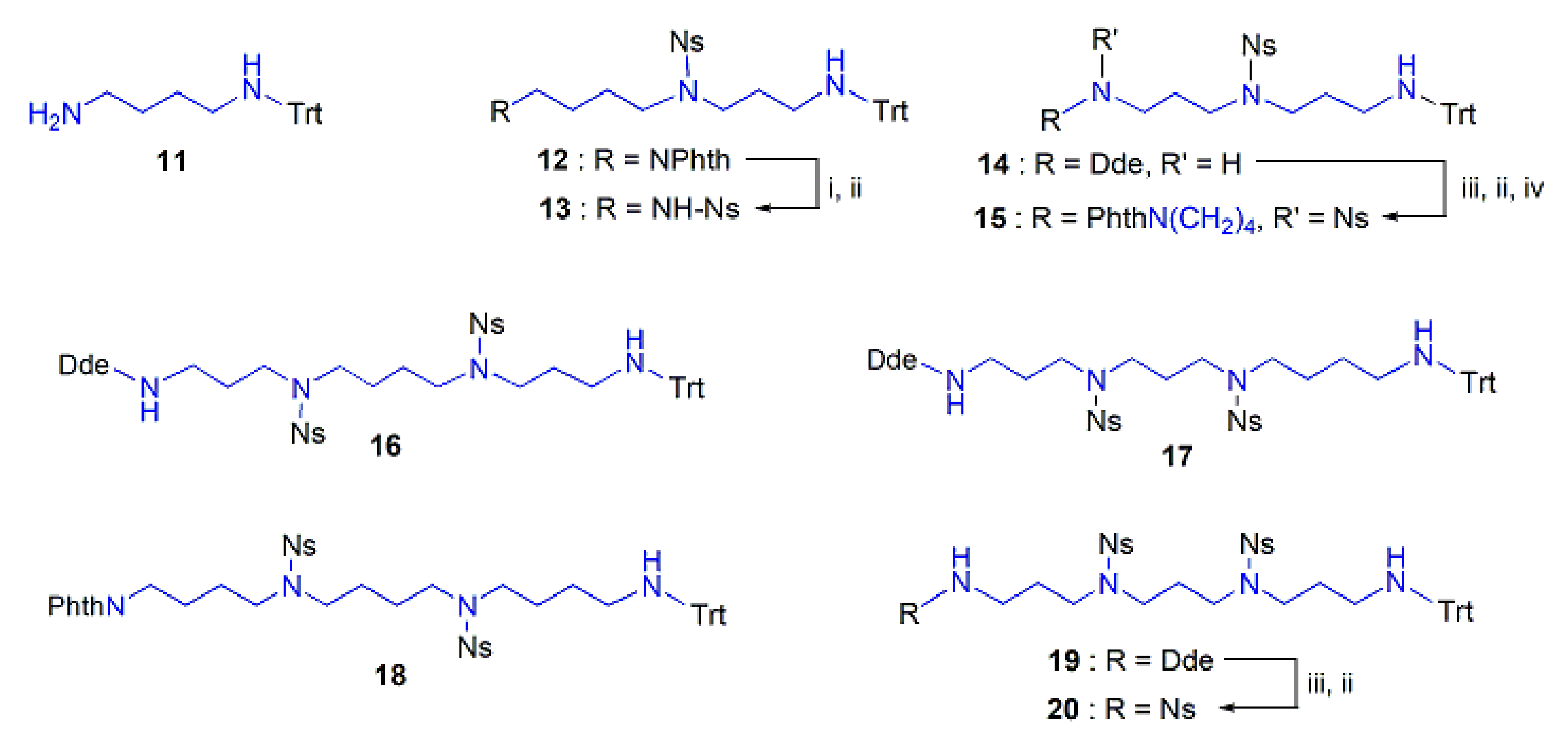
2.1.1. The Selectively Protected PA Skeleta
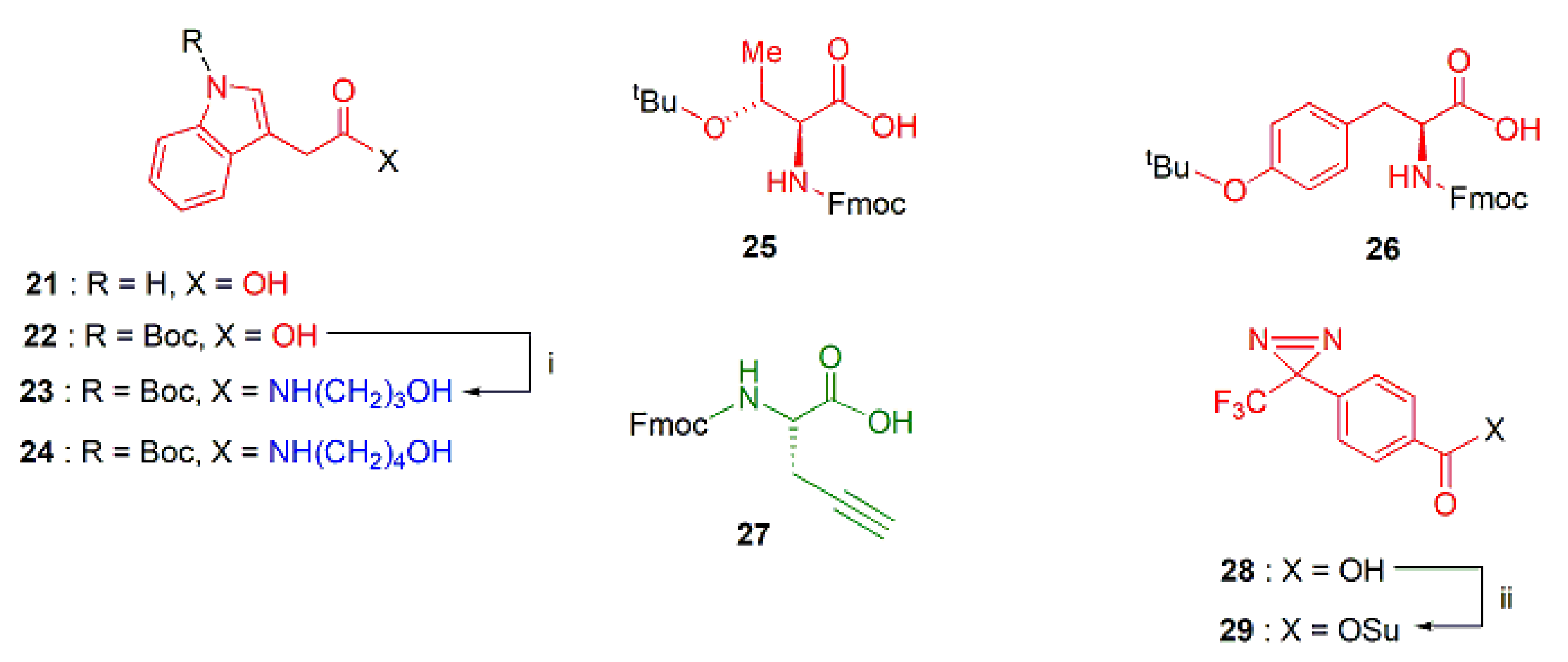
2.1.2. The Lipophilic Head Groups
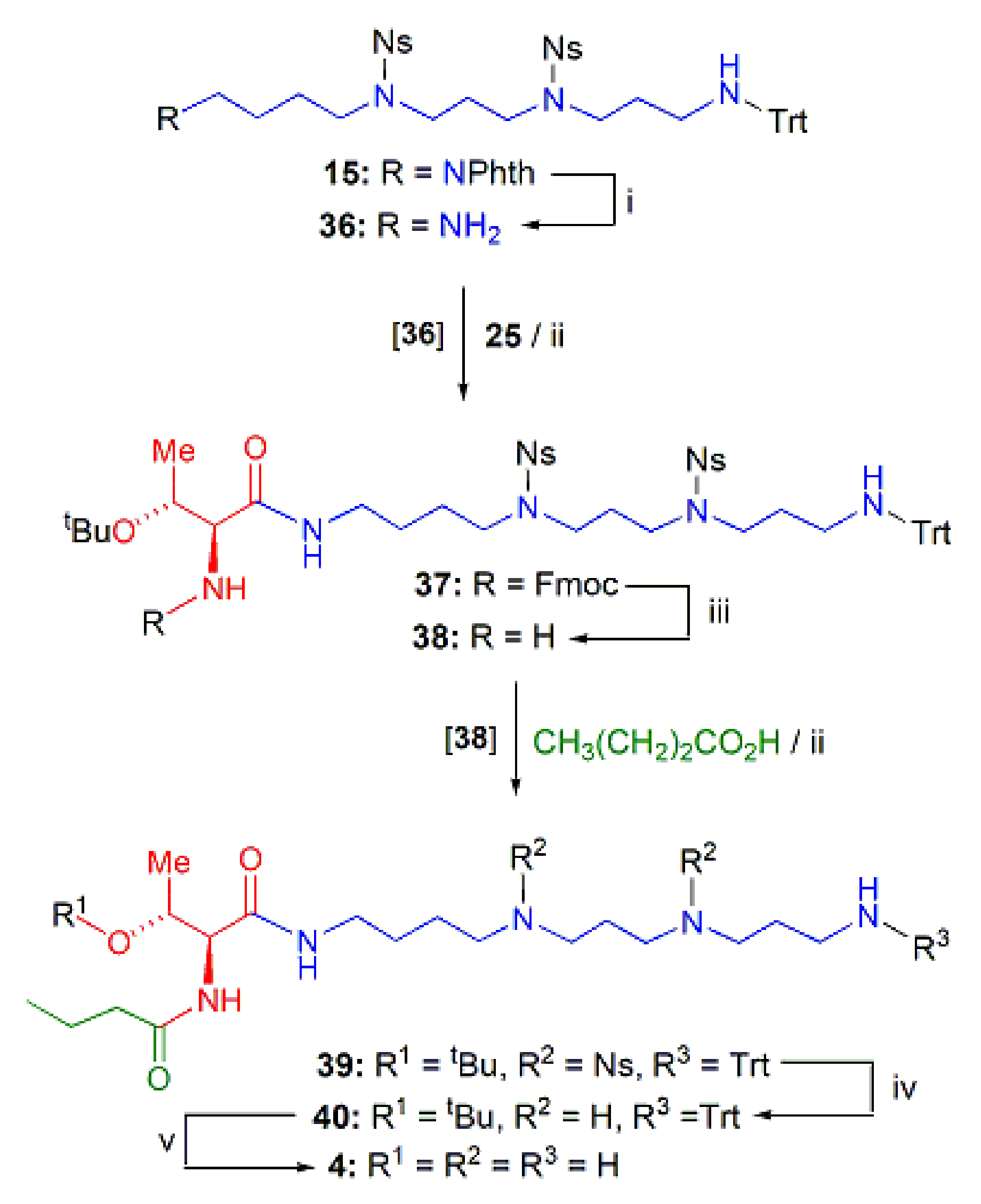
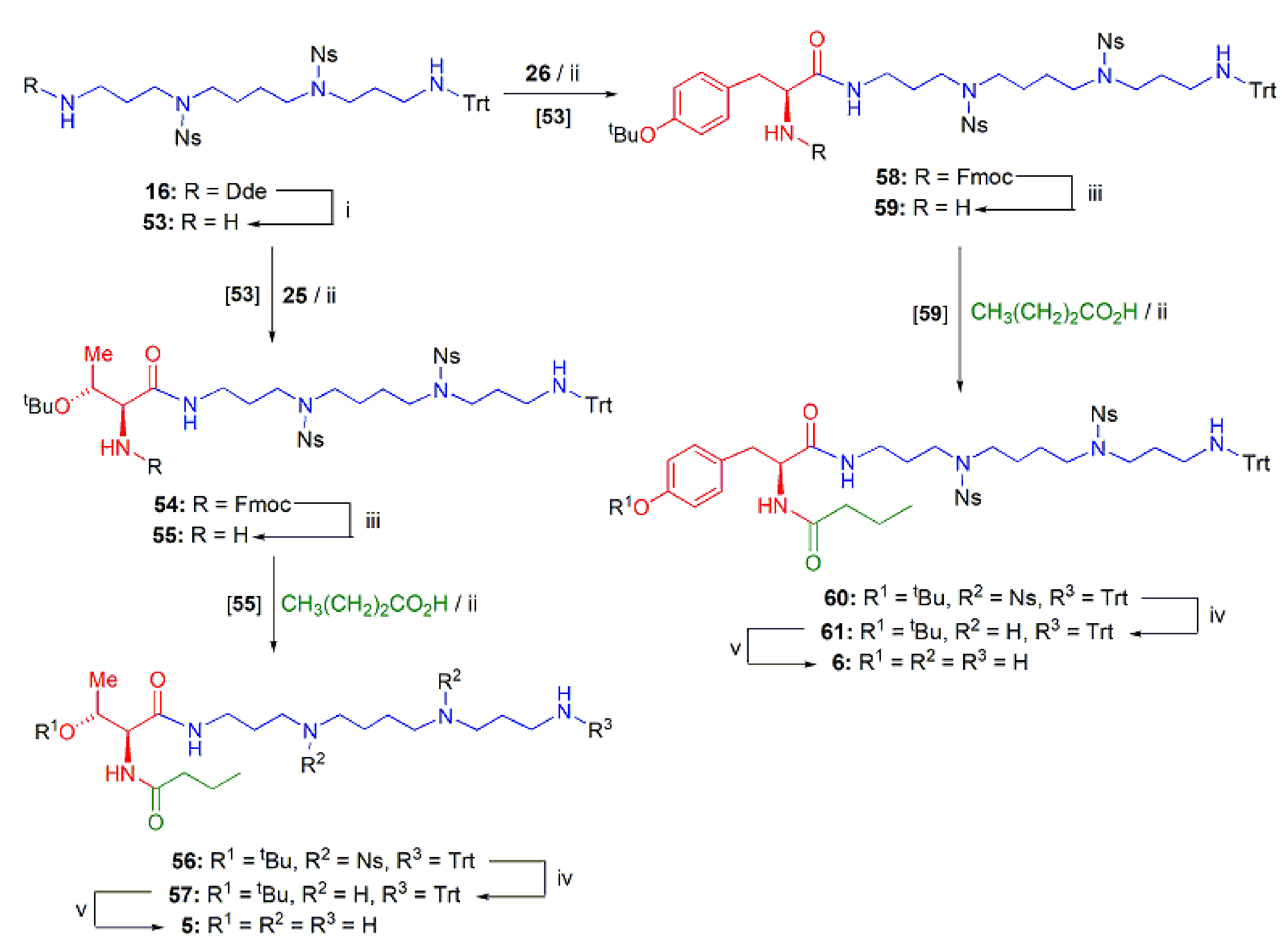
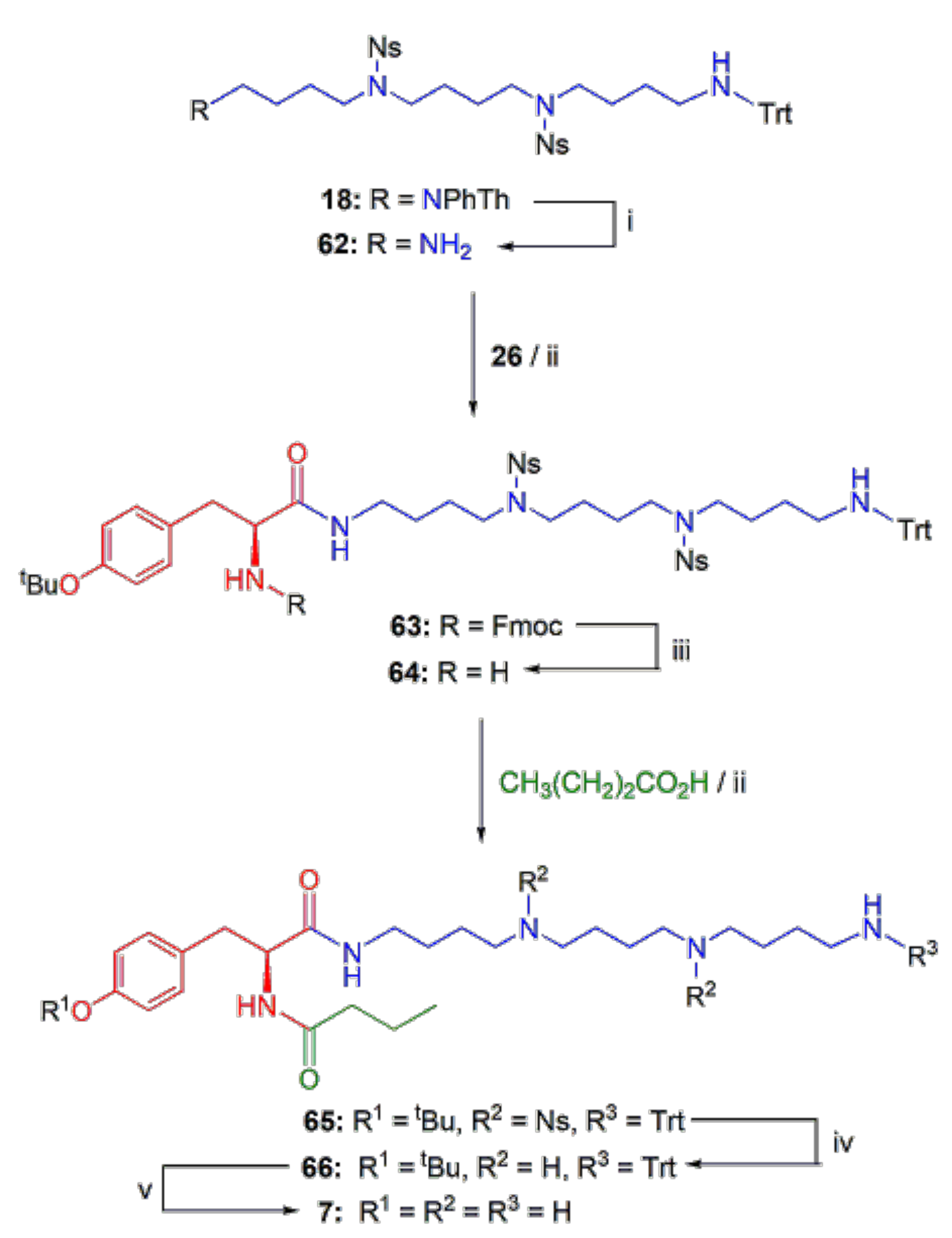
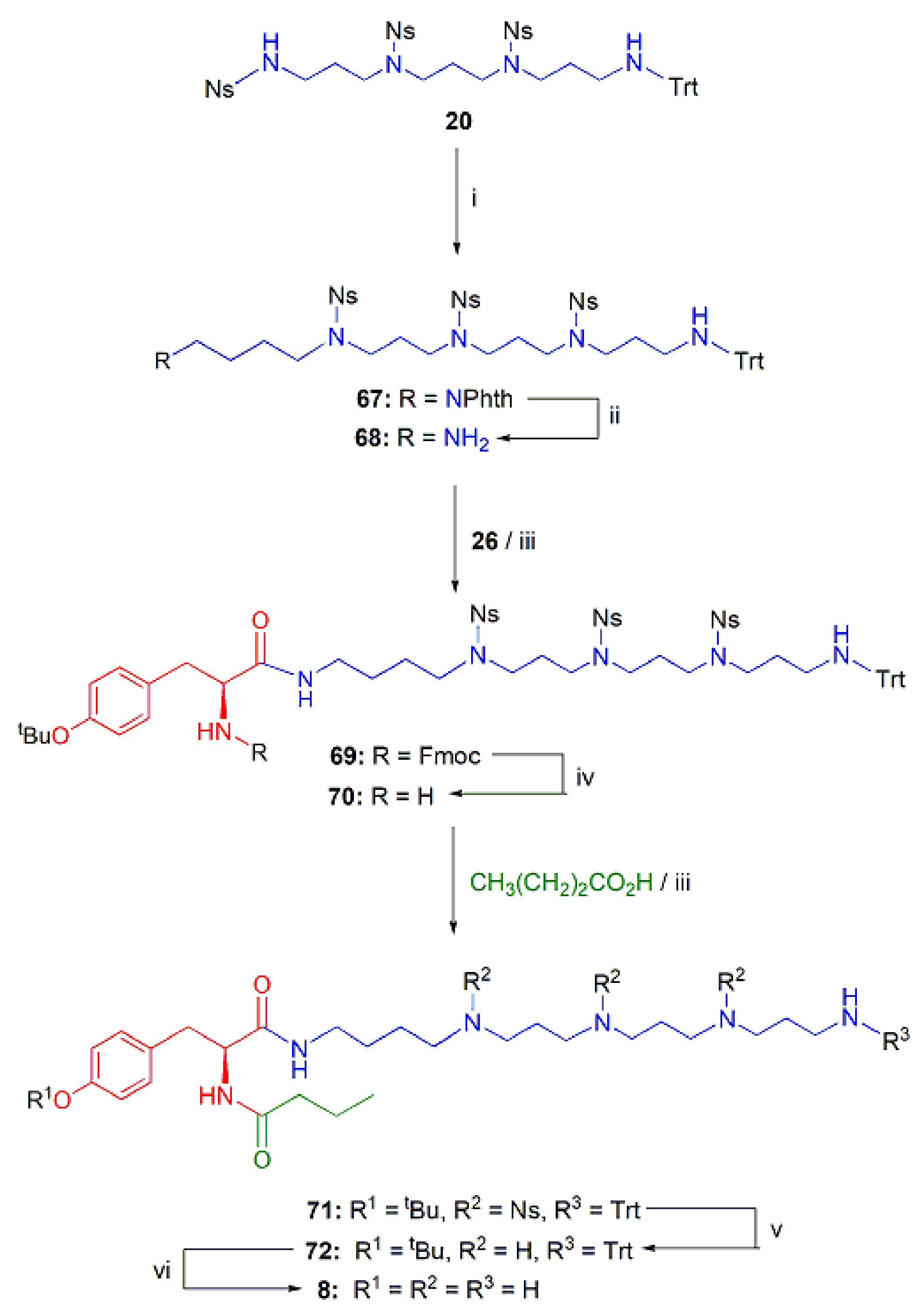
2.1.3. Assembly of the Conjugates
Shorter PA Chain Analogs 1–3 of PATs Agel 416 and HO-416b
PAT Analogs 4 and 9–10 Incorporating the Tsm Chain
PAT Analogs 5 and 6 Incorporating the Spm Chain
Analogs 7 and 8 of PhTX-433 with Longer PA Chains
2.2. Biological Evaluation
2.2.1. Antiproliferative Activity on Breast Cancer Cells
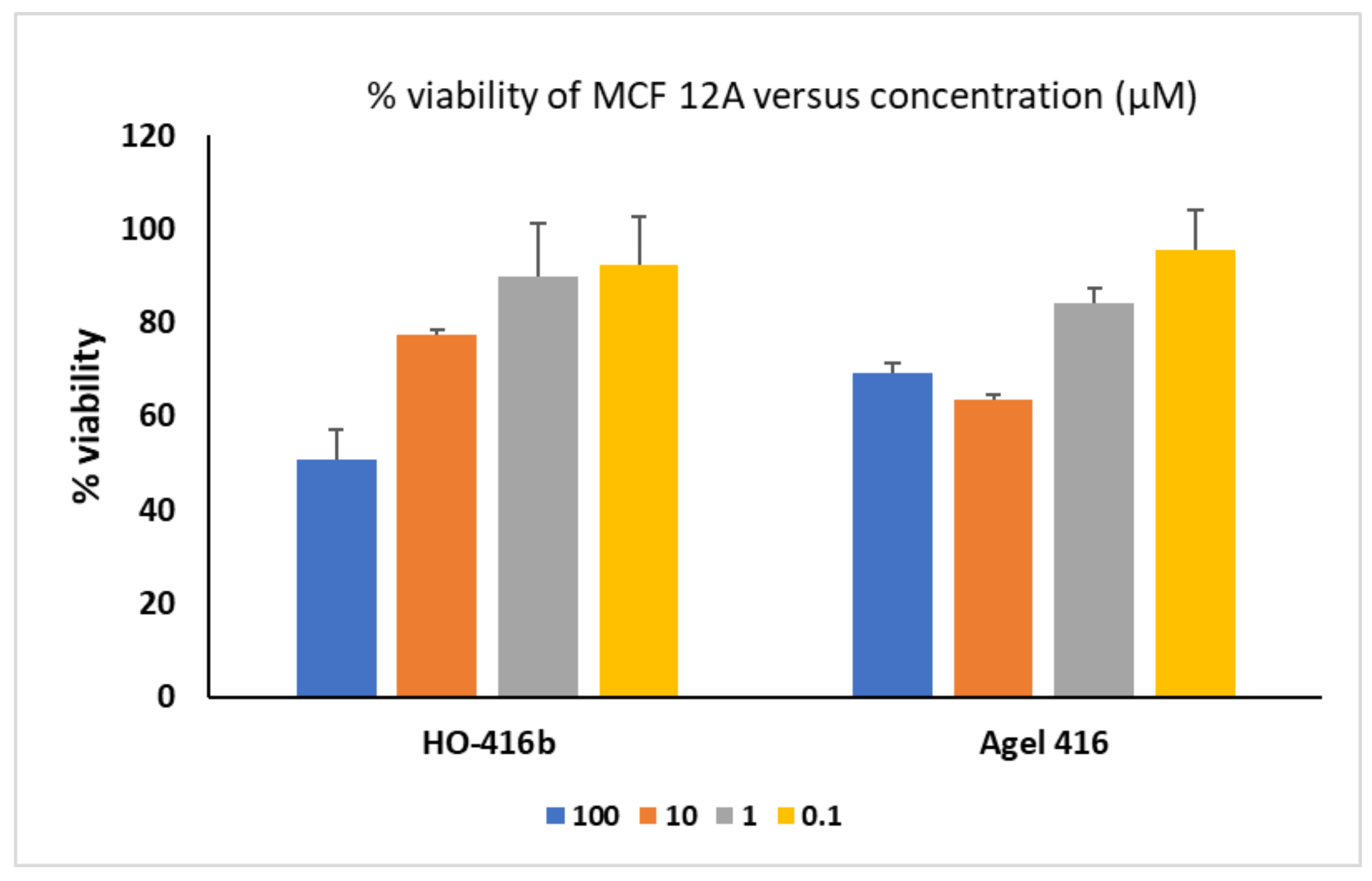
2.2.2. Cytotoxicity
2.2.3. Structure–Activity Relationships
Antiproliferative Activity on the MCF-7 Cells
Antiproliferative Activity on the MDA-MB-231 Cells
Cytotoxicity on MCF-12A Noncancerous Cells
3. Materials and Methods
3.1. General Information
3.2. Biological Evaluation
3.2.1. Cell Cultures and Conditions
Breast Cancer Cells
- Determination of IC50 values
- Cytotoxicity Evaluation in Normal Mammary Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz, S. The Chemistry of Spider Toxins and Spider Silk. Angew. Chem. Int. Ed. 1997, 36, 314–326. [Google Scholar] [CrossRef]
- Karigiannis, G.; Papaioannou, D. Structure, Biological Activity and Synthesis of Polyamine Analogues and Conjugates. Eur. J. Org. Chem. 2000, 2000, 1841–1863. [Google Scholar] [CrossRef]
- Hahn, F.; Schepers, U. Solid Phase Chemistry for the Directed Synthesis of Biologically Active Polyamine Analogs, Derivatives, and Conjugates. In Combinatorial Chemistry on Solid Supports; Bräse, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 278, pp. 135–208. [Google Scholar] [CrossRef]
- Olsen, C.A.; Kristensen, A.S.; Strømgaard, K. Small Molecules from Spiders Used as Chemical Probes. Angew. Chem. Int. Ed. 2011, 50, 11296–11311. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Boyle, G.M.; McIntyre, L.; Nolan, M.J.; Parsons, P.G.; Smith, J.J.; Tribolet, L.; Loukas, A.; Liddell, M.J.; Rash, L.D.; et al. The Aromatic Head Group of Spider Toxin Polyamines Influences Toxicity to Cancer Cells. Toxins 2017, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Kalantzi, S.; Piperigkou, Z.; Athanassopoulos, C.M.; Karamanos, N.K.; Ruonala, R.; Helariutta, Y.; Papaioannou, D. Liquid Phase Fragment Synthesis of Orthogonally Protected Polyamines and Spider Toxins with Potential Anticancer Activity. In Proceedings of the 12th AIMECS (Asian Federation of Medicinal Chemistry International Medicinal Chemistry Symposium), Instabul, Turkey, 8–11 September 2019; p. 141. [Google Scholar]
- Prakash, N.J.; Bowlin, T.L.; Davis, G.F.; Sunkara, P.S.; Sjoerdsma, A. Antitumor activity of norspermidine, a structural homologue of the natural polyamine spermidine. Anticancer Res. 1988, 8, 563–568. [Google Scholar]
- Porter, C.W.; Mc Manis, J.; Casero, R.A.; Bergeron, R.J. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987, 47, 2821–2825. [Google Scholar]
- Seiler, N. Thirty Years of Polyamine-Related Approaches to Cancer Therapy. Retrospect and Prospect. Part 2. Structural Analogues and Derivatives. Curr. Drug Targets 2003, 4, 565–585. [Google Scholar] [CrossRef]
- MacKinnon, A.L.; Taunton, J. Target Identification by Diazirine Photo-Cross-Linking and Click Chemistry. Curr. Protoc. Chem. Biol. 2009, 1, 55–73. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, K.; Choi, S.K.; Hwang, D.; Lerro, K.; Orlando, M.; Kalivretenos, A.G.; Eldefrawi, A.T.; Eldefrawi, M.E.; Usherwood, P.N.R. Bioorganic studies of transmitter receptors with philanthotoxin analogs. Pure Appl. Chem. 1994, 66, 671–678. [Google Scholar] [CrossRef]
- Kuksa, V.; Buchan, R.; Lin, P.K.T. Synthesis of Polyamines, Their Derivatives, Analogues and Conjugates. Synthesis 2000, 2000, 1189–1207. [Google Scholar] [CrossRef]
- Kalantzi, S.; Athanassopoulos, C.M.; Ruonala, R.; Helariutta, Y.; Papaioannou, D. General Approach for the Liquid-Phase Fragment Synthesis of Orthogonally Protected Naturally Occurring Polyamines and Applications Thereof. J. Org. Chem. 2019, 84, 15118–15130. [Google Scholar] [CrossRef]
- Asami, T.; Kagechika, H.; Hashimoto, Y.; Shudo, K.; Miwa, A.; Kawai, N.; Nakajima, T. Acylpolyamines Mimic the Action of Joro Spider Toxin (Jstx) on Crustacean Muscle Glutamate Receptors. Biomed. Res. 1989, 10, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Blagbrough, I.S.; Bruce, M.; Bycroft, B.W.; Mather, A.J.; Usherwood, P.N.R. Invertebrate pharmacological assay of novel, potent glutamate receptor antagonists: Acylated spermines. Pestic. Sci. 1990, 30, 397–403. [Google Scholar] [CrossRef]
- Blagbrough, I.; Brackley, P.T.; Bruce, M.; Bycroft, B.W.; Mather, A.J.; Millington, S.; Sudan, H.L.; Usherwood, P.N. Arthropod toxins as leads for novel insecticides: An assessment of polyamine amides as glutamate antagonists. Toxicon 1992, 30, 303–322. [Google Scholar] [CrossRef]
- Benson, J.; Schürmann, F.; Kaufmann, L.; Gsell, L.; Piek, T. Inhibition of dipteran larval neuromuscular synaptic transmission by analogues of philanthotoxin-4.3.3: A structure-activity study. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1992, 102, 267–272. [Google Scholar] [CrossRef]
- Goodnow, R.; Konno, K.; Niwa, M.; Kallimopoulos, T.; Bukownik, R.; Lenares, D.; Nakanishi, K. Synthesis of glutamate receptor antagonist philanthotoxin-433 (PhTX-433) and its analogs. Tetrahedron 1990, 46, 3267–3286. [Google Scholar] [CrossRef]
- Strømgaard, K.; Brier, T.J.; Andersen, K.; Mellor, I.R.; Saghyan, A.; Tikhonov, D.; Usherwood, P.N.R.; Krogsgaard-Larsen, P.; Jaroszewski, J.W. Solid-Phase Synthesis and Biological Evaluation of a Combinatorial Library of Philanthotoxin Analogues. J. Med. Chem. 2000, 43, 4526–4533. [Google Scholar] [CrossRef]
- Strømgaard, K.; Andersen, K.; Ruhland, T.; Krogsgaard-Larsen, P.; Jaroszewski, J.W. A Versatile Method for Solid-Phase Synthesis of Polyamines: Neuroactive Polyamine Toxins as Example. Synthesis 2001, 2001, 0877–0884. [Google Scholar] [CrossRef]
- Kromann, H.; Krikstolaityte, S.; Andersen, A.J.; Andersen, K.; Krogsgaard-Larsen, P.; Jaroszewski, J.W.; Egebjerg, J.; Strømgaard, K. Solid-Phase Synthesis of Polyamine Toxin Analogues: Potent and Selective Antagonists of Ca2+ Permeable AMPA Receptors. J. Med. Chem. 2002, 45, 5745–5754. [Google Scholar] [CrossRef]
- Tsopelas, F.; Giaginis, C.; Tsantili-Kakoulidou, A. Lipophilicity and biomimetic properties to support drug discovery. Expert Opin. Drug Discov. 2017, 12, 885–896. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Characteristics of cellular polyamine transport in prokaryotes and eukaryotes. Plant Physiol. Biochem. 2010, 48, 506–512. [Google Scholar] [CrossRef]
- Redmond, J.W.; Tseng, A. High-pressure liquid chromatographic determination of putrescine, cadaverine, spermidine and spermine. J. Chromatogr. A 1979, 170, 479–481. [Google Scholar] [CrossRef]




| IC50 (μM) | |||
|---|---|---|---|
| Compound | LogD 1 | MCF-7 | MDA-MB-231 |
| Agel 416 | −7.54 | 0.55 ± 0.02 | 3.31 ± 0.08 |
| HO-416b | −6.25 | 0.09 ± 0.012 | 3.98 ± 0.064 |
| 1 | −6.21 | >200 | >200 |
| 2 | −2.84 | 3.15 ± 0.25 | 12.6 ± 0.57 |
| 3 | −1.49 | 272.4 ± 11.2 | 107.2 ± 10.7 |
| 4 | −6.66 | >400 | >400 |
| 5 | −8.16 | >400 | >400 |
| 6 | −5.97 | >400 | >400 |
| 7 | −6.81 | >400 | >400 |
| 8 | −6.05 | >400 | >400 |
| 9 | −3.87 | 2.63 ± 1.58 | >200 |
| 10 | −5.34 | 2.81 ± 1.64 | >200 |
| 21 | −0.96 | 365.9 ± 73 | 262.2 ± 42 |
| 21+ Spd [21:Spd = 1:1] | 145.6 ± 96 | 282.5 ± 55 | |
| Compound | IC50 (μM) |
|---|---|
| Agel 416 | 184.14 |
| HO-416b | 97.24 |
| Time (min) | % MeCN/H2O |
|---|---|
| 0.00 | 7.0 |
| 3.50 | 95.0 |
| 6.00 | 95.0 |
| 6.50 | 100.0 |
| 7.00 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassileiou, C.; Kalantzi, S.; Vachlioti, E.; Athanassopoulos, C.M.; Koutsakis, C.; Piperigkou, Z.; Karamanos, N.; Stivarou, T.; Lymberi, P.; Avgoustakis, K.; et al. New Analogs of Polyamine Toxins from Spiders and Wasps: Liquid Phase Fragment Synthesis and Evaluation of Antiproliferative Activity. Molecules 2022, 27, 447. https://doi.org/10.3390/molecules27020447
Vassileiou C, Kalantzi S, Vachlioti E, Athanassopoulos CM, Koutsakis C, Piperigkou Z, Karamanos N, Stivarou T, Lymberi P, Avgoustakis K, et al. New Analogs of Polyamine Toxins from Spiders and Wasps: Liquid Phase Fragment Synthesis and Evaluation of Antiproliferative Activity. Molecules. 2022; 27(2):447. https://doi.org/10.3390/molecules27020447
Chicago/Turabian StyleVassileiou, Christos, Stefania Kalantzi, Eleanna Vachlioti, Constantinos M. Athanassopoulos, Christos Koutsakis, Zoi Piperigkou, Nikos Karamanos, Theodora Stivarou, Peggy Lymberi, Konstantinos Avgoustakis, and et al. 2022. "New Analogs of Polyamine Toxins from Spiders and Wasps: Liquid Phase Fragment Synthesis and Evaluation of Antiproliferative Activity" Molecules 27, no. 2: 447. https://doi.org/10.3390/molecules27020447
APA StyleVassileiou, C., Kalantzi, S., Vachlioti, E., Athanassopoulos, C. M., Koutsakis, C., Piperigkou, Z., Karamanos, N., Stivarou, T., Lymberi, P., Avgoustakis, K., & Papaioannou, D. (2022). New Analogs of Polyamine Toxins from Spiders and Wasps: Liquid Phase Fragment Synthesis and Evaluation of Antiproliferative Activity. Molecules, 27(2), 447. https://doi.org/10.3390/molecules27020447










