The Untargeted Phytochemical Profile of Three Meliaceae Species Related to In Vitro Cytotoxicity and Anti-Virulence Activity against MRSA Isolates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Profiling of Meliaceae Extracts Using UHPLC–QTOF–MS
2.2. Multivariate Statistical Discrimination Analysis of Meliaceae Species
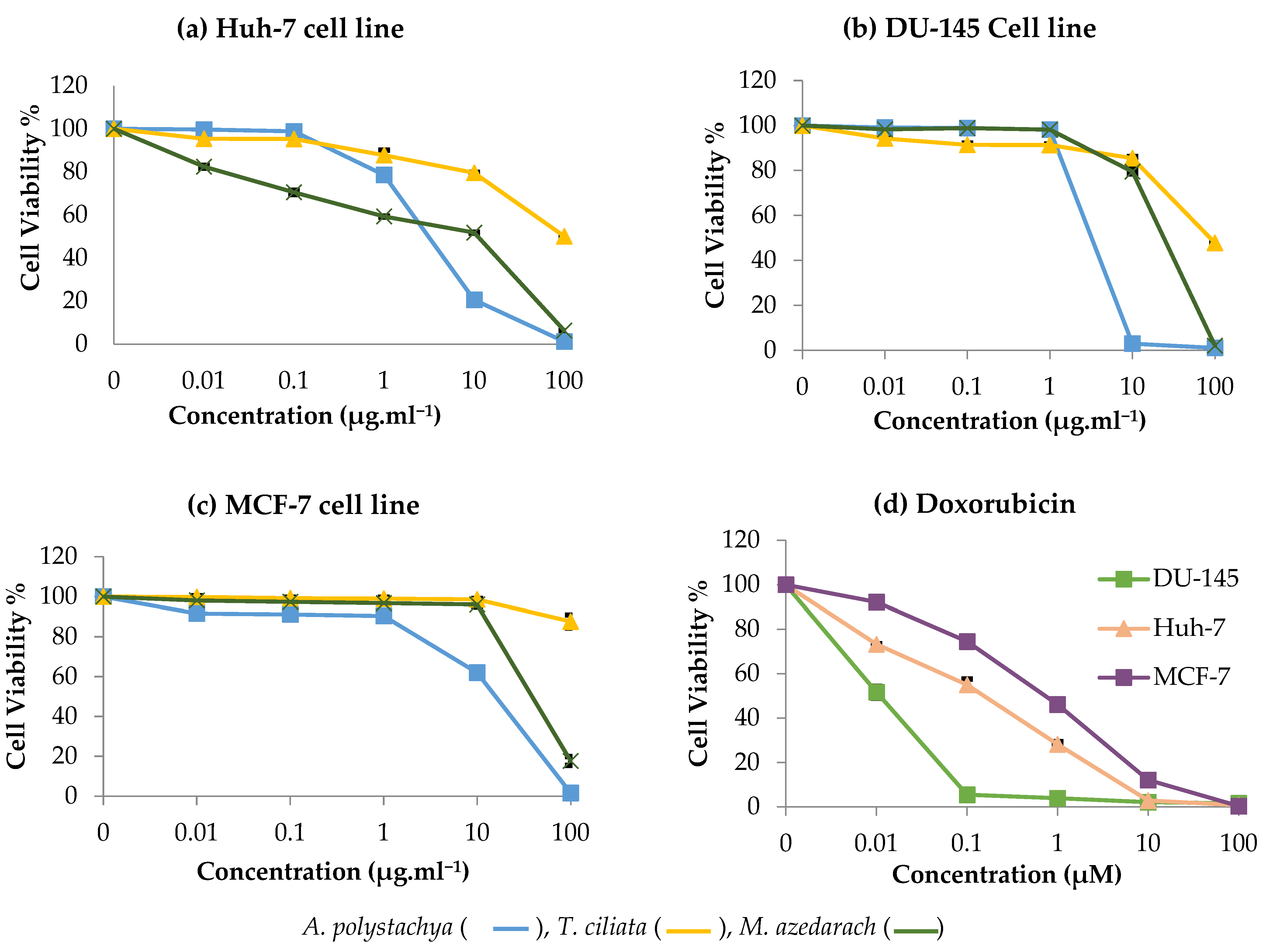
2.3. In Vitro Cytotoxic Activity of Meliaceae Extracts
2.4. Antimicrobial Testing
2.4.1. Determination of the MIC of the Tested Extracts
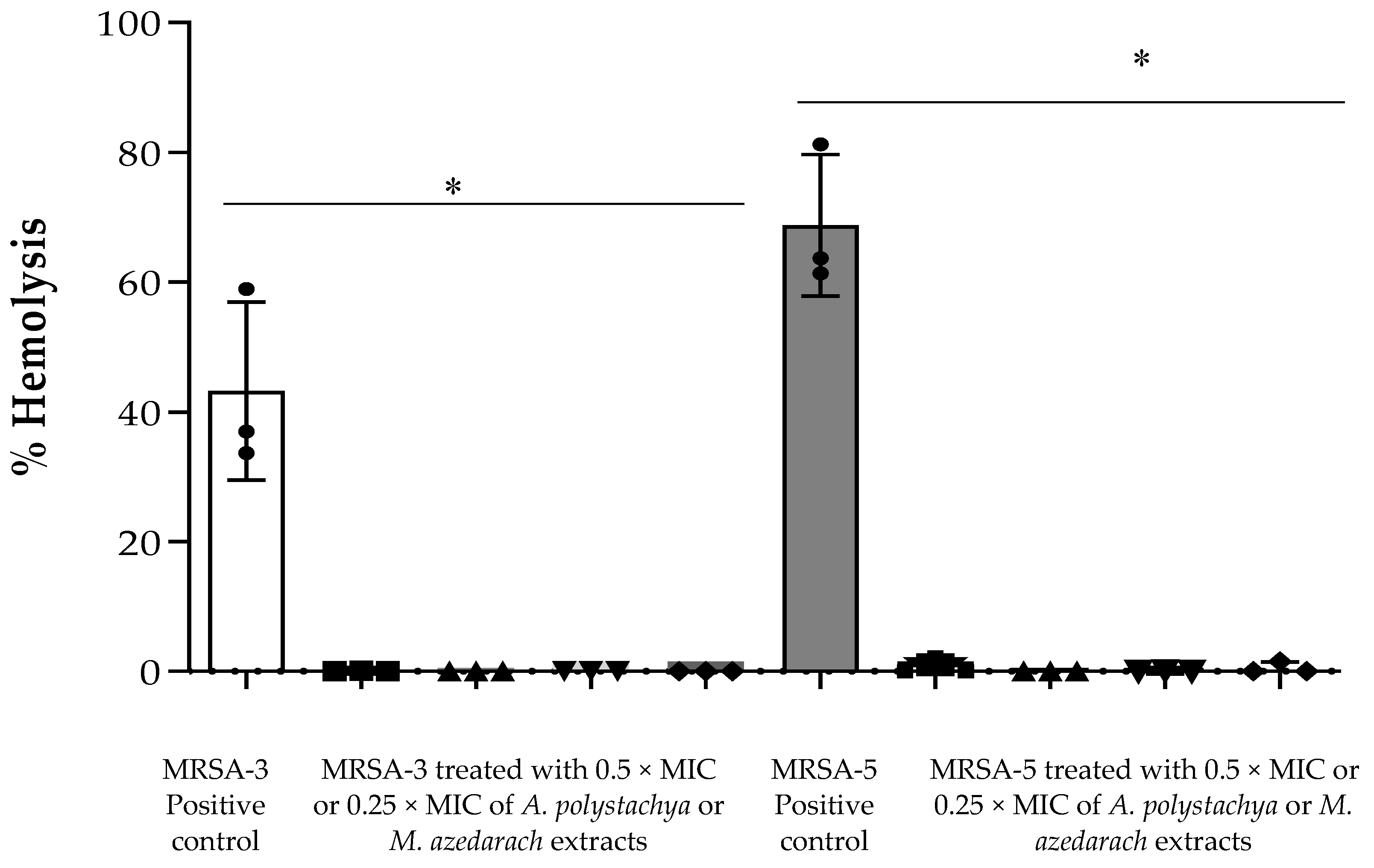
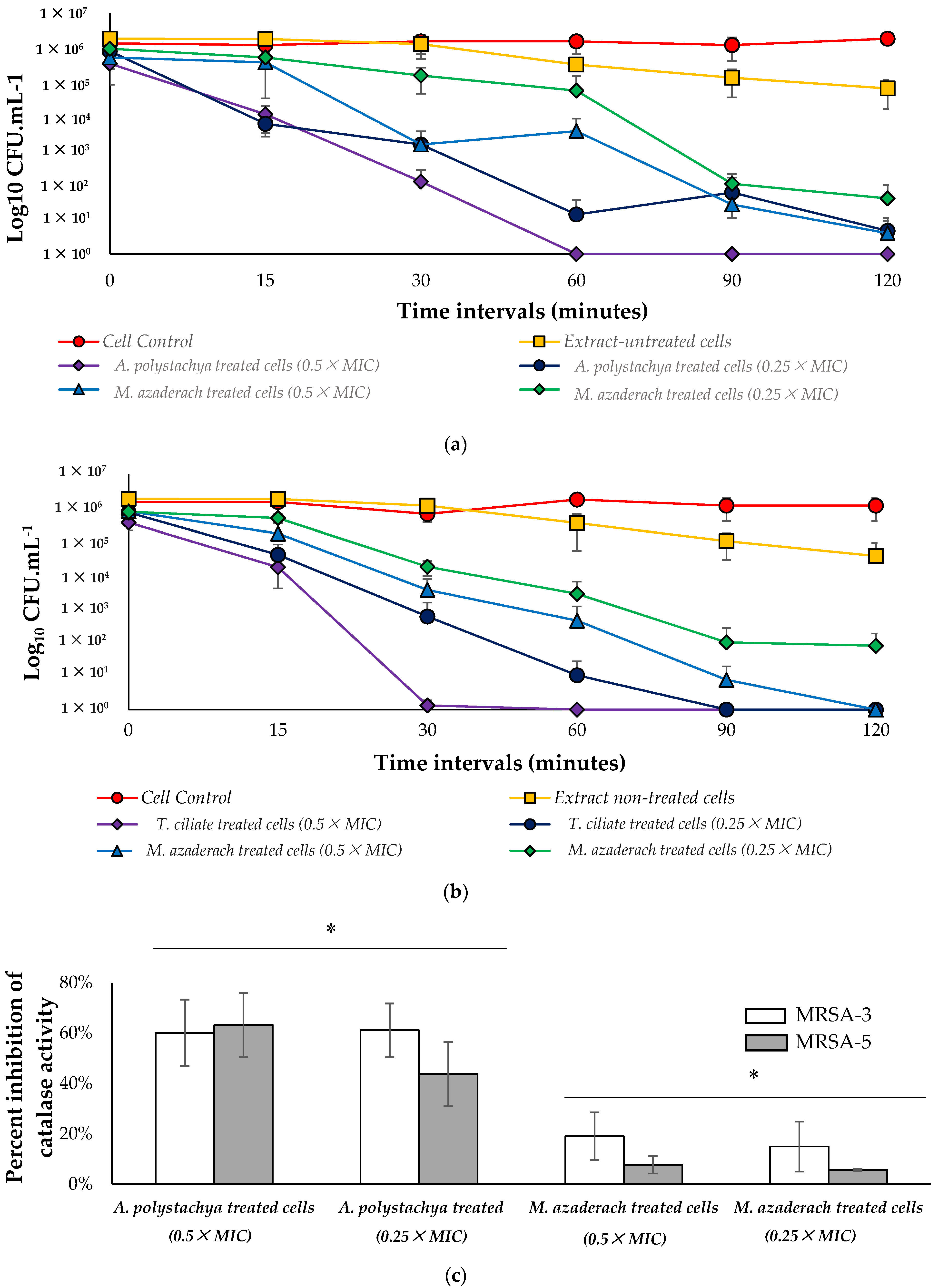
2.4.2. Anti-Virulent Activity of the Tested Extracts
2.4.3. Effect on Catalase Enzyme and Susceptibility to Killing by H2O2
2.5. Pearson’s Correlations between Phytochemical Profile and Biological Activity
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Plant Extracts
3.3. In Vitro Cytotoxic Assay
3.4. Microbiological Testing
3.4.1. Bacterial Isolates and Culture Conditions
3.4.2. Determination of the Minimum Inhibitory Concentration (MIC) of the Plant Extracts by Agar Dilution Technique
3.5. Screening of Biofilm Formation by the Tested Strains
3.5.1. Qualitative Screening Using Congo Red Agar (CRA) Medium
3.5.2. Quantitative Screening Using Flat-Bottom 96-Well Microplates
3.5.3. The Anti-Virulence Activity of the Extracts
3.6. Untargeted Profiling of Different Meliaceae Species by UHPLC-QTOF Mass Spectrometry
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef] [Green Version]
- De Silva, S.F.; Alcorn, J. Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: Chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paritala, V.; Chiruvella, K.K.; Thammineni, C.; Ghanta, R.G.; Mohammed, A. Phytochemicals and antimicrobial potentials of mahogany family. Rev. Bras. Farm. 2015, 25, 61–83. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.-H.; Su, X.-M.; Wang, C.; Du, F.; Liang, Q. The genus Amoora: A phytochemical and pharmacological review. Fitoterapia 2019, 137, 104269. [Google Scholar] [CrossRef] [PubMed]
- Nerome, K.; Ito-Kureha, T.; Paganini, T.; Fukuda, T.; Igarashi, Y.; Ashitomi, H.; Ikematsu, S.; Yamamoto, T. Potent and broad anticancer activities of leaf extracts from Melia azedarach L. of the subtropical Okinawa islands. Am. J. Cancer Res. 2020, 10, 581. [Google Scholar]
- Almubayedh, H.; Ahmad, R. Ethnopharmacology, phytochemistry, biological activities, and therapeutic applications of Cedrela serrata Royle: A mini review. J. Ethnopharmacol 2020, 246, 112206. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S. Melia azedarach L. (Meliaceae). In Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Springer International Publishing: Cham, Switzerland, 2020; pp. 1161–1170. [Google Scholar]
- Meziane, M.; Goumri, H. The antimicrobial effect of extracts of Melia azedarach on some pathogenic microorganisms. Int. J. Appl. Nat. Sci. 2014, 3, 173–180. [Google Scholar]
- Al-Khafaji, N.J.; Al-Zubaedi, R.M.; Al-Azawi, S.J. Evaluation of antibacterial effects of Melia azedarach fruit extracts against some isolated pathogenic bacteria. Vet. Sci. Dev. 2016, 6, 6080. [Google Scholar] [CrossRef]
- Khan, A.S. Trees with Antimicrobial Activities. In Medicinally Important Trees; Springer International Publishing: Cham, Switzerland, 2017; pp. 85–108. [Google Scholar]
- Khalid, M.; Hassani, D.; Bilal, M.; Butt, Z.A.; Hamayun, M.; Ahmad, A.; Huang, D.; Hussain, A. Identification of oral cavity biofilm forming bacteria and determination of their growth inhibition by Acacia arabica, Tamarix aphylla L. and Melia azedarach L. medicinal plants. Arch. Oral Biol. 2017, 81, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frieden, T. Antibiotic Resistance Threats in the United States, 2013; Centers for Disease Control Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2013; Volume 23, pp. 11–28.
- Tacconelli, E.; Magrini, N.; Kahlmeter, G.; Singh, N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; Volume 27, pp. 318–327. Available online: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 1 June 2020).
- Römling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Åkerlund, B. Microbial biofilm formation: A need to act. J. Int. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Thoendel, M.; Kavanaugh, J.S.; Flack, C.E.; Horswill, A.R. Peptide signaling in the Staphylococci. Chem. Rev. 2011, 111, 117–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, D.; Shivapriya, P.; Gautam, P.K.; Misra, K.; Sahoo, A.K.; Samanta, S.K. A review on basic biology of bacterial biofilm infections and their treatments by nanotechnology-based approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 243–259. [Google Scholar] [CrossRef]
- Holá, V.; Růžička, F.; Votava, M. The dynamics of Staphylococcus epidermis biofilm formation in relation to nutrition, temperature, and time. Scr. Med. 2006, 79, 169–174. [Google Scholar]
- WHO. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. In International Agency for Research on Cancer; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Wu, X.-Z.; Fang, F.-H.; Huang, W.-J.; Shi, Y.-Y.; Pan, H.-Q.; Ning, L.; Yuan, C.-S. Two novel nornemoralisin-type diterpenoids from Aphanamixis polystachya (Wall.) R. Parker. Fitoterapia 2020, 140, 104431. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Z.; Wei, S.; Li, Y.; Yin, Y.; Wang, X.; Luo, J.; Kong, L. Diverse limonoids from barks of Toona ciliata var. yunnanensis and their biological activities. Ind. Crop. Prod. 2020, 148, 112275. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.-S.; Gu, Y.-C.; Kong, L.-Y. Cytotoxic and anti-inflammatory triterpenoids from Toona ciliata. J. Nat. Prod. 2012, 75, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Ervina, M.; Poerwono, H.; Widyowati, R.; Matsunami, K. Bio-selective hormonal breast cancer cytotoxic and antioxidant potencies of Melia azedarach L. wild type leaves. Biotechnol. Rep. 2020, 25, e00437. [Google Scholar] [CrossRef]
- Jarvis, A.P.; Morgan, E.D.; Edwards, C. Rapid separation of triterpenoids from Neem seed extracts. Phytochem. Anal. 1999, 10, 39–43. [Google Scholar] [CrossRef]
- Chowdhury, R.; Hasan, C.M.; Rashid, M.A. Guaiane sesquiterpenes from Amoora rohituka. Phytochemistry 2003, 62, 1213–1216. [Google Scholar] [CrossRef]
- Zhu, G.-Y.; Chen, G.; Liu, L.; Bai, L.-P.; Jiang, Z.-H. C-17 lactam-bearing limonoids from the twigs and leaves of Amoora tsangii. J. Nat. Prod. 2014, 77, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Sianturi, J.; Harneti, D.; Darwati; Mayanti, T.; Supratman, U.; Awang, K. A New (–)-5′,6-dimethoxyisolariciresinol-(3″,4″-dimethoxy)-3α-O-β-D-glucopyranoside from the bark of Aglaia eximia (Meliaceae). Nat. Prod. Res. 2016, 30, 2204–2208. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Lin, C.K.; Chuang, Y.S.; Chang, F.R.; Tseng, C.K.; Wu, Y.C.; Lee, J.C. Anti-hepatitis C virus activity of 3-hydroxy caruilignan C from Swietenia macrophylla stems. J. Viral Hepat. 2012, 19, 364–370. [Google Scholar] [CrossRef]
- De Leo, M.; Milella, L.; Braca, A.; De Tommasi, N. Cedrela and Toona genera: A rich source of bioactive limonoids and triterpenoids. Phytochem. Rev. 2018, 17, 751–783. [Google Scholar] [CrossRef]
- Kikuchi, T.; Ishii, K.; Noto, T.; Takahashi, A.; Tabata, K.; Suzuki, T.; Akihisa, T. Cytotoxic and apoptosis-inducing activities of limonoids from the seeds of Azadirachta indica (neem). J. Nat. Prod. 2011, 74, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Supratman, U.; Salam, S.; Naibaho, W.; Fajar, M.; Nurlelasari; Katja, D.G.; Harneti, D.; Maharani, R.; Hidayat, A.T.; Lesmana, R.; et al. New cytotoxic limonoids from the stem bark of Chisocheton pentandrus (Blanco) Merr. Phytochem. Lett. 2020, 35, 63–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.-S.; Wei, D.-D.; Gu, Y.-C.; Wang, X.-B.; Kong, L.-Y. Bioactive terpenoids from the fruits of Aphanamixis grandifolia. J. Nat. Prod. 2013, 76, 1191–1195. [Google Scholar] [CrossRef]
- Chowdhury, R.; Hasan, C.M.; Rashid, M.A. Antimicrobial activity of Toona ciliata and Amoora rohituka. Fitoterapia 2003, 74, 155–158. [Google Scholar] [CrossRef]
- Khan, A.V.; Ahmed, Q.U.; Mir, M.R.; Shukla, I.; Khan, A.A. Antibacterial efficacy of the seed extracts of Melia azedarach against some hospital isolated human pathogenic bacterial strains. Asian Pac. J. Trop. Biomed. 2011, 1, 452–455. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.-F.; Lin, P.-C.; Zi, J.-C.; Fan, X.-N. Limonoids from seeds of Azadirachta indica and their antibacterial activity. Zhongguo Zhongyao Zazhi=China J. Chin. Mater. Med. 2019, 44, 4864–4873. [Google Scholar]
- Rahman, A.S.; Chowdhury, A.A.; Ali, H.-A.; Raihan, S.Z.; Ali, M.S.; Nahar, L.; Sarker, S.D. Antibacterial activity of two limonoids from Swietenia mahagoni against multiple-drug-resistant (MDR) bacterial strains. J. Nat. Med. 2009, 63, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Acharyya, S.; Banerjee, A.; Patra, A.; Thankamani, K.; Koley, H.; Bag, P.K. Intracellular, biofilm-inhibitory and membrane-damaging activities of nimbolide isolated from Azadirachta indica A. Juss (Meliaceae) against meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2016, 65, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Shettigar, K.; Murali, T.S. Virulence factors and clonal diversity of Staphylococcus aureus in colonization and wound infection with emphasis on diabetic foot infection. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Fleitas Martínez, O.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front. Cell. Infect. Microbiol. 2019, 9, 74. [Google Scholar] [CrossRef]
- Mohamed, J.A.; Huang, D.B. Biofilm formation by Enterococci. J. Med. Microbiol. 2007, 56, 1581–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, M.M.; Hassan, M.; Moawad, S.S.; Okba, M.M.; Ashour, R.M.; Fayek, N.M.; Saber, F.R. Exploring the Antivirulence Activity of Pulverulentone A, a Phloroglucinol-Derivative from Callistemon citrinus Leaf Extract, against Multi-Drug Resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 907. [Google Scholar] [CrossRef]
- Franco, C.M.; Vázquez, B.I. Natural Compounds as Antimicrobial Agents. Antibiotics 2020, 9, 217. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Fut. Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quelemes, P.V.; Perfeito, M.L.; Guimarães, M.A.; dos Santos, R.C.; Lima, D.F.; Nascimento, C.; Silva, M.P.; Soares, M.J.d.S.; Ropke, C.D.; Eaton, P. Effect of neem (Azadirachta indica A. Juss) leaf extract on resistant Staphylococcus aureus biofilm formation and Schistosoma mansoni worms. J. Ethnopharmacol. 2015, 175, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anju, V.; Busi, S.; Ranganathan, S.; Ampasala, D.R.; Kumar, S.; Suchiang, K.; Kumavath, R.; Dyavaiah, M. Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors. Microb. Pathog. 2021, 155, 104912. [Google Scholar] [CrossRef] [PubMed]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Ping, O.; Ruixue, Y.; Jiaqiang, D.; Kaiyu, W.; Jing, F.; Yi, G.; Xiaoli, H.; Defang, C.; Weimin, L.; Li, T. Subinhibitory concentrations of prim-O-glucosylcimifugin decrease the expression of alpha-hemolysin in Staphylococcus aureus (USA300). Evid. Based Complement. Altern. Med. 2018, 2018, 7579808. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhang, P.; Lv, H.; Deng, X.; Wang, J. A natural dietary flavone myricetin as an α-hemolysin inhibitor for controlling Staphylococcus aureus infection. Front. Cell. Infect. Microbiol. 2020, 10, 330. [Google Scholar] [CrossRef]
- Upadhyay, A.; Mooyottu, S.; Yin, H.; Nair, M.S.; Bhattaram, V.; Venkitanarayanan, K. Inhibiting microbial toxins using plant-derived compounds and plant extracts. Medicines 2015, 2, 186–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Liu, C.-I.; Lin, F.-Y.; No, J.H.; Hensler, M.; Liu, Y.-L.; Jeng, W.-Y.; Low, J.; Liu, G.Y.; Nizet, V. Inhibition of staphyloxanthin virulence factor biosynthesis in Staphylococcus aureus: In vitro, in vivo, and crystallographic results. J. Med. Chem. 2009, 52, 3869–3880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leejae, S.; Hasap, L.; Voravuthikunchai, S.P. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J. Med. Microbiol. 2013, 62, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Park, C.; Jang, H.J.; Kim, B.O.; Bae, H.W.; Chung, I.Y.; Kim, E.S.; Cho, Y.H. Antibacterial strategies inspired by the oxidative stress and response networks. J. Microbiol. 2019, 57, 203–212. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, J.-H.; Cho, M.H.; Lee, J. Flavone reduces the production of virulence factors, staphyloxanthin and α-hemolysin, in Staphylococcus aureus. Curr. Microbiol. 2012, 65, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Shouman, S.A.; Elkhoely, A.; Attia, Y.M.; Elsesy, M.S.; El Senousy, A.S.; Choucry, M.A.; El Gayed, S.H.; El Sayed, A.A.; Sattar, E.A. Anticancer potentiality of lignan rich fraction of six Flaxseed cultivars. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, A.; Satpathy, S.; Hussain, M.D. Nanodelivery and anticancer effect of a limonoid, nimbolide, in breast and pancreatic cancer cells. Int. J. Nanomed. 2019, 14, 8095. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.L.; George, S.; Ahmad, I.; Gosangari, S.L.; Abbasi, A.; Cunningham, B.T.; Watkin, K.L. Cytotoxicity Effects of Amoora rohituka and chittagonga on Breast and Pancreatic Cancer Cells. Evid-Based Complement. Altern. Med. 2011, 2011, 860605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabi, T.; Ramachandran, C.; Fonseca, H.B.; Nair, R.P.; Alamo, A.; Melnick, S.J.; Escalon, E. Novel drug amooranin induces apoptosis through caspase activity in human breast carcinoma cell lines. Breast Cancer Res. Treat. 2003, 80, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Ranjan, A.; Srivastava, A.K.; Singh, M.; Shukla, A.K.; Atri, N.; Mishra, A.; Singh, A.K.; Singh, S.K. Cytotoxic and apoptotic inducing activity of Amoora rohituka leaf extracts in human breast cancer cells. J. Ayurveda Integr. Med. 2020, 11, 383–390. [Google Scholar] [CrossRef]
- Yuan, C.-M.; Zhang, Y.; Tang, G.-H.; Di, Y.-T.; Cao, M.-M.; Wang, X.-Y.; Zuo, G.-Y.; Li, S.-L.; Hua, H.-M.; He, H.-P. Khayseneganins A–H, limonoids from Khaya senegalensis. J. Nat. Prod. 2013, 76, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Horta, B.; Calhau, C.; Morais, R.M.; Pintado, M.M. Anti-biofilm potential of phenolic acids: The influence of environmental pH and intrinsic physico-chemical properties. Biofouling 2016, 32, 853–860. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.S.; Bais, H.P.; Déziel, E.; Schweizer, H.P.; Rahme, L.G.; Fall, R.; Vivanco, J.M. Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant. Physiol. 2004, 134, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, F.M.; Ahmad, I.; Asif, M.; Tahseen, Q. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 2013, 38, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Nour El-Din, H.T.; Yassin, A.S.; Ragab, Y.M.; Hashem, A. Phenotype-Genotype Characterization and Antibiotic-Resistance Correlations Among Colonizing and Infectious Methicillin-Resistant Staphylococcus aureus Recovered from Intensive Care Units. Infect. Drug Resist. 2021, 14, 1557. [Google Scholar] [CrossRef] [PubMed]
- Pavón-Pérez, J.; Oviedo, C.A.; Elso-Freudenberg, M.; Henríquez-Aedo, K.; Aranda, M. LC-MS/MS Method For L-Dopa Quantification In Different Tissues of Vicia faba. J. Chil. Chem. Soc. 2019, 64, 4651–4653. [Google Scholar] [CrossRef]
- Kannappan, A.; Sivaranjani, M.; Srinivasan, R.; Rathna, J.; Pandian, S.K.; Ravi, A.V. Inhibitory efficacy of geraniol on biofilm formation and development of adaptive resistance in Staphylococcus epidermidis RP62A. J. Med. Microbiol. 2017, 66, 1506–1515. [Google Scholar] [CrossRef]
- Sultan, A.; Nabiel, Y. Tube method and Congo red agar versus tissue culture plate method for detection of biofilm production by uropathogens isolated from midstream urine: Which one could be better? Afr. J. Clin. Exp. Microbiol. 2019, 20, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Naves, P.; Del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Rodríguez-Cerrato, V.; Ponte, M.; Soriano, F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J. Appl. Microbiol. 2008, 105, 585–590. [Google Scholar] [CrossRef]
- Iwase, T.; Tajima, A.; Sugimoto, S.; Okuda, K.-I.; Hironaka, I.; Kamata, Y.; Takada, K.; Mizunoe, Y. A simple assay for measuring catalase activity: A visual approach. Sci. Rep. 2013, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Rocchetti, G.; Zengin, G.; Ak, G.; Yıldıztugay, E.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Lucini, L. Profiling of polyphenols and sesquiterpenoids using different extraction methods in Muscari turcicum, an endemic plant from Turkey. Ind. Crop. Prod. 2020, 154, 112626. [Google Scholar] [CrossRef]
- Zhang, L.; Rocchetti, G.; Zengin, G.; Ak, G.; Saber, F.R.; Montesano, D.; Lucini, L. The UHPLC-QTOF-MS Phenolic Profiling and Activity of Cydonia oblonga Mill. Reveals a Promising Nutraceutical Potential. Foods 2021, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. GigaScience 2013, 2, 2047–217X. [Google Scholar] [CrossRef] [PubMed]


| Tested Extract | Anthocyanins | Other Flavonoids | Lignans | LMW Phenolics | Phenolic Acids | Stilbenes | Limonoids |
|---|---|---|---|---|---|---|---|
| - Toona ciliata | 104.16 ± 2.63 c | 12.65 ± 0.65 c | 33.91 ± 1.44 a | 74.85 ± 1.53 b | 7.41 ± 0.85 a | 4.59 ± 2.7 a | 135.93 ± 13.07 a |
| - Melia azedarach | 36.35 ± 5.45 b | 3.33 ± 0.29 a | 59.25 ± 0.32 b | 31.74 ± 2.81 a | 16.68 ± 0.5 b | 3.15 ± 0.25 a | 420.63 ± 10.8 b |
| - Aphanamixis polystachya | 19.19 ± 1.35 a | 7.35 ± 0.59 b | 188.56 ± 8.1 c | 248.05 ± 8.32 c | 6.61 ± 0.56 a | 15.1 ± 0.1 b | 433.17 ± 12.63 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ismail, M.M.; Rocchetti, G.; Fayek, N.M.; Lucini, L.; Saber, F.R. The Untargeted Phytochemical Profile of Three Meliaceae Species Related to In Vitro Cytotoxicity and Anti-Virulence Activity against MRSA Isolates. Molecules 2022, 27, 435. https://doi.org/10.3390/molecules27020435
Zhang L, Ismail MM, Rocchetti G, Fayek NM, Lucini L, Saber FR. The Untargeted Phytochemical Profile of Three Meliaceae Species Related to In Vitro Cytotoxicity and Anti-Virulence Activity against MRSA Isolates. Molecules. 2022; 27(2):435. https://doi.org/10.3390/molecules27020435
Chicago/Turabian StyleZhang, Leilei, Maha M. Ismail, Gabriele Rocchetti, Nesrin M. Fayek, Luigi Lucini, and Fatema R. Saber. 2022. "The Untargeted Phytochemical Profile of Three Meliaceae Species Related to In Vitro Cytotoxicity and Anti-Virulence Activity against MRSA Isolates" Molecules 27, no. 2: 435. https://doi.org/10.3390/molecules27020435
APA StyleZhang, L., Ismail, M. M., Rocchetti, G., Fayek, N. M., Lucini, L., & Saber, F. R. (2022). The Untargeted Phytochemical Profile of Three Meliaceae Species Related to In Vitro Cytotoxicity and Anti-Virulence Activity against MRSA Isolates. Molecules, 27(2), 435. https://doi.org/10.3390/molecules27020435










